Abstract
The emotion ‘warmth-liking’ (WL) associated with feelings of affection and acceptance is regularly activated in social contexts. WL has been suggested to be more closely related to the consummatory phase of post-goal attainment positive affect than to pre-goal attainment positive affect/approach motivation and to be partly mediated by brain opioids. To validate these assumptions we employed film/imagery to induce either a neutral emotional state or WL in female participants after intake of either placebo or the opioid antagonist naltrexone. Dependent variables were emotion self-report, interpersonal trust (TRUST, i.e. a behavioral indicator of WL) and frontal asymmetry (i.e. an electroencephalogram (EEG) indicator of approach motivation/behavioral activation). We found that participants reported more WL in the placebo/WL group than in the placebo/neutral group and both naltrexone groups. In addition, TRUST increased in the WL group after placebo, but not after naltrexone, and this pattern was reversed in the neutral control groups. Consequently, opioid blockade suppressed or even reversed the effects of the WL induction on the levels of self-report and behavior, respectively. In addition, we observed reduced relative left-frontal asymmetry in the WL (vs neutral) group, consistent with reduced approach motivation. Overall, these results suggest opioidergic influences on WL and TRUST and reduced approach motivation/behavioral activation for the positive emotion WL.
Keywords: positive emotion, endogenous opioids, psychopharmacology, interpersonal trust, frontal asymmetry
INTRODUCTION
The psychophysiology and neurobiology of positive emotions has received far less attention than the investigation of negative affect (Nitschke et al., 2004; Burgdorf and Panksepp, 2006). For example, whereas the cortical activation of various negative emotions, such as anger, fear or sadness has been extensively studied, in the positive spectrum only pleasure has been considered frequently enough to be included in a meta-analysis (Murphy et al., 2003). This relative neglect of positive emotions contrasts with their important role for intra- and interpersonal assimilation, comprising the immune system and health (Danner et al., 2001; Ostir et al., 2001; Cohn et al., 2009) cognitive functioning (Fredrickson and Branigan, 2005), coping, life satisfaction and supportive (trustful) relationships (Cohn et al., 2009). The absence of positive emotions is a characteristic property of a multitude of mental disorders associated also with impaired social qualities, such as depression (Heller et al., 2009).
Extensive work in rodents and other animals suggests at least two positive emotions with overlapping, but dissociable neurobiological foundations (Berridge and Robinson, 2003; Depue and Morrone-Strupinsky, 2005; Burgdorf and Panksepp, 2006). The ‘seeking-expectancy-wanting’ system facilitates incentive-motivated behaviors, accompanied by subjective feelings of energy, positive activation and wanting-expectancy (WE) and is mainly based on the mesolimbic dopamine system (Berridge and Robinson, 2003). In contrast, the ‘care-liking’ system motivates affiliation and caring subserving the goal of attachment. Whereas distal affiliative stimuli like other incentives activate the dopaminergic ‘seeking-expectancy-wanting’ system associated with feelings of WE, more proximal affiliative stimuli such as pleasant non-sexual touch, warmth, comforting vocalizations and caring facial expressions are related to subjective feelings of affection and acceptance, relaxation, gratifying pleasure and warmth-liking (WL) (Depue and Morrone-Strupinsky, 2005). This consummatory processes initiated after successful approach to an affiliative goal are mainly based on opioidergic rather than dopaminergic neurotransmission in the ventral striatum (see Berridge and Robinson, 2003, for review) and facilitated by the neuropeptide oxytocin (see Nelson and Panksepp, 1998, for review). Similarly, liking for sweet food rewards is not mediated by the mesolimbic dopamine system in animals and accumulating evidence suggests that the pleasure of drug reward in humans is not mediated by mesolimbic dopamine either (Berridge and Robinson, 2003). In addition, opiates have been suggested to play a role in primate sociality (Nelson and Panksepp, 1998; Depue and Morrone-Strupinsky, 2005), and µ-opioid receptors (µORs) have been implicated in feelings of interpersonal warmth and decreased incentive motivation accompanying close interpersonal relationships (Depue and Morrone-Strupinsky, 2005). Trezza et al. (2011) identified nucleus accumbens (NAc) µOR stimulation as an essential mechanism for the ascription of positive value to social interactions in adolescent rats by showing that (i) blockade of NAc µOR inhibits the play-enhancing effects of the administration of the opioid agonist morphine and (ii) stimulation of these receptors is necessary and sufficient for morphine to increase social play. There is also evidence that naltrexone has a negative impact on mother–child attachment in animals (Keverne, 1996; Nelson and Panksepp, 1998) indicating that opiate activity is important for social choices (Panksepp, 2009). Based on such suggestive evidence from animal studies Depue and Morrone-Strupinsky (2005) proposed that the magnitude of state µOR is correlated with feelings of WL evoked by affiliative stimuli in humans and other animals. However, until now opioidergic influences on affiliation and WL have received only sparse attention, especially on the human level (Depue and Morrone-Strupinsky, 2005). Here, we aim to test for the first time whether pharmacological changes in opioidergic neurotransmission modulate the effects of WL on self-report, behavior and physiology.
On the behavioral level we focused on interpersonal trust as an aspect of social behavior in humans indispensible in close interpersonal bonds. Kosfeld et al. (2005) identified oxytocin as a neural substrate not only with importance for social attachment and affiliation but also enhancing interpersonal trust in humans and increasing the benefits from close interpersonal relations. Baumgartner et al. (2008) likewise observed an association between oxytocin and interpersonal trust; conversely, Bos et al. (2010) found a downregulation of human trust by testosterone. Apart from these suggestive links the neurochemical underpinnings of interpersonal trust are still largely unknown. Assuming that opioid-based WL supports interpersonal trust given appropriate interpersonal encounters, we hypothesized that experimentally induced changes in WL through either emotion induction procedures or pharmacological manipulation of opioidergic neurotransmission should result in changes in interpersonal trust.
