Abstract
Objectives
We surveyed U.S. immunization program managers (IPMs) as part of a project to improve public health preparedness against future emergencies by leveraging the immunization system. We examined immunization program policy and Immunization Information System (IIS) functionality changes as a result of the Haemophilus influenzae type B (Hib) vaccine shortage and pandemic influenza A(H1N1) (pH1N1). Evaluating changes in immunization program functionalities and policies following emergency response situations will assist in planning for future vaccine-related emergencies.
Methods
We administered three consecutive surveys to IPMs from 64 state, city, and territorial jurisdictions in 2009, 2010, and 2012. We compared IPMs' responses across either two or three years (e.g., changes in response or consistent responses across years) using McNemar's test.
Results
Immunization programs maintained increases in functionality related to communication systems with health-care providers during this period. Immunization programs often did not maintain changes to IIS functionalities made from 2009 to 2010 (e.g., identifying high-risk and priority populations, tracking adverse events, and mapping disease risk) in the post-pandemic period (2010–2012). About half of IPMs reporting additional IIS functionality in identifying high-risk populations from 2009 to 2010 reported no longer having this function in 2012. There was an 18% decline in respondents reporting geographic information systems risk-mapping capability in IIS from 2010 to 2012.
Conclusions
Because of the Hib vaccine shortage and pH1N1, immunization program needs and efforts changed to address evolving situations. The lack of sustained increases in resources or system functions after the pandemic highlights the need for comprehensive, sustainable public health emergency preparedness systems and related resources.
Pandemic influenza A(H1N1) (pH1N1) both -positively and negatively impacted existing immunization programs and policies throughout the United States, necessitating rapid changes in response to the evolving pandemic.1,2 The H1N1 influenza response required efficient action, which resulted in the vaccination of 81 million people in less than a year3 and included the collaboration of federal, state, and local partners.4 State and local immunization programs were forced to rapidly respond and evolve due to the nature of that fast-moving pandemic, and, during that time, information (e.g., disease severity and vaccine planning) was constantly changing.1,2,5,6
After-action reports, surveys, and discussion groups have summarized the perspectives of officials from all levels about the successes and challenges of the response.1,2,4–6 Funding allocated to health departments participating in the response assisted in development and implementation of response policies and activities. A major success of the H1N1 influenza vaccination program was the administration of free vaccines for the U.S. population. Additionally, after-action discussions and reports gave rise to policy recommendations regarding increased guidance on medical countermeasures and antiviral use, national vaccine registries, and improved use of the Incident Command System (ICS).7
But changes made to immunization programs during the course of the pandemic may not be sustained. The National Association of County and City Health Officials anticipated massive cuts to health departments' personnel and programs.8 Quantifying the differences in resources during emergency and nonemergency times will provide insight into the sustainability of changes made during an emergency and the ability of health departments to maintain preparation for future pandemics.
We conducted surveys of immunization program managers (IPMs) in three different years during different emergencies that would potentially affect immunization programs. In 2009, the survey questions focused on the Haemophilus influenzae type B (Hib) vaccine shortage. We administered the 2010 survey shortly after the pH1N1 response. In 2012, the survey questions reflected on the response to pH1N1. Surveying IPMs9,10 during the three time periods allowed us to assess the extent to which the immunization programs sustained the changes that occurred during pH1N1 and extended them beyond that event.
