Abstract
Changes in preference are inherently subjective and internal psychological events. We have identified brain events that presage ultimate (rather than intervening) choices, and signal the finality of a choice. At the first exposure to a pair of faces, caudate activity reflected the face of final choice, even if an initial choice was different. Furthermore, the orbitofrontal cortex and hippocampus exhibited correlations only when the subject had made a choice that would not change.
Keywords: caudate, changing mind, face preference, fMRI, gaze manipulation
INTRODUCTION
Why do we sometimes change our mind?
In the last several decades, psychologists have learned a lot about the neural basis of decision making (Kable et al., 2009; Pennartz et al., 2009). However, the distinction between sustained and transient decisions and the possibility that the decision may be overturned later have been largely neglected. We know from daily experiences that when we have to make a choice/decision, we sometimes reach a firm decision, but at other times we change our mind. However, so far we have not been able to identify the neural basis or the temporal dynamics of decision change or ‘changing the mind’. Changing mind regarding a preference is especially interesting, because of its exclusively subjective and internal nature. Recent studies have identified the so-called ‘binary attractor model’ as being behind the behavioral change process involving single decisions, but they have not revealed much about the internal process that leads to a change in an already-made decision and its neural correlates (Resulaj et al., 2009; Krajbich et al., 2010; Albantakis and Deco, 2011). It has been difficult in the laboratory to obtain meaningful behavioral and neural data about the mechanisms involved in ‘changing the mind’ phenomenon, for a variety of methodological and technical reasons, including the difficulty to generate frequent ‘real’ responses associated with a change of mind and to find a paradigm to systematically manipulate preference decisions. In this study, we aimed to reveal the temporal dynamics of the changing mind in a serial time task, especially in terms of its neural basis. To the best of our knowledge, this was the first study that employed this approach; there were little evidences that could reveal the neural basis or the temporal dynamics of the decision change; that is the changing mind. Especially, the changing mind of preference decisions is interesting due to its exclusively subjective, internal nature.
According to previous studies, attractive faces activate reward-related neural circuits. In particular, the striatum [putamen and nucleus accumbens (NAcc)]; the orbitofrontal cortex (OFC) is involved in face attractiveness judgment (Kim et al., 2007; Tsukiura and Cabeza, 2011; Mende et al., 2012). A recent study showed that there is a significant correlation between face attractiveness and hippocampal activation. This too suggests that the OFC and the hippocampus may be important for the evaluation of face attractiveness (Tsukiura and Cabeza, 2011). Furthermore, these results indicate that the evaluation of facial attractiveness is not only based on reward-related, but also on memory-related neural circuits.
The rationale behind the current study is as follows: since the preference decision and, in particular, its change are subjective and internal in nature, it is hard to explain them in terms of stimulus-driven, deterministic mechanisms. Therefore, we sought an alternative explanation as well as some physiological evidence to substantiate our rationale. We focused on the following three hypotheses regarding the neural correlates of the ‘changing the mind’ phenomenon.
Changing the mind is ‘real’, in the sense that the entire brain consistently reverses the dominance in its activity, starting from the most upstream process in the neural information-processing cascade, possibly because of sensory or attentive modulations.
Changing the mind is ‘not real’. Although reversal takes place at the behavioral (and thus the downstream decoding) levels, such as the motor cortices, possibly due to some noise, the upstream subcortical areas do not change the dominance or the relative strength of their neural activity.
There is always competition between two potential choices in neural circuits or brain activities. Alternative neural circuits are competing and suppressing each other, until one reaches the threshold to generate a choice action. The ‘changing the mind’ phenomenon reflects fluctuations in such a neural competition (cf. ‘Competition hypothesis’, Reynolds and Desimone, 1999; Resulaj et al., 2009).
Currently available data are consistent with Hypothesis 2 and, at least partly, with Hypothesis 3 as explained below. For example, Kim et al. (2007) have identified different neural components of the preference decision-making process in the time domain that indicate a signal transfer from the NAcc in the ventral striatum to the OFC (Kim et al., 2007). To test these hypotheses, we analyzed the temporal dynamics of the neural processes involved in face preference decision-making. To obtain frequent occurrences of mind changing in a systematically manipulated way, we employed the gaze manipulation paradigm (Shimojo et al., 2003).
