Abstract
Neurodevelopmental disconnections have been assumed to cause behavioral alterations in autism spectrum disorders (ASDs). Here, we combined measurements of intrinsic functional connectivity (iFC) from resting-state functional magnetic resonance imaging (fMRI) with task-based fMRI to explore whether altered activity and/or iFC of the right posterior superior temporal sulcus (pSTS) mediates deficits in emotion recognition in ASD. Fifteen adults with ASD and 15 matched-controls underwent resting-state and task-based fMRI, during which participants discriminated emotional states from point light displays (PLDs). Intrinsic FC of the right pSTS was further examined using 584 (278 ASD/306 controls) resting-state data of the Autism Brain Imaging Data Exchange (ABIDE). Participants with ASD were less accurate than controls in recognizing emotional states from PLDs. Analyses revealed pronounced ASD-related reductions both in task-based activity and resting-state iFC of the right pSTS with fronto-parietal areas typically encompassing the action observation network (AON). Notably, pSTS-hypo-activity was related to pSTS-hypo-connectivity, and both measures were predictive of emotion recognition performance with each measure explaining a unique part of the variance. Analyses with the large independent ABIDE dataset replicated reductions in pSTS-iFC to fronto-parietal regions. These findings provide novel evidence that pSTS hypo-activity and hypo-connectivity with the fronto-parietal AON are linked to the social deficits characteristic of ASD.
Keywords: autism spectrum disorders, superior temporal sulcus, functional connectivity, functional magnetic resonance imaging, emotion recognition
INTRODUCTION
Autism spectrum disorders (ASDs) encompass a group of complex neurodevelopmental conditions characterized by impairment in social-communicative skills and by repetitive and restricted interests and behaviors. Diagnosis is currently based on behavioral evaluations as neural biomarkers have proven elusive. The ‘disconnection theory of ASD’ suggests that ASD-neuropathology constitutes a developmental disconnection syndrome, where altered neuronal development leads to disconnections at the systems level (Minshew and Keller, 2010). Functional magnetic resonance imaging (fMRI) experiments have contributed to the disconnection theory of ASD with reports of altered functional connectivity or synchronizations of the low-frequency fMRI blood-oxygen-level-dependent signal (BOLD-signal) in spatially remote regions. Initial studies found altered functional connectivity during task performance (e.g. Just et al., 2004), but more recently, patterns of synchronization between regions are increasingly examined in the absence of an external task (Vissers et al., 2012). This resting-state fMRI approach is thought to provide fundamental information about the intrinsic properties of the brain and has facilitated the examination of challenging populations by bypassing the limitations of task-based fMRI (Fox et al., 2005; Kelly et al., 2012).
Considering that impairment in social cognition represents a core ASD deficit, examining brain circuits in individuals with ASD commonly involved in social processes can provide insights into the mechanisms underlying this deficient domain (Pelphrey et al., 2011; Gotts et al., 2012). Circuits based on the posterior superior temporal sulcus (pSTS) are a prime candidate as evidence exists that pSTS is structurally (Levitt et al., 2003; Boddaert et al., 2004; McAlonan et al., 2005; Barnea-Goraly et al., 2010; Noriuchi et al., 2010; von dem Hagen et al., 2011) and functionally (Di Martino et al., 2009; Philip et al., 2012) altered in ASD. The STS is highly connected with several regions of the ‘social brain’ (Brothers, 1990; Lahnakoski et al., 2012) that have been reported to be abnormally recruited in a variety of social tasks involving face processing; these include the fusiform gyrus, orbitofrontal cortex and amygdala (e.g. Critchley et al., 2000; Hadjikhani et al., 2004; Pierce et al., 2004; Dalton et al., 2005). The posterior STS also provides the main visual input to the fronto-parietal regions of the action observation network (AON) or mirror network, which has been previously implicated in ASD (‘broken mirror’ theory of autism) (Williams et al., 2001; Dapretto et al., 2006) and is known to be involved in action or emotion processing, as well as embodied cognition (Rizzolatti and Craighero, 2004).
Previous work has demonstrated ASD-related alterations in measures of functional connectivity of pSTS. These studies examined functional connectivity during the performance of cognitive (Shih et al., 2010, 2011) or social processing tasks (Kana et al., 2012; Weisberg et al., 2012) by regressing out the BOLD-signal related to the task (pseudo-rest). To date, intrinsic functional connectivity (iFC) of pSTS to regions of the AON and its relation to task-based activations or social deficits in ASD has not been examined directly. This study assessed both resting-state iFC and task-based brain-activity to investigate the role of the pSTS in emotion processing in ASD. During task-based fMRI, participants performed a ‘bodily’ emotion recognition task probing whether an emotional state can be inferred from whole-body kinematics depicted by point light displays (PLDs). Prior studies on emotion processing in ASD often focused on perception of emotional expressions from faces, reporting abnormal patterns of activation in fusiform gyrus and STS regions (e.g. Critchley et al., 2000; Hadjikhani et al., 2004; Pierce et al., 2004). However, it has been suggested that differential activation patterns in response to face processing are linked to differences in fixating on the eyes in ASD and control groups (Dalton et al., 2005; Kliemann et al., 2010; Kirchner et al., 2011).
Here, we studied emotion recognition from PLDs in which human biological motion is represented solely by tracking the motion of the major joints of a human body (Johansson, 1973). Such PLDs have the advantage that they isolate biological motion information, while limiting form information, therefore they are best recognized as human when the dots are in motion (Kaiser and Pelphrey, 2012). Prior fMRI work demonstrated that processing of PLD motion is highly salient for activating pSTS and other regions of the AON such as inferior parietal and inferior frontal areas (Saygin, 2007; Herrington et al., 2011). Furthermore, processing of biological motion is known to be critical for typical social behavior (Allison et al., 2000; Pelphrey et al., 2005; Pelphrey and Morris, 2006; Kaiser et al., 2010; Herrington et al., 2011), and the PLD paradigm has been shown to be sensitive to capturing behavioral deficits in toddlers, children and adults with ASD (for recent review, see Kaiser and Pelphrey, 2012). Most task-based neuroimaging studies examining the neural correlates of PLD perception in ASD consistently implicated the pSTS as an area of dysfunction (Herrington et al., 2007; Freitag et al., 2008; Kaiser et al., 2010; Koldewyn et al., 2011; McKay et al., 2012) (but see Weisberg et al., 2012 for contrasting results).
In this study, we adopt a multi-level approach to examine (i) whether task-based brain activity during PLD emotion recognition and resting-state iFC between pSTS and regions included in the AON underlying bodily emotion processing are altered in ASD and (ii) whether alterations in these neural measures relate to impaired social functioning as indexed by the PLD emotion recognition task. Furthermore, we explored whether alterations in resting-state pSTS iFC are replicated using a large sample included in the Autism Brain Imaging Data Exchange (ABIDE) repository (Di Martino et al., 2013) consisting of 584 (278 ASD/306 controls) resting-state fMRI scans.
MATERIALS AND METHODS
Participants
Fifteen high-functioning adult males with ASD (21.7 ± 4.0 years) and 15 typically developed (TD) controls (23.3 ± 2.9 years) were included in the original dataset (Table 1). A replication dataset (resting-state fMRI) included 278 ASD participants (15.9 ± 6.2 years) and 306 TD participants (15.9 ± 5.7 years) and was extracted from a large open-shared data repository (http://fcon_1000.projects.nitrc.org/indi/abide/; Supplementary Table S1).
Table 1.
Characteristics of the groups
| ASD (n = 15) |
TD (n = 15) |
t-value | P | |||
|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |||
| Gender | All males | All males | ||||
| Age in years | 21.7 | 4.0 | 23.3 | 2.9 | 1.2 | 0.223 |
| Verbal IQ | 109.1 | 12.9 | 117.4 | 9.9 | 2.0 | 0.055 |
| Performance IQ | 105.6 | 19.3 | 109.1 | 17.7 | 0.5 | 0.612 |
| Full scale IQ | 107.9 | 13.9 | 114.8 | 12.8 | 1.4 | 0.160 |
| Medication status (n on meds)a | 5 | |||||
| Medication status (on meds at scan) | 0 | |||||
| Total SRS—parental report (raw) | 91.5 | 28.5 | ||||
| Total SRS—parental report (T) | 77.0 | 12.1 | ||||
| Total SRS—self report (raw) | 76.5 | 24.2 | 43.6 | 21.7 | −4.3 | <0.001 |
| AQ | 29.3 | 8.2 | 12.8 | 6.2 | −6.2 | <0.001 |
a(1) Fluoxetine, Bupropion; (2) Methylphenidate, Risperidone; (3) Methylphenidate, Risperidone; (4) Lamotrigine, Lithium Carbonate; (5) Benperidol.
