Abstract
Genetic variants within the serotonin transporter gene (5-HTTLPR) impact the neurobiology and risk for anxiety-related behaviours. There are also gender differences in the prevalence of anxiety-related behaviours. Although numerous studies have investigated the influence of 5-HTTLPR genotype on the neural systems involved in emotional regulation, none have investigated how these effects are modulated by gender and anxiety. We investigated this issue using two complementary region of interest-based structural neuroimaging approaches (voxel-based morphometry and Freesurfer) in 138 healthy individuals categorized into ‘no anxiety’ and ‘subclinical anxiety’ groups based on the Hamilton Rating Scale for Anxiety (HAM-A). Preliminarily, using anxiety as a continuous variable, we found a significant interaction effect of genotype by gender on anxiety. Females homozygous for the Short allele showed the highest HAM-A scores and males the lowest. In addition, a three-way significant interaction among genotype, gender and anxiety category was found for the right amygdala volume. Post hoc tests revealed that homozygous females carrying the Short variant with a subclinical anxiety condition had larger volume. The reported interaction effects demonstrate that gender strongly modulates the relationship between 5-HTTLPR genotype and subclinical expression of anxiety acting on amygdala, one region of the emotional neural network specifically involved in the anxiety-like behaviours.
Keywords: 5-HTTLPR genotype, gender, amygdala, anterior cingulate cortex, cortical thickness, anxiety
INTRODUCTION
Serotonin (5-HT) is strongly implicated in anxiety-related disorders (Sen et al., 2004) and in the development of emotional circuitry in the brain (Gaspar et al., 2003). Even transient alterations in serotonin homeostasis during early development modify neural connections implicated in emotional processes and cause permanent elevations in anxiety-related behaviours during adulthood (Gross and Hen, 2004).
5-HT neurotransmission is regulated by the 5-HT transporter (5-HTT), which clears serotonin from the synaptic cleft. The 5-HTT gene (SLC6A4) is located on chromosome 17q11.1-q12 (Lesch et al., 1994), and its transcription is modulated by a common polymorphism (5-HTTLPR) in the upstream regulatory region. A deletion/insertion in the 5-HTTLPR was first reported to create a Short allele and a Long allele (Lesch et al., 1996). The Short allele has reduced transcriptional efficiency compared to the Long allele.
At a phenotypic level, the 5-HTTLPR polymorphism has been associated with the development of anxiety traits in humans (Lesch et al., 1996; Schinka et al., 2004; Sen et al., 2004; Canli et al., 2005; Hariri and Holmes, 2006; Homberg and Lesch, 2011) as well as in serotonin transporter knockout (5-HTT−/−) mice (Holmes et al., 2003), likely due to the Short allele conferring increased sensitivity to both aversive and rewarding (social) stimuli. In fact, anxiety-related behaviours are forms of risk assessment behaviours that are associated with a level of uncertainty or unpredictability regarding the outcome of emotionally salient events, often when both rewarding and aversive outcomes are possible. It has been proposed that these behaviours are fundamentally regulated by activity of the amygdala (Jennings et al., 2013), especially in the right side (Li et al., 2012).
However, a consistent heterogeneity is present. In fact, some studies did not find a clear association between the 5-HTTLPR genotype and anxiety phenomenology in healthy individuals. In three meta-analyses, Munafò et al. attempted to verify the role of the 5-HTT gene as a risk factor for developing anxiety personality traits. These analyses reported contrasting data: in particular that no effect was detected (Munafò et al., 2003, 2009) and, when present, it was small (Munafò et al., 2005). Minelli et al. (2011) suggested that the lack of structured psychiatric screening in the previous studies might have produced the reported inconsistencies between the association of the 5-HTTLPR variants and anxiety-like behaviours. At the intermediate phenotypic level, although a large amount of studies demonstrated that healthy individuals carrying the Short allele are characterized by neural abnormalities of critical limbic regions, such as: amygdala, orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Hariri et al., 2002; Heinz et al., 2005; Canli et al., 2005; Pezawas et al., 2005; Scherk et al., 2009; Kobiella et al., 2011; Drabant et al., 2012), important conflicting results have been reported, mainly concerning the involvement of the amygdala, a critical brain region underlying emotional regulation (Canli et al., 2005; Pezawas et al., 2005; Scherk et al., 2009). In fact, while Pezawas et al. (2005) found a clear effect of this genotype on the bilateral amygdala’s anatomy, where the carriers of the Short allele were characterized by reduced volume with respect to Long carriers, Canli et al. (2005), using the same neuroimaging method, did not detect evidence of amygdala volumetric changes. Another study (Scherk et al., 2009) described, for the first time, an inverse effect where the carriers of the Short allele were characterized by an increased right amygdala volume.
What has been suggested to explain these inconsistencies is that the biological and behavioural effects of the 5-HTTLPR polymorphism could be interfered with or modulated by two distinct factors: negative environment and/or gender (Du et al., 2000; Antypa et al., 2011; Everaerd et al., 2012). The detrimental effect of stressors occurring over the life-course has been extensively investigated (Eley et al., 2004; Caspi et al., 2010; Frodl et al., 2010; Homberg and Lesch, 2011) providing a general consensus that the Short allele is associated with emotionality or stress sensitivity. A primary mechanism of the stress response is the activation of the hypothalamus–pituitary–adrenal (HPA) axis resulting in an enhanced secretion of cortisol (Chrousos, 2001). Anxiety disorders result in down-regulation of HPA-axis activity associated with lower cortisol awakening response (Hek et al., 2013). It has been recognized that the serotonergic system, as well as 5-HTTLPR polymorphism, influences HPA axis regulation, where a sexual dimorphic effect has also been revealed (Jabbi et al., 2007; Wüst et al., 2009). Following this evidence, the interaction effects between gender and the 5-HTTLPR genotype on the neurobiological basis of anxiety-like behaviours is far from being well defined. As already demonstrated for the Catechol-O-methyltransferase (COMT) gene (Harrison et al., 2007), there is an increased interest on the dependence of genotype effects mediated by gender. In fact, functional genetic variations in COMT in humans have been associated with sexually dimorphic effects in aspects of cognitive abilities, personality, predisposition to psychiatric disorders, as well as neuroanatomy (Harrison et al., 2007). As concerns the presence of dimorphic effects of the 5-HTTLPR genotype, the recent literature demonstrated that female Short carries are more prone to develop depression (Sjöberg et al., 2006) and male Short carriers have higher values of Neuroticism (Du et al., 2000).