On the physiological level we focused on frontal EEG alpha asymmetry, for which an important role as an index of emotional and motivational states has been firmly established over the past three decades (for a review, see Coan and Allen, 2004). Whereas prior functional magnetic resonance imaging work implicated the amygdala and several prefrontal areas in aspects of interpersonal trust [e.g. the perception of trustworthiness, the establishment of a trustful relationship; see Frith (2007), Koscik and Tranel (2011), Krüger et al. (2007), Pinkham et al. (2008) and Todorov et al. (2008)], the role of asymmetrical prefrontal activity as measured with frontal EEG alpha asymmetry is currently unknown.
The valence model of frontal asymmetry postulates relatively greater left- and right-sided frontal activity for positive and negative emotions, respectively (e.g. Heller, 1990; Gotlib et al., 1998), but subsequent research also showed left-sided lateralization for the negative emotion of anger, which is usually associated with an approach tendency (e.g. Harmon-Jones and Siegelman, 2001; Wacker et al., 2003) as well as for a negative state of fear associated with a strong flight tendency (Wacker et al., 2008). Consequently, current models typically predict emotion/motivation-dependent lateralization along the dimensions of approach–withdrawal (approach–withdrawal model: approach = left, withdrawal = right; Harmon-Jones et al., 2010) or behavioral activation–behavioral inhibition (behavioral activation–behavioral inhibition model: activation = left, inhibition = right; Wacker et al., 2003, 2008). Notably, whereas these more recent models are largely based on findings with negative emotional states, both would predict stronger left-sided activity for positive emotional states associated with approach motivation/behavioral activation as well as reduced left-sided activity for positive emotional states associated with reduced approach motivation/behavioral activation. As described earlier the positive feeling of WL is more closely linked to the consummatory phase of attaining an affiliative goal, than to pre-goal attainment positive affect/approach motivation or WE. Thus, whereas the valence model of frontal asymmetry predicts increased left-sided frontal activity, current models would predict decreased left-sided or no differences in frontal activity for consummatory proximal WL (compared to a neutral control group) due to the association with low approach/behavioral activation despite positive valence, mirroring prior findings for anger (negative valence, but approach and consequently increased left-sided activity, see above).
Similarly, it is currently unknown whether EEG frontal asymmetry ASY is associated with opioidergic neurotransmission. From the perspective of the valence model one might expect that decreased opioidergic tone should be associated with decreased left-sided frontal activity (particularly after induction of positive emotions like WL) due to the association between opioids and pleasure. The predictions of the two current models are less clear, although both suggest a more direct link between frontal asymmetry and dopaminergic rather than opioidergic neurotransmission due to dopamine’s well-established role in incentive motivational processes (see Wacker et al., 2013). Consequently, both the approach–withdrawal and the behavioral activation–behavioral inhibition models imply that associations between frontal asymmetry and opioids, if present at all, should be more indirect and weaker than those with dopamine.
Summing up, this study aims to contribute to our still limited knowledge in the domain of positive emotions by examining the emotion WL from a multi-level perspective. We predicted that (i) the induction of WL would result in higher ratings of WL and higher interpersonal trust and that (ii) these effects would be reduced by a pharmacological blockade of opioidergic neurotransmission. In addition, we probed for the first time, whether the induction of WL and/or opioidergic blockade are associated with ASY.
METHODS
Due to space restrictions we will only highlight the main methodological features here. Further details can be found in the Supplementary material.
Participants and experimental design
The final sample comprised N = 95 psychiatrically healthy, non-smoking female participants currently using oral contraceptives but no other medication (mean age = 22.6 years, s.d. = 2.47, range 18–31) who were randomly allocated to one of four groups defined by the between-subject factors Emotion (neutral control, WL) and Substance (placebo, naltrexone). Additional inclusion criteria are listed in the Supplementary material. In a placebo-controlled double blind design participants received orally either placebo or the competitive opioid receptor antagonist naltrexone (25 mg). For induction of WL and an emotionally neutral state we used personal memories from childhood, including either a close person for WL or a casual acquaintance for the neutral state, respectively. In addition, a short emotional film clip was presented.
Dependent variables
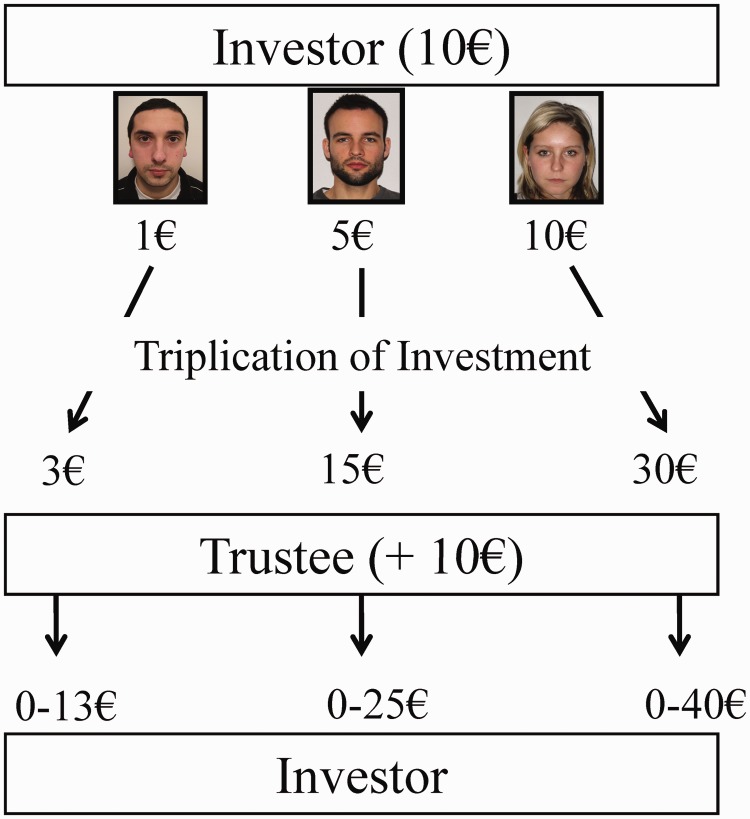
To assess the effects of the induced WL on interpersonal trust we employed an adapted version of the trust game employed by Kosfeld et al. (2005). Based on photographs of other players participants had to anticipate the other players’ behavior and were requested to decide how much money to entrust to the person in the picture. Participants were told that this task would assess the degree of their emotional intelligence (‘Menschenkenntnis’) (Figure 1). The average amount of money entrusted constituted our measure of interpersonal trust (TRUST).