In the original dataset, groups were matched for age, gender, full-scale intelligence quotient (IQ) and performance IQ (Table 1) [Ward 7-subtest of the Wechsler Adult Intelligence Scale-III (Wechsler, 1997; Girard et al., 2010)]. All ASD participants were recruited from the Autism Expertise Centre at the Leuven University Hospital. They previously had taken part in a larger family study conducted at the Leuven Autism Research center (De la Marche et al., 2012) during which a multidisciplinary team (child psychiatrist and/or expert neuro-pediatrician, psychologist, speech/language pathologist and/or physiotherapist) formulated a DSM-IV-TR diagnosis of autistic disorder (American Psychiatric Association, 1994). Diagnosis was obtained by combining information from unstructured direct observation, semi-structured parent interview [developmental, dimensional and diagnostic interview (3di), Skuse et al., 2004] as well as review of prior history and parent screening questionnaires. For all ASD participants, parents completed the Dutch version of the Social Responsiveness Scale (SRS; Constantino and Gruber, 2005; Roeyers et al., 2007), a 65-item questionnaire developed to assess a wide range of interpersonal behavior, communication and repetitive/stereotypic behavior characteristic of ASD (Constantino et al., 2003, 2004; Constantino and Gruber, 2005). For this study, only participants with prior ASD diagnosis and a parental total SRS score (raw) above 60 were included. Six (of 15; 40%) participants with ASD had a total parental SRS T-score within the mild to moderate range (60 through 75) indicating clinically significant impairment associated with mild to moderate interference in everyday social interactions. Nine (of 15; 60%) participants with ASD had a total SRS T-scores within the severe range (76 or higher), indicative of severe interference in everyday social interactions. Total parent SRS scores (raw and T-scores) are listed in Table 1.
For all participants included in this study (ASD and control), self-report SRS scores [adult-Dutch version; (Constantino and Gruber, 2005; Roeyers et al., 2007)] were assessed. As expected, total scores significantly discriminated between the two study groups (Table 1). A significant relationship was apparent between self-report total SRS scores (raw) and the previously collected parental total SRS scores (raw) (Supplementary Figure S1A). Autistic traits were also quantified by self-administration of the Autism Quotient Questionnaire (AQ) (Baron-Cohen et al., 2001), which has proven clinical validity (Woodbury-Smith et al., 2005) and has been used in autism studies to substantiate diagnosis (Yamasaki et al., 2010; Mueller et al., 2013). The AQ scale also significantly discriminated between the two study groups. As shown in Supplementary Figure S1B, significant relationships were revealed between self-report total SRS (raw) and AQ scores. Written informed consent was obtained from all participants as approved by the KU Leuven local Ethics Committee. None of the participants took psychoactive medications at the time of the scan (Table 1).
The replication dataset consisted of 584 resting-state fMRI datasets from nine sites contributing to the ‘ABIDE’ release (Di Martino et al., 2013). In this sample, all participants with ASD (278) had a clinician’s DSM-IV-TR diagnosis of ASD, which was corroborated by the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (Lord et al., 1994, 1999) for all sites except one. Supplementary Table S1 provides characteristics of this dataset. Within each site, groups were matched on age and full-scale IQ (except for two sites). Across sites, groups were matched on age, but not on full-scale IQ.
MRI data acquisition of original sample
Anatomical, resting-state and task-based fMRI images were acquired on a 3.0 T Philips MR-scanner (Best, The Netherlands) with an eight-channel phased-array head-coil. Scan sessions started with the anatomical and resting-state scans, followed by the task-related fMRI. During resting-state fMRI, participants were in a supine position with their eyes open while staring at a white cross and were instructed to lie still, stay relaxed and think of nothing in particular.
Anatomical imaging consisted of a high-resolution structural volume acquired using a coronal three-dimensional turbo field echo T1-weighted sequence with the following parameters: 182 contiguous coronal slices covering the whole brain and brainstem, slice thickness = 1.2 mm; repetition time (TR) = 9.7 ms; echo time (TE) = 4.6 ms; matrix size = 256 × 256; field-of-view (FOV) = 250 × 250 mm2; in-plane pixel size = 0.98 × 0.98 mm2; acquisition time = 6 min 38 s.
Resting-state fMRI images were acquired using a T2*-weighted gradient-echo echo planar imaging (GE-EPI) sequence with the following parameters: TR = 1700 ms; TE = 33 ms; matrix size = 64 × 64; FOV = 230 mm; flip angle 90°; slice thickness = 4 mm, no gap; axial slices = 32; 250 functional volumes; acquisition time = 7 min.
For the two task-based fMRI runs a T2*-weighted GE-EPI sequence was used with the following parameters: TR = 3000 ms; TE = 33 ms; matrix size = 80 × 80; FOV = 230 mm; flip angle 90°; slice thickness = 4 mm, no gap; axial slices = 35; 168 functional volumes; acquisition time = 8 min 33 s.
Task-based fMRI paradigm
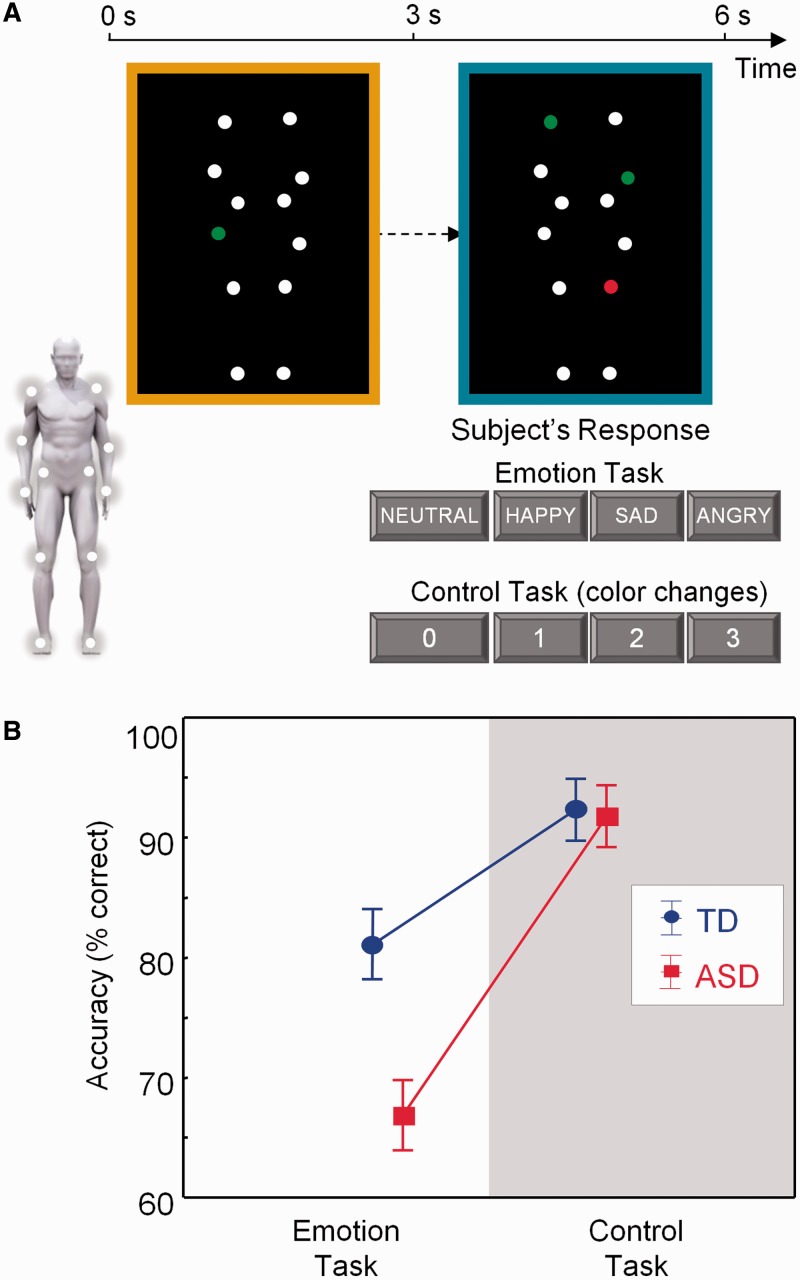
During task-based fMRI, participants completed two runs, each consisting of three blocks of an emotion recognition task, interleaved with control task blocks (40 s/block). All task blocks were separated by 16 s fixation blocks, during which participants fixated on a white cross. Each task block consisted of five trials, making a total of 30 trials (2 runs × 3 blocks × 5 trials) for each condition (emotion − control). The total duration of each trial lasted 8 s, such that stimulus presentation was jittered with respect to image acquisition (TR = 3 s). As explained in more detail in the following paragraphs, each trial consisted of two consecutive 3 s movies (yellow bordered and blue bordered), followed by a 2 s response time period showing a black screen. Prior to scanning, participants practiced the tasks inside a mock scanner.
In both tasks, stimuli consisted of moving PLDs as previously described (Alaerts et al., 2011; Nackaerts et al., 2012). In short, 12 reflective markers attached to the joints of the ankles, knees, hips, wrists, elbows and shoulders of human actors were tracked using an eight-camera VICON system (Oxford Metrics, UK). In the resulting 3 s movies, markers were visible as moving white spheres on a black background (Figure 1A). The stimuli portrayed human actions (walking; jumping; kicking) that express four bodily emotional states: anger, happiness, sadness or neutral.
Fig. 1.