To the best of our knowledge the sexually dimorphic effects of the 5-HTTLPR genotype on anxiety-like behaviours have never been directly investigated in non-clinical population. We retain that there are substantial pieces of evidence to sustain a significant effect. In fact, it is well-known that females have an increased risk to develop anxiety disorders (Altemus et al., 2006), and there is a wealth of preclinical evidence demonstrating that female brain does not process 5-HT as quickly as the male brain does (Nishizawa et al., 1997); females are also characterized by higher 5-HT binding in specific brain regions with respect to males (Christian et al., 2009) and, finally, it has been demonstrated that females have higher 5-HT transporter availability than males (Staley et al., 2001). It is therefore conceivable that if a 5-HTTLPR by gender interaction exists on the modulation of anxiety-like behaviours, the biological brain substrates influenced by 5-HTTLPR genetic variants will also interfere with or will be modulated by the gender.
For this reason, the main objective of this study is to establish whether the complex interactions among the 5-HTTLPR genotype, anxiety-like phenomenology and gender may impact the structural neuroanatomy of the underlying neural emotional network, specifically the amygdala, OFC and ACC, even in non-clinical healthy individuals. To this end, we investigated a large population by combining two complementary morphologic MR measurements: voxel-based morphometry (VBM) (Ashburner and Friston, 2005) and cortical thickness (Freesurfer) (Fischl and Dale, 2000) in a multi-method unbiased approach. We used both methods in order to receive any complementary piece of information. Indeed, whereas VBM provides a general measure of grey matter (GM) volume, which conflates the contributions of thickness and surface, cortical thickness analysis captures the columnar architecture of the cortex (Winkler et al., 2010). Although cellular characteristics cannot be quantified directly in neuroimaging data, cortical thickness may more closely reflect cytoarchitectural abnormalities than does cortical volume (Koolschijn et al., 2010), thus providing an additional neurobiological marker. Therefore, the possible converging results from cortical thickness and VBM analyses would strengthen our observations (Labate et al., 2012). We decided to employ structural brain morphology as an endophenotype for anxiety regulation, since a prior study demonstrated that the well-defined neurophysiological effects of the 5-HTTLPR genotype on amygdala reactivity are procured by changes in brain morphology (Kobiella et al., 2011).
MATERIALS AND METHODS
Subjects
Right-handed healthy individuals were recruited by local advertisements. The inclusion criterion was age between 18 and 80 years. To reduce the possibility of artifactual association caused by ethnic stratification, the final sample only included individuals of Italian-Caucasian ancestry, born and educated in Italy. Exclusion criteria were: (i) major medical illnesses and/or known or suspected history of alcoholism or drug dependency and abuse; (ii) mental disorders (i.e. schizophrenia, mood, anxiety, personality and/or any other significant mental disorders) according to the DSM-IV-TR criteria assessed by the Structured Clinical Interviews for DSM-IV-TR [SCID-I (First et al., 2002) and SCID-II (First et al., 1997)] and/or neurological disorders diagnosed by an accurate clinical neurological examination; (iii) presence of vascular brain lesions, brain tumour and/or marked cortical and/or subcortical atrophy on magnetic resonance imaging (MRI) scan; and (iv) presence of dementia. We included only subjects with Mini Mental State Examination (MMSE) score ≥24 (a cut-off point for dementia screening in the Italian population) (Measso et al., 1993) or if they did not present dementia diagnoses according to DSM-IV-TR criteria. The presence of anxiety symptoms was assessed using the Hamilton Rating Scale for Anxiety (HAM-A) (Hamilton, 1967).
After a careful evaluation of these inclusion and exclusion criteria, 138 subjects [Mean ± SD age = 41.2 ± 15.9; 62 (45%) males] were eligible for this study (Table 1). According to the 5-HTTLPR polymorphism, we categorized individuals into three groups: the homozygous Short/Short group, the Long/Short group and the remaining homozygous Long/Long group. The sample consisted of 23 smokers that were well-balanced among groups [5 (17%) smokers in the Long/Long group, 11 (16%) smokers in the Long/Short group and 6 (19%) smokers in the Short/Short group].
Table 1.
Sociodemographic and amygdala volumetric characteristics of 138 healthy Caucasian subjects carrying different 5-HTTLPR genotypes
| Demographic data | Males L/L genotype | Females L/L genotype | Males L/S genotype | Females L/S genotype | Males S/S genotype | Females S/S genotype | Genotype effect F (P) | Gender effect F (P) | Genotype by Gender interaction F (P) |
|---|---|---|---|---|---|---|---|---|---|
| N | 14 | 20 | 33 | 35 | 15 | 21 | |||
| Age (years) | 43.8 ± 15.6 | 35.6 ± 16.2 | 40.8 ± 15.7 | 44.3 ± 17.8 | 42.4 ± 15.6 | 40.0 ± 16.0 | F2,127 = 0.64 (0.52) | F2,127 = 0.07 (0.78) | F2,127 = 0.1.54 (0.21) |
| Educational level (years) | 13 (8–19) | 14 (5–20) | 14 (8–21) | 14 (8–24) | 15 (8–19) | 15 (8–21) | U = 0.61 (0.51) | U = 50 (0.61) | – |
| MMSE | 28.9 ± 1.5 | 29.5 ± 0.8 | 29.6 ± 0.6 | 29.1 ± 1.3 | 29.5 ± 0.8 | 29.6 ± 1 | F2,127 = 26 (0.77) | F2,127 = 0.17 (0.59) | F2,127 = 2.3 (0.1) |
| HAM-A | 6.7 ± 5.8 | 4.3 ± 4 | 4.03 ± 3.7 | 5.9 ± 4.1 | 3.0 ± 2.7 | 7.21 ± 5.1 | F2,127 = 0.15 (0.86) | F2,127 = 2.01 (0.16) | F2,127 = 4.2 (0.01) |
| Normalized left amygdala volume | 0.91 ± 0.09 | 0.9 ± 0.14 | 0.95 ± 0.16 | 0.89 ± 0.12 | 0.88 ± 0.13 | 1.02 ± 0.11 | F2,127 = 0.72 (0.49) | F2,127 = 1.02 (0.31) | F2,127 = 4.92 (0.008) |
| Normalized right amygdala volume | 1.05 ± 0.16 | 1.05 ± 0.17 | 1.06 ± 0.15 | 0.98 ± 0.16 | 0.95 ± 0.1 | 1.11 ± 0.13 | F2,127 = 0.36(0.69) | F2,127 = 1.19 (0.27) | F2,127 = 6.4 (0.002) |
Data are given as mean values ± SD or median values (range) when appropriate. HAM-A: Hamilton rating scale Anxiety. MMSE. Mini Mental State Examination.
Written, informed consent was obtained from all subjects participating in the study, which was approved by the local ethics committee at the Santa Lucia Foundation of Rome.
