Abstract
How children respond to social and nonsocial rewards has important implications for understanding social cognitive development. Adults find faces intrinsically rewarding. However, little is known about how children respond to face vs nonface rewards. We utilized event-related potentials (the stimulus-preceding negativity, SPN) to measure differences in reward anticipation during a guessing game in 6- to 8-year-olds. Children were presented with reward indicators accompanied by incidental face or nonface stimuli. Nonface stimuli were comprised of scrambled faces in the shape of arrows, controlling for low-level properties of the two conditions. Children showed an increased SPN when the reward stimuli were accompanied by faces, relative to nonface stimuli. This suggests that children find a face stimulus more rewarding than a nonface stimulus. The results have important implications for processing social vs nonsocial rewards in typically developing children, and allow testing of populations with deficits in social reward processing, such as autism spectrum disorder.
Keywords: event-related potentials, reward processing, faces, children, social motivation
INTRODUCTION
Most people find social interactions to be intrinsically rewarding. From birth, we have a bias to attend to faces and face-like objects (Johnson et al., 1991). Although this drive toward social stimuli is quite important for normal social functioning, we understand relatively little about the brain systems that underlie it, or how those systems develop. In addition, we know little about how social rewards are different than other kinds of rewards. The main goal of the current research is to understand how social reward systems in the brain are activated in children, and how social rewards differ from nonsocial rewards in this population.
Previous research has established that pictures of attractive faces activate reward centers of the brain (Kampe et al., 2001; Winston et al., 2007; Chatterjee et al., 2009). However, if faces themselves are rewarding, how might they compare with more concrete rewards such as money?
Multiple experiments have compared brain activity or behavioral accuracy and reaction times with faces vs money—but most of these studies have focused on between group comparisons between typically developing children and those with social impairments such as autism spectrum disorders (ASD) or attention-deficit hyperactivity disorder (ADHD) (Kohls et al., 2009b; Scott-Van Zeeland et al., 2010; Demurie et al., 2011; Kohls et al., 2011; Dichter et al., 2012;, Kohls et al., 2013). Only a few studies have directly compared responses to social and nonsocial rewards in typically developing individuals (Kohls et al., 2009a; Spreckelmeyer et al., 2009; Rademacher et al., 2010; Lin et al., 2012). In the next sections, we review previous research on behavioral, neuroimaging and electrophysiological studies.
Studies with typically developing children
Behavioral studies
To our knowledge, only one study has used behavior alone to measure reward sensitivity in typically developing children. Kohls and colleagues (2009a) used behavioral measures of reaction time and accuracy in a go/no-go task. Participants saw a stream of letters presented, and responded with button press to all letters with the exception of X. Successful inhibitions of response to X trials were rewarded with either social (happy faces) or nonsocial (monetary) feedback. Feedback for false alarms (incorrectly responding to the X) consisted of neutral faces in the social condition, or pictures of an empty wallet, signifying no money for that trial in the nonsocial condition. Response inhibition improved for both social and monetary reward conditions in this study. However, typically developing children demonstrated larger task improvement during monetary reward conditions than in the social condition.
Functional neuroimaging studies
Several studies have used functional neuroimaging to measure reward sensitivity in typically developing adults and children. Using fMRI, Spreckelmeyer et al. (2009) had participants engage in an incentive delay task with money or faces at varying degrees of reward (small, medium and large). Participants were asked to press a button as quickly as possible after seeing a target stimulus in order to get a reward. The authors found that the nucleus accumbens, putamen and thalamus were activated in a linear pattern as rewards increased for both money and face tasks. Moreover, when the authors compared brain activity patterns of males vs females, they found that monetary rewards activated a wide range of brain areas in men, while the opposite was true of women. Using the same task, Rademacher et al. (2010) found differential neural activation patterns between the social and monetary reward conditions. During cued anticipation of both reward types, the authors found activation of the nucleus accumbens. However, during the ‘consumption phase’ of reward processing (e.g. when participants saw the reward as opposed to when they anticipated the reward type), the authors found amygdala activity for social rewards, and thalamic activity during monetary rewards. Taken together, these studies suggest that the reward system is activated in response to both monetary and social rewards, but that this activation may be different between genders as well as between phases of reward processing.
