Abstract
So far, it was unclear if social hierarchy could influence sensory or perceptual cognitive processes. We evaluated the effects of social hierarchy on these processes using a basic visual perceptual decision task. We constructed a social hierarchy where participants performed the perceptual task separately with two covertly simulated players (superior, inferior). Participants were faster (better) when performing the discrimination task with the superior player. We studied the time course when social hierarchy was processed using event-related potentials and observed hierarchical effects even in early stages of sensory-perceptual processing, suggesting early top–down modulation by social hierarchy. Moreover, in a parallel analysis, we fitted a drift-diffusion model (DDM) to the results to evaluate the decision making process of this perceptual task in the context of a social hierarchy. Consistently, the DDM pointed to nondecision time (probably perceptual encoding) as the principal period influenced by social hierarchy.
Keywords: perceptual process, decision-making, social hierarchy
INTRODUCTION
The study of social interactions has provided evidence supporting the notion that comparison with others, especially upward comparison, favors self-improvement but also self-knowledge (Festinger and Hutte, 1954). Comparisons with other members of a social hierarchy are also crucial in regulating the social behavior of a group (Cummins, 2000). Less is known about how position in a social hierarchy affects different aspects of an individual’s cognitive functioning. In particular, how perceptual processing is affected by the relative position in a social hierarchy remains an open question.
Social hierarchy is important to stabilize social networks (Ridgeway, 2006), and to maintain and regulate the social welfare and health of individuals within a group (Boyce, 2004; Sapolsky, 2004). Humans are spontaneously familiarized with hierarchical patterns as young as 2 years of age (Boyce, 2004). These hierarchical patterns can be inferred automatically and early even in implicit cues such as gender, age or facial features (Chiao, 2010; Rojas et al., 2011). The ability to recognize these patterns is crucial for driving social behavior (Cummins, 2000). Different studies have attested to the impact of an individual’s social rank on some cognitive processes. On the one hand, humans gazed at high-status individuals much more often, and for longer periods than at low-status individuals (Foulsham et al., 2010). Furthermore, a handful of studies have provided evidence that the position an individual holds in a hierarchy influences some aspects of cognition mostly related to executive functions (Overbeck and Park, 2006; Smith and Trope, 2006; Guinote, 2007; Smith et al., 2008)
A recent study (Zink et al., 2008) aimed at determining the neural substrate involved in processing social hierarchies. To this end, the authors measured changes in the blood-oxygen-level-dependent (BOLD) signal when participants were viewing pictures of other (simulated) players with different hierarchical rank, represented by different numbers of stars and photographs. Brain activation was significantly higher in a network including the occipital and parietal cortices, ventral striatum and parahippocampal cortex when participants saw the superior player’s photograph. Activity in the occipital and parietal cortices, and ventral striatum is respectively associated with greater perceptual and attentional processing (Bradley et al., 2003) and salience (Zink et al., 2006). However, the authors concluded that differences in the brain activation of visual areas were merely reflecting differences in the number of stars of each picture, denoting visual hierarchy.
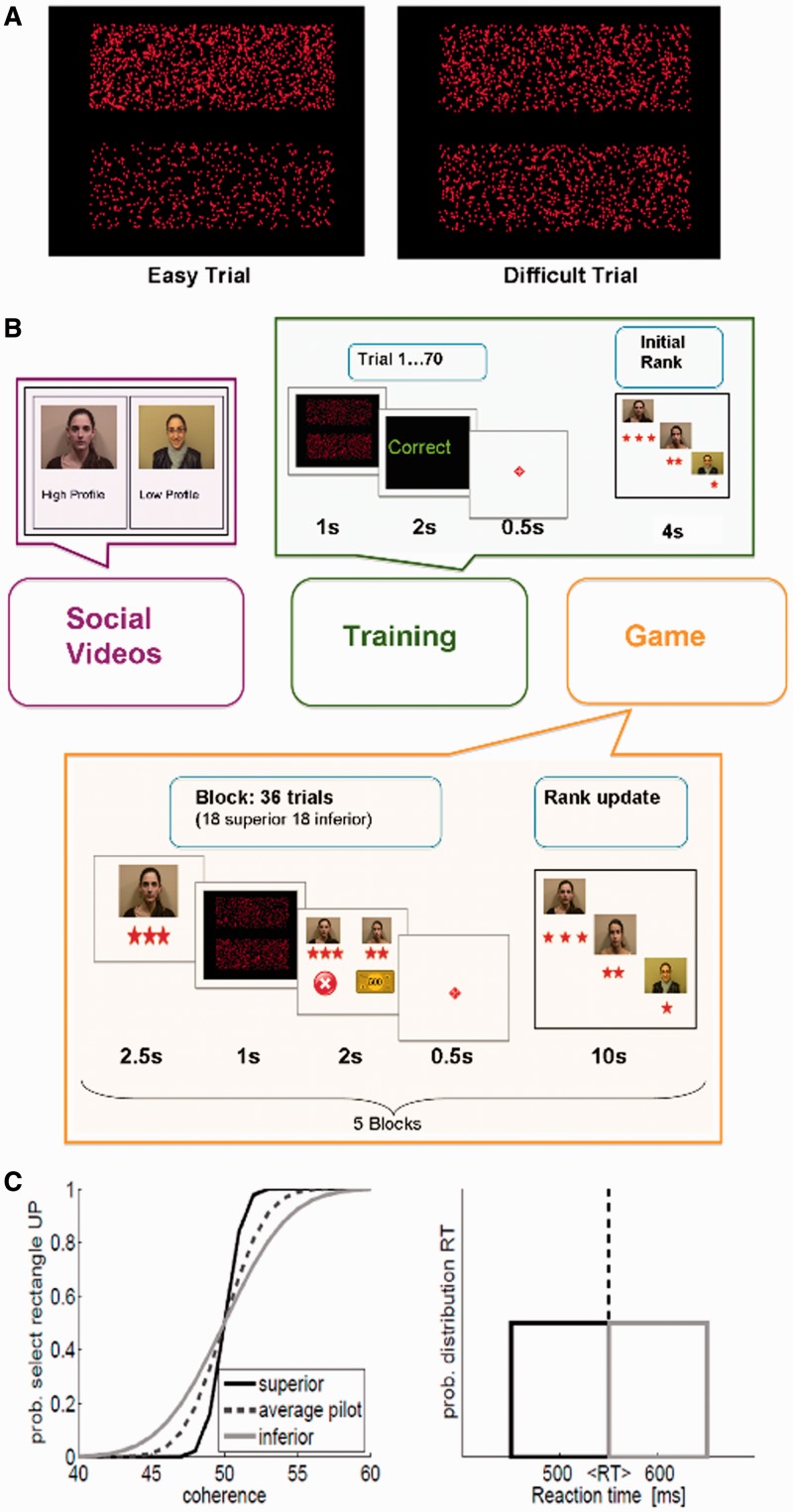
In the present research, we aim to answer two specific research questions. First, can social hierarchy influence performance on a perceptual decision task? Second, what cognitive processes are affected by this hierarchical influence and when does this influence take place? We designed an experimental game (Figure 1), following a similar procedure described in a recent study (Zink et al., 2008). Participants compared their performance on a difficult visual discrimination task with that of two other simulated players (of the same gender as the participant), one with a fixed superior rank (superior player) and the other with a fixed inferior rank (inferior player) with respect to the participant. In every trial, participants were informed of the superior and inferior players’ performance, supposedly from a previous game (unbeknown to participants, they were simulated players). The simulated players’ performance was dynamically adapted to the actual performance of each participant (see ‘Materials and Methods’ section for further description). The nature of the game was noncompetitive because the participant and the simulated players could win, lose or have different outcomes in the same trial. The whole experiment was designed to increase participants’ feeling of being involved in a realistic game to increase their motivation.