METHODS
IPMs received electronic surveys in 2009, 2010, and 2012, as described by Chamberlain et al. for the 2009 and 2010 surveys9 and by Seib et al.11 for the 2012 survey. The 2009 survey focused on management of the 2007–2009 Hib vaccine shortage and included questions on immunization programs' preparations for the fall 2009 H1N1 influenza vaccination campaign. The 2010 and 2012 surveys focused on programs' management of the H1N1 vaccination campaign and were conducted using methods similar to those used for the 2009 survey. We e-mailed or mailed surveys to IPMs in 64 jurisdictions (i.e., federal immunization program grantees), including the 50 states; the District of Columbia; Houston, Texas; New York, New York; Philadelphia, Pennsylvania; San Antonio, Texas; and U.S. territories. Each survey included qualitative and quantitative questions regarding Immunization Information System (IIS), relationships with emergency preparedness personnel, pandemic planning, relationships and communication between immunization programs and providers in the jurisdiction, identification of high-risk populations, and funding in the context of the pH1N1 vaccination campaign. Fifty-eight percent (n=37) of IPMs from the 64 jurisdictions responded to the 2009 survey, 84% (n=54) responded to the 2010 survey, and 95% (n=61) responded to the 2012 survey. Forty-five percent (n=29) responded to all three surveys (data not shown).
We identified the quantitative questions that were similar across two or more surveys. We analyzed responses using SAS® version 9.1.3.12 We compared IPMs' responses across either two or three years (e.g., changes in response or consistent responses across years) using McNemar's test, with p<0.05 considered statistically significant.
A total of 12 questions available for analysis were consistent throughout at least two of the surveys (2009 and 2012 surveys, 2010 and 2012 surveys, or across all three surveys). The 12 questions mainly focused on the IIS of each jurisdiction as an integral part of an immunization program. We analyzed these questions to identify changes in immunization programs' capabilities and practices from the height of pH1N1 to the two years following.
We asked IPMs about specific functional components of their jurisdictions' IIS. In 2009, the survey stated, “Does your state's IIS have any of the following modules or functional components?”; in 2010, we asked, “How valuable was your jurisdiction's IIS in accomplishing the following activities during the H1N1 vaccination campaign?”; and in 2012, we asked respondents to “Indicate the functionality changes that occurred to the IIS during or after the H1N1 vaccination campaign or if the functionality is planned for the future.” We recoded the result of that question to “yes” if the IPM indicated the IIS had the function and “no” if the IPM indicated the IIS did not have that function.
Questions analyzed included asking the IPMs if they had the authority to include information on adults in their jurisdictions' IIS, and asking if their IIS had the functions to do the following: track vaccine distribution, identify high-risk or high-priority populations, track vaccine administration, perform risk mapping, track vaccine-related adverse events, allow providers to place vaccine orders, push vaccine-related communication out to providers, and transfer vaccines among provider sites. We also asked IPMs about funds from the Emergency Preparedness Cooperative Agreement and other staffing support.
RESULTS
Capabilities that addressed communication with providers and strategic partners increased from 2010 to 2012. Fourteen immunization programs that reported not having the ability to push vaccine-related communication to providers in 2010 reported having that ability in 2012 (data not shown). There was an increase in the percentage of IPMs responding to the survey who indicated having the ability to push vaccine-related communication to providers, from 61% in 2010 to 83% in 2012 (Table). Only two IPMs who reported having the ability to push vaccine-related communication to providers in 2010 no longer reported having this function in 2012. Additionally, 11 immunization programs that did not have the functionality to allow providers to place vaccine orders as part of their jurisdictions' IIS in 2010 could do so in 2012, while only six immunization programs that previously allowed providers to place vaccine orders in 2010 indicated they no longer could in 2012 (data not shown).
Table.