In a study of typical adults, Lin et al. (2012) had participants engage in a probabilistic learning task. Participants were tested in one of two conditions. In the choice condition, participants saw two different slot machines, and were instructed to choose one. In this condition, participants’ choice led to positive, negative or neutral outcomes, depending on the slot machine chose. Participants were reinforced by either a social stimulus (a happy face and positive word for the positive slot machine, an angry face and a negative word for the slot machine associated with negative outcomes, or a blank screen for the machine associated with neutral outcomes) or a nonsocial stimulus (a dollar bill for positive outcomes, signifying that the participant would gain one dollar, and a crossed out dollar bill for negative outcomes, signifying that the participant would lose $1 or a blank screen for neutral outcomes). Thus, participants learned via trial and error during the task which slot machines were associated with positive, negative or neutral outcomes. The authors found that both monetary and social conditions caused activation in overlapping brain regions; in both conditions, activity was observed in the ventromedial prefrontal cortex and striatum that was correlated with reward magnitude (Lin et al., 2012). These results provide evidence that several types of rewards elicit brain activity in shared regions in typically developing individuals. However, it is important to note that the study utilized methods slightly different from previously discussed, because it involved probabilistic learning rather than an incentive delay procedure.
Regardless of the method employed it is likely that participants need to feel some control over the task to activate the reward system. In one fMRI study, Tsukamoto et al. (2006) found increased reward system activation in response to trials with feedback dependent on the participants’ response vs feedback given at random. This result suggests that reward systems are sensitive to the participants’ perceptions of their actions and are important to the outcome of the task.
Studies comparing typically developing children and clinical populations
Behavioral studies
Demurie et al. (2011) used an incentive delay task similar to Spreckelmeyer et al. (2009) and Rademacher et al. (2010) to measure reward sensitivity in typically developing children and children with ASD and ADHD. They found that children with both ASD and ADHD demonstrated faster reaction times in response to a monetary incentive delay task than a modified social incentive delay task, suggesting that they found the monetary reward more motivating than the social reward. Interestingly, however, typically developing children did not show this pattern—there were no differences in accuracy or reaction time between reward types for typically developing children.
FMRI studies
Dichter et al. (2012) reported that typically developing individuals demonstrated greater activation in reward circuits on money runs vs face runs during an fMRI incentive delay task. Kohls et al. (2009b) measure response inhibition using a go/no-go task in children with ADHD. Performance on the task improved during both monetary and social reward conditions in both the ADHD and control sample. In another study, Kohls and colleagues compared go/no go activation in typical development and ASD. In typically developing participants, Kohls et al. (2013) found increased activation in money vs face runs in an fMRI go/no-go task in the following reward circuit areas: caudate, putamen, thalamus and insula. However, social brain areas were more strongly activated in the face vs money runs (e.g. amygdala, fusiform gyrus, superior temporal sulcus, temporal pole and ventromedial prefrontal cortex). In contrast, participants with ASD showed hypoactivation in reward systems.
Electrophysiological studies
Event-related potentials (ERP) are brain potentials recorded at the scalp that reflect synchronous firing of groups of synapses. ERPs are temporally sensitive, and thus are an ideal metric of the anticipation of forthcoming rewards. In one study that used ERP to measure reward sensitivity, Kohls et al. (2011) reported increased neural activation as measured by a cued ‘go/no-go’ ERP paradigm in response to monetary vs social trials. These results highlight the complexity of the neural reward system, and suggest that some areas of the reward circuit might be especially sensitive to social rewards, whereas others may not.
The ERP literature has established that a component known as the stimulus preceding negativity (SPN) reflects brain activity that occurs before expected feedback about one’s performance (Damen and Brunia, 1987). In past decades, this component was sometimes known as the contingent negative variation (CNV) (Walter et al., 1964). Currently, the CNV and SPN are differentiated, and the CNV is thought to occur during preparation to respond to a stimulus (e.g. in ‘go/no-go’ tasks), and the SPN is thought to occur after a response is made but before feedback about whether or not the response was correct. The SPN is a slow wave that is prominent over the right hemisphere. It is typically measured during the last 200 ms before feedback is provided (e.g. Kotani et al., 2001; Ohgami et al., 2006). Previous studies have not measured the SPN in children, so it is unknown whether the amplitude and distribution of the SPN is similar in children and adults.
Multiple studies have confirmed that the SPN is sensitive to whether or not participants perceive feedback to be informative. Chwilla and Brunia (1991) were the first to investigate this, and found that the SPN was larger before trials with true feedback compared with false or no feedback. In the true feedback condition, participants were informed that the feedback was dependent upon their responses, whereas in the false feedback conditions, positive vs negative feedback was presented randomly. In the no feedback condition no feedback was presented. Ohgami et al. (2004) found that the SPN was larger before trials with feedback vs without feedback. Masaki et al. (2010) found similar results when participants either attempted to make a profitable choice (choice condition) vs trials where the participant’s choice had no bearing the results (no-choice). The SPN was larger for choice vs no-choice trials. Interestingly, ERP and fMRI evidence (reviewed above) converge to suggest that larger neural activation is observed when participants feel control over task outcomes and receive informative feedback. This is consistent with fMRI studies that suggest that perception of control is important for activation of the reward system (Tsukamoto et al., 2006). Together, these studies suggest that the SPN is sensitive to manipulations of feedback accuracy and whether or not the subject feels control over the outcome of any given trial.