Fig. 1.
Hierarchy game structure. (A) Visual discrimination task. Difficult trials: 48–52% of upper rectangle dots. (B) Procedure was subdivided into three phases: social videos (purple), training session (green) and game session (yellow). There were two social videos, one for the superior and one for the inferior player. The training session: 70 trials of a visual discrimination task without comparison with hierarchy members. The game session started right after training and consisted of five blocks. Before the first block, we presented an artificial preliminary ranking to each participant with all participants in second position. Each block (5 min) constituted 36 trials and it was followed by an update on ranking. The 5.5-s trials were subdivided into two subgroups of 18 trials presented in random order; participants played with only one of the players, superior or inferior, in each subgroup. Only one block of the game is represented. (C) Opponent behavior simulation. Left panel: psychophysical curves used to generate the outcome of the superior (black) and inferior (gray) players. The dashed line is the psychophysical curve generated from the pilot data set. Right panel: adjusted RTs and probable distributions for the superior (black) and inferior (gray) players. Vertical dashed lines represent the average participant RT.
As said, we were interested in the specific time course in which social hierarchy influences the perceptual visual task. We approached this question through two different analyses. On one hand, we measured event-related potentials (ERP) responses during the visual discrimination task. The high temporal resolution of ERP measures would allow us to determine if hierarchical influence in this kind of task could be observed in early components (related to sensory or perceptual processes) or in late components (probably related to decision-making and executive or motor processes). Following previous proposals, we assumed that modulations before 200 ms reflect either sensory processes (0–100 ms) (Di Russo et al., 2002, 2003) or perceptual processes (100–250 ms, N1 component) (Vogel and Luck, 2000; Kok, 2001). Modulations in ERP responses of later components as P300 (Polich and Criado, 2006) or Lateralized Readiness Potentials (Rangelov and Muller, 2012) should therefore reflect effects related to decision or postdecision processes. On the other hand, in a parallel analysis, we fitted the phenomenological drift-diffusion model (DDM) to participants’ behavioral data (Ratcliff, 1978). The DDM distinguishes between decision and nondecision processes (Ratcliff, 1978; Ratcliff and McKoon, 2008). Nondecision processes include predecision (i.e. sensory or perceptual processes) and postdecision (i.e. executive or motor processes). Modeling of participants’ accuracy curves and reaction times (RTs) with the DDM allows discrimination between whether social hierarchy influences mainly decision or nondecision processes by analyzing which of the parameters fit better (Ratcliff, 1978; Ratcliff and McKoon, 2008).
Both analyses (i.e. ERP modulations and fitting the DDM to behavioral data) consistently showed that the effect of social hierarchy should mainly be attributed to perceptual nondecision processes.
MATERIALS AND METHODS
Participants
Fifty-six right-handed Pompeu Fabra University students (28 females) participated in this experiment (mean age = 23.39; age range = 18–27 years). Participants were invited through an open call, voluntarily participated and received 10 Euros per hour. All participants reported normal visual accuracy and none reported psychiatric or neurological conditions. Participants gave their informed consent prior to inclusion in the study, which conforms to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Visual stimuli
On a black background, we presented two rectangles of red dots, one at the top of the screen and the other at the bottom. All dots had the same diameter, shape and brightness. Each rectangle had a different percentage of red dots with over 1000 dots in total. The percentage of red dots was complementary between the rectangles (e.g. if one had 49% of the dots, the other had 51%; Figure 1A). In every trial, we displayed screenshots of each rectangle with nine levels of dot percentages (44, 46, 48, 49–51, 52, 54, 56) for 1 s. Participants were situated ∼50 cm in front of a 19-inch screen with an angle of vision of ∼35°.
Social videos
Eight different videos (∼2 min each) depicting competitors’ profiles were created. The profiles crossed gender (male, female) and hierarchy (high, low status), and were interpreted by four confederates (two male, two female). Each confederate followed two scripts in which personal, work and academic achievements of the characters were presented. Implicit cues related to social superiority of the confederates were controlled to avoid substantial differences of age, facial expressions and attire.
Procedure
To control for possible interactions between gender and hierarchy, male participants played with male simulated participants, and female participants with females. Participants were first informed they would play a game based on a visual discrimination task, then notified that their performance would be compared with that of two players who already completed the task and that the three players would be ranked according to performance during the game. Finally, participants were told that their performance would be compared with that of future participants.
Next, participants were situated in an electrically shielded room of neuroscience laboratories (Center for Brain and Cognition, Pompeu Fabra University) where electro-encephalography (EEG) activity was registered. The experiment began after electrode application. First, participants watched a 2-min video of the other players (Figure 1) to establish the initial hierarchy. Half of the participants saw the superior then the inferior confederate while the other half saw the reverse. Participants were invited to make their own video at the end of the experiment and we had them believe it would be used with others.