Participants determined the emotional state of PLDs in which moving white dots reflected the main joints of the human body. (A) The emotional state of the blue-bordered PLD had to be indicated relative to the baseline yellow-bordered PLD (always showing a neutral emotional state). The same PLD stimuli were presented in a four-choice control task matched for cognitive and motor demands. Here, one of the dots in the yellow-bordered PLD briefly changed color to either red or green. Subsequently, participants had to indicate the number of dots that changed into the same color in the blue-bordered PLD (2 in this example). (B) The TD group was more accurate than the ASD group on the emotion recognition task, but not on the control task.
In the emotion recognition blocks (Supplementary Movie 1), each trial showed a yellow-bordered PLD (3 s movie), followed by a blue-bordered PLD (3 s movie), followed by a 2 s response time period showing a black screen. Participants were asked to indicate whether the presented point-light figure in the blue-bordered movie showed a different ‘emotional state’ compared with the point light figure in the yellow-bordered movie. The emotional state of the blue-bordered PLD could either be indicated as happier, sadder, angrier or not different (neutral) from the yellow-bordered PLD (Figure 1A). The yellow-bordered movie always showed a point-light figure in the ‘neutral emotional state’, whereas the emotional state of the blue-bordered point-light figure could either be neutral (7 of 30 trials), happy (7/30), sad (8/30) or angry (8/30).
In the control blocks (Supplementary Movie 2), participants were presented with exactly the same set of movies as those presented during the emotion recognition blocks, albeit in a different order and with a different task instruction. Instead of focusing attention on the emotional content in the PLD movies, participants were instructed to indicate color changes in the PLDs. In the yellow-bordered PLD, one dot briefly (0.5 s) changed color to ‘red’ or ‘green’ at a random time point. Participants then had to indicate the number of dots (0–1–2–3) that changed into the same color in the blue-bordered PLD (Figure 1A).
Task instructions were provided verbally and on the monitor at the start of each test block. Response options were displayed at the bottom of the screen, which corresponded to response buttons of a response box. Participants were instructed to respond as fast and accurately as possible and to use the right index, middle, ring and little finger for button pressing. Correct reaction times and accuracy rates were assessed using E-Prime-software (Psychological Software Tools).
Data analysis: task-based fMRI
SPM-8 was used for image preprocessing and statistical analyses (Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab R2008a (Mathworks). Task-based images were spatially realigned and unwarped, corrected for differences in slice acquisition time by temporal interpolation to the middle slice (reference = 17), normalized to the standard EPI-template of the Montreal Neurological Institute (MNI-152), resampled into 2 mm isotropic voxels and spatially smoothed with an isotropic 8 mm full-width-at-half-maximum Gaussian kernel.
For each subject, a general linear model (Friston et al., 1995) was calculated with the time-course of emotion recognition blocks, control blocks and fixation blocks modeled as predictors, and realignment parameters as regressors of no interest. The time series in each voxel was high-pass filtered at 1/224 Hz [1/(40 + 16 + 40 + 16) × 2)], to remove low-frequency drifts but not task-related activity. Contrast images were calculated for emotion recognition > fixation and control task > fixation and were subjected to second-level random-effects models.
Mean frame-wise displacement (FD) was assessed for each participant/run as d2 = Δx2 + Δy2 + Δz2 + (65π/180)2·(Δpitch2 + Δroll2 + Δyaw2)] (Art Repair). Mean FD did not significantly differ between groups and did not exceed 0.5 mm for any subject [Run 1: t(28) = 0.24; P = 0.81; ASD: 0.119 ± 0.068; TD: 114 ± 0.065 mm] [Run 2: t(28) = 0.89; P = 0.38; ASD: 0.116 ± 0.065 mm; TD: 0.098 ± 0.043 mm] (Statistica 9.0, Tulsa, USA).
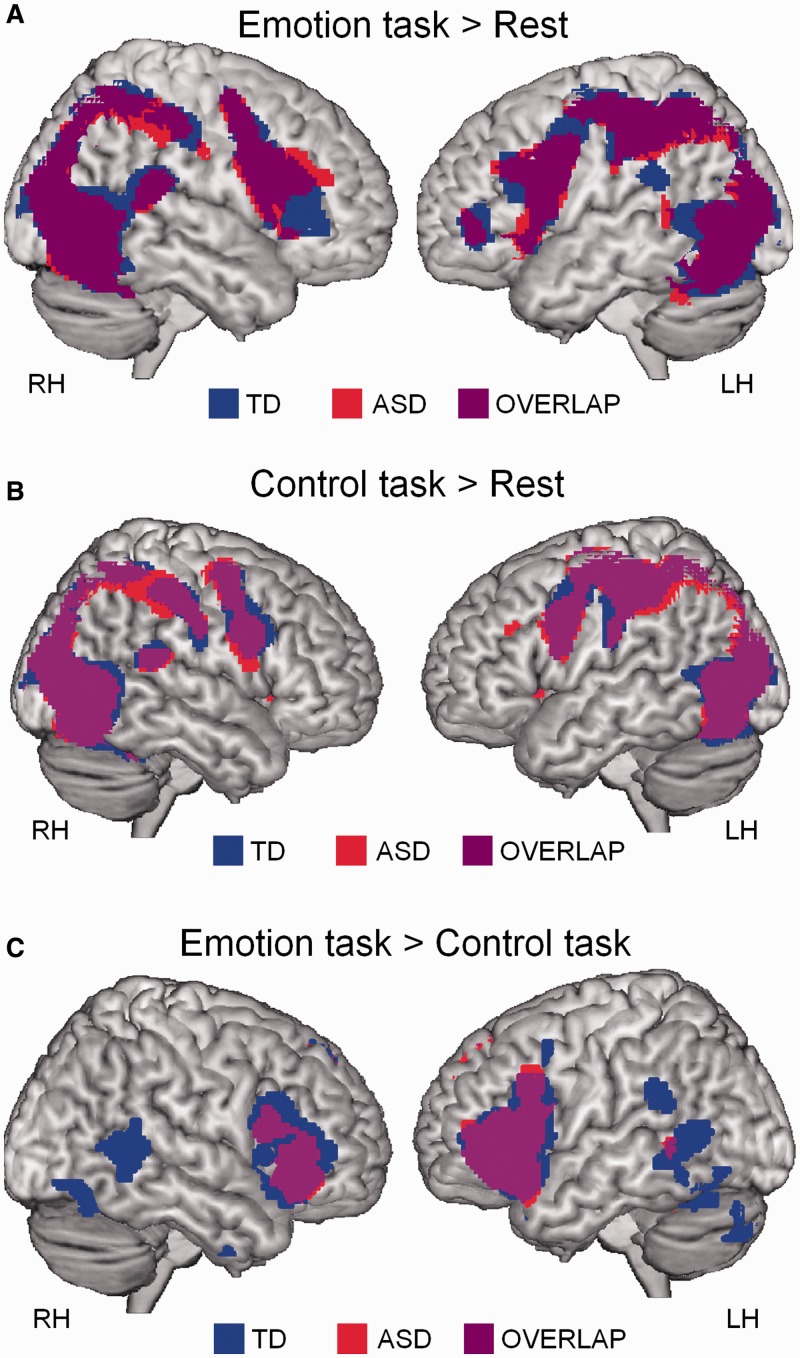
At the group level, we used one-sample t-tests within each group to identify regions with reliable activity during emotion recognition > fixation, control task > fixation or emotion recognition > control task (Figure 2). These tests were thresholded at P < 0.05 (extent threshold of 10 voxels) and family-wise error corrected for multiple comparisons at the whole-brain level. To specifically explore group differences and brain–behavior relationships in the pSTS bilaterally, a small-volume analysis was performed corrected for multiple comparisons at P < 0.05, using two 10 mm radius spheres centered on right pSTS (53, −53, 9) and left pSTS (−43, −58, 11) (Supplementary Figure S2A). These coordinates were derived a priori and represent average coordinates of 12 studies reporting pSTS activation during biological motion perception (Jastorff and Orban, 2009). Between-group differences within bilateral pSTS were explored for the contrasts (emotion > fixation) (emotion > control task).
Fig. 2.
Task-based brain activity. (A) Brain activity during performance of the emotion recognition test (>fixation) in the ASD and TD groups. (B) Brain activity during performance of the control test (>fixation) in the ASD and TD groups. (C) Brain activations during emotion recognition (>control task) in ASD and TD groups (one-sample t-tests, P < 0.05, corrected).
Brain–behavior multiple regression analysis was performed to identify correlations between brain activity [(emotion > fixation) (emotion > control task)] and emotion recognition accuracy (Figure 3; Table 2). For completeness and hypothesis-generating purposes, these analyses are additionally reported at a whole-brain uncorrected threshold of P < 0.0005 to determine the specificity within the entire brain of the identified pSTS clusters.