The SPN component is thought to reflect the expectation of reward, and related activity of the dopaminergic reward system (Van Boxtel and Bocker, 2004; Mattox et al., 2006). fMRI studies provide evidence that SPN tasks elicit activity in the insular cortex (Tsukamoto et al., 2006; Kotani et al., 2009) and caudate nucleus (Delgado et al., 2000, 2003; Tricomi et al., 2004). A spatiotemporal dipole model of the SPN (Bocker et al., 1994) suggested the SPN was likely generated from the insular cortex. Both fMRI and spatiotemporal ERP studies confirm activity in the insular cortex during SPN tasks. The anterior insula is innervated with dopamine neurons (Gaspar et al., 1989), which provides further support for the idea that the SPN is related to activity in the dopamine reward system.
If the SPN component is related to activity in the reward system, it seems likely that individuals with degradation or damage to structures involving reward would show deficits in the SPN. This is demonstrated in studies comparing individuals with Parkinson’s disease (and therefore degradation of structures responsible for dopamine production, a major neurotransmitter involved in reward pathways) and control individuals. Mattox et al. (2006) demonstrated that the SPN is less pronounced in patients with Parkinson’s disease compared with healthy individuals—even when controlling for memory performance on the Weschler Memory Scale-III. This suggests that the SPN reflects activity in response to feedback concerning rewards, and is reduced in persons who have disruptions in the dopamine systems largely responsible for processing reward.
Summary
The results of previous studies of reward motivation highlight the complexity of the neural reward system, and suggest that some areas of the reward circuit might be especially sensitive to social rewards, whereas others may not. Importantly, in all of the aforementioned studies’ money runs, participants could earn money, while during face ‘runs’ participants saw pictures of faces or saw compliments displayed on-screen (e.g. Demurie et al., 2011). Thus, in one condition, participants received a tangible item for reward, whereas in the other condition, they viewed a social stimulus, but received nothing tangible. It is not difficult to imagine why earning money might be more rewarding compared with simply looking at pictures of faces. In the present study, we aimed to hold the reward constant between ‘face’ and ‘nonface’ trial blocks. By doing so, we hoped to clarify whether faces elicit greater reward-related brain activity compared with visually matched nonface stimuli, even when the pictures do not have an effect on the outcome of the task. We utilized ERPs in a SPN paradigm to measure reward anticipation-related brain activity in young children. Previous research has examined the SPN before the subject receives feedback about whether he or she is correct and therefore whether or not he or she will receive a reward (e.g. 10 cents for each correct answer) (Ohgami et al., 2004; Mattox et al., 2006; Ohgami et al., 2006; Masaki et al., 2010). We designed the current study to examine the SPN in the same way—using goldfish crackers or an equivalent snack as a reward, with an incidental social or nonsocial stimulus manipulation.
Here, we address two aspects currently missing from the literature: controlling for rewards between ‘face’ and ‘nonface’ trial blocks, and utilizing ERP methodology in order to facilitate testing younger participants. This study sheds light on the neural underpinnings of reward anticipation in children, and is informative for how typically developing children anticipate rewards that are accompanied by either social or nonsocial stimuli.
METHODS
Participants
Twenty-six participants (17 males and 9 females) were included. Participants were between 6 and 10 years old (M = 7.49, SD = 0.91). All subjects were native English speakers with no history of developmental disabilities or psychiatric conditions, and were not taking any medications for psychiatric of neurological conditions, as reported by their caregivers. One additional male participant was tested, but was excluded because we later learned that he had a first degree relative with autism spectrum disorder. This study was reviewed and approved by the University of California, San Diego institutional review board. Participants were recruited through the UCSD subject pool, and their guardians were paid $35 for their time and participation. All participants over 7 years old signed a child assent form.