Then, training started (Figure 1B), lasting ∼6 min. This session involved 70 4-s trials of the visual discrimination task for task familiarization and performance stabilization. Each trial began with a fixation cross (0.5 s) at the center of the screen, and then the two rectangles of red dots were presented for 1 s. Within this time, participants had to decide which rectangle contained more dots, answering with the corresponding joystick movement (up or down) using their right hand. Feedback (‘correct’, ‘incorrect’ or ‘time over’ message) was then presented for 2 s. The trial ended with the fixation cross. During this session, participants did not play with others; however, at the end, participants were told that the other players had undergone a similar session and that performance would be used to rank all three. The training session concluded with the 4-s ranking presentation comprising photographs of each player alongside their ranking expressed as one (inferior player), two (participant) or three (superior player) stars to reinforce the hierarchy. For motivation, participants were told they could change ranks based on performance, but they would actually stay in second position.
The game session (Figure 1B) began immediately after training with five blocks of 36 trials (180 total, 90 with each simulated player). In each block (∼5 min), participants played nine consecutive trials twice with each player, followed by the updated ranking presentation. Rank order was fixed by manipulating the superior or inferior player’s behavior. Each 5.5-s trial started with a 2-s presentation of the opponent’s photograph with its corresponding ranking stars followed by the visual task as in training. Feedback was then presented for 2 s: pictures of the participant and opponent above, and outcome (a coin meaning correct, an ‘X’ meaning incorrect or a ‘time over’ message) below. As mentioned, both players could win or lose in a trial. The trial ended with the fixation cross for 0.5 s. Participants could rest for up to 2 min between blocks.
Opponent behavior simulation
Performance (accuracy and RT) of the players was simulated based on each participant’s performance to obtain the required ranking (Figure 1C). For the needed accuracy, we generated two psychophysical curves, one for each player, using a normal cumulative distribution. We selected the distributions’ parameters by adjusting the chance-level point to the input coherence level (difficulty level) of 50%, and by assuming SDs of 3 and 1.5 for the superior and inferior players, respectively. These values were heuristically selected comparing simulated accuracies with the performance of 20 pilot participants. The outcomes of the simulated players for each trial were determined by selecting a pseudorandom number from a uniform distribution between 0 and 1; the corresponding outcome was correct if the number was higher than the value of the accuracy curves (superior and inferior) at the corresponding difficulty level. This way, the superior player’s accuracy was superior to the participant's accuracy and the inferior player’s accuracy was inferior on average. To guarantee the required ranking in every block (superior better than inferior and worse than the participant), we fitted opponent outcomes or adjusted the reported outcome by varying some of the last 20 trials (see below) of each block, only using difficult trials (percentage of upper rectangle points = 49–51%), so participants could not detect manipulation. To maintain the hierarchy, we also adjusted the simulated players’ RTs. First, we extracted a number from a uniform distribution with the mean equal to the participant’s mean RT in the pilot experiment, then selected the range of the same order as the observed variability of the participants’ RT during the pilot (100 ms). To this value, we added (or subtracted) 50 ms for the simulated RT of the inferior (or superior) player. We programmed the visual discrimination task and game structure using MATLAB version 7.9.0 (R2009b) with the psychophysics toolbox version 3.0.8.
EEG/ERP methods
EEGs were recorded from 31 scalp sites. We placed two bipolar electrodes above and below the participant’s left eye to record eye movements, two electrodes on the mastoids and a reference electrode on the nose. EEG recordings were digitized at 250 Hz. All electrode impedances were <3 KΩ. The EEG data were low- and high-pass filtered (30–0.03 Hz), then segmented into 1100-ms epochs ranging from 100 ms before stimulus onset to 1000 ms postonset (visual discrimination task). Before averaging, segments were baseline corrected by subtracting the mean amplitude of the prestimulus interval (−100 to 0 ms) and semiautomatically screened offline for eye movements, muscle artefacts, electrode drifting and amplifier blocking. Segments containing such artefacts were rejected. The 1000-ms epochs were averaged in reference to the 100 ms prestimulus baseline.
EEG procedure of lateralized response potentials
We have followed the usual procedure used in previous studies to measure Response locked lateralized response potentials (LRPs) components (Miller et al., 1998; Ulrich and Miller, 2001; Kiesel et al., 2008; Tollner et al., 2012). LRPs were computed, using a baseline of −800 to −600 ms for Response-Locked averages. To isolate the LRP in each participant, we have created separate waveforms for the hemisphere that was contra lateral to the response and the hemisphere that was ipsilateral to the response. Given participants have used right hand to response; we have created a contralateral (C3)–ipsilateral (C4) difference waveform. Scalp distribution of LRP component was particularly focused at the lateral central sites (C3 and C4). Waveform was extracted separately for each condition, (trials with superior vs trials with Inferior). LRP amplitude and latency were measured from the resulting difference waves.
Mean amplitude in a given time window (−300 to 0 ms) relative to the baseline voltage were used to measure amplitude of Response-Locked LRP component. Onset latency of the Response-Locked LRP was measured as the time point at which the voltage reached 50% of the peak amplitude in the same time window (−300 to 0 ms) as amplitude measure.
We have controlled noise on latency measure that is highly sensitive to high-frequency using a low-pass filter prior to the latency measures (Gaussian impulse response function, half-amplitude cut-off = 23.2 Hz, full width at half maximum = 18.8 ms). Incorrect trials and trials with artefacts were excluded prior to averaging using a standardized procedure (Woodman and Luck, 2003).
RESULTS
Behavioral effects: participants were faster with the superior player
Participants (n = 56, 28 females) performed the visual task in a computerized game with two simulated players. The game was subdivided into five blocks. In each block, participants played 18 trials with each simulated player. Accuracy and RTs were analyzed as dependent variables (Figure 2). To evaluate effects on accuracy and RTs, we performed two 2 × 2 × 5 analyses of variance (ANOVAs) with the gender as a between-participants factor, and social hierarchy of competitor (superior vs inferior) and blocks (5) as within-participants factors. Power analyses indicated that a sample of (N = 40–50) would be sufficient to ensure power of 0.80 for detecting significant effects when α is set to level of P < 0.05.