Unlike previous related studies (e.g. Tsukiura and Cabeza, 2011), which employed a task of absolute attractiveness evaluation of a single face, participants in the current study performed a two-alternative forced-choice task twice. We examined differences and fluctuations in neural activity between the choice-changed cases and choice-not-changed cases. The results showed that, if the caudate showed a higher activation to a face at first sight, the participant chose that face at the time of the second decision. This is true regardless of whether the face with the higher caudate activation happened to be the first choice or not. Moreover, the OFC and the hippocampus exhibited a functional correlation at the first decision period. In contrast, in the choice-not-changed cases these two regions showed the same correlation during the second decision. The preference choice did not change in subsequent decisions when the following two conditions were met: (i) the caudate exhibited a high activity at first sight and (ii) the OFC and the hippocampus showed a high functional correlation during the first preference decision. However, if one of these two conditions was not met during the first decision, the subsequent preference decision tended to be different. In principle, we may be able to determine the likelihood of changing mind by examining the activation of the caudate at the time of the first sight, because the caudate appears to ‘know’ if one changes one’s mind.
MATERIALS AND METHODS
Participants
Thirty-six healthy volunteers gave written informed consent for this study, approved by the ethics committee of Tamagawa University. Participants were divided into three groups: Main Manip. group [Face Preference group with effective Gaze Manipulation; eight female, four male, age = 19.2 ± 0.38 (mean ± standard error of mean, SEM)], No Gaze Shift group (Face Preference group with ineffective Gaze Manipulation; six female, six male, age = 20.0 ± 0.26), and Roundness group (Face Roundness group with effective Gaze Manipulation; six female, six male, age = 19.9 ± 0.33). The No Gaze Shift and Roundness groups were used as the control groups to examine the effects of gaze manipulation.
Stimuli
One-hundred and sixty different faces (80 female, 80 male) were generated with a computer program (FaceGen; Singular Inversions). All images were presented on a 19-inch screen at 1024 × 768 pixel resolution. The viewing distance was always 57 cm. Eye movements were tracked with an eye tracking system (Arrington Research).
Prerating of faces
Before scanning, the Main Manip. and the No Gaze Shift groups were first asked to rate the facial attractiveness of the 80 female and 80 male faces on a scale from 1 to 7; the Roundness group rated the facial roundness of each face in the same way. After obtaining the ratings, faces were paired according to each participant’s rating so that faces with close ratings were paired.
Instructions
Participants in the Main Manip. and No Gaze Shift groups were asked ‘Who would you like to approach and talk to?’ and participants in Roundness group were asked ‘Which face is rounder than the other?’.
Task design and gaze manipulation
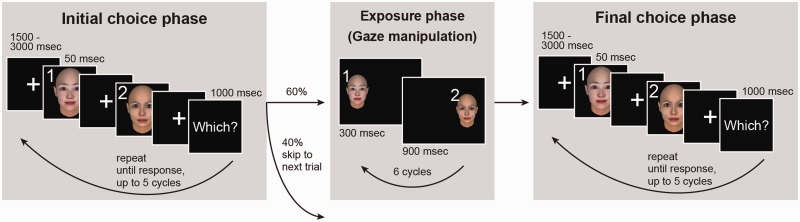
All participants underwent two functional magnetic resonance imaging (fMRI) scans, each consisting of 40 trials. A trial consisted of two-alternative forced-choice sessions (initial and final choice phases) and a gaze manipulation session (Figure 1, Supplementary Figures S1A and S1B). After the initial choice phase in each trial, participants performed the gaze manipulation and then the final choice phase. To randomize the task, the manipulation and the final choice phase were skipped in ∼40% of all trials. In the choice phases, two faces appeared sequentially on the screen, and the participants were asked to choose the face according to the instructions by pressing a button within five stimulus presentation cycles. The cycle in which the participants pressed the button was termed the ‘response cycle’, and the immediately preceding cycle was termed the ‘opening cycle’. The duration times of the fixation, the faces, and ‘Which?’ were 1500–3000, 50 and 1000 ms, respectively. In the gaze manipulation session, the Main Manip. and Roundness groups were exposed to the effective gaze manipulation and the No Gaze Shift group to the ineffective gaze manipulation. In the effective gaze manipulation, the two faces appearing in the initial choice phase were displayed six times each on the right or left side of the screen sequentially in random order, and the presentation time of each face was determined by the participant’s choice in the initial choice phase. The presentation time of the chosen face in the initial choice session was 300 ms, and that of the unchosen face was 900 ms. In contrast, in the ineffective manipulation, the two faces were displayed six times each on the center of the screen sequentially in alternate order, and the presentation time of the two faces was 600 ms.