Recording conditions
Participants wore a standard, fitted cap (Electrocap International, Eaton, OH, USA) with electrodes placed according to the international 10–20 system. Continuous EEG was recorded with a NeuroScan 4.5 System (Compumedics, Charlotte, NC, USA) with a reference electrode at Cz and re-referenced offline to the average activity at left and right mastoids. ERPs were recorded at 33 scalp locations using silver/silver-chloride (Ag/AgCl) electrodes at standard sites (Pz, Fz, O1, O2, P3, P4, T3, T4, T5, T6, C3, C4, Cz, F3, F4, F7, F8, A1, A2) and additional sites (CPz, FCz, CP5, CP6, CP1, CP2, FC1, FC2, FC5, FC6, FP1, FP2, AF7, AF8). Electrode resistance was kept under 10 kΩ. Continuous EEG was amplified with a low pass filter (70 Hz), a directly coupled high-pass filter (DC), and a notch filter (60 Hz). The signal was digitized at a rate of 250 samples per second via an Analog-to-Digital converter. Eye movement artifacts and blinks were monitored via horizontal electrooculogram (EOG) placed at the outer canthi of each eye and vertical EOG placed above and below the left eye. ERP trials were time locked to the onset of the reward stimulus. The baseline period was −2200 to −2000. Data were epoched from −2200 to 100 ms. The ITI was varied (1800–2000 ms between trials). Trials with no behavioral response, or electrophysiological artifacts, were excluded from the averages.
Artifacts were removed via a four step process. Initially, the first author visually inspected all data for drift exceeding +/−200 mV in all electrodes, high frequency noise visible in all electrodes >100 mV and all flatlined data. Following initial inspection, the data were epoched and eyeblink artifacts were identified using individual component analysis (ICA). Individual components were inspected alongside epoched data and blink components were removed. Next, we utilized a moving window peak-to-peak procedure in ERPlab (http://erpinfo.org/erplab. ERPLAB toolbox user’s manual, Markley et al., 2012). We utilized a 200 ms moving window, a 100 ms window step, and a 150 mV voltage threshold. An experimenter in an adjacent room observed participants during the task via webcam. Any trials during which participants looked away from the screen during or immediately prior to feedback were marked and excluded prior to final analysis. Participants were highly attentive, and rarely disengaged from the task. Most participants had no trials removed for this reason, and no participants had more than five trials removed due to eye movements or inattention. We excluded subjects who had fewer than 10 trials in their final average (N = 1). Thus, all of our statistics include data from the remaining 25 participants.
Stimuli and task
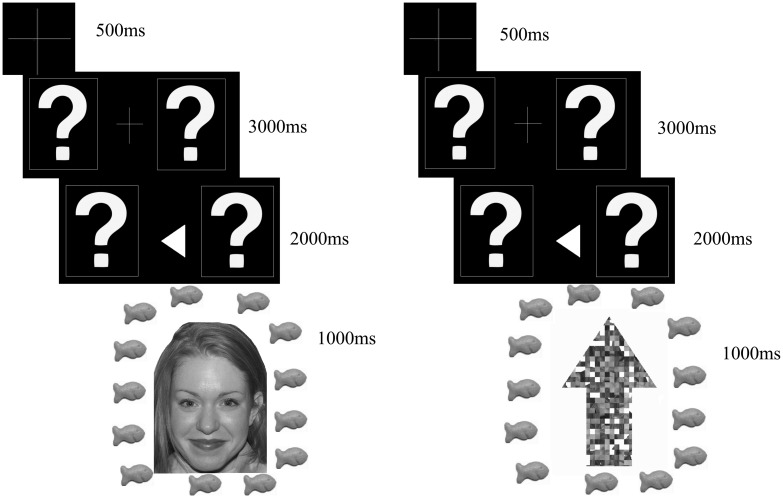
The current study had two blocks of trials, each with different feedback condition: social and nonsocial. In both blocks, at the beginning of each trial, a fixation cross appeared on screen for 500 ms. After the fixation cross, two boxes were displayed for 3000 ms; each box contained a question mark inside it. Participants were asked to guess which question mark was correct using a button pad. After participants chose the left or right box, an arrow appeared in between the question marks for 2000 ms indicating their choice (e.g. the arrow pointed left if the participant chose the left box, and right if the participant chose the right box). After 2000 ms, feedback about whether the participant guessed correctly appeared on screen for 1000 ms.