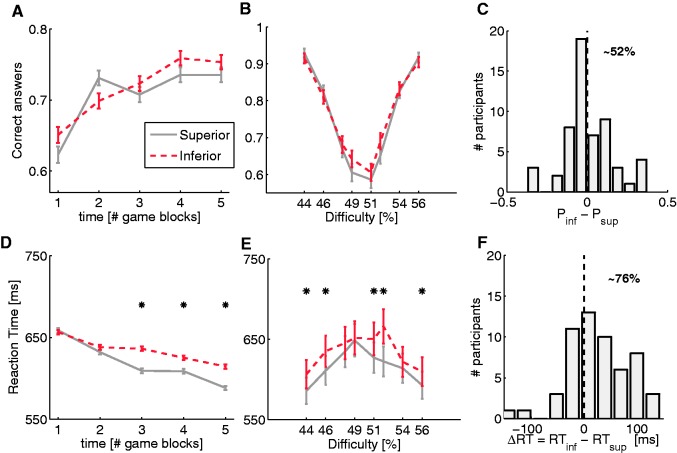
Fig. 2.
Behavioral results of the visual discrimination task. All panels show the average over all participants (28 males and 28 females). Panels (A), (B) and (C) concern accuracy; panels (D), (E) and (F) concern RT. Panels (A) and (D), respectively demonstrate accuracy and RT in each block for the two hierarchical conditions, trials played with the superior player (red) and trials played with inferior player (gray) (18 trials by each player). Panel (D) shows the main effect of hierarchy (P < 0.001) while (A) shows the absence of hierarchical effects on performance. Panels (B) and (E), respectively, demonstrate accuracy and RT for different difficulty levels for the five blocks. Asterisks in (E) show when RTs were statistically different between the two hierarchical conditions (P < 0.001). Panels (C) and (F), respectively, present the histograms of accuracy and RT differences between the two hierarchical conditions. Statistical differences were found in RT in trials played with superior player and trials played with inferior and in the neutral condition. Reported percentages in (C) and (F) refer to the positive difference. In all panels, error bars represent SEM.
Analysis of accuracy rate only yielded a significant main effect of block (F4, 216 = 15.68, P < 0.001), indicating an important learning effect (reaching asymptote at the third block). No other effects or interactions reached significant levels. Concerning RT analysis, there was again a significant main effect of the block factor (F4, 220 = 14.70, P < 0.001). In addition, we found a significant main effect of social hierarchy (F1, 54 = 18.75, P < 0.001) and an interaction between the factors (F4, 220 = 2.98, P < 0.05) (Figure 2B). The social-hierarchy effect was very robust: 76 ± 0.05% of participants were faster in trials played with the superior player (Figure 2C and F).
We have evaluated effects due to the difficulty of the task. Task difficulty was based on the dots percentage ratio between two rectangles placed in the top or bottom of the screen. An easy trial was considered one where percentage of red dots was highly different between rectangles. Thus, we subdivided analysis of task difficulty into easy trials (using the four most highly differentiated steps) and difficult trials (using the four least differentiated steps). We excluded from the analysis trials where percentage of red dots in one panel was exactly 50%. To assess if the influence of task difficulty was modulated by social hierarchy, we ran two 2 (social hierarchy) × 2 (easy vs difficult trials) ANOVAs on participants’ accuracy and RTs. Analysis of accuracy only revealed a main effect of dot distribution (F1, 55 = 406.60, P < 0.000). A parallel analysis of RTs showed a main effect of dot distribution (F1, 55 = 65.35, P < 0.001) and social hierarchy (F1, 55 = 27.49, P < 0.001) without significant interactions between factors (Figure 2B and C). This evidence supports a global effect of hierarchy in RTs. Participants were faster in trials played with superior competitor without increase error rates independently of the difficulty level.
Hierarchical effects in evoked potentials
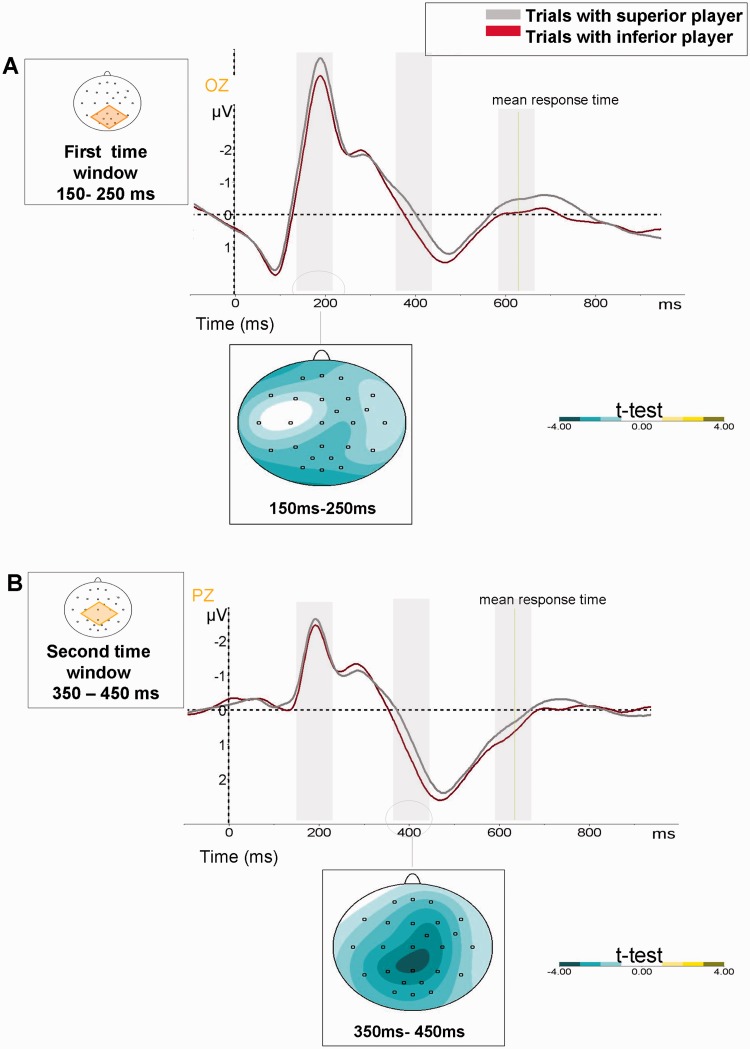
We analyzed stimulus-locked ERP recordings using multiple electrodes to study the time-course of social hierarchy effects during the perceptual task. We observed statistical differences as a function of the social hierarchy in the recordings at three time windows: an early window between 150 and 250 ms from stimulus onset (N1 component), a second window between 350 and 450 ms (early section of the P3 component–eP3) and a third window between 500 and 600 ms (late section of the P3 component–lP3). Reliable effects were obtained in the first two time windows as a function of social hierarchy.