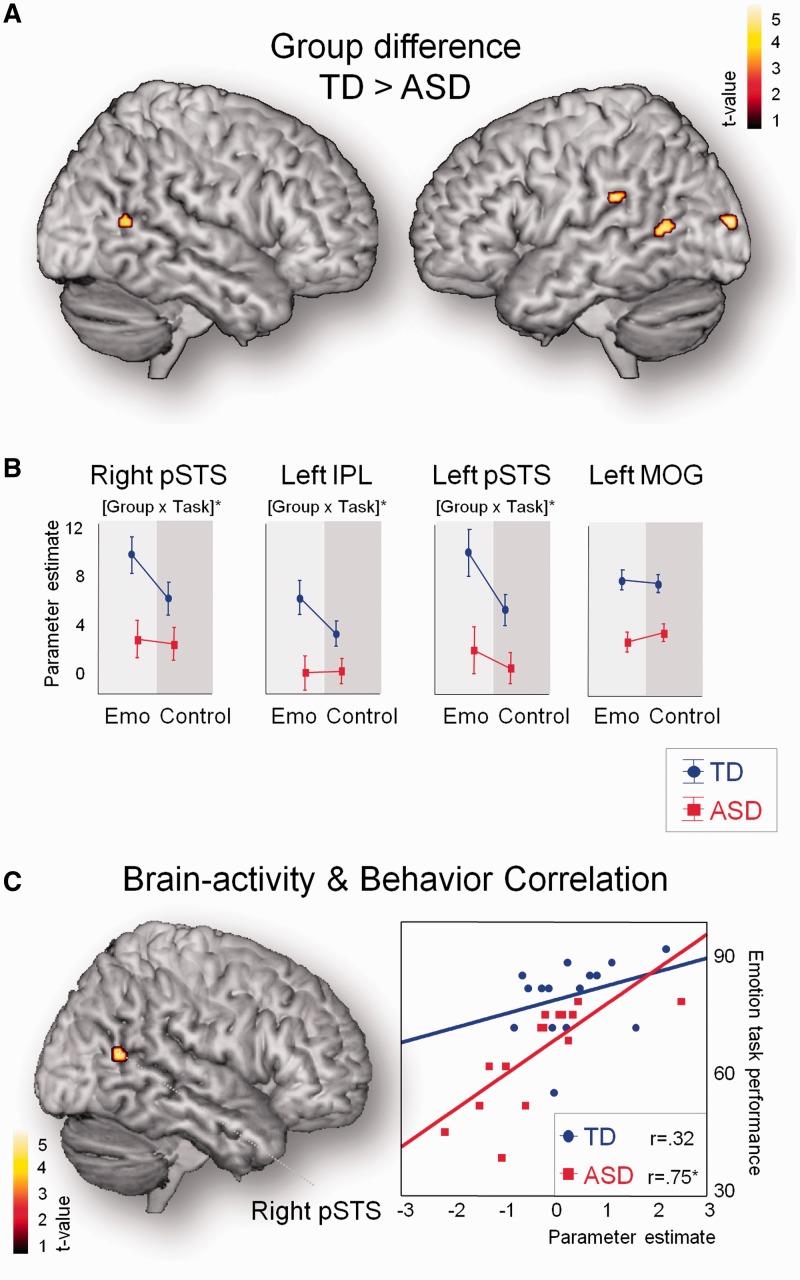
Fig. 3.
Task-based brain activity: group differences and brain–behavior relationships. (A) Between-group comparisons revealed stronger activity in bilateral pSTS in TD controls (small volume, two-sample t-test, P < 0.05, corrected). Only two additional brain regions (left IPL and left MOG) showed a similar group effect when tested at a whole-brain level (whole-brain, two-sample t-test, P < 0.0005, uncorrected), highlighting the spatial specificity of this finding. No clusters were activated more in the ASD compared with the TD group. (B) Cluster-based analysis revealed that group differences were specific to emotion recognition within bilateral pSTS and left IPL (not within left MOG). This was indicated by a significant group × task (Emo, Control) interaction. (C) Emotion recognition abilities were positively correlated with activity in right pSTS (small volume, P < 0.05, corrected). No other brain regions showed this relationship when tested at the whole-brain level (whole-brain, P < 0.0005, uncorrected).
Table 2.
Clusters showing between-group differences in task-based brain activity during emotion recognition and positive correlation between task-based brain activity and behavioral performance on the emotion recognition task
| Cluster size | Area | Hemi | x | y | z | t | P (FWE) | |
|---|---|---|---|---|---|---|---|---|
| Between-group differences in task-based brain activity during emotion recognition | ||||||||
| TD > ASD | ||||||||
| 62 | IPL | L | −45 | −32 | 24 | 4.75 | ||
| 31 | Middle temporal gyrus—pSTS | R | 53 | −58 | 12 | 3.96 | 0.0033* | |
| 35 | MOG | L | −17 | −94 | 12 | 4.59 | ||
| 22 | Middle temporal gyrus—pSTS | L | −57 | −60 | 10 | 3.92 | 0.047* | |
| ASD > TD | ||||||||
| / | ||||||||
| Positive correlation between task-based brain activity and behavioral performance on the emotion recognition task | ||||||||
| 40 | Middle temporal gyrus—pSTS | R | 47 | −60 | 16 | 5.02 | 0.027* | |
L and R refer to left and right hemispheres; x, y and z refer to the MNI coordinates corresponding to the left–right, anterior–posterior and inferior–superior axes, respectively; cluster size denotes the number of voxels; t refers to the highest t-score within a region (whole-brain, P < 0.0005, uncorrected). IPL: inferior parietal lobule; MOG: middle occipital gyrus; pSTS: posterior superior temporal sulcus.
*Denotes small volume, P < 0.05, corrected.
Data analysis: resting-state fMRI
Resting-state images were preprocessed in a similar manner to the task-based images, except images were not unwarped, resampled into 3 mm isotropic voxels and spatially smoothed with an isotropic 5 mm full-width-at-half-maximum Gaussian kernel. Resting-state images were band-pass filtered (0.009 < f < 0.08 Hz), and realignment parameters were modeled as regressors of no-interest. White matter, cerebrospinal fluid and physiological noise source estimation were also removed as confounds following the implemented CompCor-strategy (Behzadi et al., 2007). As such, no global signal regression was applied. Resting-state iFC analyses were performed using right pSTS as a seed (Functional Connectivity Toolbox). The choice for right pSTS was motivated by the task-based fMRI results, identifying right pSTS as a key region in predicting emotion recognition deficits in ASD (Figure 3C). The pSTS seed was a 10 mm radius sphere centered around MNI coordinates (47, −60, 4) and was created using coordinates that were relevant to emotion recognition in both groups (Supplementary Figure S2A).
Resting-state mean FD scores were not significantly different between groups [t(28) = 0.25; P = 0.81] (Supplementary Figure S3A) and did not exceed 0.5 mm in any of the participants. Nonetheless, considering that even small amounts of movement can produce spurious iFC (Van Dijk et al., 2012; Satterthwaite et al., 2013; Yan et al., 2013), we accounted for inter-individual differences in micro-movements by including mean FD scores as a nuisance covariate at the group level in all primary analyses (Figure 4). Furthermore, to verify that this approach effectively controlled for micro-movements, all primary analyses were repeated after ‘scrubbing’ (Power et al., 2012), i.e. censoring frames displaying FD > 0.5 mm or frame-wise changes in brain image intensity exceeding >0.5 Δ%BOLD (Supplementary Figure S4A).
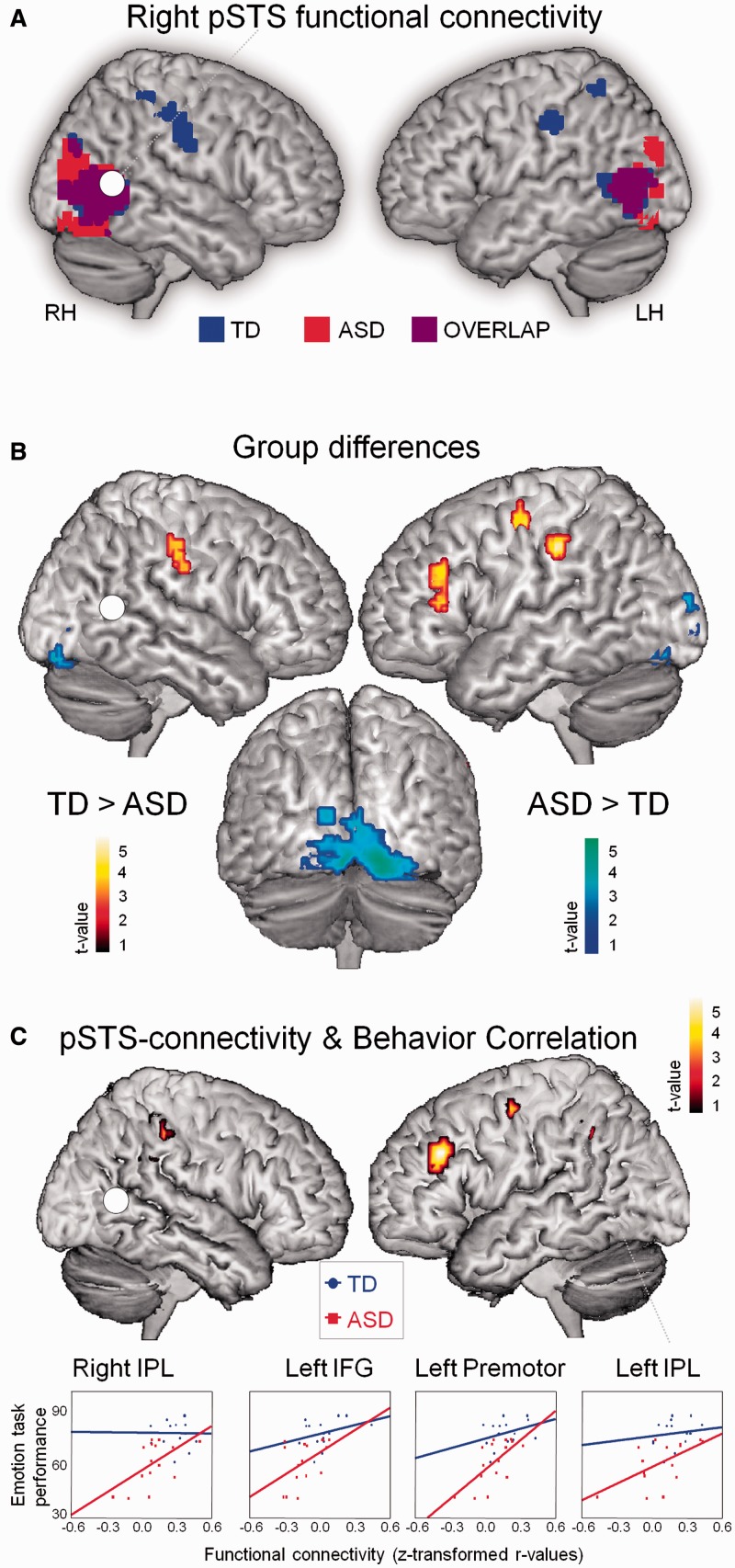
Fig. 4.