Comparison of IPM reports of immunization program and IIS functionalities, as provided in surveys conducted among U.S. IPMs surveyed in 2009, 2010, and 2012
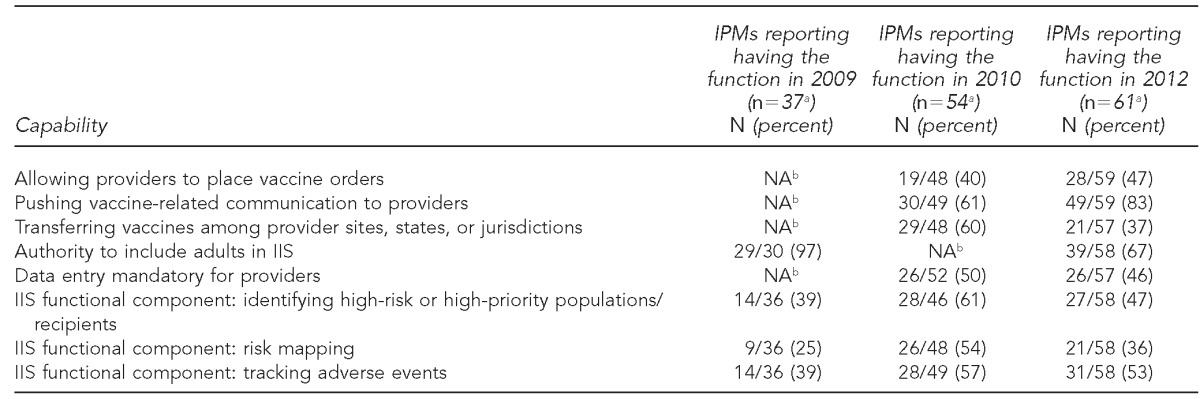
aIPMs responding to the survey did not respond to each survey question.
bQuestion not included in that year's survey
IPM = immunization program manager
IIS = Immunization Information System
NA = not available
From 2009 to 2010, about 36% of IPMs (10/28) who responded to all three surveys reported that their jurisdictions' immunization program had gained the ability to identify high-risk or high-priority populations between the two surveys, whereas only two that reported having this function in place in 2009 reported no longer having it in 2010. Of those who reported gaining this function from 2009 to 2010, 18% fewer IPMs reported having the function in 2012 (data not shown).
A similar pattern occurred for the risk-mapping capability using geographic information systems (GIS). Almost twice as many total respondents reported having this function in 2010 than in 2009. However, the proportion of IPMs who reported having this ability decreased by 18% from 2010 to 2012. The use of the IIS function to track vaccine-related adverse events also increased. Nine respondents reported gaining the function from the 2009 to the 2010 survey, while only two respondents lost this function during that time period. From the 2010 to 2012 surveys, four respondents lost this function and two gained it (data not shown).
IPMs who reported that their immunization programs had the ability to transfer vaccines among provider sites decreased from 60% (29/48) in 2010 to 37% (21/57) in 2012. Almost all of the immunization programs had the authority to include adults in their IIS in 2009 (97%, 29/30); however, in 2012, only 67% (39/58) reported having this authority (Table).
Regarding funding for public health preparedness and response, the majority of IPMs (73%) reported using funds from the Centers for Disease Control and Prevention (CDC) Public Health Emergency Preparedness Cooperative Agreements13 in 2009. In 2012, 41% of IPMs reported receiving funding, staffing support, and other resources from the emergency preparedness program for their immunization programs after the H1N1 vaccination campaign (data not shown).
DISCUSSION
Through the analyses of IPM responses to similar questions in surveys spanning three years, we found that vaccine shortages and the efforts to vaccinate communities for pandemic influenza not only called for changes to existing programs, but also required increased resources to respond. Despite some limited successes that have been sustained or strengthened over time (e.g., the capabilities for IPM-immunization provider communication), other key functions (e.g., the ability to identify populations at elevated risk for severe illness or to map the risk or prevalence of disease using GIS) were not used from the end of the pandemic to 2012, potentially due to a lack of sufficient staffing. These functionalities are especially important during a public health emergency, but they should not be lost or left entirely inactive during nonemergency situations. These capabilities remain important to public health departments in non-pandemic situations, such as shortages of routine vaccines (e.g., 2007–2009 Hib vaccine shortage and 2004 influenza vaccine shortage),14 vaccine recalls (e.g., December 2009 quadrivalent human papillomavirus vaccine recall),15 localized epidemics (e.g., pertussis outbreaks), and natural disasters. There is a need to identify high-priority groups for vaccination in standard practice. For example, including adults in the IIS for vaccinations where the risk factors for severe illness may be similar for an influenza pandemic (e.g., adult pneumococcal vaccine for individuals ≥65 years of age) may allow physicians to identify high-risk populations during an emergency.5 Using functions such as high-risk patient identification on a regular basis will enable practitioners to keep their skills current for use in an emergency.
