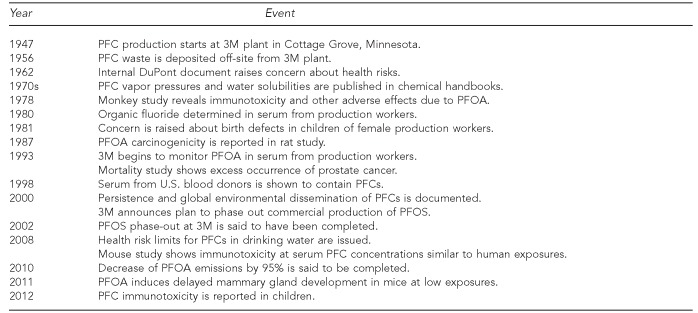
Perfluorinated compounds (PFCs) have been in use for more than 60 years.1 Perfluorooctanoic acid (PFOA) was a primary PFC product at the 3M facility in Cottage Grove, Minnesota, but perfluorooctane sulfonate (PFOS) and other PFCs were also produced. The PFCs show high thermal, chemical, and biological inertness—properties that make them useful for certain industrial purposes, but at the same time also resulted in environmental persistence and potential human health risk.2 Little was published in scientific journals on PFC toxicology until the 1980s, perhaps because compounds resistant to breakdown were erroneously considered inert.3 Gradually, evidence for persistent, bioaccumulative effects has emerged, raising warning signs. A chronology of important events in understanding PFC health risks is provided in the Figure.
Figure.
Time course of important developments regarding PFC exposure and health risks
PFC = perfluorinated compound
PFOA = perfluorooctanoic acid
PFOS = perfluorooctane sulfonate
PERVASIVE EXPOSURES TO PFCS
The existence of PFCs in the human body was first suspected in the late 1960s when fluoride in blood samples was found to be partially bound to organic compounds of unknown structure.4 High concentrations in exposed workers were documented in the 1970s,5 and specific PFCs were later identified in serum samples from workers at production facilities.6 Laboratory animal studies soon confirmed that PFCs are readily absorbed from oral intake and inhalation.7
A variety of PFCs and polyfluorinated compounds are applied in water-, soil-, and stain-resistant coatings for clothing and other textiles; oil-resistant coatings for food wrapping materials; and other products. Human exposures also include precursor compounds that may be broken down into PFOA and PFOS from PFC-containing products or from environmental sources, including house dust and groundwater.1
Analysis of serum samples from the National Health and Nutrition Examination Survey (NHANES) in 2000 showed that PFOS and PFOA were detectable in all Americans.8 Median concentrations in serum were about 30 nanograms per milliliter (ng/mL) (PFOS) and 5 ng/mL (PFOA). The average had decreased 8–10 years later to less than half for PFOS that had been phased out in the U.S. by 2002, while PFOA had changed to a much lesser extent.9 Overall, serum concentrations in children tend to be higher than in adults.10
Serial analyses of serum samples from former 3M production workers after retirement suggested elimination half-lives for long-chain PFCs to be about three years for PFOA and about five years for PFOS.11 Environmental dissemination and human exposure to PFCs are anticipated to continue for the foreseeable future due to their persistence in the environment, their continued formation from precursor compounds, and continued production elsewhere.1
DISCOVERY OF ADVERSE EFFECTS
The main evidence on adverse effects in humans comes from observational studies of occupational cohorts and community studies of subjects exposed either at background levels or through contaminated drinking water. Some studies are hampered by imprecise estimates of long-term PFC exposures and may for this reason have underestimated the effects.12–16
New evidence is emerging, as a settlement agreement in 2005 established the C8 Health Project, where data on approximately 70,000 exposed Ohio and West Virginia residents provided information on drinking water intake, serum-PFOA concentrations, and a variety of possible clinical outcomes.17 Additional evidence on associations between PFC exposure and disease parameters in the general population comes from the NHANES database.
Most published toxicity reports are based on the rat, which eliminates PFCs much more rapidly than humans and, therefore, is not an ideal species.18 Chronic toxicity studies in other species are lacking, and a formal cancer bioassay has not yet been completed. In addition, insufficient attention had been paid so far to exposures during sensitive developmental stages.