Genotyping
DNA was extracted from of a 5 ml blood sample using a kit (QIAamp Blood Isolation Kit QIAGEN) following the instructions from the supplier. The 5HTT regulatory gene region was amplified by polymerase chain reaction (PCR) with oligonucleotide primers: forward 5′-GGCGTTGCCGCTCTGAATGC-3′; reverse 5′-GAGGGACTGAGCTGGACAACCAC-3′. PCR was performed using Taq DNA Polymerase Quiagen Kit protocol containing Q-Solution, able to amplify the GC-rich regions (Quiagen). PCR started with an initial denaturation at 94°C for 4 min, followed by 30 s at 94° C, 30 s at 64° C, 30 s at 72° C for 30 cycles and final extension at 72°C for 10 min. PCR products were separated on 2% agarose gel supplemented with ethidium bromide allowing differentiation of the 5-HTTLPR Long (528 base pairs) and Short (484 base pairs) variants. Bands were visualized by ultraviolet illumination.
MRI
All MRI analyses were carried out using an anatomical a priori approach. Indeed, since the goal of the study was to explore the impact of the 5-HTTLPR genotype, anxiety-like phenomenology and gender on the structural neuroanatomy of the neural emotional network, we decided to use the amygdala, the ACC (BA 32) and the OFC (including both lateral and medial parts, BA 11/47) as bilateral a priori regions-of-interest (ROIs) given their consolidated role in anxiety-like emotional behaviours (Hariri et al., 2002; Heinz et al., 2005; Canli et al., 2005; Pezawas et al., 2005; Christian et al., 2009; Jedema et al., 2010; Kobiella et al., 2011; Drabant et al., 2012).
Sequences
Participants underwent the same imaging protocol with a whole-brain T1-weighted scan using a 3 T Allegra MR imager (Siemens, Erlangen, Germany) with a standard quadrature head coil. Whole-brain T1-weighted images were obtained in the sagittal plane using a modified driven equilibrium Fourier transform (MDEFT) sequence (TE/TR = 2.4/7.92 ms, flip angle 15°, voxel-size 1 × 1 × 1 mm3).
Amygdala volumetry
Automated labelling and quantification of amygdala volume were performed using FreeSurfer 4.05 installed on a Red Hat Enterprise Linux v.5. The automated procedures for volumetric measures of several deep GM structures have been previously described (Fischl et al., 2002; Cerasa et al., 2011b). This procedure automatically provided segments and labels for up to 40 unique structures and assigned a neuroanatomical label to each voxel in an MRI volume based on probabilistic information estimated automatically from a manually labelled training set.
The automated subcortical segmentation performs by Freesurfer requires these steps: first, an optimal linear transform is computed that maximizes the likelihood of the input image, given an atlas constructed from manually labelled images. A non-linear transform is then initialized with the linear one, and the image is allowed to further deform to better match the atlas. Finally, a Bayesian segmentation procedure is performed, and the maximum a posteriori estimate of the labelling is computed. This approach provides advantages similar to manual ROI drawing (Jovicich et al., 2009; Morey et al., 2009) without the potential for rater bias, offering an anatomically accurate rendering of regional volumes (Fischl et al., 2002). Intracranial volume (ICV) was calculated and used to correct the regional brain volumes analyses (Buckner et al., 2004). ICV includes biological material such as meninges and cerebrospinal fluid in addition to brain tissue. Normalized right and left amygdala values were calculated as follows: [raw amygdala volume/ICV] × 1000. No significant difference was detected for ICV values among genotyped groups.
Voxel-based morphometry
Data were processed and examined using VBM5 toolbox (http://dbm.neuro.uni-jena.de), which utilizes and extends the new unified segmentation approach implemented in Statistical Parametric Mapping (SPM5) (Ashburner and Friston, 2005; Wellcome Department of Imaging Neuroscience, London, http://www.fil.ion.ucl.ac.uk/spm). Unified segmentation provides a generative model of VBM pre-processing that integrates tissue classification, image registration and MRI inhomogeneity bias correction. The VBM5 toolbox extends the unified segmentation model as it increases the quality of segmentation by applying a Hidden Markov Random Field (HMRF) model on the segmented tissue maps (Cuadra et al., 2005). The Montreal Neurological Institute (MNI) brain was used as a template for normalization. The normalized and segmented GM images were then modulated by the Jacobian determinants derived from the spatial normalization, with the aim of preserving the volume within each GM voxel. Finally, for whole-brain analysis the modulated volumes were smoothed with a Gaussian kernel of 10 mm full-width at half maximum (FWHM).
Cortical thickness
To corroborate voxel-based findings we further performed cortical thickness analysis of the cortical mantle by using Freesurfer (Dale, 1999; Fischl and Dale, 2000; Cerasa et al., 2011a). In particular, we were interested in confirming findings provided by VBM analysis. Brain images for cortical thickness analysis were first corrected for intensity of non-uniformity and registered via affine transformation (12 parameters) to MNI space. Then, images underwent a further intensity normalization using a different automated algorithm and were automatically skull stripped (Dale, 1999). Next, the entire cortex was visually inspected prior to analysis, and data from 138 subjects were deemed to require manual correction, which included: (i) manually realigning each subject’s image to the MNI template, (ii) setting intensity normalization control points where brain matter was erroneously skull stripped, (iii) adjustment watershed parameters of the skull strip, and (iv) visual inspection and correction of the automatic subcortical segmentation. All subjects were inspected by a neuroradiologist with a high level of neuroanatomical expertise who was blinded to the MRI results. MRIs of inferior quality, not suitable for reliable tissue segmentation with Freesurfer even after manual editing of cortical surfaces and subcortical regions, were discarded.
For each subject, thickness measures across the cortex were computed by finding the point on the grey–white boundary surface that was closest to a given point on the estimated pial surface (and vice versa) and averaging between these two values (Fischl and Dale, 2000). The accuracy of the thickness measures derived from this technique has been validated by direct comparisons with manual measures on post-mortem brains (Rosas et al., 2002) as well as direct comparisons with manual measures on MRI data (Kuperberg et al., 2003). The surface representing the grey–white border was ‘inflated’ (Fischl et al., 1999a), differences among individuals in the depth of gyri–sulci were normalized, and each subject’s reconstructed brain was then morphed and registered to an average spherical surface representation that optimally aligned sulcal and gyral features across subjects (Fischl et al., 1999a,b). This spherical morphing procedure was used to map the thickness measurements at each vertex on each subject’s cortical surface into a common spherical coordinate system (Fischl et al., 1999a,b). Finally, cortical maps were smoothed with a 10-mm FWHM Gaussian kernel.
Statistical analysis
Assumptions for normality were tested for all continuous variables by using the Kolmogorov–Smirnov test. All variables were normally distributed, except for the number of years of formal education (K–S = 0.2, P < 0.05). In the absence of a normal distribution of data, the Mann–Whitney U-test and χ2 were employed.
