Abstract
Objective
To determine if supplemental intra-articular alpha-2 macroglobulin (A2M) has a chondroprotective effect in a rat OA model.
Methods
A2M was identified as a potential therapeutic agent by comparing A2M concentrations in serum, synovial fluid (SF), and cartilage from normal and osteoarthritic (OA) patients by Western blotting, mass spectrometry, ELISA, and immunohistochemistry (IHC). The effects of A2M on IL-1-induced cartilage catabolic enzymes were evaluated by Luminex and ELISA in cultured chondrocytes. In vivo effects on cartilage degeneration and MMP-13 concentration were evaluated in male rats (N=120) randomized to four treatments: (1) CLT+saline, (2) ACLT+A2M (1IU/kg), (3) ACLT+A2M (2IU/kg) or (4) sham surgery+saline. Intra-articular injections were given for 6 weeks. The concentration of MMP-13 in SF lavages was measured using ELISA. OA-related gene expression was quantified by RT-qPCR. Histology was performed to grade OA.
Results
In both normal and OA patients, the levels of A2M were lower in SF compared to serum, and MMP-13 was higher in SF than serum of OA patients. In vitro, A2M inhibited the induction of MMP-13 by IL-1 in a dose-dependent manner in human chondrocytes. In the rat ACLT OA model, supplemental intra-articular injection of A2M reduced the concentration of MMP-13 in SF, had a favorable effect on OA-related gene expression, and attenuated OA progression.
Conclusion
A2M is a plasma protease inhibitor that is not present in sufficient concentrations to inactivate the high concentrations of catabolic factors found in OA SF. Our findings suggest that supplemental intra-articular A2M provides chondral protection for post traumatic OA.
Introduction
Anterior cruciate ligament (ACL) injury is one of the most frequent musculoskeletal injuries in adolescents and young adults, and it is known to place the injured knee at risk for early post-traumatic osteoarthritis (PTOA) (1). Evidence suggests that the current gold standard of treatment, surgical ACL reconstruction, does not appreciably reduce this risk (2–7). Discovery of mechanisms responsible for PTOA in this patient population would enable clinicians to identify markers and targets to aid in the diagnosis, treatment, and prevention of PTOA. OA progression is due, at least in part, to the up-regulation of inflammatory mediators and proteases (8) (9–11). Since elevated levels of catabolic enzymes in synovial fluid are associated with chondrocyte death and cartilage matrix degeneration within one week of injury (8) (12) (13, 14) (15), early intervention strategies should focus on modulating these cartilage degrading enzymes within this time frame. Evidence from our group (11) (16) (17) (18) (19) and others (8) (13) (14) suggests that new molecular interventions targeting these enzymes can potentially arrest these adverse events and preserve joint health. It is unlikely however, that blocking only one of these catabolic factors would be enough to repress PTOA after injury.
Our initial hypothesis was that endogenous serum protease inhibitors are not adequately present in the joint. A2M is a serum protease inhibitor that was identified as a potential therapeutic agent by screening serum, synovial fluid (SF), and cartilage from normal and osteoarthritic (OA) patients with Western blotting, mass spectrometry, ELISA, and immunohistochemistry (IHC). A2M, a major serum protease inhibitor, inhibits all classes of endoproteases (20, 21). Our hypothesis is that A2M injected intra-articularly could potentially slow cartilage damage following a traumatic knee injury by neutralizing cartilage catabolic degrading enzymes. In order to establish a functional role for A2M in OA development, the concentrations of cartilage catabolic factors and their gene expression were quantified after A2M supplementation in cultures of human OA chondrocytes and cartilage organ cultures. The changes in cartilage catabolic enzymes were monitored in vivo by fluorescence molecular tomography (FMT) using a mouse partial medial meniscectomy (PMM) OA model. To assess the effects of A2M on cartilage damage in vivo, we used the rat ACLT model treated with supplemental intra-articular injections of A2M shortly after injury. We also characterized the endogenous expression of A2M in human knee joint tissues. Our results strongly indicate that A2M is a negative regulator of cartilage catabolic enzymes, but it is not present in vivo at sufficient levels to counteract the increased concentrations of catabolic factors that appear after injury. Therefore, supplemental intra-articular injection of A2M shortly after injury may provide chondral protection to the ACL injured knee by reducing catabolic enzymes.