In regard to cancer, evidence from animal studies dates to the late 1970s, specifically pancreatic tumors and hepatocellular carcinomas in animals as a result of peroxisome proliferation.19 For Leydig cell tumors, the first evidence describing the tumor mechanisms was published in 1992.20 The U.S. Environmental Protection Agency (EPA) draft risk assessment of PFOA concluded in 2005 that the evidence was suggestive of a cancer risk in humans, but peer review recommended that PFOA be considered “carcinogenic to humans.”21 This conclusion is supported by the recent C8 Health Project results,15 which found a significant positive exposure-response relationship between PFOA and kidney cancer. A population-based case-control analysis supports the association between PFOA exposure and both kidney and testicular cancer and suggests an association with prostate and ovarian cancer and non-Hodgkin lymphoma.16 For PFOS, the evidence of carcinogenicity is less extensive and less conclusive.
Immunotoxicity was already considered a main effect of PFOA in an unpublished rhesus monkey study sponsored by 3M in 1978.22 Routine parameters in later studies failed to show any significant effects.23 However, immunotoxicity effects of PFOA and PFOS have recently been demonstrated in vitro and in a variety of species and models.24 When childhood vaccination responses were used as a clinically relevant outcome, PFC concentrations in maternal pregnancy serum showed a strong negative correlation with vaccine antibody concentrations in children at 5 years of age.25 While maternal PFOS and PFOA concentrations were not associated with hospitalization rates for infectious disease in their children,26 another study showed increased rates of common infections.27 The combined human and experimental evidence therefore strongly supports adverse effects on certain immune functions at current exposure levels.
DISCUSSION
This review of the PFCs emphasizes that early toxicity information was not published or went unheeded at first. Their toxic properties were first examined in the 1970s, although not in a systematic way. Thus, despite the fact that PFCs have been in use for more than 60 years, risks to public health attracted increased attention only after the environmental dissemination of the PFCs became known. Because PFC persistence in the environment was apparent by the 1990s, regulatory control of these compounds should have been a higher priority by 2000, if not before. Only recently, published research has reported carcinogenicity and immunotoxicity as health risks that may be relevant at exposure levels prevalent in the U.S. and elsewhere. Even today, chronic toxicity studies are incomplete, and a carcinogenicity assay has not yet been carried out.
Existing exposure limits appear insufficiently protective, as they are based on evidence available before 2008. Thus, the EPA issued in 2009 provisional health advisories of 0.4 micrograms per liter (μg/L) (400 ng/L) drinking water for PFOA and 0.2 μg/L (200 ng/L) for PFOS. These limits were based on liver toxicity and other adverse effects in animal studies, and the EPA concluded that “[e]pidemiological studies of exposure to PFOA and adverse health outcomes in humans are inconclusive at present.”2 The same toxicology data were used for the derivation of drinking water limits authorized by U.S. states and European Union (EU) countries as well as the EU Tolerable Daily Intakes for PFOA and PFOS.
The aforementioned recent data allow the calculation of updated exposure limits. Thus, serum PFOA concentrations from an experimental study of endocrine disruption after developmental exposure have been used to calculate benchmark dose levels.18,28 Serum concentrations from the recent human immunotoxicity study25 showed similar benchmark results.29 While taking into account an interspecies uncertainty factor of 10 routinely used in standards setting, these benchmark dose results are about 1,000-fold lower than the Benchmark Dose Lower Limits used by the EPA. Current exposure limits therefore do not protect against adverse effects. A draft risk assessment of PFOA was released by the EPA in 2006, but a final version has yet to appear and would likely require substantial revision.
PUBLIC HEALTH IMPLICATIONS
Although the Toxic Substances Control Act (TSCA) has been in force since the late 1970s, the law did not require testing of substances already in commerce at the time; therefore, toxicology studies of the PFCs were not mandated. It is even possible that the TSCA may have discouraged chemicals producers from testing substances that had already received blanket approval.30
PFOA and PFOS provide an example of what a National Research Council committee called the “untested-chemical assumption” (i.e., the lack of documentation means that they do not require regulatory action).31 Thus, risk assessment has relied on existing animal models and outcome variables, while other outcomes and vulnerable subgroups were ignored. This tradition has resulted in exposure limits that may be 1,000-fold too high to adequately protect against adverse health effects.
Ideally, conclusions on risks to human health should consider what could realistically be known, given the research studies completed so far, and what can appropriately be ignored. The absence of documentation from published epidemiologic studies does not exclude the possibility of adverse effects.32 Thus, in agreement with the National Research Council conclusions,31 the recent and much delayed insights into PFC exposure and health risks would support the call for a revision of the U.S. risk assessment and chemicals policy.