To define the independent and interaction effects of anxiety, we further split our cohort of healthy individuals into two groups following previous evidence (Bishop et al., 2007; Antypa et al., 2011). This step was performed in order to convert the continuous distribution of HAM-A scores into a categorical variable useful to perform additional ANOVA analysis where the variable ‘anxiety’ has been considered as an independent variable. Participants were allocated into ‘no-anxiety’ and ‘subclinical anxiety’ groups based on HAM-A median score (above score 6 coded as subclinical; below or equal to score 6 coded as no). HAMA scores ranged from 0 to 20, and no subject was diagnosed with any anxiety disorder.
Analysis of covariance (ANCOVA) was used to assess main/interaction effects of 5-HTTLPR allelic variants (Long/Long vs Long/Short vs Short/Short), gender (males vs females) and anxiety category (no-anxiety vs subclinical anxiety) on the brain anatomy using age and total ICV as covariates of no-interest. All statistical analyses had a two-tailed α level of <0.05 for defining significance. Main and interactive effects were followed by post hoc tests (Duncan t-test). Only significant post hoc tests are reported.
VBM statistics
The GM volume maps were statistically analysed using the general linear model based on Gaussian random field theory. As above-specified, to assess main/interaction effects of 5-HTTLPR allelic variants, gender and anxiety category on GM volume, the 138 smoothed GM images were entered into a second-level ANCOVA model, with age and total ICV as covariates of no-interest. Since our sample population was characterized by a large age range, in order to further control for the effect of this variable we re-analysed the data excluding age as a covariate of no-interest in the statistical model, but the overall pattern of findings remained the same.
As previously stated, we decided to use the amygdala, the ACC and the OFC as left and right a priori ROIs. All ROIs were created with the ‘aal.02’ atlas included in the Wake Forest University Pickatlas software version 1.04 (Functional MRI Laboratory at the Wake Forest University School of Medicine, Winston-Salem, NC, USA; http://www.fmri.wfubmc.edu/download.htm). All analyses were thresholded by using correction for multiple comparisons [false discovery rate (FDR) P < 0.05] within ROIs (Genovese et al., 2002).
Cortical thickness statistics
For each hemisphere, differences in cortical thickness were tested by computing a general linear model of genotype, gender and anxiety category effects on structural neuroanatomy at each vertex. A FDR of P < 0.05 was applied to correct for multiple comparisons within ROIs: the left and right ACC (including the caudal and rostral parts of the ACC) and OFC (including the lateral and medial parts). Age was included in the models (ANCOVA and simple regression) as a covariate of no-interest.
RESULTS
Socio-demographic and clinical data
The allelic distribution of 5-HTTLPR genotypes was in Hardy–Weinberg equilibrium (df = 2; χ2 = 0.7; P > 0.05). No significant differences were detected for demographic variables (Table 1).
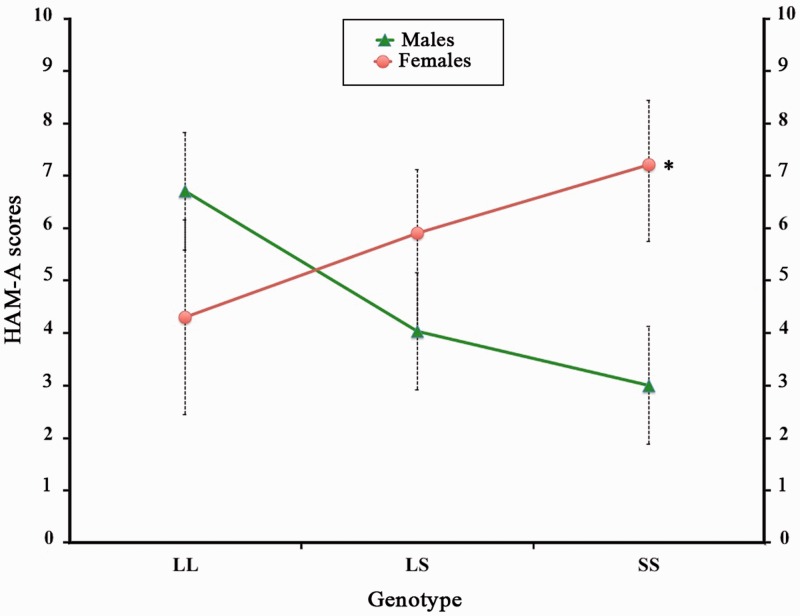
A significant result only emerged when considering the genotype by gender interaction effect for anxiety scores treated as continuous variable, where homozygous females for the Short allele showed the highest values and males the lowest (F2,132 = 4.2; P = 0.01). For exploratory purposes, we performed a further post hoc analysis (Duncan t-test). Results confirmed the expected significant difference between males and females in the Short/Short group (P = 0.01), whereas in the Long/Long group, although we detected an opposite interaction effect where males showed the highest anxiety values, this difference did not reach a significant threshold (Duncan t-test, P = 0.1), nor did it in the Long/Short group (Duncan t-test, P-level = 0.21) (Figure 1).
Fig. 1.
Subclinical anxiety severity (HAM-A scores) is different in males and females carrying different serotonin transporter variants. Error-bars represent standard errors. *Post hoc analysis was significant at P-level < 0.05.; L/L: homozygous Long variant; L/S: Heterozygous Long Variant; S/S homozygous Short variant.
To better disentangle the effects of anxiety from those of genotype and gender on the neural networks involved in emotional regulation, participants were further grouped according to anxiety categories, changing the meaning of HAM-A scores from a dependent (continuous values) to independent (categorical) variable. The distribution of ‘no-anxiety’ and ‘subclinical anxiety’ individuals in the three genotype groups (Table 2) was not significantly different (χ2 = 0.377; P-level = 0.82; Long/Long group: 21 healthy individuals had no-anxiety and 13 healthy individuals had subclinical anxiety; Long/Short group: 38 healthy individuals had no-anxiety and 30 healthy individuals had subclinical anxiety; Short/Short group: 20 healthy individuals had no-anxiety and 16 healthy individuals had subclinical anxiety).
Table 2.
Demographic, global cognitive and genetic characteristics of healthy subjects grouped according to anxiety category
| Demographic data | No-Anxiety | Subclinical Anxiety | P-value |
|---|---|---|---|
| N | 79 | 59 | |
| Gender (M/F) | 42 / 37 | 30 / 29 | 0.92a |
| Age (years) | 38.2 ± 15.7 | 41.3 ± 16.2 | 0.12b |
| Educational level (years) | 15 (8–21) | 13.5 (5–24) | 0.25c |
| MMSE | 29.2 ± 1.6 | 29.5 ± 1 | 0.88b |
| 5-HTTLPR genotype | |||
| Long/Long | 21 | 13 | 0.82a |
| Long/Short | 38 | 30 | |
| Short/Short | 20 | 16 | |
Data are given as mean values ± SD or median values (range) when appropriate. MMSE: Mini Mental State Examination.
aChi-square test, bUnpaired t-test, cMann–Whitney test.