Fig. 1.
Iterated choice task design. A trial, using the same face pair throughout, consisted of an initial choice phase, a manipulated exposure phase, and a final choice phase. To randomize the task, 40% of trials ended after the initial choice. In choice phases, the two faces were presented in sequence, followed by an option to respond. The cycle was repeated, up to five times, until the subject indicated which person they would rather ‘approach and talk to’. In the exposure phase, durations were biased toward the initially unchosen face to subconsciously bias subject preferences.
Changing ratio
After the scans, we counted the number of the changing choices between the first choice session and second choice session, and calculated the changing ratio of the choice in each subject.
Imaging procedures
Functional imaging was conducted on a 3-Tesla Siemens Trio Tim MRI scanner. For each participant, we acquired whole-brain T1-weighted anatomical scans and gradient echo T2 weighted echo planar images (EPI) with BOLD contrast (TR = 2000 ms; TE = 25 ms; slice gap, 0.6 mm; FOV, 192 mm; slice thickness, 3.0 mm; 34 oblique axial slices). We used a tilted acquisition sequence at 30° to the AC–PC line to recover signal loss in the medial orbitofrontal cortex (mOFC; Deichmann et al., 2003). The first 5 volumes of images were discarded to allow for equilibration effects.
Imaging data analysis
Image data were analyzed by using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). To correct for participants’ motion, the images were realigned to the mean volume image and spatially normalized to a bias-corrected T1 image, and spatial smoothing was applied by using a Gaussian kernel with a full width at half maximum (FWHM) of 8 mm.
Trials were separated into Firm Choice and Changed Choice trials on the basis of whether the initial and final choices matched or differed. We sorted the trials by the number of cycles required to make decisions and selected only the trials with two cycles to examine the temporal fluctuation of the neural activities and to compare the data with a previous report (Kim et al., 2007). All MRI data in this article were limited to the Main Manip. group’s data because the number of the Changed Choice trials in the other two groups were very small; thus, we could not detect any statistical differences in neural activity among the two groups. Each face presentation was treated as an event and categorized into chosen and unchosen faces as well as whether it was part of a Firm or Changed Choice trial. To avoid confusion, we refer to the faces on the basis of their status having been chosen or unchosen in the final choice phase.
Linear contrasts of regression coefficients (parameter estimates) were computed at the individual participant level in contrast to the final choice or the other face. The results from each participant were taken to a random effects level by including contrast images from each single participant into a paired t test. A statistical threshold at P < 0.001 or < 0.005 (uncorrected) was used.
Region of interest extraction
We used the MarsBar tool for SPM (http://marsbar.sourceforge.net/) to extract activations from the spherical regions of interest (ROIs) centered on the peak coordinates for the significant caudate, hippocampus and OFC contrasts (arrows in Figures 3 and 4).
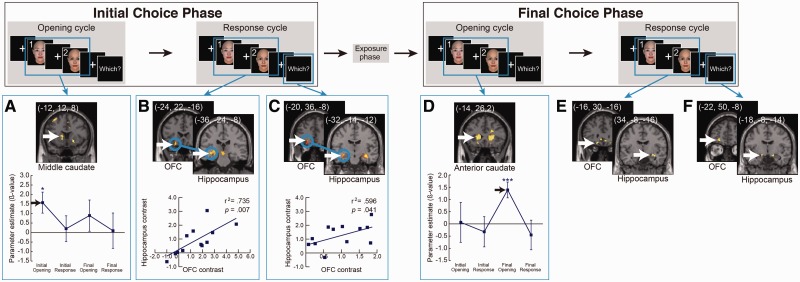
Fig. 3.
Significant activity contrasts and correlations at successive cycles of the Firm Choice trials. Initial choice phase and Final choice phase trials were analyzed separately. White arrows indicate the peak voxels in the caudate, the hippocampus, and the OFC. (A, D) The bottom graphs show the time course of caudate activity. (B, C) Blue circles indicate a significant correlation of contrast levels between the hippocampus and OFC, and subject-by-subject plot of contrast levels in hippocampal vs OFC ROIs, indicating significant correlations. To define the activated regions, a statistical threshold at P < 0.005 (uncorrected) was used. ***P < 0.001, **P < 0.005, *P < 0.01.