Fig. 1.
The trust game. Both players receive a starting capital of 10€. The investor then decides how much of this money he wants to give to the trustee presented in a photograph. The trustee receives the investment after triplication by the experimenter and is given the choice to return any amount between 0 and the total sum of money available to him (investment*3 + 10€). The back transfer is not tripled again. As an example, the investor decides to invest 5€, the trustee’s total amount is 25€, because of the triplication and his own starting capital. The trustee can now return any amount between 0 and 25€. Therefore, the investor’s profit depends on his investment and the trustee’s back transfer. The next trail starts with a new trustee and a new starting capital of 10€. Participants completed 10 trials as investor in each of the two trust games.
To distinguish interpersonal trust from mere effects in risk taking we additionally presented a real-estate game, which closely mirrors the structure of the trust game with the difference that money had to be invested in buildings with allegedly objectively determined relative market values rather than people.
Participants provided a nine-point intensity rating of several emotional states at different times of the main session (Figure 2; Supplementary material). Here, we focus on the two most relevant scales: ‘WL’ (cozy, comforting, liked and secure) and ‘WE’ (pleasant anticipation, eager, inspired, drive and confident).
Fig. 2.
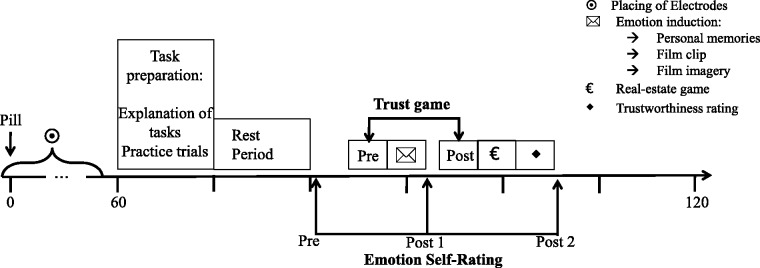
Schematic of the procedure during the main testing session. The initial rest period started about 60 min after intake of the pill and was followed by repeated administrations of an emotional self-report and the trust game, before and after an emotion induction through personal memories and brief film clips. Subsequently, participants completed a real-estate game and rated the trustworthiness of the trustees encountered in the trust game.
Frontal alpha asymmetry
For a detailed description of EEG recording, data reduction and analysis, see Supplementary material. EEG was recorded during three different recording times (initial rest period, personal memory and post-film imagery) and for each phase asymmetry scores (ASY) were computed as the difference of ln-transformed alpha power at homologous right and left sites [e.g. ASY = ln(alpha power@F4) − ln(alpha power@F3)]. To provide a focused test of our a priori hypotheses, we averaged asymmetry scores across the two sites for which Wacker et al. (2013) previously observed consistent dopaminergic personality and gene effects (AF4–AF3 and F4–F3), and—for comparison purposes—across two parietal sites (PO4–PO3 and P4–P3). All analyses were performed with these measures of frontal and parietal asymmetry within each of two alpha bands (low alpha, 8–10 Hz and high alpha, 11–13 Hz).
Procedure
A schematic illustration of the testing procedure is shown in Figure 2. After task preparation and an initial rest period, participants provided the emotion self-rating scales, followed by the pre-induction trust game. The subsequent induction of emotions was followed by a post-1 self-rating and the post-induction trust game. In addition, the real-estate game and a trustworthiness-rating were presented. The testing session was finalized by a post-2 self-rating.
RESULTS
Side effects and blindness to pharmacological treatment
Eight of 107 participants reported adverse side effects such as nausea and/or dizziness and were consequently excluded from further analyses. The two substance groups differed in the percentage of participants who in the post-experimental interview in a forced choice question guessed that they had taken the drug [naltrexone: 25.5%, placebo: 6.3%, Chi2(df = 1) = 6.67, P = 0.036]. Twelve participants reported being more than 90% sure of their guess, the average of all participants was 64.8% (s.d. = 21.14). All ‘sure’ participants guessed that they had received placebo, although seven of them had in fact received naltrexone. Thus, it can be concluded that participants in the analysis sample were reasonably blind to the substance received, with only a few of those in the naltrexone group having a hunch that they had received an active substance.