On the one hand, hierarchical differences were observed in the N1 component. This component is usually reported at occipital electrodes and its typical peak has been reported ∼150–200 ms poststimulus presentation (Vogel and Luck, 2000; Kok, 2001). We have found hierarchical differences starting at 150 ms after stimulus presentation in the usual occipital electrodes (Oz, O1 and O2). The N1 component was larger at these electrodes when participants played with the superior player (Figure 3). We performed an ANOVA with gender as the between-participants factor, and social hierarchy and electrode (Oz, O1 and O2) as within-participants factors. The analysis only revealed a significant main effect of hierarchy (F1, 54 = 4.57, P < 0.05, Bonferroni corrected). We did not find other significant effects or interactions.
Fig. 3.
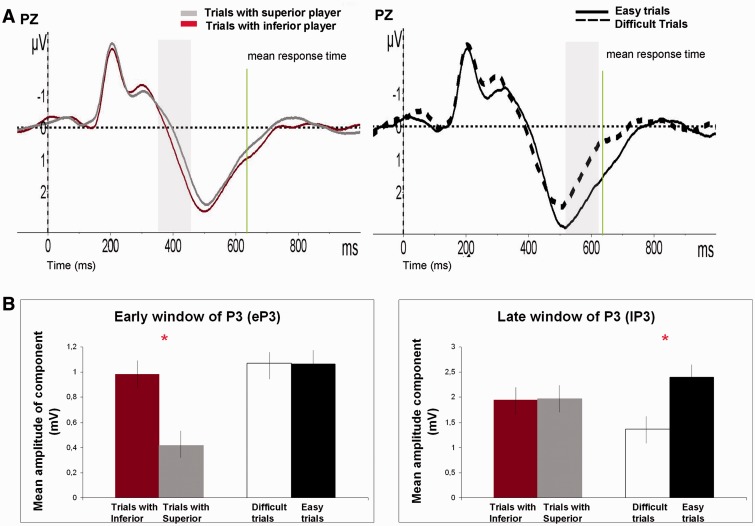
Hierarchical effects in evoked potentials. ERP results (stimulus-locked): differences in modulation of ERP components in trials performed with superior (gray line) vs inferior (red line) player. The green line shows the mean participant RT. (A) Results started 150 ms after onset of visual stimuli. Differences in windows (150–250 ms) are related to modulation of the N1 component in OZ electrode. The shaded areas demonstrate statistical differences (see t-test values represented by green areas). (B) Effects in the second time window (350–450 ms) are related to modulation of the early window of P300 component in PZ electrode.
On the other hand, an effect of social hierarchy was also found on the amplitude of parieto-central P3 component. This effect was seen in the early window of the P3 component (eP3) (350–450 ms) in the parietal electrodes (PZ, P3 and P4), the usual place where the P3 component is reported (Kok, 2001; Polich and Criado, 2006). We ran an ANOVA analyzing the amplitudes of the P3using gender, social hierarchy and electrode as factors. Only significant differences of social hierarchy factor (F1, 54 = 6.24, P < 0.05, Bonferroni corrected) were found. We observed larger amplitudes of eP3 in trials played with inferior player. No other effects or interactions reached statistical significance (Figure 3). We have analyzed possible differences due to social hierarchy in the late window of P3 component (lP3) (500–600 ms time window). Analysis did not yield any amplitude effects of social hierarchy at this time window.
Finally, we have considered that the found late differences in registers starting at 600 ms were strongly related with behavioral responses time as confirmed by the mean of response time in this task around to 620 ± 80 ms. Differences in register at this point could be reflecting the differences in RT in trials played between superior and inferior players.
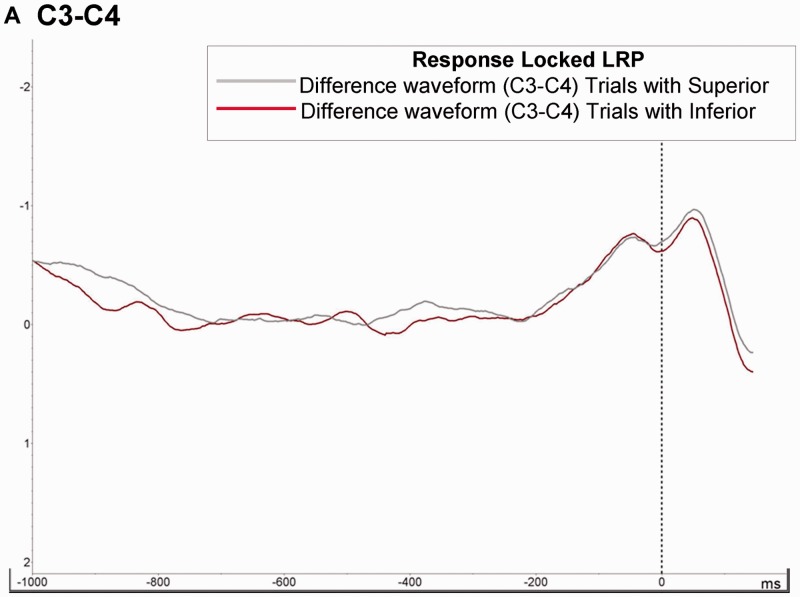
Response—LRPs
We have analyzed modulation on Lateralized readiness potentials to control effects in motor preparation process The analysis of variance of the social hierarchy factor showed no significant effects either on the latency or on the amplitude of the response locked-LRP (Figure 4). Additionally, we did not observe any significant correlation between RTs and latency (P > 0.56) or amplitude (P > 0.83; see also Table 1).
Fig. 4.
LRP (Response-Locked). Panel (A) shows Waveforms in response locked LRP. Waveform was extracted from the difference between contralateral vs ipsilateral (C3–C4) side of motor behavior following procedure proposed in previous studies. We did not observe statistical differences between trials played with Superior and Inferior competitor.
Table 1.