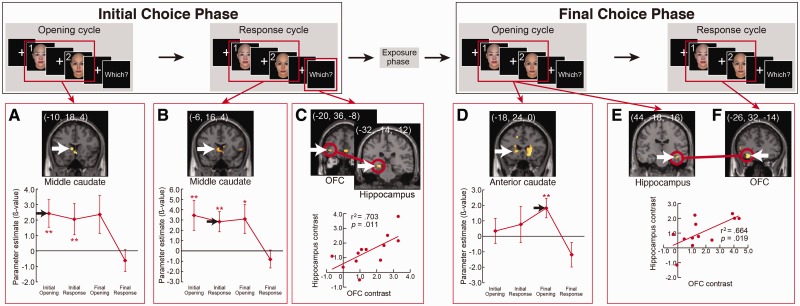
Fig. 4.
Significant activity contrasts and correlations at successive cycles of the Changed Choice trails. Initial choice phase and Final choice phase trials were analyzed separately. White arrows indicate the peak voxels in the caudate, the hippocampus and the OFC. (A, D) The bottom graphs show the time course of caudate activity. (C, E, F) Red circles indicate a significant correlation of contrast levels between the hippocampus and OFC, and subject-by-subject plot of contrast levels in hippocampal vs OFC ROIs. To define the activated regions, a statistical threshold at P < 0.005 (uncorrected) was used. ***P < 0.001, **P < 0.005, *P < 0.01.
Correlation analysis
In phases, where significant activity was found in the hippocampus and OFC, we used SPSS (IBM) software to run Pearson correlation analysis between contrast levels in the ROIs of the OFC and hippocampus.
Statistical analysis
All statistical analyses were performed with SPSS software.
RESULTS
Behavioral results
One procedural complication posed by compressed laboratory experiments such as this one is the typical paucity of reported changes due to consistency bias and rote behavior patterns. Thus, during the middle exposure phase, we employed a gaze manipulation paradigm, which has been shown to subliminally bias subject preferences (Shimojo et al., 2003). Control experiments with unmanipulated exposures and a ‘roundness’ choice task verified that this manipulation was successful in leading subjects to change their minds; however, only in the preference task (P < 0.001, Supplementary Figure S1C).
The temporal dynamics of the changing mind
We analyzed fMRI activity in response to face presentations by performing comparisons for the face of final choice vs the other face. These comparisons were performed for data from the first stimulus presentation cycle (opening cycle) and the last cycle prior to choice response (response cycle) separately (Figures 2, 3, 4, Supplementary Figures S2 and S3, and the main effects were shown in Supplementary Tables S1–S4). We divided the trials into Firm Choice trials, where subjects made the same choice in both phases, and Changed Choice trials, where the initial and final choices differed. As shown in Supplementary Figures S2 and S3, these final chosen vs unchosen face comparisons were statistically significant regardless of the size choice of ROIs.
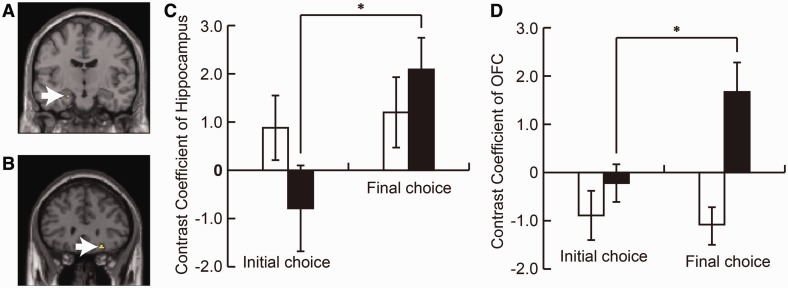
Fig. 2.
Gaze manipulation increased the activation of the hippocampus and the OFC. The contrast between the response cycles of the initial and final choice phase showed activations in the left hippocampus (A, −22, −12, −20) and the right OFC (B, 24, 30, −16). ROI analysis showed that these regions were activated only in the Changed Choice trials (C, D). These two regions were considered to play an important role in face attractiveness judgment. White arrows indicate the peak voxels in the hippocampus (A) and the OFC (B). White bars are Firm Choice, and black bars are Changed Choice trials. To define the activated regions, a statistical threshold at P < 0.005 (uncorrected) was used. Each peak voxel was used as the center of 4-mm radius spherical ROI. Error bars mean SEM; *P < 0.05. All MRI data are from the Main Manip. group.
