Abstract
Repeating a hepatitis C virus antibody test for a person who previously tested positive provides no new information, wastes resources, and may reflect poor coordination of medical care. Using public health surveillance data collected by the New York City Department of Health and Mental Hygiene, we evaluated the magnitude of duplicate antibody testing and assessed patient-level and facility-level risk factors for duplicate testing. From 2006 to 2010, 70,257 duplicate tests were performed for 58,886 individuals in New York City, costing an estimated $1.4 million. Analyses using a polytomous logistic regression model indicated that individuals in correctional and substance abuse treatment facilities were more likely to undergo duplicate testing. Future efforts should focus on coordinating medical information and care for hepatitis C antibody-positive individuals to ensure that the recommended diagnostic follow-up and treatment services are provided.
Hepatitis C virus (HCV) is the leading cause of chronic liver disease and cirrhosis in the United States.1 Approximately 1.3% of the U.S. population is HCV antibody-positive (indicating exposure to the virus during the individual's lifetime) and 1.0% have chronic HCV.2 In New York City (NYC), the estimated prevalence of HCV infection is 2.4%.3 Many people with HCV are unaware of their infection status.4–6 Identification and education of people with HCV infection is critical to appropriate medical care and reducing the risk of liver disease progression.6
Screening with an HCV antibody test is recommended for individuals with HCV risk factors and people born between 1945 and 1965.7 Those who are antibody-positive should be tested for HCV ribonucleic acid (RNA) to distinguish past (resolved) from current infection.8 Repeat HCV antibody testing for people who previously tested positive provides no new information, wastes resources, and may reflect poor care coordination. Appropriate testing for HCV infection may be especially challenging when patients receive care in multiple health-care facilities. Adherence to HCV RNA testing guidelines is not well studied.
The aim of this study was to evaluate the magnitude of duplicate HCV antibody testing and evaluate risk factors for duplicate testing among individuals with positive HCV antibody tests reported to the NYC Department of Health and Mental Hygiene as part of routine public health surveillance.
METHODS
The NYC Health Code requires the reporting of positive HCV antibody tests with a high signal-to-cut-off ratio, positive recombinant immunoblot assay results, and positive HCV RNA results.9,10 Eligibility criteria for this analysis included unique people with a positive HCV antibody test result in the HCV surveillance database from 2006 to 2010, a valid birth date, age ≥1 year, and residing in NYC at the time of testing. We used a de-duplication algorithm to identify unique individuals; individuals reported prior to 2006 were excluded, along with all HCV antibody test results for those individuals. The algorithm uses identifiers such as patient name, date of birth, address, and Social Security number. The majority of reports are de-duplicated automatically through this algorithm; the possible matches are manually reviewed.
For each individual, we calculated the number of duplicate positive antibody tests by subtracting 1 from the total number of positive antibody tests. The distribution of this variable clustered in the lower end, so we created four categories: 0, 1, 2–3, and 4–37 duplicate tests. Individuals testing positive for HCV antibodies in one of 23 jails, prisons, or detention centers in NYC were coded as “correctional,” and those testing positive in one of 339 drug or alcohol treatment facilities were coded as “substance abuse treatment”; individuals could be coded as testing positive in both settings. We used the following age groups: 1–40 (reference group), 41–52, 53–65, and ≥66 years of age.
We calculated the overall number of duplicate antibody tests by subtracting the number of unique individuals from the total number of positive antibody tests. We estimated the cost of duplicate antibody testing using the 2006 Centers for Medicare & Medicaid Services reimbursement of $19.94 for an enzyme immunoassay test.11
We conducted bivariate analyses to assess associations between each predictor and duplicate antibody testing. We used polytomous logistic regression to examine associations between predictors and duplicate antibody testing and obtain odds ratios and 95% confidence intervals. We performed statistical analyses using SAS® version 9.2.12
RESULTS
Of 65,110 HCV antibody-positive individuals in NYC's HCV surveillance database from 2006 to 2010, 58,886 were eligible for analysis; 50% (n=29,431) had only one positive HCV antibody test (and, therefore, no duplicate tests), 23% (n=13,586) had one duplicate test, 18% (n=10,404) had 2–3 duplicate tests, and 9% (n=5,465) had 4–37 duplicate tests. From 2006 to 2010, 70,257 duplicate positive HCV antibody tests were performed, costing an estimated $1,400,925. Overall, 13% (n=7,854) of people in our analysis tested positive for HCV antibodies in a correctional facility and 25% (n=14,564) tested positive for HCV antibodies in a substance abuse treatment facility (Table 1).