Response-Locked measures in each Hierarchical condition
| Superior Trials | Inferior Trials | Statistics | Correlations with reaction time | |
|---|---|---|---|---|
| Response-Locked LRP amplitude (−300 to 0 ms) | −0.28 mV | −0.31 mV | F(1,55) = 0.56 | r = −1.86 P = 0.17 (RT in trials with Inferior) |
| P = 0.81 | r = 0.12 P = 0.34 (RT in trials with Superior) | |||
| Response-Locked LRP peak onset (−300 to 0 ms) | −218 ms/Amplitude mV (0.487) | −212 ms/Amplitude mV (0.480) | F(1,55) = 0.33 | r = −1.86 P = 0.17 (RT in trials with Inferior) |
| P = 0.56 | r = 0.12 P = 0.34 (RT in trials with Superior) |
Difficulty effects in evoked potentials
There is abundant literature reporting modulation of the P3 component as a function of task difficulty (Kok, 2001; Polich and Criado, 2006). The amplitude of the P3 is attenuated when stimuli are processed in the context of a difficult situation, allegedly reflecting processing devoted to stimuli encoding. Here, we obtained reliable effects of task difficulty only in the later time window (500–600 ms; lP3) at parieto-central electrode, the usual electrode where these effects are reported (Pz) (Pz; F1, 55 = 9.53, P < 0.001, Bonferroni corrected). No interaction between social hierarchy and task difficulty was observed. We have obtained the expected modulation of P3component due to difficulty of the task. Difficult trials had shorter amplitudes of P3 specifically in the late window of P3 (500–600 ms). We did not observe modulation of amplitudes at this time window due to social hierarchy (Figure 5).
Fig. 5.
Hierarchical vs Difficulty effects in evoked potentials. Effects on P3 component according to social hierarchy vs task difficulty. (A) Graph in the left side: effects of social hierarchy in the eP3 (350–450 ms). Right side of the panel: differences in P3 amplitude due to task difficulty lP3 (500–600 ms). Panel (B) shows the effects in amplitude of P3 due to social hierarchy ΔERPSH vs effects due to task difficulty ΔERPD in both eP3 and lP3. Error bars represent s.e.m. Asterisks in (B) show when conditions were statistically different in each time of window of P3 (P < 0.001).
In an additional analysis, we analyzed the relation between social hierarchy and task difficulty in the eP3 and lP3 time windows. We used the ΔERPSH and the ΔERPD (= ERPeasy – ERPdifficult) to evaluate effects due to social hierarchy and task difficulty in the eP3 (350–450 ms) and lP3 (500–600 ms) time windows respectively. We defined an ANOVA with the factors time window of P3 (eP3 vs lP3), and effects due to social hierarchy (ΔERPSH) vs effects due to task difficulty (ΔERPD). The analysis revealed a significant interaction between both factors (Figure 5; F1, 55 = 3.88, P < 0.05), reflecting that the effects due to social hierarchy are placed in most early windows of P3 regarding to the difficulty effects.
Correlations between behavioral and ERP measurements
We analyzed the relation between ERP components and behavioral measures. In particular, we considered whether the fast RTs in trials with the superior player could be associated with different modulations of ERP components related to perceptual processing. Arguably, the influence of social hierarchy on the decision-making process may be connected to modulation of the perceptual stimulus-encoding phase. To assess this hypothesis, we defined two sets of variables, ΔERPSH = ERPsup – ERPinf and ΔRTSH = RTsup – RTinf, expressing the magnitude of the social hierarchy effect on ERPs (N1 and eP3 amplitudes) and RTs, respectively. We found significant correlations between ΔERPSH and ΔRTSH for the first two time windows in ERP registers (N1 time window: r = 0.38, P < 0.01; eP3 time window: r = 0.39, P < 0.01). Additionally, we found a significant correlation (r = 0.40, P < 0.01) between ΔERPSH calculated in N1 and eP3, suggesting that both ERP effects may be interdependent.
Social hierarchy effects in perceptual decision task occur in nondecision period
The ERP analyses revealed an early effect (150–250 ms from stimulus onset) due to the hierarchical environment (Figures 2, 3 and 4). Previous studies have linked effects in this time window to perceptual processing (Vogel and Luck, 2000; Kok, 2001). However, given the nature of the task and observation of an amplitude difference in the eP3 component, it is not possible to discard the hypothesis that processes related to decision-making may also be involved in the observed modulation. One useful way to separate decision from perceptual processes is by modeling participants’ responses through a DDM (Figure 6). This model is optimal for our needs as it has three essential features: (i) it is a highly successful model and widely used to fit behavioral data (both of performance and RT) in tasks where (perceptual) information is accumulated over time (instead of subitized) as in our task (Ratcliff, 1978; Ratcliff and McKoon, 2008); (ii) it is feasible in computational cost as it is quite easy to fit its parameters and find those that influence the process more (Vandekerckhove and Tuerlinckx, 2007, 2008); and (iii) crucially, even though the structure of this model is purely phenomenological, it allows interpretation of the dynamics of task processing in terms of decision vs nondecision phases. As opposed to other models, the distinction between decision and nondecision components can be easily extracted in terms of the fitted parameters.
Fig. 6.
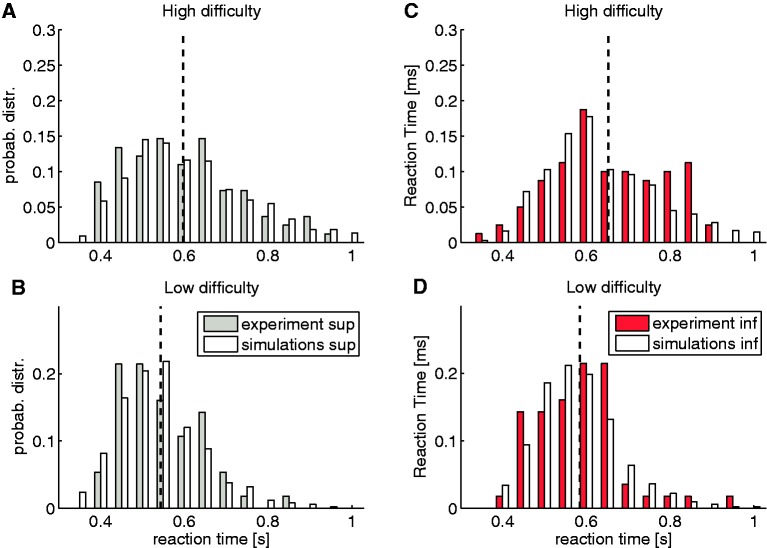
Simulated and empirical RT distributions in the visual task. Examples of simulated (white bars) and empirical (gray and red bars) RT distributions for the visual discrimination task. Participants belong to the intermediate-RT group. Simulated RT distributions were obtained from the DDM with the HF-free nondecision time parameter (see text). Panels (A) and (B) show the RT distribution for a trial with the superior opponent; likewise, panels (C) and (D) with the inferior opponent. From top to bottom, the RT distributions range from high difficulty to low difficulty. Dashed lines show the average experimental RT for the corresponding difficulty level.