Resting-state iFC. (A) Resting-state iFC pattern of right pSTS in ASD and TD groups (one-sample t-test, P < 0.05, cluster-wise corrected). (B) Between-group comparisons showed that pSTS-iFC with bilateral IPL, left premotor and IFG was stronger in the TD group than in the ASD group (two-sample t-test, P < 0.05, cluster-wise corrected) (red–yellow). Stronger pSTS-iFC in the ASD group was observed with lingual/calcarine gyrus and superior occipital gyrus (blue–green). (C) Emotion recognition ability was positively correlated with the strength of pSTS-iFC with left/right IPL, left IFG, premotor area and left SMA (not shown) (P < 0.05, cluster-wise corrected).
For each subject, we extracted the residual BOLD time course from the pSTS seed and computed bivariate correlation coefficients between its time course and the time course of all other brain voxels. Correlation coefficients were converted to normally distributed z-scores using Fisher’s transform to conduct group analyses.
To specifically explore iFC of pSTS with regions that are relevant to PLD emotion recognition, all resting-state analyses were conducted within a broad mask encompassing brain regions that are activated during task-based fMRI in both groups (emotion recognition > fixation contrast) (Supplementary Figure S2B). All resting-state iFC analyses were thresholded at P < 0.05, cluster-wise corrected for multiple comparisons (extent threshold of 10 voxels): (i) one-sample t-tests, calculated separately for each group to identify regions with significant pSTS iFC; (ii) two-sample t-tests, to identify between-group differences in pSTS-iFC and (iii) brain–behavior multiple regression, to identify brain regions where pSTS-iFC positively correlated to emotion recognition abilities (Figure 4; Table 3). Z-scores were extracted to visualize brain–behavior correlations separately for the ASD group and TD group (Figure 4C). A debriefing-questionnaire about the participants’ experience and spontaneous thoughts during the resting state scan revealed no significant group differences (Supplementary Table S4).
Table 3.
Clusters showing between-group differences in resting-state functional connectivity with right pSTS and positive correlation between pSTS resting-state functional connectivity and behavioral performance on the emotion recognition task
| Cluster size | Area | Hemi | x | y | z | t | |
|---|---|---|---|---|---|---|---|
| Between-group differences in resting-state functional connectivity with right pSTS | |||||||
| TD >ASD | |||||||
| 41 | IPL | L | −49 | −27 | 40 | 4.81 | |
| 49 | IPL | R | 32 | −36 | 49 | 3.63 | |
| 45 | Precentral gyrus (BA 6) | L | −37 | −12 | 55 | 3.13 | |
| 35 | Supramarginal gyrus | R | 59 | −21 | 37 | 3.29 | |
| 27 | IFG (pars triangularis) (BA 45) | L | −40 | 30 | 28 | 3.23 | |
| ASD >TD | |||||||
| 389 | Lingual gyrus | R | 14 | −90 | −11 | 4.63 | |
| Lingual gyrus | L | −16 | −84 | −8 | 4.27 | ||
| L | −22 | −81 | −14 | 4.20 | |||
| Calcarine gyrus | L | −1 | −84 | −8 | 4.48 | ||
| L | 2 | −90 | 10 | 3.95 | |||
| Fusiform gyrus | R | 29 | −75 | −2 | 3.65 | ||
| Positive correlation between pSTS resting-state functional connectivity and behavioral performance on the emotion recognition task | |||||||
| 38 | IPL | R | 32 | −36 | 52 | 4.52 | |
| Supramarginal gyrus | R | 29 | −39 | 43 | 4.10 | ||
| 23 | SMA (BA 6) | L | −1 | 18 | 46 | 3.96 | |
| 43 | IPL | L | −28 | −42 | 37 | 3.83 | |
| 29 | IFG (pars triangularis) (BA 45) | L | −40 | 33 | 31 | 3.51 | |
| 35 | Precentral gyrus (BA 6) | L | −28 | −3 | 49 | 3.37 | |
| L | −25 | −6 | 58 | 3.34 | |||
L and R refer to left and right hemispheres; x, y and z refer to the MNI coordinates corresponding to the left–right, anterior–posterior and inferior–superior axes, respectively; cluster size denotes the number of voxels; t refers to the highest t-score within a region (P < 0.05, cluster-wise corrected). IPL: inferior parietal lobule; IFG: inferior frontal gyrus; SMA: supplementary motor area.
Replication using the Autism Brain Imaging Data Exchange release
For the ABIDE-replication dataset (278 ASD/306 TD participants) (Supplementary Table S1), we computed iFC and mean FD scores using the same procedures as described above (Supplementary Figure S3B). Within each site, groups displayed comparable mean FD scores (except for one site). Across sites, micro-movements were greater within the ASD than in the TD group [F(1,566) = 4.23, P = 0.041] (Supplementary Table S1 and Figure S3B). As for the original sample, we corrected for micro-movements by including mean FD scores as a nuisance covariate in the primary group analysis (Figure 6); and by performing a secondary analysis using individual ‘scrubbed’ data (Power et al., 2012) (Supplementary Figure S4B).
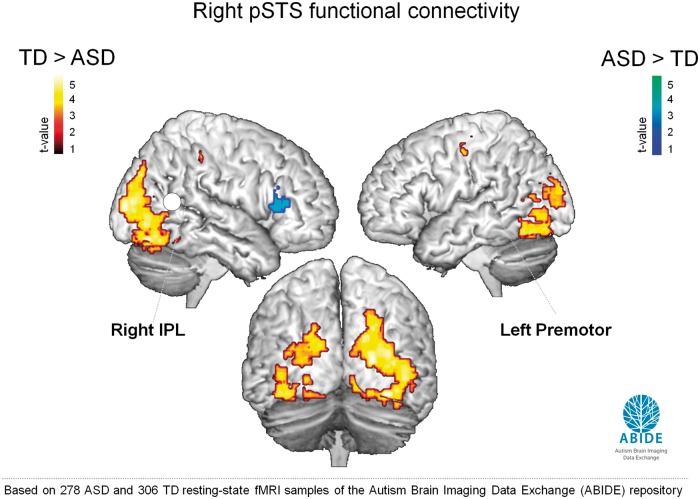
Fig. 6.
ABIDE: resting-state iFC. Brain regions showing differences in resting-state iFC with right pSTS between the TD group (n = 306) and ASD group (n = 278) of the ABIDE sample (ABIDE) (two-sample t-test, P < 0.05, cluster-wise corrected). Stronger pSTS-iFC was observed in the TD group with right IPL, left premotor, bilateral fusiform gyrus and bilateral SOG (red–yellow) (regions indicated in bold font were previously identified in the original sample of 30 participants). Stronger pSTS-iFC in the ASD group was observed with right IFG (part triangularis) (blue–green) and left thalamus (not shown).
RESULTS
Behavioral performance
The TD group was more accurate in recognizing emotional states from PLDs than the ASD group. No accuracy differences were observed for the control task [group × task: F(1,28) = 7.69; P = 0.01] (Figure 1B). Reaction times on both tasks were comparable between groups [F(1,28) = 0.19; P = 0.67] (Supplementary Figure S5).
Task-based brain activity
During emotion recognition (>fixation) both groups activated a bilateral fronto-parietal network and visual areas including pSTS (one-sample t-test, P < 0.05, corrected) (Figure 2A). Both groups recruited a similar network during performance of the control task (>fixation) (Figure 2B), although several regions were activated more strongly during emotion recognition than during the control task, especially in the TD group (Figure 2C).