In the social block, feedback was an image of a smiling face surrounded by goldfish crackers for correct answers, and an image of a frowning face surrounded by crossed out goldfish crackers for incorrect answers. Faces were obtained from the NimStim database (Tottenham et al., 2009). In order to avoid confounds specific to gender or race, 33 faces (18 female, 15 male) from the NimStim database were utilized in the social condition. The faces were presented in pseudorandom order, with no face appearing more than once in a row. Figure 1a depicts a detailed schematic exemplar of our stimuli and timeline in the social block. In the nonsocial trial block feedback was an image of an upward facing arrow (made of scrambled face images from the social condition) surrounded by a ring of goldfish for correct answers and an image of a downwards facing arrow surrounded by a ring of crossed out goldfish crackers for incorrect answers. Figure 1b depicts a detailed schematic of our stimuli in the nonsocial block. In order to control for visual differences between the social and nonsocial feedback trials, the arrows were composed of scrambled fragments of the faces used in the social trials.
Fig. 1.
(a) Schematic of the stimuli and timing used in the ‘social’ or ‘face’ block for correct answers. Stimuli and timing for incorrect answers was identical except the goldfish were crossed out and the face was frowning. Note the arrow points in the direction of the question mark the participant selected (e.g. it points left if the participant chose the left question mark, and right if the participant chose the right question mark). (b) Schematic of the task and timing used in the ‘nonface’ or ‘arrow’ block for correct answers. Stimuli and timing was identical for incorrect answers except the goldfish were crossed out and the arrow pointed downwards.
If no choice was made, the trial ended, and the fixation cross appeared again signaling the beginning of the next trial. Participants were told that the reward for correct answers was a goldfish cracker. If participants did not want goldfish, they were told that they could trade in goldfish crackers for fruit snacks. Importantly, in both the social and nonsocial feedback trials, the face/arrow information was incidental. It was not necessary for the participant to determine whether or not their response was correct. The participants were told that correct vs incorrect responses were signaled by whether or not the goldfish were intact or crossed out. In order to control for differences in accuracy between participants, correct vs incorrect answers were predetermined by the computer program. That is, each trial was marked to be correct vs incorrect regardless of the participant’s response. There were equal numbers of ‘correct’ and ‘incorrect’ answers in pseudorandom order, with no more than three of the same answer in a row.
The two kinds of feedback trials (‘face’/‘social’ trials and arrow/‘non-social’ trials) were tested in separate blocks, each composed of 80 trials. Within each block of 80 trials, there were 30-s breaks every 15 trials. During these breaks, participants were told to relax, or move if they felt restless. Between blocks, a longer break (5–10 min) was available if the participant wished to take it.
As a manipulation check, immediately after the completion of each block, 19 of the participants rated how much they enjoyed each block of the task as well as whether they felt as though they were able to figure out the correct answers during the task on a scale from 1 to 7. We included this in order to insure that participants were equally motivated and engaged in the task across conditions.
RESULTS
All results were analyzed using JMP (version 10.0). We used repeated measures ANOVA to test for differences between conditions, hemisphere and caudality (anterior–posterior differences).
Behavioral measures
Participant’s responses about liking the guessing game, as well as their responses about getting correct answers were analyzed using matched-pairs t-tests. There was no difference between conditions for participant’s enjoyment of the game, t(18) = −0.66, P = 0.52, ns, or their perceived ability to obtain correct answers, t(18) = −0.52, P = 0.61, ns.
EEG results
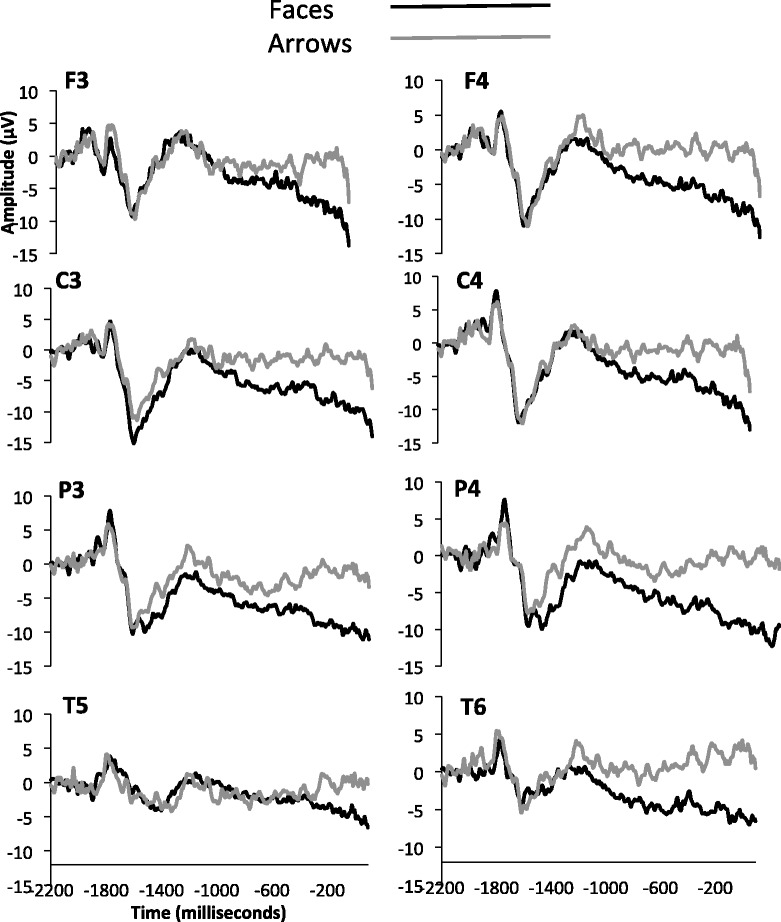