Emotion self-report
As expected, a three-way repeated measures ANOVA (Time × Emotion × Substance) showed a significant Time × Emotion interaction for WL ratings, indicating that WL changes across time differed between emotion groups, F(2,91) = 9.75, P < 0.001. No significant differences were observed between the four groups at baseline pre-memory/film. As shown in Figure 3 and Table 1, the induction of WL under placebo resulted in a significant increase in WL ratings from baseline (Pre) to directly after the emotion induction (Post 1), t(91) = 4.06, P < 0.001, as well as to after the second trust game, t(91) = 2.19, P = 0.03. The increase in WL ratings after induction of WL was also significant under naltrexone, t(91) = 2.03, P = 0.045, but by the end of the second trust game no longer existent, t(91) = −1.33, P = 0.19. No effects were observed within the two neutral CO groups, all Ps > 0.10. Under placebo, the increases in the WL group were larger than in the CO group, t(91) = 3.55 and 2.33, P = 0.0006 and 0.02, for Post 1–Pre and Post 2–Pre, respectively. In addition, relative to placebo, naltrexone significantly reduced the increases in the WL groups measured after the second trust game (Post 2–Pre), t(91) = 2.47, P = 0.015, although not significantly so directly after the emotion induction (Post 1–Pre), t(91) = 1.34, P = 0.18. Furthermore, as expected, WL ratings in the WL groups were lower under naltrexone than placebo after the second trust game (POST 2), t(91) = 2.18, P = 0.032, and marginally lower directly after the emotion induction (POST 1), t(91) = 1.72, P = 0.089. WL ratings in the WL group under placebo were higher than those in both CO groups directly after the emotion induction and after the second trust game, all Ps < 0.05.
Fig. 3.
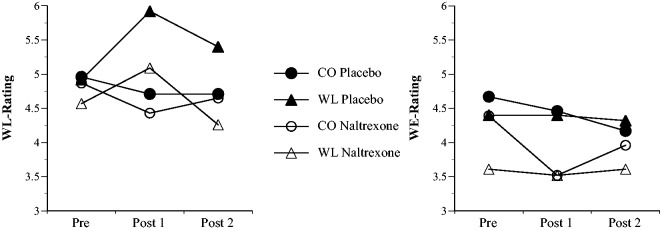
Mean WL ratings (left) and WE ratings (right) in the four experimental groups over time. CO, neutral control; Pre, before emotion induction; Post 1, after emotion induction; Post 2, after second trust game.
Table 1.
Means (s.d.) of WL and WE ratings
| Emotion | Substance | Time |
||
|---|---|---|---|---|
| Pre | Post 1 | Post 2 | ||
| WL rating | ||||
| Neutral control | Placebo | 4.96 (1.57)a1 | 4.71 (1.52)a1 | 4.71 (1.9)ab1 |
| Naltrexone | 4.87 (1.60)a1 | 4.43 (1.73)a1 | 4.65 (1.82)ab1 | |
| WL | Placebo | 4.92 (1.58)a1 | 5.92 (1.44)b2 | 5.40 (1.73)b3 |
| Naltrexone | 4.57 (1.62)a1 | 5.09 (2.00)ab2 | 4.26 (1.79)a1 | |
| WE rating | ||||
| Neutral control | Placebo | 4.67 (1.69)a1 | 4.46 (2.06)a12 | 4.17 (2.12)a2 |
| Naltrexone | 4.39 (1.70)ab1 | 3.52 (1.68)a2 | 3.96 (1.72)a12 | |
| WL | Placebo | 4.40 (1.63)ab1 | 4.40 (1.66)a1 | 4.32 (1.82)a1 |
| Naltrexone | 3.61 (1.64)b1 | 3.52 (1.31)a1 | 3.61 (1.78)a1 | |
Pre, rating before emotion induction; Post 1, rating directly after emotion induction; Post 2, rating after second trust game, real estate game and trustworthiness ratings. For each rating, values within each column (row) not sharing any superscript letters (digits) differ significantly, P < 0.05.
In sum, (i) the emotion induction resulted in higher WL ratings in the WL group, but not in the neutral CO groups immediately after the emotion induction and (ii) WL ratings post-memory/film in the WL group were lower under naltrexone than under placebo.
Although WL ratings were substantially correlated with ratings of the approach-related positive emotion WE [r(93) = 0.49, 0.52 and 0.59, Ps < 0.0001, for Pre, Post 1 and Post 2, respectively], an analogous three-way repeated measures ANOVA computed for WE ratings only revealed an unexpected trend for the main effect of Substance, F(1,91) = 3.82, P = 0.053, due to slightly decreased scores in self-reported WE under naltrexone vs placebo, mean (s.d.) = 3.77 (1.64) and 4.40 (1.82), respectively (Figure 3; Table 1; P ≥ 0.064 for all other F-tests). Notably, no significant changes from baseline were observed for either of the two WL groups, |t(91)| ≤ 0.33, P ≥ 0.74 (see Table 1 for a summary of contrast tests).
Interpersonal trust
Preliminary analyses confirmed that for both the first and the second trust game the 10 trustees differed considerably in the average amount they were entrusted with by participants, F(9,94) ≥ 13.22, P < 0.0001. Whereas the trustee who appeared most trustworthy on average received 5.82€ (s.d. = 2.06), the trustee who appeared least trustworthy on average only received 3.52€ (s.d. = 1.97). Across the 20 trustees the average amount of money entrusted and the average rating of trustworthiness were almost perfectly correlated, r(18) = 0.98, P < 0.0001. Obviously, our cover story had successfully concealed the true purpose of the game, that is, to assess interpersonal trust rather than ‘Menschenkenntnis’ and participants’ behavior in the game was indeed guided by the trustees’ perceived trustworthiness.