Automated amygdala volume
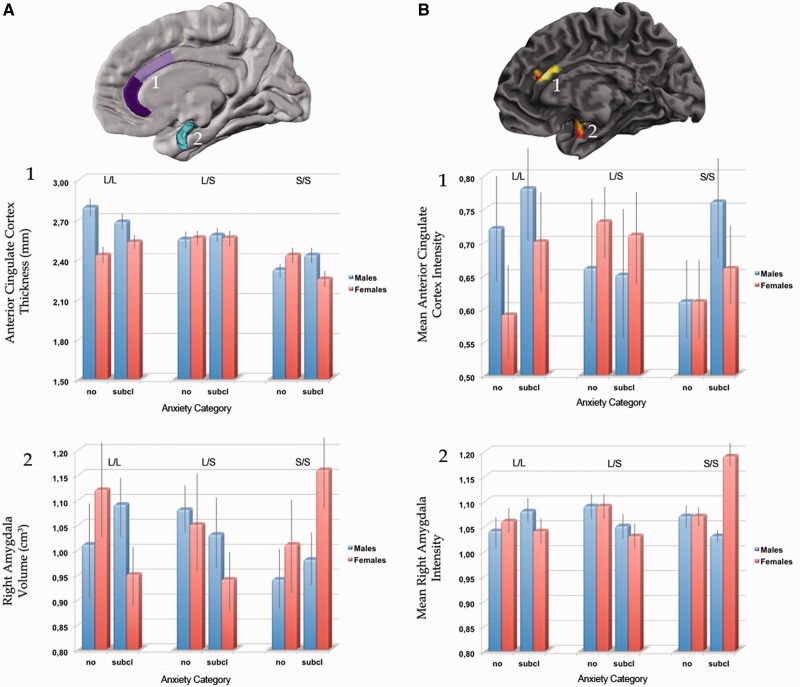
We found a significant interaction effect of anxiety category with genotype and gender on the amygdala volume in the right side (F2,124 = 3.5; P = 0.03) (Figure 2A). When further exploring the three interactions by computing a two-way analysis with the factors genotype × anxiety category in females and males, separately, we found a significant interaction in females (F2,69 = 6.2; P = 0.002) but not in males. (F2,55 = 1.02; P = 0.365). Post hoc analysis (Duncan t-tests) demonstrated that the right normalized amygdala volume of the homozygous females carrying the Short variant with subclinical anxiety was significantly larger than other groups: with respect to subclinical anxiety males in the Short/Short group (t = 2.9; P = 0.02); to no-anxiety males in the Short/Short (t > 4.6; P = 0.007); to subclinical anxiety females in the Long/Short group (t > 4.5; P = 0.008); to subclinical anxiety females in the Long/Long group (t = 3.4; P = 0.01). Moreover, additional interaction effects were detected in the right amygdala. In particular, we revealed significant genotype by gender (F2,124 = 4.6; P = 0.01) and genotype by anxiety interaction effects (F2,124 = 4.4; P = 0.01). Finally, investigating the main effect of single independent variables (genotype, gender, anxiety), no significant findings within amygdala volume were found.
Fig. 2.
Multimodal neuroanatomic findings as a function of 5-HTTLPR genotype, gender and anxiety level. (A) 3D model of the amygdala (light-blue), together with the pial view highlighting the 3D gyral and sulcal anatomy of the anterior cingulate cortex (violet) as automatically parcellated by Freesurfer. (B) VBM maps related to statistical effects within the right amygdala and the ACC have been plotted on a standard 3D template. Bar graphs refer to mean values extracted from brain surface analysis of the ACC (A 1 cortical thickness, mm), automated labelling of the right amygdala (A 2, normalized volume, cm3), as provided by Freesurfer (A) and GM mean intensity values of ACC (B1) and amygdala (B2) as provided by VBM. Error bars represent standard errors. L/L: homozygous Long variant; S/S homozygous Short variant. Subcl: subclinical.
VBM data
Similar to previous volumetric analysis, VBM revealed a significant three-way interaction effect (i.e. anxiety by genotype by gender) on the right amygdala (F2,124 = 8.89; PFDR–ROI = 0.03; x: 26, y: 6, z: −15)(Figure 2B). When further exploring the three interactions by computing a two-way analysis with the factors genotype by anxiety in females and males, separately, we found a significant interaction in the right amygdala in females (F2,69 = 7.34; PFDR–ROI = 0.03; x: 30, y: −1, z: −15) but not in males. Moreover, additional interaction effects were detected in the amygdala. In particular, we revealed significant genotype by gender (F2,69 = 5.83; PFDR–ROI = 0.04; x: 32, y: −6, z: −14) and genotype by anxiety (F2,69 = 9.94; PFDR–ROI = 0.004; x: 32, y: −6, z: −15) interaction effects. Finally, investigating the main effect of single independent variables (genotype, gender, anxiety), no significant findings within amygdala volume were found.
We did not detect a three-way interaction effect relative to other specific ROIs, that is ACC and OFC. For exploratory purpose, we investigated the presence of further interaction effects using two-way analysis. Only the significant genotype by gender interaction effect was detected in a bilateral cluster within the ACC (F2,124 = 9.32; PFDR–ROI = 0.04; x: 4, y: 33, z: 18)(Figure 2B), while no evidence of significant genotype by anxiety or gender by anxiety interaction effects were detected. Investigating the main effect of single independent variables, we found a significant bilateral effect of genotype on the ACC (F1,124 = 10.07; PFDR–ROI = 0.03; x: −8, y: 28, z: −5) and significant effects of genotype (F1,124 = 9.19; PFDR–ROI = 0.04; x: 30, y: 57, z: 3) and anxiety (F1,124 = 32.31; PFDR–ROI < 0.001; x: 28, y: 38, z: −14) on right OFC volume. In particular, the Short/Short group exhibited the lowest GM volume in the bilateral ACC and right OFC, whereas individuals with subclinical anxiety had the highest GM volumes in the right OFC.
Cortical thickness data
The VBM-derived imaging genetic findings were supplemented with point-by-point measurements of thickness across the entire mantle. We did not detect a three-way interaction effect relative to specific ROIs, which are ACC and OFC. For exploratory purposes, we investigated the presence of further interaction effects using two-way analyses. One only significant genotype by gender interaction effect was detected in a bilateral area within the ACC (surviving a statistical threshold of FDR < 0.05, at whole brain; F2,124 = 10.25; x: 8, y: 41, z: 11) (Figure 2B), while no evidence of significant genotype by anxiety or gender by anxiety interaction effects was detected. Post hoc analyses (Duncan t-test) demonstrated that extracted thickness measurements from the ACC of the homozygous females carrying the Short variant were significantly different from that of other groups (with respect to males in the Long/Long group, P = 0.000004; to females in the Long/Long group, P = 0.002; to males in the Long/Short group, P = 0.00004; to females in the Long/Short group, P = 0.00004; and to males in the Short/Short group, P = 0.047). Investigating the main effect of single independent variables, we detected significant effects of the genotype on the bilateral ACC (F1,124 = 18.7; PFDR < 0.05; x: 8, y: 39, z: 5) and right OFC (F1,124 = 14.5; PFDR < 0.05; x: 25, y: 35, z: −10) and of the anxiety on the right OFC (F1,124 = 12.8; PFDR < 0.05; x: 16, y: 18, z: − 23).