Group differences
A direct comparison between groups for the emotion > fixation contrast, revealed that activity in bilateral pSTS was significantly higher in the TD group (small volume, two-sample t-test, P < 0.05, corrected). Only two other brain areas in the left inferior parietal lobule (IPL) and right middle occipital gyrus (MOG) showed a similar group difference (TD > ASD) when tested at a more liberal threshold at the whole-brain level (two-sample t-test, P < 0.0005, uncorrected) (Figure 3A; Table 2). No clusters showed greater activation in the ASD compared with the TD group. Direct comparison between groups for the emotion > control task contrast failed to reveal any significant clusters at the whole-brain level. Only a cluster-based ANOVA analysis, exploring group × age interaction effects, specifically for the parameter estimates extracted from bilateral pSTS, left IPL and left MOG (clusters shown in Figure 3A), revealed that group differences within pSTS bilaterally and left IPL (but not within right MOG) were more pronounced during the emotion recognition task, than during the control task [group × task: F(1,28) > 5.38; P < 0.05] (Figure 3B). Specifically, it was revealed that—only in the TD group—brain activity increased during performance of the emotion recognition task, compared with the control task, whereas in the ASD group, brain activity remained relatively constant across tasks.
Brain–behavior relationship
We then tested whether brain activity during emotion recognition > fixation correlated with task performance. Small-volume analysis, restricting analysis to bilateral pSTS, showed that participants with high emotion recognition accuracy exhibited stronger activation (>fixation) in right pSTS (r = 0.65, P < 0.05, corrected). Notably, no other brain region showed this relationship even when tested at a whole-brain level with a more liberal threshold (P < 0.0005, uncorrected) (Figure 3C; Table 2). As seen in Figure 3C, the effect was mainly driven by variance in the ASD group reflecting the marked heterogeneity in the expression of ASD emotion recognition deficits. Importantly, the relationship between task-based pSTS activity and emotion recognition performance remained unchanged when corrected for full-scale IQ, age and accuracy on the control task (all: r = 0.63; ASD: r = 0.74; TD: r = 0.36]. We also explored whether a relationship with behavior exists for the extent of differential brain activity between the emotion and control task (emotion recognition > control task contrast). However, this analysis failed to reveal any significant results (P < 0.0005, uncorrected).
Resting-state intrinsic functional connectivity
Task-based fMRI analyses identified right pSTS as a key region in predicting emotion recognition in ASD. Here, we specifically determined the whole-brain iFC pattern of right pSTS using resting-state fMRI.
In the TD group, right pSTS showed reliably high resting-state iFC with bilateral IPL and several visual areas. In the ASD group, strong pSTS-iFC was only found with occipital areas (one-sample t-test, P < 0.05, cluster-wise corrected) (Figure 4A).
Group differences
A direct comparison between groups confirmed that iFC was significantly higher in the TD group between pSTS and bilateral IPL (two-sample t-test, P < 0.05 cluster-wise corrected) (Figure 4B; Table 3). Posterior STS-iFC in the TD group was also stronger with left inferior frontal gyrus (IFG) and left premotor area. Stronger pSTS-iFC in the ASD group compared with the TD group was observed with several visual areas (lingual and superior occipital gyrus) (Figure 4B; Table 3).
Brain–behavior relationship
Brain–behavior correlations were then performed to test whether pSTS-iFC strength is correlated with emotion recognition ability. Strikingly, lower iFC of right pSTS with bilateral IPL, and left IFG/premotor/supplementary motor area (SMA) was highly predictive of poor emotion recognition ability (P < 0.05, cluster-wise corrected) (Figure 4C; Table 3).
Secondary analyses
To verify that our primary results were not confounded by micro-movements, iFC analysis was repeated on ‘scrubbed’ data. Supplementary Figure S4A displays group differences for the primary (unscrubbed) and secondary (scrubbed) analyses using the same statistical threshold (two-sample t-test, P < 0.05 cluster-wise corrected). The overall pattern of results remained unchanged, showing robust reductions in pSTS-iFC with bilateral IPL in ASD. However, primary findings of reductions in pSTS-iFC with left IFG/premotor were only identified subthreshold in the secondary analysis. As recent work demonstrated more impact of motion on prefrontal cortex compared to inferior parietal and superior temporal cortex (Satterthwaite et al., 2013; Yan et al., 2013), the present IFG/premotor results need to be interpreted with caution. Findings of ASD > TD group differences in pSTS-iFC with visual areas and brain–behavior correlation results were also replicated in the secondary analysis (not shown).
Relationship between task-based brain activity and resting-state functional connectivity
Although right pSTS task-based activity and resting-state iFC were sampled and measured independently, they both reliably correlated with emotion recognition performance. Here, we directly explore the relationship between these measures.
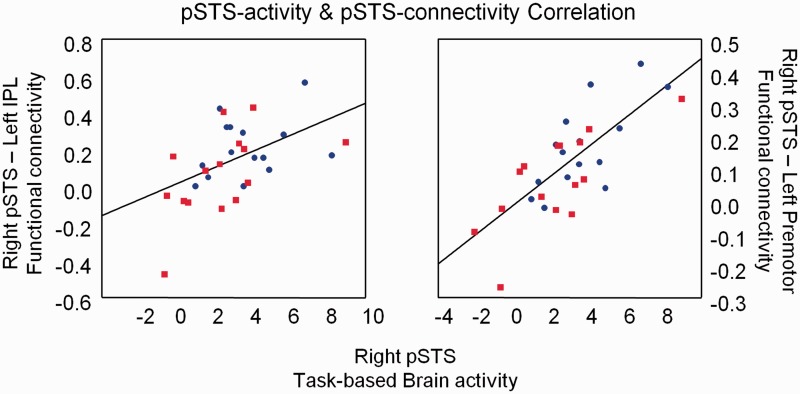
Participants with high task-based pSTS activity during emotion recognition were also those that exhibited stronger pSTS-iFC with left IPL (r = 0.43, P = 0.025) and left premotor (r = 0.72, P < 0.001) (Figure 5) (controlled for group, age and full-scale IQ). A non-significant tendency existed for the relationship between pSTS activity and pSTS-iFC with right IPL (r = 0.35, P = 0.07). No relationship existed between pSTS activity and pSTS-iFC with left IFG or SMA.
Fig. 5.
Relationship between task-based brain activity and resting-state iFC. Participants with high pSTS activity during emotion recognition also exhibited strong iFC of pSTS with left IPL and left premotor.
Next, we conducted a multiple regression analysis to test the extent to which the independent variables (i) pSTS activity and (ii) pSTS-iFC, predicted accuracy on the emotion recognition task (dependent variable). Activity in pSTS alone significantly predicted emotion recognition accuracy [ß = 0.37, t(28) = 4.11] [Whole model: R2 = 0.37, F(1,28) = 16.92, P = 0.0003], but the proportion of variance explained (R2) significantly increased when including the variable ‘pSTS-iFC’ in the regression model (Supplementary Table S2). Specifically, iFC of pSTS with left IFG, right IPL, left SMA and left IPL, significantly improved the overall model (explaining an additional 12%, 11.5%, 9% or 5.5% of the variance, respectively, all P < 0.05). Overall, these findings indicate that pSTS activity and pSTS-iFC explain a unique portion of variance in emotion recognition performance.
Replication using the Autism Brain Imaging Data Exchange repository
To test for reproducibility, we explored group differences in pSTS-iFC between 278 ASD and 306 TD resting-state fMRI datasets included in the ABIDE repository.
ABIDE group differences
Similar to findings emerging from the original dataset, we observed a significant reduction in pSTS-iFC with right IPL and left premotor area in the ASD compared with the TD group. Reduced pSTS-iFC was also observed with the fusiform gyrus and bilateral superior occipital gyrus (Figure 6; Supplementary Table S3). Stronger pSTS-iFC in the ASD group was observed with left thalamus and right IFG (Supplementary Table S3).
ABIDE secondary analyses
TD > ASD group differences remained similar in the secondary ‘scrubbed’ analyses (Supplementary Figure S4B), whereas primary findings of ASD > TD group differences were only identified subthreshold.
DISCUSSION
Individuals with ASD exhibited reduced resting-state iFC between the pSTS and fronto-parietal regions of the AON. Notably, resting-state pSTS hypo-connectivity was related to the degree of hypo-activity in pSTS during performance of an emotion recognition task and both neural measures were strongly predictive of emotion recognition abilities. Furthermore, our findings of ASD-related reductions in pSTS-iFC to fronto-parietal regions were replicated using a large independent sample of the ABIDE repository.