Enhanced communication with providers and the ability to transfer vaccine among providers may be useful in settings other than an emergency. For example, during a recall of one lot of the Gardasil® vaccine in December 2013, the use of vaccine tracking and provider communication tools within IIS could have provided an efficient way to ensure the recall was communicated rapidly and handled appropriately.16 While the ability to transfer vaccines among provider sites is most critical during an emergency, due to the routine nature of shortages of vaccines or other products,1 there may be a need to transfer products to a higher volume practice to better serve the demand. In 2013, during a shortage of tuberculin skin tests, CDC recommended prioritizing the use of this product for tuberculosis contact investigations for high-risk individuals.17 Such a recommendation might require the transfer of the product from providers who have low-risk patients to providers who have high-risk patients, or to public health authorities. The ability to transfer products among provider sites more frequently may allow providers to do the same on a larger scale during an emergency. Public health departments would benefit from additional resources to maintain these utilities. Improving the functionality profile of immunization programs may assist in improving the standard of care during non-emergencies.
Through our analysis, we learned that IPMs' communication with community partners was an area of improvement since 2009. We observed that enhancements made to communication capabilities during the 2009 pH1N1 response were sustained following the pandemic. This result mirrors other conclusions about partnership strengthening between and within health departments, schools, and emergency responders as a result of responding to pH1N1.4 Our results also show that IPMs reported that improved communication among immunization programs, providers, emergency preparedness personnel, and other key partners was sustained. Running successful and efficient mass vaccination clinics required joint participation of health departments, health-care providers, schools, and pharmacies,18,19 and these partnerships that arose out of necessity are likely to be the most useful during future emergencies.10
Because funding for public health programs is limited, it is unrealistic to expect all capabilities established in response to a national vaccine-related emergency to be maintained post-emergency. These functions often require increased staffing or funding levels, and in the era of budget limitations, it may not be feasible to maintain all capabilities at maximal levels during non-emergency periods.20 Health departments need to prioritize programs based on jurisdictions' needs. Resources, including personnel, need to adapt as funding climates and demands change;8 however, it remains important not to lose programmatic familiarity with the functionalities, management policies, and budgetary decisions that proved effective during a large-scale emergency. One way to maintain preparedness for vaccine-related emergencies both large and small is by continuing to foster ties between immunization programs and emergency preparedness programs. Through concerted efforts to maintain close working relationships between immunization and emergency preparedness programs, functionalities can be shared, costs can be diffused, and an institutional memory of what has been successful in the past can persist over time and across multiple programs. In 2012, the Association of Immunization Managers (AIM) developed a set of collaboration principles for immunization programs and emergency preparedness programs that explicitly describes activities the two programs can do to maintain their collaborations across time and between emergencies.21 From the perspective of maintaining preparedness during a tight budgetary climate, AIM's principles remain valuable.
After the initial shortage of vaccine, vaccine manufacturing increased, allowing the H1N1 influenza vaccine to be distributed quickly in mass quantities.22,23 The federal government purchased the pH1N1 vaccine and allocated it to the states, and the states were responsible for allocating the vaccine in a way that made sense for each state. For example, some states used the health-care system to distribute vaccine.24,25 Working with providers to distribute vaccine required effective communication with providers.9,10,13,18 This enhanced communication with providers, especially those who cared for high-risk patients, might have also increased the ability to push out vaccine-related communication to providers.2 The tracking of adverse events associated with this new vaccine was a priority for the federal government and impacted states and providers through enhanced tracking of adverse events associated with the vaccine.26,27 The increased priority at the federal level likely improved the ability of immunization programs at the state or local level to track adverse events during the pandemic.