Methods
This study was approved by the IRB and IACUC at Rhode Island Hospital.
Human samples
OA cartilage samples were obtained from patients at the time of total joint arthroplasty (N=17, 11 female, 6 male, age 68.6±8.6 (mean±SD), range 55–79). Normal cartilage samples were obtained from patients undergoing tumor resections (N=6, 6 male, age 23.8±13.6, range 15–51). These samples were a subset of those used for a previous study (22). Serum and OA SF samples were also obtained prior to and during knee joint arthroplasty, respectively (N= 39, 20 female, 19 male, age 65.4±9.6, range 48–80) in another set of patients. OA diagnosis was made by the clinician’s assessment using American College of Rheumatology (ACR) criteria. Normal serum samples were also collected (N=43, age 37.5±10.2, range 20–56). Cartilage damage in knee joints was classified during arthroscopy before arthroscopic debridement or by direct surgical observation during joint replacement, using the Outerbridge cartilage damage score (23) (Scores 1 and 2 were designated as early stage, and Scores 3 and 4 as end stage). Normal SF samples were collected from the contralateral uninjured knees of patients undergoing unilateral ACL reconstruction, normal arthroscopy patients, and one healthy volunteer (n=33, age 26.3±11.0, range 15–54) who had no previous history of knee injury and normal standing radiographs. Human cartilage samples were divided into OA cartilage, severely fibrillated, from the more affected compartment (usually medial, Mankin score 9–14) and “relatively normal” or non-fibrillated cartilage from the uninvolved compartment (usually lateral, Mankin score 0–2) (24).
Human serum and SF collection and analysis
Human serum and SF samples were aliquoted and frozen at −80°C until analysis. Before performing the experiments, the SF samples were treated with 15 U/ml of bovine testicular hyaluronidase as described previously (22). The levels of A2M and MMP-13 in human serum and SF were measured by ELISA (A2M: Cat: Kt-499, Seattle, WA, USA; MMP-13: Cat: E90099Hu, USCN, Wuhan, China).
Western blotting
Total proteins (14μg) were separated by SDS PAGE (10% polyacrylamide) under reducing conditions, as previously reported (22). The membrane was probed with an antibody against A2M (1:1000 dilution) (sc-8513, Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibody IgG (H+L) (Bio-Rad, Hercules, CA) was diluted 1:3,000. Visualization of immunoreactive proteins was achieved by ECL (Amersham, Arlington Heights, IL). Alpha-1-antitrypsin (A1AT) was used as loading control.
Chondrocyte isolation and primary culture
Human chondrocytes were isolated as previously described (22) and plated either in 8-well chambers at 1 × 105 cells/well or in 6-well culture plates at 1 × 106 cells/plate. At 90% confluence, the cells were cultured overnight under serum-free conditions and then treated with recombinant human IL-1β (10ng/ml) for two hours before treatment with different concentrations of A2M protein (Sigma, St. Louis, MO). Culture medium was collected after 24 hours and analyzed for catabolic cytokines and MMPs. The same experiments were also performed using the human chondrocyte cell line, C-28/12 and cartilage tissues (25).
Luminex assay
Catabolic cytokines and MMPs in the culture medium were quantified by Luminex Human Inflammatory 5-Plex Panel (Invitrogen, Cat: LHC0003) and Luminex Human MMP 3-Plex Panel (Invitrogen, Cat: LHC6002) respectively. The inflammatory panel measured GM-CSF, IL-1β, IL-1RA, IL-6, IL-8, and TNF-α; and the MMP panel measured MMP-3, -9, and -13. The 5-Plex or 3-Plex beads were incubated with 100 μl of either standards or samples for 2 hours. Biotinylated antibodies were added and incubated for 1 hour. After washing and addition of R-phycoerythrin-labeled streptavidin, the plates were analyzed using a Luminex xMAP instrument (Luminex Technologies, Inc., Austin, TX). The concentration of MMP-13 activity in the medium was quantified by ELISA (R&D Systerms, Cat: F13M00). APMA activates any potentially active forms of MMP-13 present in the sample. Since we wished to measure the endogenous levels of active, but not inactive MMP-13 in samples, we did not add APMA to the sample wells.
Mouse partial medial meniscectomy (PMM) OA model
The mouse PMM OA model was used to determine the kinetics of the expression of inflammatory mediators(26) because FMT in our facility can only be used for mice. Cathepsin is a family of proteases. The changes in cathepsin mediated inflammation in vivo were monitored by fluorescence molecular tomography (FMT) at different time points post-menisectomy (N=4).