Table 1.
Characteristics of HCV antibody-positive patients (n=58,886) in New York City, 2006–2010
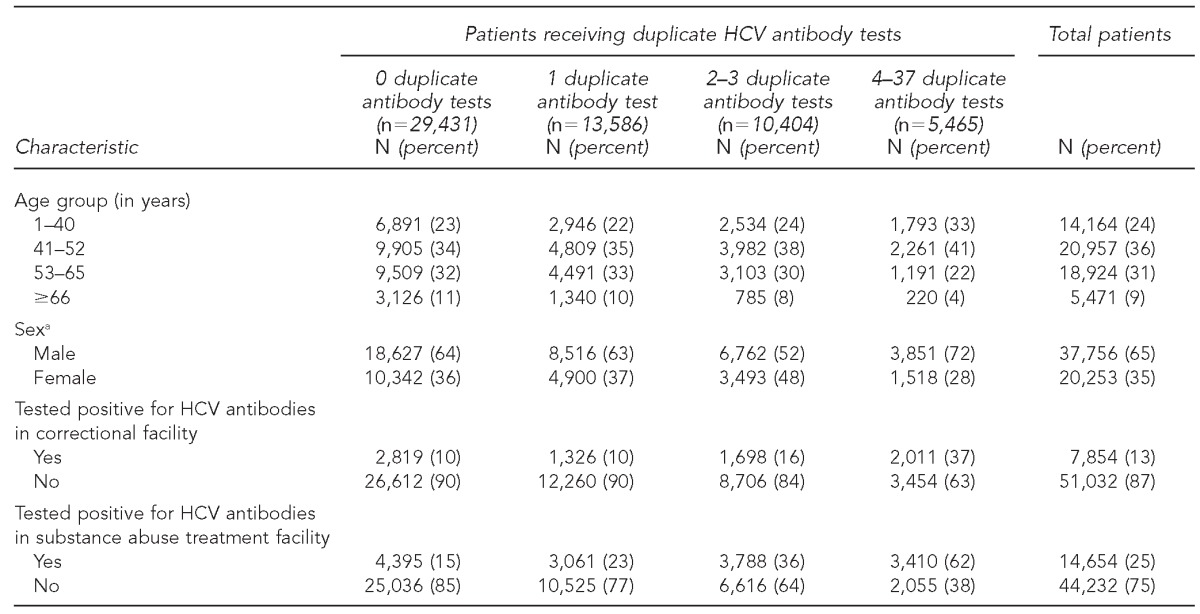
aMissing sex data for 877 (1%) patients
HCV = hepatitis C virus
In bivariate analyses, younger age (i.e., those aged ≤52 years), male sex, and testing positive for HCV antibodies in a correctional facility and substance abuse treatment facility were significant predictors of having a duplicate test (p<0.001 for each). Cross-tabulations of the substance abuse treatment and sex variables showed that more males than females had tested positive for HCV antibodies in substance abuse treatment facilities. An interaction term between sex and substance abuse treatment added to the regression model was significant (p<0.001) (data not shown).
Sex appeared to be an effect modifier of the association between substance abuse treatment and duplicate antibody tests; therefore, we constructed two polytomous logistic regression models, one for each gender (Table 2). Males and females who tested positive for HCV antibodies in a substance abuse treatment facility were more likely to have duplicate tests than those who did not test positive for HCV antibodies in a substance abuse treatment facility. Males and females who tested positive for HCV antibodies in a correctional setting were more likely to undergo ≥2 duplicate tests than those who did not test positive in a correctional setting. Age 41–65 years was a highly significant predictor of receiving duplicate antibody tests among both males and females; age ≥66 years was protective of receiving duplicate antibody tests.
Table 2.