The SPN was measured as mean amplitude between −210 and −10 ms before feedback onset. The final 200 ms prior to feedback onset has been utilized in previous studies (Kotani et al., 2001; Ohgami et al., 2006). Here, we chose to analyze mean amplitude between −210 and −10 ms in order to avoid artifacts associated with feedback onset (i.e. 0 ms). We analyzed electrode F3/F4, C3/C4, P3/P4 and T3/T4, as these are the typical electrodes with maximal SPN amplitudes (Kotani et al., 2003). Grand average waveforms for the face and arrow conditions are plotted for the eight electrodes associated with the SPN in previous literature (F3, F4, C3, C4, P3, P4, T5, T6) in Figure 2. A 2 (condition) × 2 (hemisphere) × 4 (caudality) within subjects ANOVA was conducted for the eight aforementioned electrodes. Significant block effects were found such that the SPN was larger (more negative) in the face vs nonface condition, F(1, 24.1) = 7.46, P = 0.01. Significant electrode effects were observed, F(3, 70.95) = 5.27, P = 0.002. Tukey HSDs post hoc tests revealed that SPN amplitude in temporal electrodes was significantly smaller than observed in central and parietal electrodes (P < 0.05). No significant amplitude difference was observed between frontal and temporal electrodes (P > 0.05). Furthermore, there was no significant amplitude difference between frontal, temporal and parietal electrode sites. No significant effect of hemisphere was observed, F(1, 24.29) = 0.001, ns. Because there was no effect of hemisphere, we re-ran the analysis collapsed across hemispheres. All previously reported significant effects remained. In order to assess for effects of gender, we re-ran the analysis with gender as a factor. No significant effect of gender was observed, F(1, 23.06) = 0002, ns.
Fig. 2.
Grand averaged ERP waveforms from the electrodes analyzed for the SPN. Face trials are depicted with a black line, and arrow trials are depicted with a grey line.
DISCUSSION
Our results showed that children’s brain response in anticipation of a social stimulus was larger than in anticipation of a nonsocial stimulus, even when that stimulus was incidental to the expected reward. Previous studies that have used a variety of methods for measuring responses to social and nonsocial feedback (typically in functional imaging paradigms) have contrasted tangible, monetary rewards with a social (but nontangible) reward consisting of the chance to look at a face. The current study is the first to investigate neural response to social vs nonsocial rewards while keeping the rewards and visual stimuli constant between conditions. By telling participants they would earn goldfish or an equivalent snack for right answers irrespective of block type, we assured that differences between conditions were not due to varying reward values (e.g. a picture of a face vs physical money after the experiment).
Our results differ from those of Demurie et al. (2011) and Kohls et al. (2009b, 2011, 2013). These studies have generally found that performance and brain activation are enhanced when the expected reward is monetary. However, it is important to note the critical differences between these previous studies and our own. In previous studies, tangible monetary reward was contrasted with intangible, but social rewards (viewing faces). Thus it is possible that the results from the previous studies were driven by the tangibility of the reward, and that this effect masked effects of social motivation. Thus, while we found results different from previous authors, we suspect that those differences are largely accounted for by task differences.
The current study presents a novel use of the SPN component. We utilized the SPN component in order to better understand reward anticipation of social vs nonsocial stimuli. Previous research has provided important information about the location of brain structures that may respond differently to social and nonsocial rewards. However, many of these studies have generally lacked the temporal resolution necessary for clearly delineating the brain functions that anticipate the acquisition of reward. In contrast, previous SPN studies, which are ideal for measuring reward anticipation with exceptional temporal resolution, have not directly compared responses with social and nonsocial rewards.