DISCUSSION
Although the mechanisms of anxiety-like behaviours are particularly complex, three principal predictors have been proposed: genetics, environment and gender (Hariri and Holmes, 2006; Canli and Lesch, 2007; Homberg and Lesch, 2011; Everaerd et al., 2012). Studies mainly investigated either the single effects of genetic factors and negative environment or their combined effects, describing homozygosity for the Long variant as the less vulnerable for anxiety disorders. Nevertheless, several conflicting findings have been reported. Part of this variability has been ascribed to the influence of gender. In fact, females react differently to negative environments than males (Wüst et al., 2009). Also, the serotonergic system is regulated differently in males and females (Canli and Lesch, 2007; Cosgrove et al., 2007). For these reasons, the present study was aimed at determining how gender might impact the neurobiological effects of the 5-HTTLPR genotype on known brain markers for vulnerability to anxiety disorders: (i) amygdala, (ii) ACC and (iii) OFC (Domschke and Dannlowski, 2010).
We obtained several pieces of evidence: first, the amygdala, with specific involvement of the right side, was the only region where we detected a three-way genotype by gender by anxiety category interaction effect, which was driven by the fact that only homozygous Short female carries exhibited an association between subclinical anxiety and larger right amygdala volume (Figure 2). On the contrary, homozygous females carrying the Long variant showed an inverse relationship between subclinical anxiety and smaller right amygdala volume. This important morphological finding, which also persists using a voxel-based approach, demonstrates a gender-specific dissociation in genetic and anxiety-like behaviours on the right amygdala volume in healthy individuals. In other words, what clearly emerged from our study was that the relationship between the 5-HTTLPR genotype and anxiety-like behaviours, as expressed by neurobiological vulnerability of right amygdala morphology, has an inverse pattern in males and females. Notably, our results showed that the three-way genotype by gender by anxiety category interaction effect was confined to the right amygdala. This hemispheric lateralization is novel and therefore it has to be interpreted with caution since no studies so far investigated the structural lateralization of the amygdala in relation to gender effects in anxiety and serotonin functions. A recent study by Li and colleagues (Li et al., 2012) found that the significant association between surface metrics of the amygdala and trait anxiety levels was confined to the right lateral and central nuclei, thus supporting the notion that right amygdala is in charge of reacting to negative emotion inputs. There are also studies that, investigating the biological abnormalities concomitant with impulsivity/self-aggression, described the presence of increased right amygdala volume (Spoletini et al., 2011) more evident in females (Monkul et al., 2007). From a functional point of view, a novel study investigated the modulation of sex and serotonin transporter genotype on resting-state cerebral blood flow (rCBF) in amygdala and blood-oxygen-level-dependent (BOLD) amygdala response to fearful dynamic faces in the same sample of healthy subjects (El-Hage et al., 2013). Results showed an interaction between sex and genotype in rCBF in right amygdala (higher values of rCBF in the right amygdala in males’ Short allele carriers compared with females) and a significant negative correlation between the rCBF and BOLD response in the right amygdala, and more so in Short allele carriers. Overall our findings are in agreement with previous imaging genetic studies investigating the strict relationship between the 5-HTTLPR genotype and gender on brain regions implicated in anxiety and mood related illnesses, either considering human or animal models (Christian et al., 2009).
Second, in bilateral ACC, a critical region involved in emotional regulation (Drevets et al., 2008) and highly innervated and modulated by the serotonergic system (Gaspar et al., 2003), we detected a genotype by gender interaction effect. Overall, differently to those found in the right amygdala morphology, the 5-HTTLPR per se influences the anatomy of ACC, but this effect is hampered by gender. Indeed, females carrying the Short allele have thinner bilateral ACC cortices with respect to other groups. Results of our study are in line with recent evidence suggesting the key role of 5-HTTLPR genotype in regulating the physiology of cortical development (Gaspar et al., 2003; Pezawas et al., 2005; Jedema et al., 2010; Kobiella et al., 2011). What seems to be unique in our work is that this described phenomenon in ACC is independent from anxiety. In fact, as revealed by both VBM and cortical thickness analysis, the relationship between anxiety and the bilateral ACC volume is similar in the genotyped groups, with individuals having subclinical anxiety condition showing a trend towards lower GM volumes (Figure 2).
Third, the OFC is the only region where we did not detect any significant interaction. We only found significant main effects of genotype and anxiety. These findings are in agreement with those reported in previous studies; in particular, Canli et al. (2005) described reduced volume in healthy Short allele carriers in the medial prefrontal cortex, a key region previously shown to be involved in the functional integration of emotional information and strongly modulated by the 5-HTTLPR polymorphism (Heinz et al., 2005); while Blackmon et al. (2011) found a significant association between thickening of the OFC and increased anxiety in healthy individuals. Again it is important to emphasize the laterality effect on the right hemisphere revealed when considering the main effect of genotype or anxiety. The right hemispheric dominance of emotional processing has long been a subject of great interest (Gazzaniga and LeDoux, 1978). In the last years, a plethora of neuroimaging and neuropsychological studies have shown asymmetrical dominance of the right OFC for emotional processing. For instance, patients with right hemisphere damage have disinhibition syndrome with aggressive outbursts, euphoria, hyperphagia and irritability and exhibit poor performance on a gambling task compared with those with left OFC damage (Bechara et al., 2004). Finally, Shaw and colleagues, investigating the typical pattern of development of cortical asymmetry in human brain, found a disrupted evolution of typical asymmetry in the right OFC in patients with Attention-Deficit/Hyperactivity Disorder (Shaw et al., 2009).