Task-based brain-activity
Performance on the bodily emotion recognition task was reduced in the ASD compared with the TD group. This is in line with previous work showing behavioral deficits in children (Moore et al., 1997; Blake et al., 2003; Parron et al., 2008) and adults (Hubert et al., 2007; Atkinson, 2009; Nackaerts et al., 2012) with ASD in biological motion or emotion recognition from PLDs. Previous research using the same PLD stimuli showed that impairments in the emotion recognition task are not entirely attributable to more basic deficits in biological motion detection in ASD. Instead, this task was shown to tap into ASD-specific deficits in recognizing the emotional dimension of PLDs (Nackaerts et al., 2012). Compared to fixation, emotion recognition activated several fronto-parietal regions of the AON, and visual cortex, including bilateral pSTS. A similar network was activated during the control task, during which exactly the same PLDs where displayed, but task instructions were directed at indicating color changes in the PLDs instead of directing attention to the PLD emotional states. These results suggest that the control task still evoked an unconscious or implicit perception of the emotional content within the PLDs which activated the fronto-parietal AON. Interestingly, it appeared that—at least for the TD group—several regions, including bilateral pSTS, were activated more strongly during the explicit emotion recognition task, compared to the implicit processing of emotional content during the control task. However, no direct group differences were revealed for this contrast (emotion > control task), indicating that the extent of differential brain activity during explicit and implicit emotional processing did not differ between groups. When brain activity during explicit emotion recognition is compared to the baseline condition (fixation), direct group differences were revealed in bilateral pSTS and a region in left IPL, and these group effects were more pronounced for explicit emotion recognition (emotion > fixation), than during implicit emotional processing in the control task (control > fixation). Previous studies exploring explicit and implicit social processing in the context of ASD suggested that alterations in ASD may be primarily implicit in nature (Deeley et al., 2007; Frith and Frith, 2008). However, based on the current task paradigm, we could not confirm this hypothesis, as here, alterations in brain activity of pSTS and left IPL were more pronounced during explicit, compared to implicit emotional processing. Brain–behavior correlation analyses further emphasized a specific role of right pSTS in explicit emotion recognition such that subjects with good performance recruited right pSTS to a greater extent. No relationship with behavior was revealed for the extent of differential brain activity during ‘explicit’ and ‘implicit’ emotional processing.
Identification of pSTS as a locus of abnormal activation in ASD extends previous studies demonstrating structural alterations and hypo-activation in pSTS when testing the response to a variety of social cognition tasks (Philip et al., 2012). Present findings of pSTS hypo-activity during emotion recognition from PLDs specifically extend previous reports of altered recruitment of pSTS in ASD during basic biological motion perception tasks (Herrington et al., 2007; Freitag et al., 2008; Kaiser et al., 2010; Koldewyn et al., 2011) (but see Weisberg et al., 2012). Furthermore, this study demonstrates a crucial link between brain and behavior by providing evidence that pSTS hypo-activations in ASD are strongly related to emotion recognition performance.
Resting-state intrinsic functional connectivity
The task-based fMRI analyses identified right pSTS as a key region in predicting emotion recognition deficits in ASD. From the resting-state fMRI scans, we determined the whole-brain iFC-pattern of right pSTS in the same group of participants. In the ASD group we noted abnormal iFC decreases between pSTS and several regions of the fronto-parietal AON (Rizzolatti and Craighero, 2004), including bilateral IPL, left IFG and left premotor area. These findings of pSTS hypo-connectivity to fronto-parietal regions support the hypothesis that reduced functionality of the AON may contribute to the pathophysiology of ASD (‘broken-mirror theory’) (Dapretto et al., 2006; Williams et al., 2006). However, our data extend this account by providing strong indications that emotion perception deficits in ASD may not arise from hypo-activations in the fronto-parietal AON per se, but rather from hypo-connectivity of pSTS, the main visual input area to this system. Therefore, our findings may reconcile the underconnectivity theory of ASD (Just et al., 2004; Minshew and Keller, 2010; Vissers et al., 2012) with the influential ‘broken-mirror’ account (Williams et al., 2001; Rizzolatti and Craighero, 2004; Dapretto et al., 2006), by demonstrating pronounced hypo-connectivity between visual area pSTS and ‘downstream’ fronto-parietal ‘mirror-motor’ regions.
These results extend findings from a previous study in which granger causality mapping was used to measure connectivity during PLD perception. Although groups were not compared directly, only the control group, not the ASD group was shown to utilize a network with information passing from inferior temporal (in close proximity of pSTS) to parietal AON regions (McKay et al., 2012). Our resting-state iFC results also extend previous studies exploring functional connectivity of STS during task-based fMRI. Similar to our results, Kana et al. (2012) reported ASD-related reductions in functional connectivity between the temporal-parietal junction (in close proximity to pSTS) and premotor cortex/IFG (Kana et al., 2012) during performance of a social task (intentional attribution). In a study by Shih et al.(2010), functional connectivity of bilateral pSTS and other regions of the fronto-parietal simulation network were measured during a semantic decision task (Shih et al., 2010). Although no direct group differences in iFC were revealed, the level of ‘pSTS-interconnectivity’ with other nodes was less robust in ASD. In contrast, the same group reported marked hyper-connectivity of distinct pSTS-subregions during performance of a visual-search task, but with the BOLD-signal related to the task regressed out (Shih et al., 2011). The apparent inconsistency of the latter study with the present findings of pSTS-fronto-parietal hypo-connectivity is probably related to several methodological differences. Here, we used iFC measures of task-free resting-state fMRI, whereas previous studies measured pSTS-functional connectivity during task performance. The task-free nature of resting-state fMRI can be advantageous because it is insensitive to strategic, motivational or attention-related differences between groups. This may be of particular importance, considering that residual effects of task-evoked variations may persist in the low frequency BOLD-fluctuations (Hasson et al., 2009), and signal-to-noise ratios of task-based fMRI may be reduced in ASD (Dinstein et al., 2012). A weakness of resting-state fMRI is that one cannot exclude differences in spontaneous thoughts during the measurements. However, debriefing of our original sample on their experience in the scanner provided no direct indications of differences between groups (Supplementary Table S4).
Replication using the Autism Brain Imaging Data Exchange repository
Findings of pSTS-fronto-parietal underconnectivity with right IPL and left premotor regions were replicated using a large independent sample of the ABIDE repository (Di Martino et al., 2013). This replication is important because previous iFC fMRI studies often yielded mixed results using substantially smaller samples. For example, in a recent survey of 32 studies, 22 were identified as supporting the account of general hypo-connectivity in ASD, whereas 11 were not consistent with this hypothesis (Muller et al., 2011). Although differences in methodological approach may contribute to varying results, small sample sizes constitute an important cause for empirical inconsistencies (Button et al., 2013). The consistent finding of hypo-connectivity of pSTS with right IPL and left premotor regions across our original sample and the ABIDE sample as well as across unscrubbed and scrubbed analyses, therefore signifies the robustness of the effect. Using the much larger ABIDE sample, we additionally revealed pSTS hypo-connectivity with bilateral fusiform gyrus extending to superior occipital gyrus. This finding is in agreement with the hypothesized role of the fusiform gyrus in ASD (e.g. Schultz et al., 2000; Dalton et al., 2005), and specifically corroborates a recent report of ASD-related reductions in functional connectivity between the right lateral fusiform gyrus and right pSTS during a social perception task (observing social interactions as depicted by geometrical shapes) (Weisberg et al., 2012).
From our original sample, we revealed pSTS hyper-connectivity in ASD with several visual areas. This finding may be indicative of altered neural processing routes in ASD tentatively implicating an increased reliance on visual processing, instead of connecting with fronto-parietal ‘mirror-motor’ areas. However, caution should be taken as findings of increased pSTS-visual iFC from our original sample were not replicated using the large ABIDE sample. Here, hyper-connections in ASD were only revealed between right pSTS and left thalamus/right IFG, and the latter results were not robust to extensive motion correction in the secondary analyses of the ABIDE sample.
Relating task-based and resting-state fMRI to behavioral measures
By combining task-based and resting-state fMRI, we demonstrated that reductions in pSTS resting-state iFC are related to reductions in task-based pSTS-activation, and more importantly, that reductions in pSTS-iFC were predictive of emotion recognition over and above the effects explained by task-based pSTS-activity. Both neural measures therefore explained a unique portion of behavioral variance in emotion recognition. This finding demonstrates that the emotion recognition deficits characteristic of ASD directly relate not only to deficiencies within pSTS itself (hypo-activity) but also to deficits in its intrinsic connections to other brain regions (hypo-connectivity). While prior work has explored the relationship between iFC and task activation in healthy adults (Mennes et al., 2010, 2011), this is the first study to explore this relationship in ASD.
Limitations
Because our original sample size was small (15 participants/group), replication of the task-based group analyses may be warranted. However, findings regarding pSTS-underconnectivity in ASD appear to be rather robust since they were confirmed using a much larger, independent sample of the ABIDE-repository (278 ASD/306 TD participants).
Furthermore, we contrasted the emotion task against a low-level fixation baseline, or with the control task, which may still have evoked implicit emotional processing. Future research should compare explicit emotional processing from PLDs with a more neutral ‘high-level’ baseline, e.g. by presenting only neutral trials (without emotional content) during the control task.
Finally, for one of the two samples with ASD (original sample, n = 15) we missed gold standard diagnostic instruments (ADOS and/or Autism Diagnostic Interview—Revised) thus limiting comparisons with the larger community. However, a multidisciplinary clinical team confirmed the DSM-IV-TR diagnosis and clinically significant autistic traits were quantified with standardized screening instruments including the SRS and AQ (Baron-Cohen et al., 2001; Constantino et al., 2003, 2004). Of note, in contrast with our original sample, diagnosis of ASD in the replication ABIDE dataset was obtained with gold standard diagnostic assessments (Lord et al., 1994, 1999), thus reducing concerns regarding specificity of findings.