During the fall 2009 peak of pH1N1, when the vaccine was first produced and distributed, there were shortages of the H1N1 influenza vaccine.18 The lessons learned from the Hib vaccine shortage served as a starting point for developing plans to maximize H1N1 influenza vaccine delivery to high-risk populations.10 While health departments attempted to prioritize high-risk groups, reallocation of the vaccine was likely necessary because of (1) the way the vaccine was packaged for delivery (i.e., shipment sizes too large for a given practice's needs), (2) the vaccine formulation recommended or contraindicated for certain groups, and (3) the elevated need in some places but not others.18 This shortage called for the ability of jurisdictions to move vaccines between provider sites.28 This policy change was necessary in the context of an emergency. Further research is needed to investigate whether or not this policy change, if made permanent, would affect funding or would be useful in non-emergency settings.
The addition of capabilities during emergencies and then their subsequent loss, along with the current budget climate, highlights the need for comprehensive emergency preparedness programs in which immunization plays a role along with other key preparedness activities. By viewing components of public health preparedness across a continuum rather than as dissociated activities with little or no routine coordination across departments, officials can leverage these activities for the most effective use of limited resources. This incorporation of emergency preparedness and routine functionality can provide a framework for more rapid coordination when public health emergencies arise.
Strengths and limitations
This study was subject to several limitations. For one, while the response rates for the 2010 and 2012 surveys were high, the response rate for the 2009 survey was less than optimal. Because we administered the 2009 survey from July through October, IPMs were likely preoccupied with preparing for the fall 2009 mass vaccination campaign. However, the timing of the surveys did enable us to gather data on programmatic capabilities both during the height of the pandemic and post-pandemic. These measurements allowed us to evaluate not only the changes made in response to the pandemic, but also the sustainability of changes across three distinct time periods. With high survey response rates in 2010 and 2012, we were able to evaluate changes in programmatic functionalities even without data from the mid-pandemic period from certain programs.
Second, because the wording of the questions in each survey was not always consistent, it was challenging to compare each question over time; however, we were able to use questions that were very similar, if not worded exactly the same, for comparisons between each survey. The findings of reduced IIS functionality at later time points, among IPMs that previously reported having these functionalities, may be due in part to a difference in perception relative to the questions asked, as increased technological components of IIS are not routinely uninstalled. If an IIS had the program modules installed, but the modules were not actively being used after the pandemic, it is possible that respondents may not have perceived these modules as available functions within their IIS and responded that the function was not in place. Further research is needed to identify the reasons behind these perceived functionality losses.
Third, the surveys were aggregated at a demographic level, and issues in specific jurisdictions were not described. Some areas may not have the ability or need to maintain certain functions. The use of public health preparedness systems as encompassing immunization programs may negate the need for immunization programs to have the capacity to perform tasks on a regular basis that may exist elsewhere in a health department and could be used in an emergency. This analysis did not examine the prioritization of immunization programs' capabilities specific to certain jurisdictions.
CONCLUSIONS
Our analysis across three surveys administered during four years suggests that some of the programmatic capabilities established within immunization programs during both routine vaccine shortages and a response to a pandemic have not been sustained. The loss of these functional capabilities may limit the ability of health departments to be adequately prepared for the next vaccine-related public health emergency. A sustained balance between available resources and the need to maintain comprehensive public health emergency preparedness programming is necessary in the face of limited resources and future public health threats. When resources are not sustained, capabilities may not be sustained; constant rebuilding of infrastructure and capabilities needed in responding to vaccine shortages and public health emergencies may not be an effective use of resources. Further investment in immunization systems as a part of comprehensive emergency preparedness programs across health departments could maintain preparedness in strategic and cost-effective ways, thereby potentially reducing the strain on the immunization system and boosting its success during future public health emergencies.