Associations between the number of duplicate positive HCV antibody tests and age, testing positive in a substance abuse treatment facility, and testing positive in a correctional facility, stratified by sex: New York City, 2006–2010 (n=58,009)
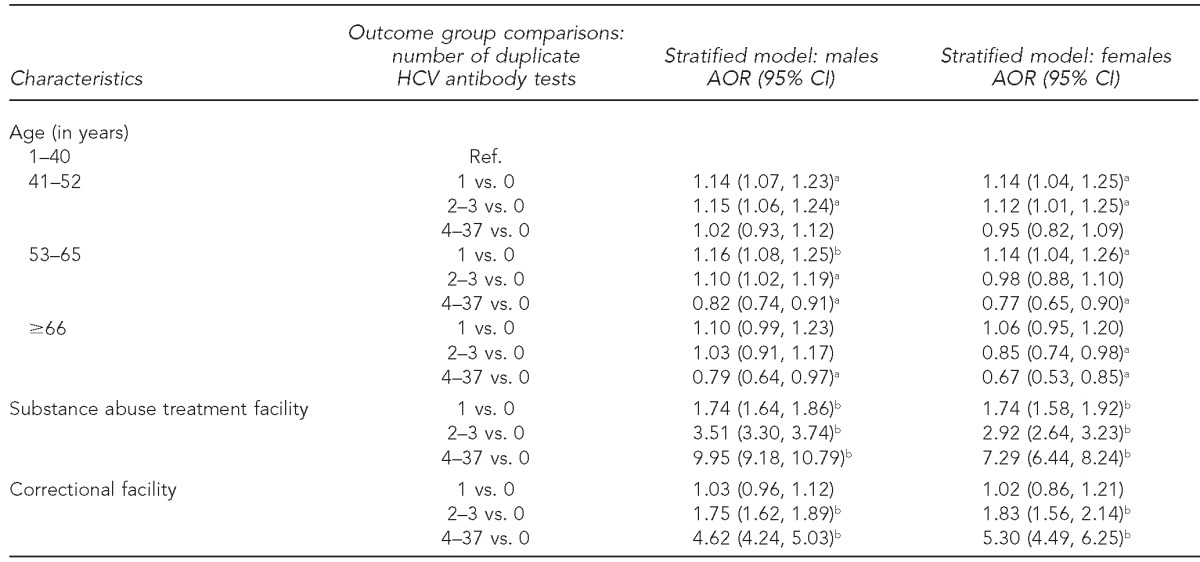
aStatistically significant at p<0.05
bStatistically significant at p<0.001
HCV = hepatitis C virus
AOR = adjusted odds ratio
CI = confidence interval
Ref. = reference group
DISCUSSION
Half of all HCV antibody-positive individuals in this surveillance dataset had duplicate antibody testing. A previous analysis found that among HCV antibody-positive veterans, 40% had ≥1 duplicate positive HCV antibody test, slightly lower than the 50% in the current analysis.11 This finding may reflect missed opportunities for follow-up steps recommended for HCV-positive individuals, such as RNA testing, counseling, linkage to medical care, evaluating liver damage, and considering antiviral treatment. Ensuring such follow-up care can prevent or slow liver disease progression. Although we did not assess patients' knowledge and understanding of their HCV antibody status, our findings suggest that patients may be unaware of prior positive HCV antibody test results. Patients should be educated about the difference between antibody status and infection status, and the need for HCV RNA testing after a positive antibody test. Previous studies have also demonstrated gaps in provider knowledge to appropriately diagnose, treat, and refer patients with HCV to specialists.13–15
Individuals who tested positive in a correctional or substance abuse treatment facility were more likely to have duplicate antibody tests than those who did not test positive in these settings. Substance abuse treatment facilities are an important source of access to HCV screening (accounting for one-fourth of all antibody tests in this dataset), but also contribute to the high level of duplicate antibody testing. Maintaining HCV screening at all points of care that serve injection drug users, including hospitals, clinics, substance abuse treatment facilities, harm-reduction and syringe exchange programs, and within the correctional system, is essential.
Most of the NYC correctional population is in a jail (not a prison), where standard practice is to conduct a medical intake examination, including HCV testing when indicated.16,17 Before August 2011, NYC correctional facilities did not have a comprehensive electronic health record (EHR) system in place (Personal communication, Farah Parvez, NYC Department of Health and Mental Hygiene [DOHMH], August 2013). Providers could not easily look up previous test results at intake, relying on self-report of results from detainees. Because the median length of stay is seven days for NYC jails, detainees may not receive their HCV antibody test result before leaving (Personal communication, Farah Parvez, NYC DOHMH, August 2013). These factors likely contribute to the high number of duplicate positive tests observed in correctional facilities. With a comprehensive EHR in place as of August 2011, providers at NYC correctional facilities can access previous test results at intake and share the information with hospitals that provide care to detainees after their release from the correctional system.