In contrast with previous SPN literature, the current study did not observe larger SPN amplitudes in the right hemisphere (Kotani et al., 2001; Ohgami et al., 2006; Mattox et al., 2006; Masaki et al., 2010). However, Brunia et al. (2011) suggested that SPN paradigms that do not employ punishment conditions often do not find larger SPN amplitudes in the right hemisphere (Chwilla and Brunia, 1991; Kotani et al., 2003; Ohgami et al., 2004). The present study did not utilize punishment for incorrect responses, which we believe accounts for the lack of amplitude difference between hemispheres. Previous literature varies in the observed amplitudes of the SPN. Our observed mean amplitudes for the nonsocial condition are similar to those of Ohgami et al. (2006), but smaller than those reported by Kotani et al. (2003). Our observed mean amplitudes for the social condition, however, are larger than those reported in previous SPN literature. Amplitude differences between studies are likely due to variation in task requirements and stimuli. Furthermore, the previously reported SPN paradigms have utilized adult participants, while our participants were young children. It is not uncommon for ERP components to be larger in children when compared with adult participants (e.g. Taylor et al., 1999). It cannot be ruled out, therefore, whether observed amplitude differences might be due to participant age.
Finally, our study has provocative implications for the development of the reward system in young children. As the SPN is thought to reflect activity from the dopamine reward system, this study suggests that this system is highly developed in children as young as 6 years of age. Several theories of social cognitive development suggest that motivation to attend to social stimuli, or the reward that accompanies encountering social stimuli, plays a pivotal role in the development of social cognition and understanding. It is somewhat unclear, however, when this type of motivation and anticipation begins in children. Interestingly, in an ERP version of a Posner cued location paradigm, Perchet and Garcia-Larrea (2005) found that while adults demonstrated a slow negative potential akin to the CNV prior to the target, children did not demonstrate this neural pattern. The authors suggest that this CNV activity in adults reflects highly developed executive functioning, and the lack-thereof in children. In contrast, in a four choice ERP gambling task, Carlson et al. (2009), found that 8-year-old children demonstrated a clear SPN in the time between decision making and feedback.
However, it is important to note that our paradigm, as well as the gambling task used by Carlson et al. (2009) are both very different from the task used by Perchet and Garcia-Larrea (2005). In both our task and the task used by Carlson et al. (2009), participants were asked to choose a correct answer by guessing between various options. Furthermore, these paradigms were designed to elicit the SPN response, which require anticipation of an outcome. In Perchet and Garcia-Larrea’s (2005) task, however, participants were told to respond to a star, which was either correctly or incorrectly cued with a preceding rectangle. In this way, participants could use the rectangle in order to anticipate the location of the star. The task did not involve a response and anticipation of feedback, but rather a cue and then a target. Furthermore, as the task was not designed to elicit the SPN, the time between the cue (rectangle) and target (star) was only 500 ms, as opposed to typical SPN paradigms which have anticipatory periods of 2000–3000 ms. It perhaps is not surprising, then, that Perchet and Garcia-Larrea (2005), did not find evidence of anticipatory brain activity in children. Future studies might benefit from examining how early the SPN component can be measured in children and the role that task differences and complexity plays in these measurements.
Future directions
This paradigm demonstrates a novel method of successfully comparing the reward value of social vs nonsocial stimuli in young children. Several developmental disorders, including autism spectrum disorder and ADHD, are thought to involve deficits or differences in social motivation or general reward processing. In the future, studies should attempt to utilize this paradigm to better understand disorders of social or reward processing deficits. Additionally, social motivation may play a role in a number of problems of childhood and adolescence (e.g. social anxiety, substance abuse). These problems and disorders may benefit from the present methodology.
Conflict of Interest
None declared.
Acknowledgments
K.K.M.S. is supported by Autism Speaks’ Dennis Weatherstone Predoctoral Fellowship (7844). L.J.C. is supported by the National Institute of Health/ National Institute of Child Health and Human Development (NIH/NICHD R01 HD052804-01A2) and the National Institute of Neurological Disorders and Stroke/National Institute of Health (NINDS/NIH R01NS071580-01).
We thank the children and their parents for participation in this study, and the members of the DN Lab for assistance.