Fluorescence Molecular Tomography (FMT)
FMT is a noninvasive and quantitative fluorescence-based technology with high molecular specificity and sensitivity for 3D tissue imaging in live animals. Biologic processes can be probed dynamically on timescales of hours to days (27, 28). ProSenseTM 750-fluorescence agents become fluorescent when activated by cathepsins (Cathepsins B, L, S, and plasmin), but are optically silent in the inactivated state. Mice were injected with ProSense 750EX and imaged with the FMT system (ViSen, Waltham, MA) 24 hours after injection.
Rat ACLT OA model and treatment with supplemental intra-articular A2M injection
One hundred and twenty 10-week-old rats (180–230g) were randomized to four groups (N=30 per group): (1) ACLT + Saline, (2) ACLT + A2M(1 IU/kg), (3) ACLT + A2M(2 IU/kg) and (4) Sham + Saline. ACL transection and sham operations were performed on the right knees, as published previously (18). 1 IU/kg or 2 IU/kg of A2M (Sigma-Aldrich, St Louis, MO) dissolved in 20 μl of saline were used to treat rats in groups (2) and (3). Intra-articular injections were performed immediately and 3 days after ACLT, and then weekly for six weeks. Animals in groups (1) and (4) received an equivalent volume of saline at identical time points as the experimental groups (2) and (3) in their right knees to control for any procedural effects. All animals were euthanized at week 8 after the operation. Fifteen rats were used for histology study and fifteen for RT-PCR per group.
Rat SF collection and analyses
SF lavage was collected as published previously (11). MMP-13 content was measured in the SF samples by ELISA following the manufacturer’s instructions (Catalog No. E90099Ra, Uscn Life Science Inc., Wuhan, China). Colorimetric density on the developed plates was determined using a microplate reader set to 450 nm (Model BF10000, Packard Bioscience, Meridian, CT). The ELISA assay was performed in duplicate.
Real Time PCR (qPCR)
The cartilage samples were ground with a mortar and pestle under liquid nitrogen and total RNA was isolated from human and rat knee joint cartilage, using RNeasy isolation kit (Cat. No. 74704, Qiagen, Valencia, CA) (22) (22). Cartilage samples from 3 rat tibial plateaus and femur condyles were dissected with a scalpel and pooled together. Five pooled samples per group were used for this study. (N=15/group). 1μg of total RNA was reversed transcribed to cDNA using the iScript™ cDNA synthesis Kit (Bio-Rad, Hercules, CA). 40 ng/ul of the resulting cDNA was used as the template to quantify the relative content of mRNA using the QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA) with DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA). Human A2M forward primer - CTT TCC TTG ATG ACC CAA GCG CC, reverse primer - GTT GAA AAT AGT CAG CGA CCT; Rat Col2a1 forward primer - AAG GGA CAC CGA GGT TTC ACT GG, reverse primer - GGG CCT GTT TCT CCT GAG CGT; Rat Acan forward primer - CAG TGC GAT GCA GGC TGG CT, reverse primer - CCT CCG GCA CTC GTT GGC TG; Rat Col10a1 forward primer - CCA GGT GTC CCA GGA TTC CC, reverse primer - CAA GCG GCA TCC CAG AAA GC; Rat Mmp3 forward primer - TTG TCC TTC GAT GCA GTC AG, reverse primer - AGA CGG CCA AAA TGA AGA GA; Rat Mmp13 forward primer - GGA CCT TCT GGT CTT CTG GC, reverse primer - GGA TGC TTA GGG TTG GGG TC; Rat Runx2 forward primer - CCGCAC GAC AAC CGC ACC AT, reverse primer - CGC TCC GGC CCA CAA ATC TC; 18S RNA forward primer - CGG CTA CCA CAT CCA AGG AA, reverse primer - GCT GGA ATT ACC GCG GCT. Relative transcript levels were calculated as x =2-ΔΔCt, in which ΔΔCt = ΔCt E - ΔCt C, and ΔCt E = Ctexp-Ct18S, and ΔCt C = CtC-Ct18S as previously described (22).