Relevant to our goals, the DDM interprets ‘nondecision phases’ including both sensory-perceptual processes (stimuli encoding) and postdecision processes (motor responses). Therefore, we did not expect that parameters related to the decision phase would account for the effects of social hierarchy in participants’ responses.
In our implementation, DDM had seven parameters: boundary, starting point and its across-trial variability, nondecision time and its across-trial variability, and drifts and their across-trial variability (for a further review see Supplementary Data).
We subdivided the analysis into two steps. First, we identified the parameters that fitted better (performance and RTs; Figure 6) if left free with regard to the hierarchical factor (HF) compared with a model of HF-fixed parameters (baseline model; BLM). Second, we looked for individual parameters that fitted the data better, by generating one model for each parameter and comparing them (see Supplementary Data for more details).
The analyses showed that out of all tested models, only the model with fixed nondecision time with regard to the HF was significantly better than the BLM (Model N) [χ2(1) = 10.87, P < 0.001], and the model with fixed boundary with regard to the HF was only slightly better than the BLM [χ2(1) = 7.01, P < 0.1]. Finally, to determine which parameter explained the hierarchical effect best, we generated one more model with both parameters HF-free. This last model performed significantly better than the model with only the boundary HF-free [χ2(1) = 3.98, P < 0.05] but not better than the model with only nondecision time HF-free [χ2(1) = 0.23, P > 0.5]. Therefore, the DDM predicted that nondecision time was the most-relevant parameter to explain hierarchical differences. We interpret this result as supporting our claim that hierarchical effects affect perceptual processes.
DISCUSSION
In this study, we explored two main questions. First, can social hierarchy influence performance on a perceptual decision task? Second, how does social hierarchy modulate the decision-making process during a perceptual task?
To answer the first question, we observed a strong influence of social hierarchy on participants’ responses in a perceptual decision task: participants were faster while keeping the same accuracy level overall when they performed the task in presence of a superior player. This result could be explained by the implicit social reward reported in this type of context. Arguably, participants received a greater social reward when they improved their performance in trials played in presence of the top player. This interpretation is consistent with evidence showing enhanced activation of the reward circuitry when processing photographs of superior individuals (Zink et al., 2008).
We were interested in isolating the underlying cognitive processes influenced by hierarchical aspects during the perceptual decision task. For this, we analyzed ERPs when participants performed the visual discrimination task in presence of the superior or inferior player using stimulus-locked analyses. Our main result demonstrated modulation of early perceptual processing of visual stimuli by an induced social hierarchy. Larger amplitudes in trials played with the superior player were observed in the N1 window. Several authors have suggested that the N1 reflects changes in the modulation of visual attention resulting in enhanced perceptual processing of some stimuli or stimulus features (Vogel and Luck, 2000; Wascher et al., 2009). The fact that we did not observe changes in the earlier ERP components such as P1 (or C1) indicates that the impact of social hierarchy on early sensory-perceptual processes are not determined by nonspecific changes in arousal (or distraction) during task performance (Di Russo et al., 2002, 2003). Playing with the superior player, in comparison to playing with the inferior player, visual attention is altered by specifically increasing the gain on sensory processing, therefore reducing and optimizing encoding time. We observed an effect on the amplitude of the P3 component as a function of social hierarchy but only in an early window. Our hypothesis is that this difference in amplitude reflects differences in perceptual processing. The pattern of correlations between social hierarchy effects in participants’ RTs, and of the amplitudes of the N1 and P3 components support this assumption. We found that the changes in activity in both the N1 and eP3 windows are correlated with social-hierarchy-induced performance changes. Importantly, modulations of the N1 amplitude and eP3 window due to social hierarchy were also significantly correlated. Therefore, we argue that this correlation suggests that the complex nature of our visual task required different resources and times in perceptual processing.
Importantly, we observed dissociation between task difficulty and social hierarchy in the early and late windows of the P3 component (Figure 5). There is abundant literature describing the reverse relationship between task difficulty and amplitude of the P3 component (Polich and Criado, 2006). In our study, we also observed this relationship in the late window of the P3 component: trials with similar percentages of dots in the upper and lower panels yielded both slower RTs and smaller P3 responses. However, in clear contrast with this well-known relationship between task difficulty and amplitude of the P3 component, we observed that faster RTs induced by high-rank social hierarchy were accompanied by reduced P3 responses in the eP3 time window. The contrasting patterns between RTs, and early and late windows of the P3 response nicely fit with the explanation that the eP3 window echoes the benefits of enhanced perceptual processing induced by the hierarchical context (therefore requiring less computational demands), and the lP3 window demonstrates the already described attenuation of the P3 response in the case of higher perceptual or memory load (Polich and Criado, 2006). The lack of effects in the central section of the P3 likely is results from the opposing effects of social hierarchy and task difficulty. Therefore, the answer to our second question concerning the locus of social hierarchy modulation is: at early perceptual encoding stages.
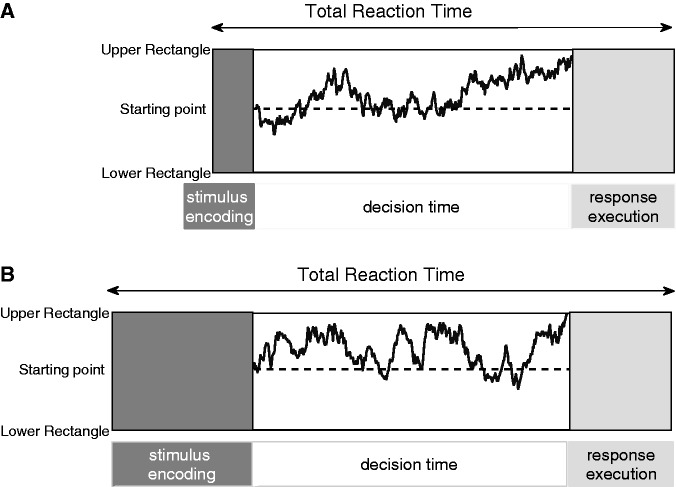
DDM analysis (Ratcliff, 1978) of participants’ responses provides converging support for this conclusion. Following common practice, we subdivided the processing of the visual discrimination task into three phases: predecision, decision and postdecision (Figure 7). Results of the DDM analysis showed that social hierarchy influences the nondecision phase, which means that the hierarchical effects likely take place before or after the decision is made. As discussed, the ERP results let us conclude that effects of social hierarchy on performance take place before the decision phase, specifically during perceptual stimulus encoding. The absence of effects in response-locked LRPs also supports this conclusion (Figure 4).