REFERENCES
- Böcker KBE, Brunia CHM, van den Berg-Lenssen MC. A spatiotemporal dipole model of the stimulus preceding negativity (SPN) prior to feedback stimuli. Brain Topography. 1994;7:71–88. doi: 10.1007/BF01184839. [DOI] [PubMed] [Google Scholar]
- Brunia CHM, Hackley SA, van Boxtel GJM, Kotani Y, Ohgami Y. Waiting to perceive: reward or punishment? Clinical Neurophysiology. 2011;122:858–68. doi: 10.1016/j.clinph.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Zayas V, Guthormsen A. Neural correlates of decision making on a gambling task. Child Development. 2009;80:1076–96. doi: 10.1111/j.1467-8624.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Thomas A, Smith SE, Aguirre GK. The neural response to facial attractiveness. Neuropsychology. 2009;23:135–43. doi: 10.1037/a0014430. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Brunia CHM. Event-related potentials to different feedback stimuli. Psychophysiology. 1991;28:123–32. doi: 10.1111/j.1469-8986.1991.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Damen EJ, Brunia CH. Changes in heart rate and slow brain potentials related to motor preparation and stimulus anticipation in a time estimation task. Psychophysiology. 1987;24:700–13. doi: 10.1111/j.1469-8986.1987.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52:1164–73. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Neurophysiology. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012;42:147–60. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by immunohistochemistry of tyrosine hydroxlase and dopamine-beta-hydroxylase. Comparative Neurology. 1989;279:249–71. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. 2009a;12:614–25. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Kohls G, Herpertz-Dahlmann B, Konrad K. Hyperresponsiveness to social rewards in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) Behavioral and Brain Functions. 2009b;5 doi: 10.1186/1744-9081-5-20. doi: 10.1186/1744-9081-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Rüther M, et al. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41:1523–33. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2013;8:565–72. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani Y, Hiraku S, Suda K, Aihara Y. Effect of positive and negative emotion on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology. 2001;38:873–8. doi: 10.1111/1469-8986.3860873. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Kishida S, Hiraku S, Suda K, Ishii M, Aihara Y. Effects of information and reward on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology. 2003;40:818–26. doi: 10.1111/1469-8986.00082. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Ohgami Y, Kuramoto Y, Tsukamoto T, Inoue Y, Aihara Y. The role of the right anterior insular cortext in the right hemisphere preponderance of stimulus-preceding negativity (SPN): An fMRI study. Neuroscience Letters. 2009;450:75–9. doi: 10.1016/j.neulet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience. 2012;7:274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley C, Luck S, Lopez-Calderon J. ERPlab toolbox users manual version 3.0. 2012. Retrieved from http://erpinfo.org/erplab/erplab-documentation/manual/index.html. (10 October 2012, date last accessed)
- Masaki H, Yamazaki K, Hackley SH. Stimulus-preceding negativity is modulated by action-outcome contingency. Neuroreport. 2010;21:277–81. doi: 10.1097/WNR.0b013e3283360bc3. [DOI] [PubMed] [Google Scholar]
- Mattox ST, Valle-Inclán F, Hackley SA. Psychophysiological evidence for impaired reward anticipation in Parkinson’s disease. Clinical Neurophysiology. 2006;117:2144–53. doi: 10.1016/j.clinph.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Kotani Y, Hiraku S, Aihara Y, Ishii M. Effects of reward and stimulus modality on stimulus-preceding negativity. Psychophysiology. 2004;41:729–38. doi: 10.1111/j.1469-8986.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Kotani Y, Tsukamoto T, et al. Effects of monetary reward and punishment on stimulus-preceding negativity. Psychophysiology. 2006;43:227–36. doi: 10.1111/j.1469-8986.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Perchet C, Garcia-Larrea L. Learning to react: Anticipatory mechanisms in children and adults during a visuospatial attention task. Clinical Neurophysiology. 2005;116:1906–17. doi: 10.1016/j.clinph.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Poli S, Sarlo M, Bortoletto M, Buodo G, Palomba D. Stimulus-Preceding negativity and heart rate changes in anticipation of affective pictures. International Journal of Psychophysiology. 2007;65:32–9. doi: 10.1016/j.ijpsycho.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49:3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Mochizuki Y, Masaki H, Takasawa N, Yamazaki K. Stimulus preceding negativity represents arousal induced by affective picture. International Congress Series. 2005;1278:385–8. [Google Scholar]
- Taylor MJ, McCarthy G, Saliba E, Degiovanni E. ERP evidence of developmental changes in processing of faces. Clinical Neurophysiology. 1999;110:910–5. doi: 10.1016/s1388-2457(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Kotani Y, Ohgami Y, Omura K, Inoue Y, Aihara Y. Neuroscience Letters. 2006;399:39–44. doi: 10.1016/j.neulet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Van Boxtel GJ, Böcker KB. Cortical measures of anticipation. Journal of Psychophysiology. 2004;18:61–76. [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensory-motor association and expectation in the human brain. Nature. 1964;203:380–4. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kliner JM, Perrett DI, Dolan RJ. Neuropsychologica. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]