Histology
Gross morphological lesions on the rat femur condyles and tibia plateau (N=15/group) were visualized by Indian ink staining (29). The femurs and tibiae were hemi-sected in the mid-sagittal plane, and each half was embedded in a single block of Paraplast X-tra (Fisher, Santa Clara, CA). Blocks were trimmed to expose cartilage. Ten adjacent sections were collected at intervals of 0μm, 100 μm, and 200 μm. Two serial 6-μm thick sections from each interval were stained with Safranin O. Cartilage degradation was quantified using the OARSI grading system (30). Three independent and blinded observers scored each section, and the scores for all of the sections cut from the medial and lateral tibial plateaus were averaged within each joint.
Immunohistochemistry
Immunohistochemistry was performed on all specimens after india ink staining to detect type II and type X collagen, and MMP-13 using the Histostain-SP Kit (Zymed-Invitrogen, Carlsbad, CA). The sections were digested with 5 mg/ml of hyaluronidase in PBS (Sigma-Aldrich, St Louis, MO) for 20 min. Nonspecific protein binding was blocked by incubation with a serum blocking solution (LICOR, Lincoln, NE). The sections were incubated with antibody against rat type X collagen (2 μg/ml) (EMD, Gibbstown, NJ), MMP-13 (2μg/ml) (Santa Cruz, CA) and type II collagen (2 μg/ml) (Santa Cruz, CA) respectively at 4°C overnight. Thereafter, the sections were treated sequentially with biotinylated secondary antibody and streptavidin-peroxidase conjugate, and then were developed in DAB chromogen.
To detect the distribution of A2M in human cartilage and the synovial membrane, 6 μm sections were analyzed by immunofluorescent staining with a polyclonal antibody against A2M (sc-8513, Santa Cruz, CA). The negative control sections were incubated with isotype control antibody (sc-8514-P, Santa Cruz, CA) in PBS. The sections were incubated with primary antibody at 4°C overnight. After washing, affinity-purified TRITC conjugated donkey anti-goat secondary antibody (1:500) (Jackson, West Grove, PA) was applied with Hoechst nuclear dye (0.5 mg/ml) (Pierce, Rockford, IL).
Statistical analyses
Analyses of variance (ANOVA) was used to compare the in vitro concentrations of A2M, cartilage catabolic factors (GM-CSF, IL-1β, IL-1RA, IL-6, IL-8, TNF-α, MMP-3, MMP-9, and MMP-13) in different groups, and the in vivo concentrations of MMP-13 and the Col2a1, Acan, Mmp3, Mmp13, Runx2, and Col10a1 mRNA levels. A two-way mixed absolute intraclass correlation coefficient (ICC) for the cartilage damage score was calculated. Follow-up pair-wise comparisons between multiple experimental groups were carried out with orthogonal contrasts using the Scheffe’s test (α=0.05) and a test of homogeneity. Adjusted p-values for the multiple comparisons were reported. Differences were considered significant at p<0.05. Statistics were performed using SPSS software (SPSS Inc, Chicago, IL).
Results
Identification of A2M in human OA and normal knee synovial fluid
An SDS-PAGE gel stained with Coomassie Blue showed a band (approximately 180 kDa) present in higher amounts in OA SF than normal knees (Figure 1A). The band was further analyzed by mass spectrometry. The top 4 candidate proteins, Alpha-2-Macroglobulin (A2M), Fibronectin, Apolipoprotein B (APOB) and Complement component 3 (C3) were chosen for further analysis. An increase in A2M was validated by Western blotting in the SF from OA patients (N=3) compared with aged-matched normal controls (N=2). Alpha-1-antitrypsin (A1AT) was used as loading control (Figure 1B) (16).
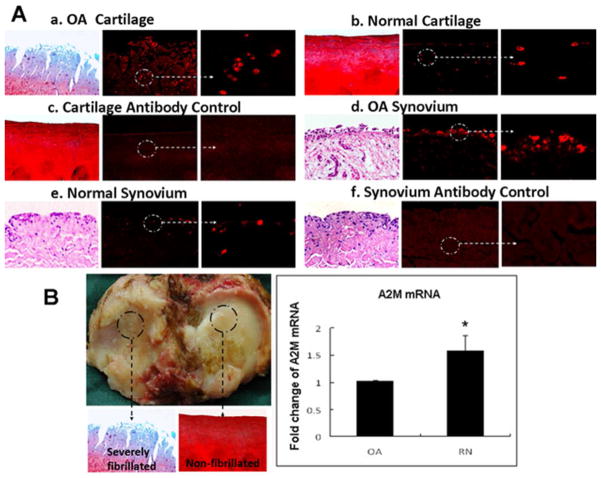
Figure 1. A2M is elevated in OA compared to normal synovial fluid.