A three-way repeated measures ANOVA (Time × Emotion × Substance) revealed a significant main effect for Time, F(1,91) = 26.07, P < 0.001, due to a general increase in the amount invested by participants post-emotion-induction. More importantly, a significant Time × Emotion × Substance interaction indicated that the changes across the two measurements differed between the four groups, F(1,91) = 29.95, P < 0.001. Whereas no group differences were observed at baseline (Pre), TRUST significantly increased in the WL group under placebo, but neither in the neutral CO group under placebo nor in the WL group under naltrexone (Table 2; Figure 4). Furthermore, the increase in TRUST in the WL group under placebo was significantly larger than in the neutral CO group under placebo, t(91) = 3.24, P = 0.0017, and the WL group under naltrexone, t(91) = 4.71, P < 0.001. In addition, as expected, after memory/film the WL group showed higher trust than the neutral CO group under placebo, t(91) = 2.21, P = 0.03. However, we also observed an unexpected increase in TRUST in the neutral CO group after naltrexone, which surpassed both the changes in the neutral CO group under placebo, t(91) = 3.04, P = 0.003, and in the WL group under naltrexone, t(91) = 4.48, P < 0.001.
Table 2.
Means (s.d.) of interpersonal trust (TRUST)
| Emotion | Substance | Time |
|
|---|---|---|---|
| TRUST Pre | TRUST Post | ||
| Neutral control | Placebo | 4.41 (1.24)a1 | 4.52 (1.46)a1 |
| Naltrexone | 4.38 (1.23)a1 | 5.00 (1.25)ab2 | |
| WL | Placebo | 4.77 (1.41)a1 | 5.41 (1.57)b2 |
| Naltrexone | 5.06 (1.32)a1 | 4.92 (1.33)ab1 | |
TRUST Pre, trust game before emotion induction; TRUST Post, trust game after emotion induction. Values within each column (row) not sharing any superscript letters (digits) differ significantly, P < 0.05.
Fig. 4.

Mean interpersonal trust in the four experimental groups over time.
A two-way ANOVA for the investments in the real-estate game with the between-subjects factors Emotion and Substance only revealed a significant Emotion × Substance interaction, F(1,91) = 4.25, P = 0.042. Contrasts revealed that this effect was largely due to higher real-estate investments in the neutral CO group after naltrexone vs placebo, t(91) = 2.08, P = 0.04. No differences were observed between the two substance groups (naltrexone vs placebo) under WL nor between the two emotion groups (WL vs neutral) under placebo, all Ps > 0.10. Thus, only the unexpected effect of higher TRUST in the neutral CO group after naltrexone was mirrored by higher real-estate investments, indicating that this particular effect may be driven by the risk-taking component shared by the two tasks rather than by interpersonal trust.
EEG frontal alpha asymmetry
A three-way ANOVA (Recording Time × Emotion × Substance) for frontal ASY in the low alpha band revealed no significant main effects or interactions of substance group on frontal asymmetry, Ps ≥ 0.37, indicating no influence of opioidergic blockade on this measure. However, we observed a significant Recording Time × Emotion interaction, F(2,87) = 3.37, P = 0.039. Computation of post hoc contrasts showed that this effect was due to significant differences between WL and neutral CO groups in the asymmetry changes from rest to personal memories, t(87) = 2.53, P = 0.03, but not from rest to film imagery, t(87) = 0.12, P = 1.00 (both P-values Bonferroni-corrected for the two comparisons of changes from rest, see table 3, Figure 5). Further follow-up contrasts revealed that personal memories vs rest were associated with shifts toward more left-frontal cortical activity in the neutral CO groups, t(87) = 3.20, P = 0.004, but with non-significant shifts toward more right-frontal cortical activity in the WL groups (P-values Bonferroni-corrected for the two tests for changes from rest to personal memories within groups). As in our previous work, group differences were only observed in ASY changes but not in the absolute ASY values at any of the three measurements.
Table 3.
Means (s.d.) frontal low alpha asymmetry
| Emotion | Time |
||
|---|---|---|---|
| Rest period | Personal memories | Film imagery | |
| Neutral control | −0.115 (0.146)a1 | −0.047 (0.154)a2 | −0.107 (0.135)a12 |
| WL | −0.094 (0.097)a1 | −0.100 (0.140)a1 | −0.082 (0.151)a1 |
Values within each column (row) not sharing any superscript letters (digits) differ significantly, P < 0.05.
Fig. 5.
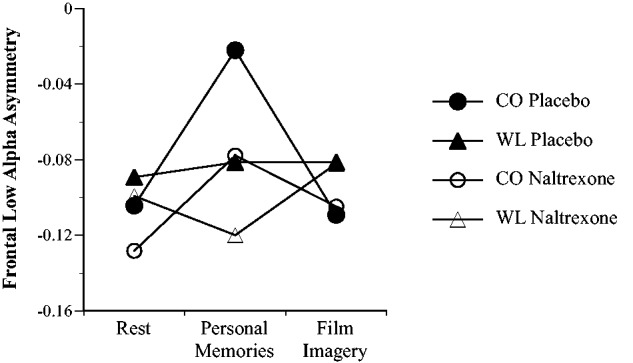
Frontal low alpha asymmetry in the four experimental groups for initial rest period, personal memories and film imagery EEG recordings.
A series of supplementary analyses computed to probe the specificity of the effect of Emotion on memory-induced changes showed a similar (although not statistically reliable) effect in the high alpha band for frontal ASY, t(87) = 1.58, P = 0.12, but not in either alpha band for parietal ASY, |t(87)| ≤ 1.11, P ≥ 0.27.
EEG frontal alpha asymmetry, emotion self-report and interpersonal trust
We computed correlations to probe whether the absence of a significant overall change in frontal ASY from baseline within the WL groups was due to individual differences in the extent to which our emotion induction successfully induced WL. Indeed, in the WL groups, but not the neutral CO groups, self-reported WL after the emotion induction [average of ratings directly after emotion induction (Post 1) and after second trust game, real estate game and trustworthiness ratings (Post 2)] was significantly correlated with the changes in frontal low alpha ASY after personal memories, r = −0.35 and −0.02, P = 0.02 and 0.92, for the WL and CO groups, respectively. Moderated regression analyses with frontal low alpha ASY changes as the dependent variable and Substance, self-reported WL after the emotion induction (centered within Substance groups) and the Substance × self-reported WL interaction as predictors computed separately for the WL and CO groups did not reveal moderating influences of Substance group on either of these associations. Furthermore, the association observed in the WL groups persisted, even after partialling the concurrent measurement of self-reported WE suggesting considerable specificity, rpartial = −0.37, P = 0.01. Thus, higher levels of self-reported WL were associated with greater shifts toward lower left-frontal cortical activity after personal memories designed to induce WL even after controlling for individual differences in approach-related positive affect (i.e. WE ratings).