Footnotes
The Emory University Institutional Review Board approved all surveys as exempt studies. This project was supported by Centers for Disease Control and Prevention (CDC) grant #5P01TP000300 for the Emory University Preparedness and Emergency Response Research Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
REFERENCE
- 1.Government Accountability Office (US) Washington: GAO; 2011. Influenza pandemic: lessons from the H1N1 pandemic should be incorporated into future planning. Also available from: URL: http://www.gao.gov/assets/330/320176.pdf [cited 2014 Jan 6] [Google Scholar]
- 2.Institute of Medicine. Washington: National Academies Press; 2010. The 2009 H1N1 influenza vaccination campaign: summary of a workshop series. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) Final estimates for 2009–10 seasonal influenza and influenza A (H1N1) 2009 monovalent vaccination coverage—United States, August 2009 through May 2010. 2011 [cited 2014 Jul 3] Available from: URL: http://www.cdc.gov/flu/fluvaxview/coverage_0910estimates.htm.
- 4.Stoto MA, Nelson C, Higdon MA, Kraemer J, Hites L, Singleton CM. Lessons about the state and local public health system response to the 2009 H1N1 pandemic: a workshop summary. J Public Health Manag Pract. 2013;19:428–35. doi: 10.1097/PHH.0b013e3182751d3e. [DOI] [PubMed] [Google Scholar]
- 5.Association of State and Territorial Health Officials. Arlington (VA): ASTHO; 2010. Assessing policy barriers to effective public health response in the H1N1 influenza pandemic: project report to the Centers for Disease Control and Prevention. Also available from: URL: http://www.astho.org/Programs/Infectious-Disease/H1N1/H1N1-Barriers-Project-Report-Final-hi-res [cited 2014 Jan 6] [Google Scholar]
- 6.National Association of County and City Health Officials Washington: NACCHO; 2010. -NACCHO H1N1 policy workshop report. Also available from: URL: http://www.naccho.org/topics/h1n1/upload/naccho-workshop-report-in-template-with-chart.pdf [cited 2014 Jan 6] [Google Scholar]
- 7.Stoto MA, Nelson C, Higdon MA, Kraemer J, Singleton CM. Learning about after action reporting from the 2009 H1N1 pandemic: a workshop summary. J Public Health Manag Pract. 2013;19:420–7. doi: 10.1097/PHH.0b013e3182751d57. [DOI] [PubMed] [Google Scholar]
- 8.National Association of County and City Health Officials. Washington: NACCHO; 2010. Local health department job losses and program cuts: findings from January/February 2010 survey. Also available from: URL: http://www.naccho.org/topics/infrastructure/lhdbudget/upload/Job-Losses-and-Program-Cuts-5-10.pdf [cited 2014 Jan 6] [Google Scholar]
- 9.Chamberlain AT, Seib K, Wells K, Hannan C, Orenstein WA, Whitney EA, et al. Perspectives of immunization program managers on 2009–10 H1N1 vaccination in the United States: a national survey. Biosecur Bioterror. 2012;10:142–50. doi: 10.1089/bsp.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain AT, Wells K, Seib K, Kudis A, Hannan C, Orenstein WA, et al. Lessons learned from the 2007 to 2009 Haemophilus influenzae type B vaccine shortage: implications for future vaccine shortages and public health preparedness. J Public Health Manag Pract. 2012;18:E9–16. doi: 10.1097/PHH.0b013e31821dce27. [DOI] [PubMed] [Google Scholar]
- 11.Seib K, Chamberlain A, Wells K, Hannan C, Curran E, Whitney EAS, et al. Changes in the U.S. immunization system since the H1N1 pandemic response: immunization program managers share perspectives in 2012 national survey. Hum Vaccin. 2015 doi: 10.4161/21645515.2014.972798. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAS Institute, Inc. SAS®: Version 9.1.3. Cary (NC): SAS Institute, Inc.; 2004. [Google Scholar]
- 13.Centers for Disease Control and Prevention (US), Office of Public Health Preparedness and Response. Funding, guidance, and technical assistance to states, localities, and territories [cited 2014 Jan 6] Available from: URL: http://www.cdc.gov/phpr/coopagreement.htm.