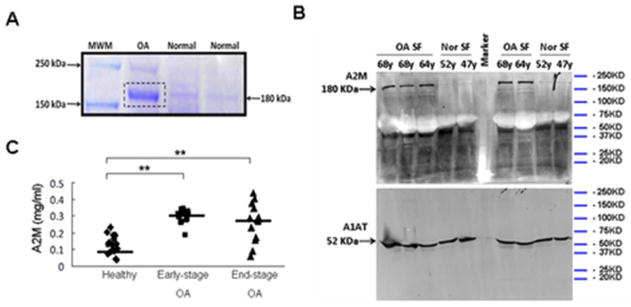
(A) Proteins in SF were separated by SDS-PAGE (10% polyacrylamide). Staining with Coomassie Blue showed a more prominent band (~180 kDa) in OA synovial fluid compared with normal controls. OA: 64y male. Normal: 52y male, 50y male. Sequencing of this band by mass spectrometry showed that 37 of the unique peptides matched A2M. (B) Higher A2M expression in OA synovial fluid was confirmed by Western blotting. Alpha-1-antitrypsin (A1AT) was used as loading control. OA: 68y male, 68y male, 64y female. Normal: 52y male, 47y male. (C) A2M content quantified by ELISA in synovial fluid from healthy individuals (N=16), early-stage OA patients (N=18), and end-stage OA patients (N=14). **, p<0.01.
ELISA results show that the concentration of A2M is higher in OA SF (Outerbridge score 1–2, 0.302±0.04mg/ml, N=18; Outerbridge score 3–4, 0.264±0.11mg/ml, N=14) than in healthy knees (Outerbridge score 0) (0.126±0.06mg/ml, N=16). (Figure 1C). We notice that the Western analysis suggests an obvious difference of A2M concentration between OA and normal SF whereas the ELISA gives a 3-fold-difference.
A2M is expressed in human cartilage and synovial membrane
Immunohistochemical (IHC) staining showed positive staining of A2M in cartilage (Figure 2A-a, 2A-b) and synovium (Figure 2A-d) from OA patients and normal controls. Quantification of mRNA from human knee joints with OA indicated that A2M mRNA levels were lower in the cartilage from the involved compartment compared to the uninvolved compartment of the joint, which we called “relatively normal” (RN) cartilage, from the same patient (N= 7 patients) (Figure 2B). Our data show that A2M is synthesized by chondrocytes and synovial membrane de novo. However, OA chondrocytes appeared to have reduced ability to produce A2M compared with those in the adjacent relatively normal cartilage (Figure 2B).
Figure 2. A2M is expressed in cartilage and synovium.
Positive staining of A2M (red fluorescence) was observed in human OA cartilage (2A-a) and human OA synovium (2A-d) (N=5, age 64.8 ± 8.7(mean±SD), range55–77), normal cartilage (2A-b) and synovium (2A-e) (N=6, age 23.8±13.6(mean±SD), range 15–51), (2A-c, negative control), indicating that A2M was produced in joint tissue. (2B) Total RNA was isolated from human severely fibrillated OA cartilage (Mankin score 9–14) and the adjacent “relatively normal” cartilage (non-fibrillated cartilage) (Mankin score 0–2) from the same OA patients (N=7, age 73.7± 7.3 (mean±SD), range 58–79). Cartilage damage was evaluated using Safranin O-stained. Real time PCR results demonstrate that A2M mRNA levels were lower in OA cartilage compared to the relatively normal cartilage from the same patient. Mean±SEM. *, p<0.05.
A2M in SF is lower than A2M in normal and OA serum and MMP-13 is elevated in OA SF
We compared the protein levels of A2M in SF and serum and found that, although A2M is higher in OA compared to normal knees, the levels are much lower in SF than in serum (Figure 3A-a). We further found that A2M protein expression is opposite to the protein levels of MMP-13 in the serum and SF in OA patients (1.53±0.052mg/ml A2M in serum and 0.24±0.002mg/ml A2M in SF, p=0.002; compared to 91.07±16.12ng/ml MMP-13 in serum and 251.01±19.23ng/ml MMP-13 in SF, p=0.007) (N=20) (Figure 3A-b).