Finally, we also probed whether changes toward greater TRUST are associated with both self-reported WL and the degree to which personal memories induced changes toward less left-frontal cortical activity. To this end we computed a general linear model in which changes in TRUST (Post–Pre-emotion induction) were predicted by Emotion, Substance, Emotion × Substance, self-reported WL (again averaged across Post 1 and Post 2), frontal ASY change scores (personal memories minus rest) and the interactions of each of the two continuous predictors with Emotion, Substance and Emotion × Substance (both self-reported WL and frontal ASY change scores were centered within Emotion × Substance groups). This analysis revealed two additional significant effects apart from the Emotion × Substance interaction, F(1,79) = 30.38, P < 0.0001: first, a main effect of self-reported WL, F(1,79) = 5.47, P = 0.02, mirroring a significant positive correlation between self-reported WL and changes in TRUST in the whole sample, r = 0.23, N = 91, P = 0.03, and, second, an interaction of Substance and frontal ASY change, F(1,79) = 7.28, P < 0.01, due to a significant negative correlation between frontal ASY change and changes in TRUST in the placebo group, r = −0.40, N = 48, P = 0.005, and a significant positive correlation between the two variables in the naltrexone group, r = 0.37, N = 43, P = 0.01.
DISCUSSION
Combining emotional personal memories and film clips to induce emotional states this study showed for the first time that pharmacological changes in opioidergic neurotransmission modulate the positive emotion of WL and its influence on a behavioral measure of interpersonal trust. In addition, we provide initial evidence for an association between WL and reduced left-sided frontal cortical activity. These findings (i) validate a novel procedure for a highly specific emotion induction, (ii) support Depue and Morrone-Strupinsky’s (2005) theoretical implication of opioids in WL and (iii) provide further evidence against the valence model of frontal EEG asymmetry from the realm of positive emotions complementing prior findings with negative emotions (Wacker et al., 2003, 2008; Harmon-Jones et al., 2010). We will now discuss each of these points in more detail.
Validity and specificity of the warmth-liking induction
Under placebo the WL group showed stronger memory/film-induced increases in WL ratings as well as higher WL ratings both directly after the induction (Post 1) and more than 10 min later (Post 2) relative to the neutral CO group. In addition, although WL ratings and WE ratings were substantially correlated, effects of the emotion induction were only observed for the former. Finally, under placebo the emotion induction also resulted in a stronger increase in interpersonal trust (but not higher risk-taking in the investment game) and a stronger reduction in relative left-frontal cortical activity (but not in changes in asymmetric cortical activity in more posterior regions) in the WL group relative to the neutral group, although it should be noted that the effect of the emotion induction of frontal asymmetry was only significant for personal memories but not for the film clips. Taken together, these converging observations from the levels of self-report, behavior and physiology indicate that the present approach to combine personal memories of loved ones with carefully selected film clips was successful in specifically inducing WL vs WE (i.e. an appetitive pre-goal attainment form of positive affect). Future studies aiming to induce WL may benefit from adopting the present emotion induction procedure (possibly even without complementing the personal memories by a film clip).
Opioidergic influences on warmth-liking and interpersonal trust
Pharmacological blockade of opioidergic neurotransmission through administration of naltrexone resulted in lower WL ratings both directly after the emotion induction (Post 1) and more than 10 min later (Post 2) in the WL groups. Such effects of naltrexone on WL ratings were neither observed before the emotion induction nor in the neutral CO groups, indicating considerable specificity to the experimentally induced state of WL. In contrast, for WE ratings we only observed a general tendency toward decreased values under naltrexone that might be due to mild side effects or a general emotional blunting effect of naltrexone (e.g. increased fatigue and consequently reduced WE). This specificity of the naltrexone effects is consistent with the idea that WL is more closely linked to post-goal attainment (consummatory) positive affect and the hedonic impact of reward, which has been previously shown to be sensitive to opioid blockade in humans (Petrovic et al., 2008).
Furthermore, administration of the opioid blocker naltrexone also resulted in a complete blockade of the memory/film-induced increase in interpersonal trust in the WL groups: Under placebo, the 10 trustees in the game after the emotion induction on average received over 6€ more than the 10 trustees in the game before the induction, whereas after naltrexone the same 10 trustees after the induction on average received around 1€ less than those before. This significant reduction in the memory/film-induced investments in other people by naltrexone was not mirrored by a similar reduction in real estate investments in the real-estate game played directly after the second trust game, indicating that the effect was driven by interpersonal trust rather than by the risk-taking component shared by both games (high risk-taking should be associated with increased investments in both games). Because a significant difference between naltrexone and placebo was again observed for ratings of the trustee’s trustworthiness obtained after the real-estate game, it seems unlikely that a reduction of the pharmacological effect of naltrexone between second trust game and real-estate game underlies the absence of a significant naltrexone effect in the latter. These findings concord with Kosfeld et al. (2005), who suggested specific effects of oxytocin on trust but not risk taking, as well as with Houser et al. (2010), who found converging evidence for the discrimination of trustful behavior and risk attitudes.