In light of the recent Everaerd et al. (2012) study demonstrating that gender modulates the interaction effect of the 5-HTTLPR genotype on the hippocampal volume, thus increasing the vulnerability for depression, our study extends these findings demonstrating the key role of gender in modulating the biological effect of the 5-HTTLPR polymorphism on the neural network underlying emotional anxiety-like regulation in healthy subjects. Interestingly, 5-HTTLPR per se is not associated with anxiety-like behaviours or amygdala volumetric changes (Canli et al., 2005; Munafò et al., 2005; Minelli et al., 2011), but a significant association emerges when the interaction effect with gender is taken into account. In fact, healthy females carrying the at-risk Short allele have both the highest anxiety scores and highest right amygdala volumes, while males have the lowest. This phenomenon is only valid for females with both Short/Short homozygosity, whereas homozygous/heterozygous males carrying the Long variant presented an opposite effect. Moreover, the inclusion of the anxiety category as a factor modulating the previous interaction effect, contributes to disentangle the gender-driven effects on right amygdala morphology. The opposite effects between genders are not surprising since previous studies found a similar phenomenon considering the genetic susceptibility mechanisms for depression driven by the 5-HTTLPR genotype (Sjöberg et al., 2006; Everaerd et al., 2012). As suggested by these authors, the opposite effects may reflect a true difference between the sexes, which in turn might reflect a difference in the interaction between the 5-HTTLPR polymorphism and gonadal and/or adrenocortical hormones (HPA-axis activity). This explanation would be consistent with previous observations that these hormones are likely to interact with both gender and anxiety-like behaviours (Wüst et al., 2009). As concerns the opposite effects between genotyped groups, the issue of the homozygous vs heterozygous effect of the Short allele remains unclear. The heterozygous genotype performed comparably to the Short/Short genotype at a phenotypic anxiety level or in between the two homozygous genotypes in structural neuroimaging data. Since the vast majority of the previous structural imaging genetic studies (Pezawas et al., 2005; Canli et al., 2005; Canli et al., 2006; Scherk et al., 2009; Selvaraj et al., 2011) grouped Long/Short and Short/Short genotypes together, we were unable to determine the real inconsistency of data. Thus, future research should continue to examine differential effects of the three genotypes separately.
An important strength of our study involves the employment of two distinct but matching advanced MRI techniques to provide a wider picture of neuroanatomical substrates modulated by the 5-HTTLPR polymorphism. The advantage of combining these two techniques lies in the complementary nature of the two methods. In fact, VBM provides a mixed measure of cortical GM, including cortical surface area and/or cortical folding as well as cortical thickness; whereas, cortical thickness has the advantage of providing a quantitative value that represents a physical property of the cortical mantle. Therefore, the fact that both VBM and cortical thickness provide similar results speaks to the robustness of our findings and suggests that neuroanatomical changes driven by genetic variation in the 5-HTTLPR polymorphism involve an abnormal increase/decrease in the dendritic arborization, neuronal sprouting and neuropil as a function of gender. Of note, some differences could be detected with respect to the evidence provided by previous studies. In fact, while the impact of the Short allele on the ACC found here is perfectly in agreement with that found in previous studies (Canli et al., 2005; Pezawas et al., 2005; Frodl et al., 2008; Selvaraj et al., 2011), our findings on amygdala morphology appear to be in partial contradiction with respect to part of the literature. Many studies used samples of healthy volunteers where the presence of males was predominant (Canli et al., 2006; Frodl et al., 2008; Kobiella et al., 2011; Selvaraj et al., 2011). Therefore, some of these studies reported a reduced amygdala volume in healthy individuals carrying the Short allele (Frodl et al., 2008; Kobiella et al., 2011), while others did not find evidence of GM changes considering the main effect of genotype (Canli et al., 2006; Selvaraj et al., 2011); only one study, describing increased right amygdala volume in Short carriers, was mostly comprised of females (61%) (Scherk et al., 2009). Thus, our findings that males carrying the Short allele are characterized by reduced volume of the entire amygdala while females present an inverse effect, now appear in line with those previously reported.
Three issues that could limit results of this study need to be discussed. First, to investigate the complex impact of the 5-HTTLPR genotype on anxiety-like behaviours we employed an individual scale (Hamilton, 1967). To improve the understanding of this issue, both self-reporting and objective assessments of anxiety-related traits and behaviours (Minelli et al., 2011) must be measured. However, in psychology many questionnaires have been created to measure personality traits or behaviours. One specific weakness is that the vast majority of these questionnaires are self-reporting, thus relying heavily on participants’ ability to estimate their own personality, that is, metacognition. Second, we did not consider the effect of negative environment, such as stressful life events, which has been demonstrated to interact with 5-HTTLPR genotype in hampering vulnerability to affective disorders (Lesch et al., 1994; Canli and Lesch, 2007) and impact hippocampal volume (Everaerd et al., 2012). Thus, further studies are needed in order to extend evidence provided by our study in the environmental interaction. Third, epistatic interactions are likely to exist among 5-HT system components (Stoltenberg, 2005), which may suggest that our findings could be related to other biological pathways enhanced by the low/high transcriptional activity: i.e. neurotrophic factors (Cole et al., 2011). Again, an important limitation of our study is the lack of investigation of the triallelic rs25531 polymorphism, which has been demonstrated to mitigate the transcriptional efficacy of the Short/Long variants of the diallelic 5-HTTLPR polymorphism in the promoter region of the SLC6A4 gene (Hu et al., 2006). Future studies, are strongly warranted to define if the reported interaction effect on brain anatomy and anxiety-like behaviours are modulated by the presence of this additional 5-HTTLPR polymorphism. In reality, it is important to bear in mind that the triallelic 5-HTTLPR polymorphism has been found to be associated with different anatomical changes (Frodl et al., 2008; Kobiella et al., 2011; Cole et al., 2011) with respect to the diallelic 5-HTTLPR variations (Pezawas et al., 2005; Canli et al., 2005; Scherk et al., 2009) and that a recent in vivo PET study did not find evidence of significant different expression of the 5-HTT in the adult human brain as function of the diallelic or the triallelic 5-HTTLPR variants (Murthy et al., 2010). All these imaging genetic studies reported only one specific convergent finding related to the involvement of the ACC. Thus, we can speculate that our findings highlighting the interaction effect between the diallelic 5-HTTLPR polymorphism and gender on the anatomy of the ACC could be moderated by the uninvestigated effect of the triallelic rs25531 polymorphism. This evidence together with the sample size (n = 138 individuals) employed in this work, limited our possibility to test all these possible genetic effects.
In conclusion, our study reports new evidence on the strict relationship between 5-HTTLPR polymorphism and neural networks underlying anxiety-like behaviours, highlighting the crucial role of the amygdala, which is known to be strongly involved in the elicitation and experience of emotions, particularly anxiety (Domschke and Dannlowski, 2010). The reported interactive effects between genotype, gender and anxiety might constitute a new model for biological vulnerability toward subclinical expression of anxiety disorders. Generally, in anxiety disorders evidence has been provided about the presence of altered neuroanatomy of brain areas involved in emotional control functions (Freitas-Ferrari et al., 2010). This evidence suggests the need to understand whether the phenomenon is a consequence of the disease or is due to antecedent genetic factors that makes the individual more vulnerable to emotional disorders. To be a female carrier of the 5-HTTLPR Short allele may represent a risk factor for developing anxiety disorders throughout mechanisms linked with the morphology of critical regions involved in the emotional network (Gaspar et al., 2003; Pezawas et al., 2005; Jedema et al., 2010; Kobiella et al., 2011). It remains to be assessed whether these findings in healthy individuals could be also generalized within the clinical manifestations of anxiety disorders.