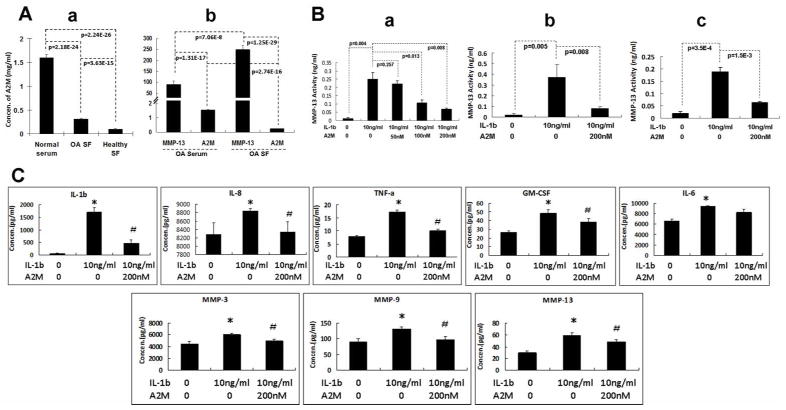
Figure 3. A2M negatively regulates cartilage catabolic cytokines and MMPs.
(A) Although A2M concentrations are higher in OA SF than in normal SF, the levels are much lower than in normal serum (Figure 3A-a) (Normal serum: N=43, age 37.5±10.2 (mean±SD), range 20–56); OA SF: N=39, age 65.4±9.6 (mean±SD), range 48–80); Normal SF (N=33, age 26.3±11.0(mean±SD), range 15–47). Higher A2M concentration and lower MMP-13 content were detected in the serum from OA patients, when compared with OA synovial fluid (same patients. N=20, age 67.0±7.1 (mean±SD), range 55–79) (Figure 3A-b). (B) MMP-13 activity was induced by IL-1 (10 ng/ml), and inhibited by A2M in a dose-dependent manner in human OA chondrocytes (B-a). The more potent inhibition was achieved by 200nM of A2M. Similarly, IL-1-induced MMP-13 activity was reduced by A2M (200nM) treatment of human OA cartilage explants cultures (B-b) and the human chondrocytic C28/12 cells (B-c). (C) IL-1β (10 ng/ml) induced the expression of IL-8, TNF-α, GM-CSF, and MMPs, while A2M (200nM) inhibited the increase induced by IL-1 in human OA chondrocytes. Mean±SEM. *, compared with control group, p<0.05. #, compared with IL-1 group, p<0.05. Human OA chondrocytes and explants were collected from the same patients (N=5, age 65.2±8.1, range 58–79).
A2M suppresses catabolic cytokines and MMPs
ELISA results showed that exogenous A2M inhibited the induction of MMP-13 activity by IL-1 in a dose-dependent manner in human primary OA chondrocytes (Figure 3B-a), in human OA cartilage explants (Figure 3B-b), and in a human chondrocyte cell line C-28/12 (Figure 3B-c) (25). Our data from the Luminex Human Inflammatory Panel and Luminex Human MMP Panel further demonstrated that treatment of human primary OA chondrocytes with A2M decreased protein levels of the majority of cartilage catabolic cytokines and enzymes induced by IL-1β, including IL-1β, IL-8, TNF-α, GM-CSF, MMP-3, -9, and -13 (Figure 3C). Thus, these results suggest that A2M supplementation beyond the endogenous levels may inhibit OA cartilage degradation in vivo through decreasing cartilage catabolic and inflammatory factors, in addition to inhibiting protease activity.