Moreover, the effect of opioid blockade on the memory/film-induced changes in TRUST was strongly modulated by the emotion induction: In contrast to its TRUST-reducing effect in the WL group, naltrexone even increased interpersonal trust in the neutral CO group. Whereas the modulating influence of the WL induction on the naltrexone effect supports the hypothesized link between WL and opioidergic neurotransmission, the trust-increasing effect of naltrexone observed for the neutral CO group was unexpected. Note, however, that a similar effect was also observed for the investments in the real-estate game, suggesting that this particular effect may be due to the risk-taking component shared by the two games rather than due to interpersonal trust specific to the trust game. Possibly participants in the relatively uneventful neutral CO group were more inclined to make use of the stimulating effects of taking bigger risks to ameliorate or distract themselves from naltrexone’s mild side effects such as fatigue.
The converging evidence of a modulatory influence of changes in opioidergic neurotransmission on WL effects on the levels of both self-report (WL rating) and behavior (TRUST) strongly support Depue and Morrone-Strupinsky’s (2005) suggestion of a link between opioid receptor stimulation and sensations of interpersonal warmth and affection. The inhibition of µ-OR by naltrexone reduced (or even eliminated) the subjective experience of WL and trustful interpersonal behavior induced by affiliative stimuli. The present findings, thus, suggest that µ-OR stimulation not only plays an important role for social behavior in primates and animals (Nelson and Panksepp, 1998; Depue and Morrone-Strupinsky, 2005; Trezza et al., 2011) but also in humans.
Warmth-liking, opioids and frontal EEG asymmetry
Reliving brief emotional film clips designed to induce either a neutral emotional state or WL did not have any significant effects on frontal EEG asymmetry. Possibly, the emotional states induced by the WL clip were too weak or too variable across subjects to produce reliable EEG effects. However, the induction of WL through personal memories in the WL groups resulted in a significant reduction in the shift toward relative left-frontal cortical activity seen in the neutral CO groups. As expected, this finding was significant in the low alpha band (8–10 Hz), but not in the high alpha band (10–13 Hz) supporting previous suggestions that the low alpha band is more sensitive to affective states (e.g. Goncharova and Davidson, 1995; Davidson et al., 2000; Wacker et al., 2003). In addition, the effect also showed the expected specificity to frontal regions.
The significant change in frontal asymmetry from resting baseline in the neutral CO groups (toward more left-frontal cortical activity) may be due to activation of a left-lateralized network during autobiographical memories (Svoboda et al., 2006). Possibly the left-lateralizing effect of autobiographical memories was cancelled out by a right-lateralizing effect of WL (due to the association with consummatory goal achievement) leading to no significant overall changes from resting baseline in the emotion groups. Most importantly, it is less likely that the difference in asymmetry changes between the CO and WL groups are driven by higher approach motivation/behavioral activation in the CO groups rather than higher WL in the WL groups for several reasons. First, the CO and WL groups differed only in WL ratings but not in WE ratings (i.e. the affective state associated with approach motivation/behavioral activation). Second, if anything, WE ratings decreased from baseline in the neutral CO groups, indicating reductions rather than increases in approach motivation/behavioral activation. Third, within the WL groups higher WL ratings after the emotion induction were associated with changes toward less left-frontal cortical activity even after partialling concurrent WE ratings. Finally, under placebo higher increases in TRUST after the emotion induction (i.e. a behavioral indicator of WL) were likewise associated with changes toward less left-frontal cortical activity.
In sum, the present pattern of frontal asymmetry findings tentatively suggests that the net effect of WL on frontal asymmetry is a reduction of relative left-frontal cortical activity, because WL eliminated the left-lateralizing effects of personal memories. This proposition is consistent with Depue and Morrone-Strupinsky’s (2005) assumption that WL is associated with the consummatory rather than the anticipatory phase of goal attainment. In addition, the present findings provide further evidence against the valence model of frontal EEG asymmetry (positive = left, negative = right; e.g. Heller, 1990; Gotlib et al., 1998) by demonstrating that both individual differences in self-reported WL (i.e. a positive emotional state presumably related to low approach motivation/behavioral activation) and the net effect of the induction of WL are associated with decreased relative left-frontal cortical activity. It should, however, be noted that we only observed a modulatory effect of opioidergic neurotransmission on the effects of the WL induction for WL ratings and TRUST, but not for frontal asymmetry, indicating that the latter is only indirectly linked to the opioidergic basis of WL. In fact, given our substantial sample size we can effectively rule out a ‘large’ main effect of naltrexone (25 mg) on frontal asymmetry (type II error < 0.05 for Cohen’s d = 0.8) despite significant substance effects on the levels of self-report and behavior. On the other hand, we did observe a modulatory effect of opioid receptor blockade on the association between frontal asymmetry and TRUST changes. Whereas frontal asymmetry and TRUST changes were negatively correlated under placebo, a significant positive association was found under the opioid blocker naltrexone. Possibly, opioids modulate WL and its more direct behavioral expressions (including TRUST), whereas WL, in turn, only reduces left-frontal asymmetry and approach motivation/behavioral activation in some individuals (e.g. depending on individual levels of state/trait approach motivation and/or dopaminergic system parameters; see Wacker et al., 2013). In fact, in this study minimization of opioid-related frontal asymmetry and TRUST variance under naltrexone may have functioned to unveil a positive association between the two (e.g. due to a shared approach/activation vs inhibition-component associated with both left-frontal cortical activity and financial risk taking in the trust game).