The effects of gaze manipulation on the face preference decision
To examine the effects of gaze manipulation on the preference decision, we compared the contrast between the initial choice and the final choice phase. The fMRI data revealed that there was an increase and inversion of the activity in the hippocampus and OFC only in the Changed Choice trials but not in the Firm Choice trials (Figure 2).
The temporal dynamics of caudate activity during changing of mind
The caudate showed a significant contrast toward the face of the final choice in the opening cycles of both choice phases, and this was true for both Firm and Changed Choice trials (Figures 3A and 4A). The caudate activity exhibited a dynamic spatial pattern as the trial progressed. Significant activity began in the middle caudate and then moved to the anterior caudate (Figures 3A, D, 4A, B, D, and Supplementary Figure S4). In the Firm Choice trials, this pattern was more distinct with the middle caudate being significant only at the opening of the initial choice phase and the anterior caudate being significant only at the opening of the final choice phase. The Changed Choice trials showed a more sustained version of the same pattern (Figure 4B and D). The middle caudate extended its significant activity throughout the initial choice phase and into the opening of the final choice phase. The pattern of the anterior caudate began to increase in the initial choice phase and became significant at the opening of the final choice phase (Figures 3D and 4D).
The functional correlation of the hippocampus and the OFC
The hippocampus and the OFC (Figures 3B, C, 4C, E and F) also showed significant contrast at selected phases and during the ‘Which’ response, which is the time to decide which face is preferable to the other and indicate it by pressing a button. We calculated the correlations between these regions during each choice phase. We found significant activity and correlations in phases where subjects made a firm or final choice, but not in phases where their choice was tentative and was going to change. In the Firm Choice trials, significant contrast and correlation was present in the initial choice phase (Figure 3B and C, blue circles and bottom scatter-plot). In the final choice phase, where subjects stuck to their earlier choice, there was also a significant contrast in both areas (Figure 3E and F), but they lacked the correlation between the hippocampus and the OFC. In Changed Choice trials, there was no significant contrast in the response cycle in the initial choice phase in which the subjects responded with a choice that was later reversed. However, there was a correlation between the hippocampus and the OFC in the ‘Which’ response timing (Figure 4C). Significant contrast and correlation was present in the final choice phase (Figure 4E and F, red circles and bottom scatter-plot). Finally, we also found a significant contrast in the ACC and the DLPFC; however, only in the Changed Choice trials (Supplementary Figure S5).
DISCUSSION
We identified the neural basis underlying the changing of mind, and the dynamic process appears be as follows: At first sight (initial opening cycle), (i) the caudate is activated in response to 1 face (we call it ‘Caudate-dominant face’) over the other (‘Caudate-nondominant face’). In other words, caudate activity reflects the final choice even at the very beginning of the trial, even when the subject is about to make an initial choice favoring the opposite face. This might lead to (ii) a functional correlation between the hippocampus and the OFC, which provides the conditions for the choice. The Caudate-dominant face is typically selected for the initial choice; however, occasionally the Caudate-nondominant face is selected possibly due to noise or fluctuations in competition. Indeed, only in the Choice Changed trials, the ACC and the DLPFC, which are well-known regions to correlate with a decision conflict and resolution, (MacDonald et al., 2000; Milham et al., 2001; Pochon et al., 2008) showed high activation (Supplementary Figure S5). While we admit that this is a post hoc interpretation of the activity, the known functions of these areas and the specificity to the Choice Changed trials seem to be highly consistent.
It is likely that when (i) and (ii) above are satisfied, the choice is maintained in the final judgment. When these conditions are not met, a change of mind is likely to occur. Interestingly, even in such cases, there is an increase in the activity of both the hippocampus and the OFC as well as in the functional correlation in response to a newly chosen face. However, there were some laterality differences in the connected regions (Figures 3B, C, 4C, E and F) and the increase in activity (Figure 2). This is logical if we consider that the second choice is firmer than the first one in the Choice Changed trials. Either way, our findings add a significant constraint on the manipulation effect—it reverses the choice, only (or mostly) when the neural response to the finally chosen object is high in the memory-related brain circuits (such as the hippocampus–OFC network) from the beginning. Meanwhile, the transitions from the middle to the anterior caudate (Figures 3A, D, 4A, B and D) might reflect a progression in consolidation of a preference tendency into firm preferences. Along with the shift in caudate activity, the hippocampal and OFC activity might also possibly be involved in the consolidation of a preference tendency into a firm decision. Alternatively, the activity could reflect ancillary processes such as a memory process that was engaged because of the decision (Tsukiura and Cabeza, 2011).