Fig. 7.
Schematic diagram of the visual task represented with DDM. In the figure, the two panels (A), (B) show two possible lengths of the predecision (stimulus encoding) phase. When the visual stimulus (rectangles of dots) was presented, there was a first phase when the stimulus was encoded (dark gray block). Then, the decision process started with differential evidence accumulation that ended when the path touched one of two thresholds (upper or lower rectangle). The last phase was dedicated to the executive response (light gray block). The total RT constitutes the sum of the decision and nondecision components, such as stimulus encoding and response execution. The four example paths represent the differential evidence accumulation for different trials: darker paths represent faster trials and lighter paths represent slower trials. However, all the paths of both panels represent only one difficulty.
The present results contribute in a significant and novel way to our understanding of the complex ways in which the brain integrates different kinds of information: social and cognitive. Previous research has measured participants’ responses when processing hierarchically marked stimuli (faces linked to different numbers of stars, or voices from higher vs lower social class, among others). In contrast with previous studies, the stimuli and task in our experimental situation did not have any intrinsic social cues (participants were simply deciding which panel had more dots). In doing so, we have been able to unravel the specific influence of social information by eliminating the perceptual (and attentional) processing of hierarchically marked stimuli. The observation of significant effects of social contexts on task performance is certain to greatly benefit our understanding of how social groups influence the way individuals perceive and more importantly, learn. This knowledge has clear consequences on our understanding of political decisions in education and may eventually inform those decisions.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This research was supported by grants from the Spanish Ministerio de Economía y Competitividad (JCI-2009-04492; SEJ2009-09072; SAF2010-16085; Consolider-Ingenio2010-CDS-2007-00012) and the Catalan Government (SGR 2009-1521). N. Sebastián-Gallés received the “ICREA Acadèmia” prize for excellence in research, funded by the Generalitat de Catalunya.
REFERENCES
- Boksem MA, Kostermans E, Milivojevic B, De Cremer D. Social status determines how we monitor and evaluate our performance. Social Cognitive and Affective Neuroscience. 2012;7:304–13. doi: 10.1093/scan/nsr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzel K, Grusser OJ. Electric brain potentials evoked by pictures of faces and non-faces: a search for ‘face-specific’ EEG-potentials. Experimental Brain Research. 1989;77:349–60. doi: 10.1007/BF00274992. [DOI] [PubMed] [Google Scholar]
- Boyce WT. Social stratification, health, and violence in the very young. Annals of New York Academy of Sciences. 2004;1036:47–68. doi: 10.1196/annals.1330.003. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioural Neuroscience. 2003;117:369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Neural basis of social status hierarchy across species. Current Opinion in Neurobiology. 2010;20:803–9. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Cummins D. How the social environment shaped the evolution of the mind. Synthese. 2000;122(1/2):3–28. [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex. 2003;13:486–99. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human Brain Mapping. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L, Hutte HA. An experimental investigation of the effect of unstable interpersonal relations in a group. Journal of Abnormal and Social Psychchology. 1954;49:513–2. doi: 10.1037/h0058604. [DOI] [PubMed] [Google Scholar]
- Foulsham T, Cheng JT, Tracy JL, Henrich J, Kingstone A. Gaze allocation in a dynamic situation: effects of social status and speaking. Cognition. 2010;117:319–31. doi: 10.1016/j.cognition.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Guinote A. Power and goal pursuit. Personality and Social Psychology Bulletin. 2007;33:1076–87. doi: 10.1177/0146167207301011. [DOI] [PubMed] [Google Scholar]
- Kiesel A. Measurement of ERP latency differences: a comparison of single participant and Jackknife-based scoring methods. Psychophysiology. 2008;45(2):250–74. doi: 10.1111/j.1469-8986.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Miller JT. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35(1):99–115. [PubMed] [Google Scholar]
- Overbeck JR, Park B. Powerful perceivers, powerless objects: flexibility of powerholders’ social attention. Organizational Behaviour and Human Decision Process. 2006;99:227–43. [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway CL. Status construction theory. In: Burke PJ, editor. Contemporary Social Psychological Theories. Stanford University Press; 2006. pp. 301–23. [Google Scholar]
- Rojas M, Masip D, Todorov A, Vitria J. Automatic prediction of facial trait judgments: appearance vs. structural models. PLoS One. 2011;6:e23323. doi: 10.1371/journal.pone.0023323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Annual Review of Anthropology. 2004;33:393–418. [Google Scholar]
- Smith PK, Jostmann NB, Galinsky AD, van Dijk WW. Lacking power impairs executive functions. Psychological Science. 2008;19:441–7. doi: 10.1111/j.1467-9280.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- Smith PK, Trope Y. You focus on the forest when you're in charge of the trees: power priming and abstract information processing. Journal of Personality and Social Psychology. 2006;90:578–96. doi: 10.1037/0022-3514.90.4.578. [DOI] [PubMed] [Google Scholar]
- Tollner TD. How the speed of motor-response decisions, but not focal-attentional selection, differs as a function of task set and target prevalence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28) doi: 10.1073/pnas.1206382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Miller J. Using the jackknife-based scoring method for measuring LRP onset effects in factorial designs. Psychophysiology. 2001;38(5):816–27. [PubMed] [Google Scholar]
- Vandekerckhove J, Tuerlinckx F. Diffusion model analysis with MATLAB: a DMAT primer. Behaviour Research Methods. 2008;40:61–72. doi: 10.3758/brm.40.1.61. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J, Tuerlinckx F. Fitting the Ratcliff diffusion model to experimental data. Psychonomic Bulletin & Review. 2007;14:1011–26. doi: 10.3758/bf03193087. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Wascher E, Hoffmann S, Sanger J, Grosjean M. Visuo-spatial processing and the N1 component of the ERP. Psychophysiology. 2009;46:1270–7. doi: 10.1111/j.1469-8986.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology.Human Perception and Performance. 2003;29(1):121–38. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–22. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–83. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–83. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.