- 14.Centers for Disease Control and Prevention (US) Current vaccine shortages & delays [cited 2014 Jan 8] Available from: URL: http://www.cdc.gov/VACCINes/vac-gen/shortages/default.htm.
- 15.Centers for Disease Control and Prevention (US) Recalled vaccines [cited 2014 Jan 8] Available from: URL: http://www.cdc.gov/VACCINes/recs/recalls/default.htm.
- 16.Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31:452–60. doi: 10.1016/j.vaccine.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J Clin Periodontol. 2013;40(Suppl 14):S170–80. doi: 10.1111/jcpe.12082. [DOI] [PubMed] [Google Scholar]
- 18.Rambhia KJ, Watson M, Sell TK, Waldhorn R, Toner E. Mass vaccination for the 2009 H1N1 pandemic: approaches, challenges, and recommendations. Biosecur Bioterror. 2010;8:321–30. doi: 10.1089/bsp.2010.0043. [DOI] [PubMed] [Google Scholar]
- 19.Kun KE, Zimmerman J, Rose DA, Rubel S. State, territorial, and local health departments' reporting of partnership strength before and after the H1N1 response. Prehosp Disaster Med. 2013;28:508–5. doi: 10.1017/S1049023X13009011. [DOI] [PubMed] [Google Scholar]
- 20.National Association of County and City Health Officials. Washington: NACCHO; 2011. Local health department job losses and program cuts: findings from January 2011 survey and 2010 National Profile Study. Also available from: URL: http://www.naccho.org/topics/infrastructure/lhdbudget/upload/Final.pdf [cited 2014 Jul 3] [Google Scholar]
- 21.Association of Immunization Managers. Rockville (MD): AIM; 2012. Preparing for the next pandemic: immunization program & emergency preparedness program pandemic preparedness collaboration principles. Also available from: URL: http://www.immunizationmanagers.org/resource/resmgr/files/collaboration_principles.pdf [cited 2014 Jan 8] [Google Scholar]
- 22.Centers for Disease Control and Prevention (US) H1N1 flu: table and graph of 2009 H1N1 influenza vaccine doses allocated, ordered, and shipped by project area [cited 2014 Jan 6] Available from: URL: http://www.cdc.gov/h1n1flu/vaccination/vaccinesupply.htm.
- 23.World Health Organization. Pandemic influenza vaccine manufacturing process and timeline [cited 2014 Jan 6] Available from: URL: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090806/en.
- 24.Centers for Disease Control and Prevention (US) H1N1 flu: allocation and distribution Q&A [cited 2014 Jan 6] Available from: URL: http://www.cdc.gov/h1n1flu/vaccination/statelocal/centralized_distribution_qa.htm.
- 25.Centers for Disease Control and Prevention (US) H1N1 flu: vaccine against 2009 H1N1 influenza virus [cited 2014 Jan 6] Available from: URL: http://www.cdc.gov/h1n1flu/vaccination/public/vaccination_qa_pub.htm.
- 26.Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, et al. Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) system, 2009–2010. Am J Epidemiol. 2012;175:1120–8. doi: 10.1093/aje/kws197. [DOI] [PubMed] [Google Scholar]
- 27.Federal Immunization Safety Task Force (US) Federal plans to monitor immunization safety for the pandemic 2009 H1N1 influenza vaccination program [cited 2013 Dec 26] Available from: URL: http://www.flu.gov/planning-preparedness/federal/fed-plan-to-mon-h1n1-imm-safety.pdf.
- 28.Centers for Disease Control and Prevention (US) H1N1 flu: reallocating influenza A (H1N1) 2009 monovalent vaccine [cited 2014 Jan 6] Available from: URL: http://www.cdc.gov/h1n1flu/vaccination/reallocating.htm.