Limitations
This analysis was subject to certain limitations. Not every individual who tested positive for HCV antibodies in correctional and substance abuse treatment facilities was categorized as such due to inaccurate addresses or addresses that could not be distinguished from a larger facility (e.g., substance abuse treatment center within a hospital). We did not assess the reasons for duplicate antibody testing within and across facilities. We did not assess the potential overlap among patients who tested positive both in substance abuse treatment and correctional facilities; there may be a collinear relationship between these variables. Lastly, our dataset did not include reports from the Veterans Affairs system.
CONCLUSION
We estimate that duplicate antibody tests cost more than $1.4 million from 2006 to 2010 in NYC. This figure is likely an underestimate, as private laboratories may charge more than the Centers for Medicare & Medicaid Services. Duplicate antibody testing is only one example of the waste generated by lack of coordination among providers and health-care systems. These findings highlight the importance of developing systems to share medical information within and across facilities. Establishing EHRs and promoting data sharing through projects such as regional health information organizations can reduce unnecessary testing. However, the solution to the issue of duplicate testing is not simple, especially in facilities without EHRs, or if patients do not provide accurate and complete histories. Future efforts should focus on identifying and understanding reasons for inappropriate duplicate testing and how best to improve HCV testing practices.
Footnotes
The authors thank Sharon Balter, New York City Department of Health and Mental Hygiene (NYC DOHMH); Mary Ann Chiasson, Columbia University Mailman School of Public Health, Public Health Solution; Sharon Greene, NYC DOHMH; and Farah Parvez, NYC DOHMH, for their contributions to this article.
REFERENCES
- 1.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balter S, Stark JH, Kennedy J, Bornschlegel K, Konty K. Estimating the prevalence of hepatitis C infection in New York City using surveillance data. Epidemiol Infect. 2014;142:262–9. doi: 10.1017/S0950268813000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55:1652–61. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornschlegel K, Berger M, Garg RK, Punsalang A, McKinney CM, Gwynn RC, et al. Prevalence of hepatitis C infection in New York City, 2004. J Urban Health. 2009;86:909–17. doi: 10.1007/s11524-009-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Hepatitis C and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington: National Academies Press; 2010. [PubMed] [Google Scholar]
- 7.Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965 [published erratum appears in MMWR Morb Mortal Wkly Rep 2012;61(43):886] MMWR Recomm Rep. 2012;61(14):1–36. [PubMed] [Google Scholar]
- 8.Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62(18):362–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2013. Hepatitis C information for health professionals. Guidelines for laboratory testing and result reporting. Also available from: URL: http://www.cdc.gov/hepatitis/HCV/LabTesting.htm#section1 [cited 2013 Jun 7] [Google Scholar]
- 10.New York City Department of Health and Mental Hygiene. Hepatitis A, B and C surveillance report: New York City, 2008 and 2009 [cited 2014 Jul 7] Available from: URL: http://www.nyc.gov/html/doh/downloads/pdf/cd/cd-hepabc-surveillance-report-08-09.pdf.
- 11.Groom H, Dieperink E, Nelson DB, Garrard J, Johnson JR, Ewing SL, et al. Outcomes of a hepatitis C screening program at a large urban VA medical center. J Clin Gastroenterol. 2008;42:97–106. doi: 10.1097/MCG.0b013e31802dc56f. [DOI] [PubMed] [Google Scholar]
- 12.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 13.Ferrante JM, Winston DG, Chen PH, de la Torre AN. Family physicians' knowledge and screening of chronic hepatitis and liver cancer. Fam Med. 2008;40:345–51. [PubMed] [Google Scholar]
- 14.Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000–2007. Am J Manag Care. 2011;17:548–55. [PubMed] [Google Scholar]
- 15.Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol. 2003;98:639–44. doi: 10.1111/j.1572-0241.2003.07331.x. [DOI] [PubMed] [Google Scholar]
- 16.New York Department of Health and Mental Hygiene. Physical health services in New York City's correctional facilities. 2008. [cited 2014 Jul 7]. Available from: URL: http://www.nyc.gov/html/doh/downloads/pdf/public/testi/testi20081028.pdf.
- 17.Jordan AO. Linkages and care engagement: from NYC jail to community provider. New York State Department of Health AIDS Institute HIV Quality of Care Advisory Committee Meeting; 2013 Dec 12; New York. Also available from: URL: http://www.hivguidelines.org/wp-content/uploads/2013/12/9-NYC-Corrections-Linkage-and-Care-Engagement.pdf [cited 2014 Jul 20] [Google Scholar]