Summary
Our data provide converging experimental evidence that underconnectivity between key areas in the temporal and fronto-parietal lobes underlie the social deficits in emotion recognition associated with ASD. Considering that pSTS is a major neural hub connecting several social processing networks (including the fronto-parietal AON), we propose that deficiencies in the neural connections of visual area pSTS may precede alterations in downstream fronto-parietal ‘mirror-motor’ regions as hypothesized by the broken-mirror theory of ASD. Furthermore, the strong demonstration of a link between neural abnormalities in pSTS and the emotion perception deficits in ASD provides an interesting anatomical target for therapeutical approaches aimed at improving social perception processes.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We are grateful to all the subjects who voluntarily participated in this research and to E. Nackaerts for her help with data collection. We thank I. Noens, J. Wagemans and other members of the Leuven Autism Research Consortium (LAuRes) for discussion and aid in subject recruitment. This work was supported by grants from the Flanders Fund for Scientific Research (FWO project 0749.09), and by IAP grant P7/21 from the Interuniversity Attraction Poles program of the Belgian federal government. The study was conducted in collaboration with the LAuRes funded by the Research Council of the University of Leuven (IDO/08/013). K.A. is supported by a FWO postdoctoral research fellowship grant. D.G.W. is supported by grant G.0404.12/G.0758.10 from the Flanders Fund for Scientific Research.
We would also like to thank all the members of the Autism Brain Imaging Data Exchange Consortium (ABIDE; http://fcon_1000.projects.nitrc.org/indi/abide/) and Michael P. Milham and the INDI team (http://fcon_1000.projects.nitrc.org/) supporting the ABIDE effort. We especially thank the sites whose data were included in these analyses and their funding sources: (i) Olin, Institute of Living at Hartford Hospital [Autism Speaks (to M.A.), Hartford Hospital (to M.A.)], (ii) Oregon Health and Science University [R00 MH091238 (Fair), R01 MH096773 (Fair), R01 MH086654 (Nigg), Simon Foundation, Inc. (Nigg)], (iii) Trinity Centre for Health Sciences [The Meath Foundation, Adelaide and Meath Hospital, incorporating the National Children's Hospital (AMNCH), Tallaght, and travel fellowship by the Kyulan Family Foundation], (iv) University of Utah, School of Medicine [National Institutes of Health (grant numbers: K08 MH092697, RO1MH080826, P50MH60450, T32DC008553, R01NS34783), Autism Speaks Mentor-based Predoctoral Fellowship (grant number: 1677), University of Utah Multidisciplinary Research Seed Grant, NRSA Predoctoral Fellowship (grant number: F31 DC010143), Ben B. and Iris M. Margolis Foundation], (v) Yale Child Study Center [Simons Foundation (KP), Autism Speaks (KP), John Merck Scholars Fund (KP), Autism Science Foundation, NICHD (KP), NIMH], (vi) University of Leuven: Sample 2 [Fund for Scientific Research-Flanders (F.W.O.) (research grant G.0354.06, doctoral mandate to JV, research grant 1841313N, senior clinical investigator grant to SS); Belgian Inter University Attraction Pole (grant 6/29); KU Leuven Research Council (grant IDO/08/013)], (vii) NYU Langone Medical Center [NIH (K23MH087770; R21MH084126; R01MH081218; R01HD065282), Autism Speaks, The Stavros Niarchos Foundation, The Leon Levy Foundation, An endowment provided by Phyllis Green and Randolph Cōwen], (viii) University of California, Los Angeles: Sample 1 (UCLA Autism Center of Excellence, NICHD P50 HD055784, NIMH 1R01 HD065280-01) and (ix) University of Michigan: Sample 2 [Autism Speaks (CM), NIH (U19 HD035482 and MH066496 (CL)), Autism Speaks Pre-doctoral Fellowship 4773 (JW), Michigan Institute for Clinical and Health Research (MICHR) Pre-doctoral Fellowship UL1RR024986 (JW), NIH R21 MH079871 (SP)].
REFERENCES
- Alaerts K, Nackaerts E, Meyns P, Swinnen SP, Wenderoth N. Action and emotion recognition from point light displays: an investigation of gender differences. PLoS One. 2011;6:e20989. doi: 10.1371/journal.pone.0020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47:3023–9. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings a diffusion tensor imaging study using tract-based spatial statistics. Archives of General Psychiatry. 2010;67:1052–60. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14:151–7. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–26. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–12. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Marche W, Noens I, Luts J, Scholte E, Van Huffel S, Steyaert J. Quantitative autism traits in first degree relatives: evidence for the broader autism phenotype in fathers, but not in mothers and siblings. Autism. 2012;16:247–60. doi: 10.1177/1362361311421776. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly EM, Surguladze S, et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biological Psychiatry. 2007;62:207–17. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Li Q, Yan C, et al. The autism brain imaging data exchange: towards large-scale evaluation of the intrinsic brain in autism. Molecular Psychiatry. 2014;19:659–67. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75:981–91. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haberlen M, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–94. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–89. [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60:503–10. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Girard TA, Axelrod BN, Wilkins LK. Comparison of WAIS-III short forms for measuring index and full-scale scores. Assessment. 2010;17:400–5. doi: 10.1177/1073191110369763. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–25. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–50. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10841–6. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Baron-Cohen S, Wheelwright SJ, et al. The role of MT+/V5 during biological motion perception in Asperger Syndrome: an fMRI study. Research in Autism Spectrum Disorders. 2007;1:14–27. [Google Scholar]
- Herrington JD, Nymberg C, Schultz RT. Biological motion task performance predicts superior temporal sulcus activity. Brain and Cognition. 2011;77:372–81. doi: 10.1016/j.bandc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Hubert B, Wicker B, Moore DG, et al. Brief report: recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:1386–92. doi: 10.1007/s10803-006-0275-y. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Orban GA. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. Journal of Neuroscience. 2009;29:7315–29. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G. Visual-perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–11. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21223–8. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Pelphrey KA. Disrupted action perception in autism: behavioral evidence, neuroendophenotypes, and diagnostic utility. Developmental Cognitive Neuroscience. 2012;2:25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Social Cognitive and Affective Neuroscience. 2014;9(1):98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: promise and pitfalls. Trends in Cognitive Sciences. 2012;16:181–8. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner JC, Hatri A, Heekeren HR, Dziobek I. Autistic symptomatology, face processing abilities, and eye fixation patterns. Journal of Autism and Developmental Disorders. 2011;41:158–67. doi: 10.1007/s10803-010-1032-9. [DOI] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Steimke R, Heekeren HR. Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. Journal of Neuroscience. 2010;30:12281–7. doi: 10.1523/JNEUROSCI.0688-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14:1075–88. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, et al. Naturalistic fMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in Human Neuroscience. 2012;6:233. doi: 10.3389/fnhum.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, et al. Cortical sulcal maps in autism. Cerebral Cortex. 2003;13:728–35. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Service; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorder. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–76. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- McKay LS, Simmons DR, McAleer P, Marjoram D, Piggot J, Pollick FE. Do distinct atypical cortical networks process biological motion information in adults with Autism Spectrum Disorders? Neuroimage. 2012;59:1524–33. doi: 10.1016/j.neuroimage.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–9. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Current Opinion in Neurology. 2010;23:124–30. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DG, Hobson RP, Lee A. Components of person perception: an investigation with autistic, non-autistic retarded and typically developing children and adolescents. British Journal of Developmental Psychology. 1997;15:401–23. [Google Scholar]
- Mueller S, Keeser D, Samson AC, et al. Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS One. 2013;8:e67329. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? a survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackaerts E, Wagemans J, Helsen W, Swinnen SP, Wenderoth N, Alaerts K. Recognizing biological motion and emotions from point-light displays in autism spectrum disorders. Plos One. 2012;7:e44473. doi: 10.1371/journal.pone.0044473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, et al. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Research. 2010;1362:141–9. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Parron C, Da Fonseca D, Santos A, Moore DG, Monfardini E, Deruelle C. Recognition of biological motion in children with autistic spectrum disorders. Autism. 2008;12:261–74. doi: 10.1177/1362361307089520. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP. Brain mechanisms for interpreting the actions of others from biological-motion cues. Current Directions in Psychological Science. 2006;15:136–40. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Wyk BCV. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip RCM, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2012;36:901–42. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–16. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Roeyers H, Thys M, Druart C, et al. SRS—Screeningslijst voor autismespectrum stoornissen, Dutch translation. Amsterdam, The Netherlands: Hogrefe Uitgevers BV; 2007. [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin AP. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–61. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Muller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biological Psychiatry. 2011;70:270–7. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Muller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–9. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D, Warrington R, Bishop D, et al. The developmental, dimensional and diagnostic interview (3di): a novel computerized assessment for autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:548–58. doi: 10.1097/00004583-200405000-00008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews. 2012;36:604–25. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EAH, Nummenmaa L, Yu RJ, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale-Third Edition Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Weisberg J, Milleville SC, Kenworthy L, et al. Social perception in autism spectrum disorders: impaired category selectivity for dynamic but not static images in ventral temporal cortex. Cerebral Cortex. 2014;24(1):37–48. doi: 10.1093/cercor/bhs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–95. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S. Screening adults for Asperger Syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders. 2005;35:331–5. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Yamasue H, Abe O, et al. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biological Psychiatry. 2010;68:1141–7. doi: 10.1016/j.biopsych.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76C:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.