More precisely, our findings provide a new insight into the temporal neural processing during face preference decisions, especially regarding the function of the caudate, OFC and hippocampus. The ventral striatum and the OFC are two major subcortical and cortical regions, respectively, which have been strongly implicated in reward-related processing (Knutson et al., 2001; Cardinal et al., 2002; O’Doherty et al., 2001). In general, the ventral striatum is involved in encoding errors in predicting future rewards (i.e. the reward anticipation), whereas the OFC is involved in encoding stimulus-reward value and in representing expected future rewards (O’Doherty et al., 2004). The present data indicate a distinct contribution of these two reward-related regions in terms of temporal dissociation and consolidation during face preference decision-making. As shown by the caudate activity at first sight (the opening cycle of the initial choice phase), the relative evaluation of two faces was made instantly without any delay. The information was then transferred from the caudate to the OFC and the hippocampus, which are anatomically connected (Barbas and Blatt, 1995; Carmichael and Price, 1995; Lavenex et al., 2002). This functional connectivity between the OFC and hippocampus during face preference judgment reflects the positive signals generated by an attractive face (Tsukiura and Cabeza, 2011) and is possibly involved in the consolidation of a preference tendency into a firm decision. The current study demonstrates such a serial transfer of the preference signal from the subcortical level to the cortical level for the first time. At the same time, it also indicates that such a signal transfer often does not work; for example, in the Choice Changed cases. In a sense, the process of changing of mind is very simple; signal transfer is disrupted by some conflicting signals representing the activity of the ACC and the DLPFC, and the participant’s choice is not consistent with a Caudate-dominant face. In such cases, the choice is changed rapidly.
Moreover, our data may be interpreted as, changing of mind does not really occur in the narrower sense. It can be argued that the future decision has already been made implicitly at first sight and never been changed (as indicated by the early caudate activity). It may be because of random noise or fluctuation in the downstream processes, or overridden by the cortical deliberation system and, therefore, the ‘dominant’ face was not chosen in the initial decision. In the final decision, the original evaluation is transferred from the caudate (implicit, subcortical level) to the hippocampus and OFC (explicit, cortical level) because of the gaze manipulation, and this transfer enables the so-called changing of the mind. This interpretation is different from a more integrated/consistent view of decision making, but rather reminiscent of the ‘neural competition of choices’ idea (Reynolds et al., 1999; Resulaj et al., 2009). The results may be applicable to various other cases of decision changes in the laboratory and in everyday life.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by Tamagawa University global Center of Excellence (GCOE) program of the Japanese Ministry of Education, Culture, Sports, and Technology. SS has been supported by the Japanese Science and Technology Agency (JST) CREST program (Implicit Interpersonal Communication). TI has been supported by the Grant-in-Aid for Young Scientists (B) (No. 24730628).
REFERENCES
- Albantakis L, Deco G. Changes of mind in an attractor network of decision-making. PLOS Computational Biology. 2011;7:e1002086. doi: 10.1371/journal.pcbi.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–33. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–45. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Adolphs R, O'Doherty JP, Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proceedings of the National Academy of Sciences. 2007;104:18253–8. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nature Neuroscience. 2010;13:1292–8. doi: 10.1038/nn.2635. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. The Journal of Comparative Neurology. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Cai Y, Todorov A. The neural dynamics of updating person impressions. Social Cognitive and Affective Neuroscience. 2013;8:623–31. doi: 10.1093/scan/nss040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, et al. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–73. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Berke JD, Graybiel AM, et al. Corticostriatal interactions during learning, memory processing, and decision making. Journal of Neuroscience. 2009;29:12831–8. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. Journal of Neuroscience. 2008;28:3468–73. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. The role of neural mechanisms of attention in solving the binding problem. Neuron. 1999;24:19–29. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–66. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo S, Simion C, Shimojo E, Scheier C. Gaze bias both reflects and influences preference. Nature Neuroscience. 2003;6:1317–22. doi: 10.1038/nn1150. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Remembering beauty: roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage. 2011;54:653–60. doi: 10.1016/j.neuroimage.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.