Limitations and future research
First, because in this initial study we only investigated young females in heterosexual relationships with use of oral contraceptives, who were currently in a heterosexual relationship, it remains an open question whether the present findings generalize to more diverse samples. Future research may also benefit from incorporating potential correlates of individual differences in WL, for instance, trait affiliation (Depue and Morrone-Strupinsky, 2005) or opioid-receptor genes previously shown to moderate social reward (Trezza et al., 2011). Second, because we did not obtain separate WL ratings for the two induction methods, the relative success in inducing the targeted emotion cannot be fully evaluated. However, as the frontal asymmetry effects observed for personal memories were not detectable for the film clips as well, it is possible that despite extensive pre-testing our WL film clips may have been less suitable than anticipated. Third, although our measure of interpersonal trust is based on a well-established paradigm (Kosfeld et al., 2005) and also includes a control for potential confounds of risk behavior, the inclusion of monetary winnings in the trust game represents an approach motivational context that may or may not interact with the observed WL effects. Consequently, a transposition to a non-monetary trust game, e.g. a prisoners dilemma game (Insko et al., 2005), with days in prison instead of money or points (Schipper and Petermann, 2011), could be conducted in future research. Finally, although naltrexone acts specifically on µORs, it remains unclear whether the observed effects are direct consequences of changes in opioidergic transmission or instead attributable to side effects of naltrexone or indirect effects on other neuropeptides, such as oxytocin. This limitation can be partly overcome by comparing naltrexone’s effects to other pharmacological substances in future work.
CONCLUSIONS
This study showed for the first time that a pharmacological change in opioidergic neurotransmission modulates both the emotion WL and interpersonal trust and suggests that the positive emotion WL is associated with reduced left-frontal asymmetry. These findings support neurobiological theories gleaned from basic neuroscience work in animals that distinguish between positive feelings associated with approach motivation/wanting expectancy and consummatory pleasure/WL (Depue and Morrone-Strupinsky, 2005) and suggest several new opportunities for research on positive emotions in humans.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (grant number WA 2593/5-1). The authors wish to thank Annelie Tuchscherer for her help with scoring and analyzing the EEG data.
REFERENCES
- Baumgartner T, Heinrichs M, Vonlanten A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Berridge K, Robinson T. Parsing reward. Trends in Neuroscience. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bos PA, Terburg D, van Honk J. Testosterone decreases trust in socially naïve humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(22):9991–5. doi: 10.1073/pnas.0911700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and Biobehavioral Reviews. 2006;30:173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohn M, Fredrickson B, Brown S, Mikels J, Conway A. Happiness unpacked: positive emotions increase life satisfaction by building resilience. Emotion. 2009;9(3):361–8. doi: 10.1037/a0015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D, Snowdon D, Friesen W. Positive emotions in early life and longevity: findings from the nun study. Journal of Personality and Social Psychology. 2001;80:804–13. [PubMed] [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- Depue R, Morrone-Strupinsky J. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–95. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Fredrickson B, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion. 2005;19:313–32. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The social brain? Philosophical Transactions of the Royal Society B. 2007;362:671–8. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, Davidson RJ. The factor structure of EEG: differential validity of low and high alpha power asymmetry in predicting affective style. Psychophysiology. 1995;32:S35. [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition and Emotion. 1998;12(3):449–78. [Google Scholar]
- Harmon-Jones E, Gable P, Peterson C. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84:451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80:797–803. [PubMed] [Google Scholar]
- Heller A, Johnstone T, Shackman A, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22445–50. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. The neuropsychology of emotion: developmental patterns and implications for psychopathology. In: Stein NL, Leventhal B, Trabasso T, editors. Psychological and Biological Approaches to Emotion. Hillsdale, NJ: Erlbaum; 1990. pp. 167–211. [Google Scholar]
- Houser D, Schunk D, Winter J. Distinguishing trust from risk: an anatomy of the investment game. Journal of Economic Behavior & Organisation. 2010;74:72–81. [Google Scholar]
- Insko CA, Kirchner JL, Pinter B, Efaw J, Wildschut T. Interindividual-intergroup discontinuity as a function of trust and categorization: the paradox of expected cooperation. Journal of Personality and Social Psychology. 2005;88(2):365–85. doi: 10.1037/0022-3514.88.2.365. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Psychopharmacology of maternal behaviour. Journal of Psychopharmacology. 1996;10:16–22. doi: 10.1177/026988119601000104. [DOI] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia. 2011;49:602–11. doi: 10.1016/j.neuropsychologia.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krüger F, McCabe K, Moll J, et al. Neural correlates of trust. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20084–9. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nitschke J, Nelson E, Rusch B, Fox A, Oakes T, Davidson R. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ostir G, Markides K, Peek K, Goodwin J. The associations between emotional well-being and the incidence of stroke in older adults. Psychosomatic Medicine. 2001;63:210–5. doi: 10.1097/00006842-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience. New York: Oxford University Press; 2009. [Google Scholar]
- Petrovic P, Pleger B, Seymour B, Klöppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. The Journal of Neuroscience. 2008;28:10509–16. doi: 10.1523/JNEUROSCI.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99:164–75. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper M, Petermann F. Trust—a subject for social neuroscience? Zeitschrift für Neuropsychologie. 2011;22(4):245–55. [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience. 2008;3:119–27. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens-opioid receptors mediate social reward. The Journal of Neuroscience. 2011;31(17):6362–70. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Leue A, Stemmler G. Is running away right? The behavioral activation-behavioral inhibition model of anterior asymmetry. Emotion. 2008;8:232–49. doi: 10.1037/1528-3542.8.2.232. [DOI] [PubMed] [Google Scholar]
- Wacker J, Heldmann M, Stemmler G. Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion. 2003;3(2):167–93. doi: 10.1037/1528-3542.3.2.167. [DOI] [PubMed] [Google Scholar]
- Wacker J, Mueller EM, Pizzagalli D, Hennig J, Stemmler G. Dopamine D2 receptor blockade reverses the association between trait BAS and frontal asymmetry in an approach motivational context. Psychological Science. 2013;24:489–97. doi: 10.1177/0956797612458935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.