Conflict of Interest
None declared.
Acknowledgments
This work has been funded by the Italian Ministry of Health (Grants RC-07-08-09-10/A; and RF 07-08).
REFERENCES
- Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Hormones and Behavior. 2006;50:534–8. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Antypa N, Cerit H, Kruijt AW, Verhoeven FE, Van der Does AJ. Relationships among 5-HTT genotype, life events and gender in the recognition of facial emotions. Neuroscience. 2011;172:303–13. doi: 10.1016/j.neuroscience.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex. 2007;17:1595–603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Carlson C, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Research. 2011;194:296–303. doi: 10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–9. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of the National Academy of Science USA. 2005;102:12224–9. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K. Neural correlates of epigenesis. Proc Natl Acad Sci USA. 2006;103:16033–8. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A, Gioia MC, et al. Dysbindin C-A-T haplotype is associated with thicker medial orbitofrontal cortex in healthy population. Neuroimage. 2011a;55:508–13. doi: 10.1016/j.neuroimage.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A, Gioia MC, et al. MAO A VNTR polymorphism and amygdala volume in healthy subjects. Psychiatry Research. 2011b;191:87–91. doi: 10.1016/j.pscychresns.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J. Serotonin transporter binding and genotype in the nonhumane primate brain using [C-11]DASB PET. Neuroimage. 2009;47:1230–6. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Glucocorticoid therapy. In: Flig P, Frohman L, editors. Endocrinology and Metabolism. 4th edn. New York: McGraw-Hill; 2001. pp. 609–32. [Google Scholar]
- Cole J, Weinberger DR, Mattay VS, et al. No effect of 5HTTLPR or BDNF Val66Met polymorphism on hippocampal morphology in major depression. Genes Brain and Behavior. 2011;10:756–64. doi: 10.1111/j.1601-183X.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Transactions on Medical Imaging. 2005;24:1548–65. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53:822–31. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Ramel W, Edge MD, et al. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. American Journal of Psychiatry. 2012;169:397–405. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrum. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bakish D, Hrdina PD. Gender differences in association between serotonin transporter gene polymorphism and personality traits. Psychiatric Genetics. 2000;10:159–64. doi: 10.1097/00041444-200010040-00002. [DOI] [PubMed] [Google Scholar]
- El-Hage W, Zelaya F, Radua J, et al. Resting-state cerebral blood flow in amygdala is modulated by sex and serotonin transporter genotype. Neuroimage. 2013;15(76C):90–7. doi: 10.1016/j.neuroimage.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Everaerd D, Gerritsen L, Rijpkema M, et al. Sex modulates the interactive effect of the serotonin transporter gene polymorphism and childhood adversity on hippocampal volume. Neuropsychopharmacology. 2012;37:1848–55. doi: 10.1038/npp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High resolution intersubject and a coordinate system for the cortical surface. Human Brain Mapping. 1999b;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010;34:565–80. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry. 2008;13:1093–101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–90. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Review Neuroscience. 2003;4:1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, LeDoux J. The Integrated Mind. New York, NY: Plenum Press; 1978. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nature Review Neuroscience. 2004;5:545–52. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Clinical Psychology. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-Methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2007;33:3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hek K, Direk N, Newson RS, et al. Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology. 2013;38:300–5. doi: 10.1016/j.psyneuen.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behaviour in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain and Behavior. 2003;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–9. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American journal of Human Genetics. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, et al. Cognitive impact of genetic variation of the serotonin in primates is associated with differences in brain morphology rather than serotonin neurotrasmission. Molecular Psychiatry. 2010;52:1059–69. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–28. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshöfer DE, et al. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Translational Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Schnack HG, Janssen J, Hulshoff Pol HE, Kahn RS. Cortical thickness and voxel-based morphometry in depressed elderly. European Neuropsychopharmacology. 2010;20:398–404. doi: 10.1016/j.euroneuro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Mula M, et al. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2012;53:377–85. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, et al. Organization of the human serotonin transporter gene. Journal of Neural Transmission Genetic Section. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Y, Xu P, et al. Surface morphology of amygdala is associated with trait anxiety. PLoS One. 2012;7:e47817. doi: 10.1371/journal.pone.0047817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measso G, Cavarzeran F, Zappalà G, et al. The mini-mental state examination: normative study of an Italian random sample. Developmental Neuropsychology. 1993;9:77–85. [Google Scholar]
- Minelli A, Bonvicini C, Scassellati C, Sartori R, Gennarelli M. The influence of psychiatric screening in healthy populations selection: a new study and meta-analysis of functional 5-HTTLPR and rs25531 polymorphisms and anxiety-related personality traits. BMC Psychiatry. 2011;31:11–50. doi: 10.1186/1471-244X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular Psychiatry. 2007;12:360–6. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, et al. Rebuttal to Hasan and Pedraza in comments and controversies: ‘improving the reliability of manual and automated methods for hippocampal and amygdala volume measurements’. Neuroimage. 2009;48:499–500. doi: 10.1016/j.neuroimage.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, et al. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. American Journal of Medical Genetics B Neuropsychiatric Genetics. 2009;150B:271–81. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Molecular Psychiatry. 2005;10:415–9. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Moore LR, Payne E, Walton R, Flint J. Genetic polymorphisms and personality in healthy adults: a systematic review and meta-analysis. Molecular Psychiatry. 2003;8:471–84. doi: 10.1038/sj.mp.4001326. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52:50–4. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Science USA. 1997;94:5308–13. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Scherk H, Gruber O, Menzel P, et al. 5-HTTLPR genotype influences amygdala volume. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:212–7. doi: 10.1007/s00406-008-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Molecular Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of.Medical Genetics B Neuropsychiatric Genetics. 2004;127:85–9. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Godlewska BR, Norbury R, et al. Decreased regional gray matter volume in S' allele carriers of the 5-HTTLPR triallelic polymorphism. Molecular Psychiatry. 2011;16:472–3. doi: 10.1038/mp.2010.112. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, et al. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9:443–9. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2009;66:888–96. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoletini I, Piras F, Fagioli S, et al. Suicidal attempts and increased right amygdala volume in schizophrenia. Schizophrenia Research. 2011;125:30–40. doi: 10.1016/j.schres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-sarin S, Zoghbi S, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–84. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF. Epistasis among presynaptic serotonergic system components. Behavioral Genetics. 2005;35:199–209. doi: 10.1007/s10519-004-1019-4. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst S, Kumsta R, Treutlein J, et al. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–82. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]