Footnotes
This work was funded, in part, by the National Institute of Environmental Health Sciences, National Institutes of Health (ES012199), and the Danish Council for Strategic Research (09-063094).
Philippe Grandjean prepared an expert report in 2012 on human health risks from exposure to perfluorinated compounds for the Minnesota Department of Health. Richard Clapp provided paid expert testimony in 2004 on the epidemiology of perfluorinated compounds for law firms Taft, Stettinius & Hollister in Ohio and Hill, Peterson, Carper, Bee & Dietzler in West Virginia, in civil suits.
REFERENCES
- 1.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45:7954–61. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 2.Environmental Protection Agency (US) Provisional health advisories for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) Washington: EPA; 2009. [Google Scholar]
- 3.Sargent JW, Seffl RJ. Properties of perfluorinated liquids. Fed Proc. 1970;29:1699–703. [PubMed] [Google Scholar]
- 4.Taves DR. Evidence that there are two forms of fluoride in human serum. Nature. 1968;217:1050–1. doi: 10.1038/2171050b0. [DOI] [PubMed] [Google Scholar]
- 5.Ubel FA, Sorenson SD, Roach DE. Health status of plant workers exposed to fluorochemicals—a preliminary report. Am Ind Hyg Assoc J. 1980;41:584–9. doi: 10.1080/15298668091425310. [DOI] [PubMed] [Google Scholar]
- 6.Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med. 1998;40:614–22. doi: 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Griffith FD, Long JE. Animal toxicity studies with ammonium perfluorooctanoate. Am Ind Hyg Assoc J. 1980;41:576–83. doi: 10.1080/15298668091425301. [DOI] [PubMed] [Google Scholar]
- 8.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) Environ Sci Technol. 2007;41:2237–42. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol. 2011;45:8037–45. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ Sci Technol. 2009;43:2641–7. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- 11.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander BH, Olsen GW. Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann Epidemiol. 2007;17:471–8. doi: 10.1016/j.annepidem.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol. 2008;18:15–22. doi: 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009;20:921–8. doi: 10.1097/EDE.0b013e3181b5f395. [DOI] [PubMed] [Google Scholar]
- 15.Steenland K, Woskie S. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol. 2012;176:909–17. doi: 10.1093/aje/kws171. [DOI] [PubMed] [Google Scholar]
- 16.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121:318–23. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010;118:1100–8. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Svoboda DJ, Azarnoff DL. Tumors in male rats fed ethyl chlorophen-oxyisobutyrate, a hypolipidemic drug. Cancer Res. 1979;39:3419–28. [PubMed] [Google Scholar]
- 20.Cook JC, Murray SM, Frame SR, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol. 1992;113:209–17. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- 21.Environmental Protection Agency (US), Science Advisory Board. SAB review of EPA's draft risk assessment of potential human health effects associated with PFOA and its salts. Washington: EPA; 2006. [Google Scholar]
- 22.Goldenthal EI, Jessup DC, Geil RG, Jefferson ND, Arceo RJ, Ruecker FA. Final report: ninety day subacute rat toxicity study on Fluorad® fluorochemical, FC-143. International Research and Development Corporation, Study No. 137- 089, 3M Reference No. T-3141, November 6, 1978, U.S. EPA Administrative Record, AR226-0441. [Google Scholar]
- 23.Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, et al. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci. 2002;69:244–57. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 24.Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, et al. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2012;258:248–55. doi: 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds [published erratum appears in JAMA 2012;307:1142] JAMA. 2012;307:391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ Res. 2010;110:773–7. doi: 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10:373–9. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 28.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandjean P, Budtz-Jorgensen E. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ Health. 2013;12:35. doi: 10.1186/1476-069X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sass J. The chemical industry delay game: how the chemical industry ducks regulation of the most toxic substances. Washington: Natural Resources Defense Council; 2011. [Google Scholar]
- 31.National Research Council. Science and decisions: advancing risk assessment. Washington: National Academies Press; 2009. [PubMed] [Google Scholar]
- 32.Grandjean P. Science for precautionary decision-making. In: Gee D, Grandjean P, Hansen SF, van den Hove S, MacGarvin M, Martin J, et al., editors. Late lessons from early warnings: science, precaution, innovation. Copenhagen: European Environment Agency; 2013. pp. 623–42. [Google Scholar]