A peak in joint Cathepsin/Plasmin activity occurs at day 2 in the mouse PMM OA model
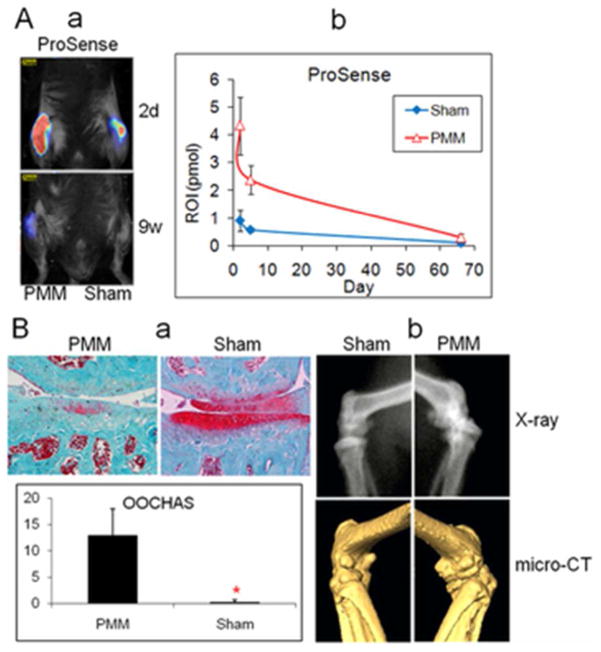
In order to determine the optimal timing for administering exogenous A2M, cathepasin was characterized in a mouse model, in which FMT data showed that the strongest joint cathepsin mediated inflammation occurs 2 days after surgery (Figure 4A). This model results in OA that can be seen by histology (Figure 4B-a) and x-ray (Figure 4B-b) after 9 weeks.
Figure 4. Cathepsin peaks 2 days after knee joint injury.
(A) The highest levels of cathepsin activity, detected by FMT during the 9 weeks after mice were subjected to partial medial meniscectomy (PMM), were observed 2 days after surgery (A-a), indicating an early catabolic response that subsided thereafter. Data were quantified by average ROI, and are shown as intensity at each time point over the 9-week period (A-b) (N = 4). (B) Safranin O staining and quantification of histological results using the OOCHAS grading system indicated articular cartilage damage and loss of PG staining (B-a). *, p<0.05. X-ray and micro-CT (B-b) illustrated the morphological changes in the entire knee joint at 9 weeks.
Supplemental intra-articular A2M attenuated PTOA pathogenesis in rat ACLT model
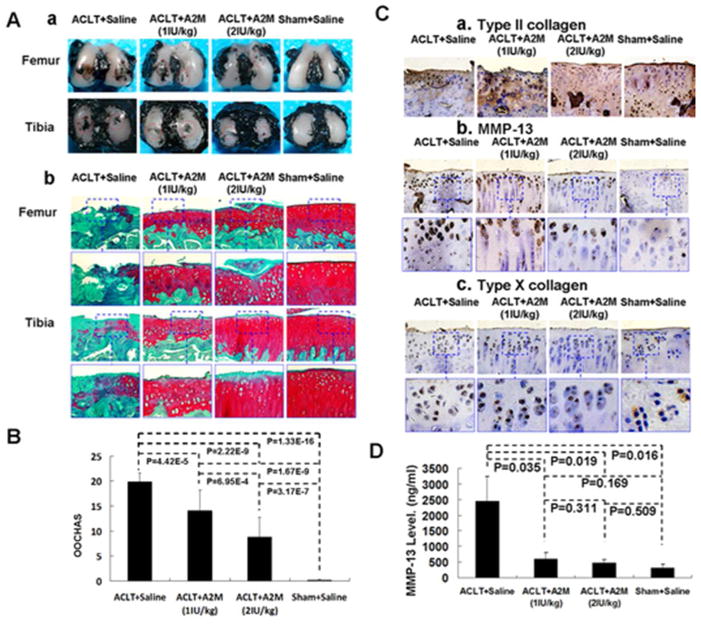
We found a significant decrease in OA score in the A2M-treated rats compared with the saline-treated group (Figure 5A). After treatment with A2M at both concentrations, stronger SafraninO staining, more cellularity but less chondrocyte cloning, and less fibrillation were observed than in the saline-treated groups. The cartilage in the higher A2M (2IU/kg) group had a stronger staining and more intact surface than the lower A2M (1IU/kg) group, but weaker than in the Sham + Saline control group (Figure 5B). The OARSI Histological Grading scores in both A2M groups suggested mild degeneration (14.1.0±4.2 and 8.8±3.9, respectively. p=0.001) (Figure 5C), while cartilage damage in the ACLT + saline group was significantly more severe (19.9±1.8. p <0.01). The cartilage in the Sham+Saline group had the least amount of damage (0.2±0.2. p <0.01). Histologic changes were evaluated at 8 weeks only. Collagen II staining in both A2M-treated groups was stronger than the ACLT + saline groups (Figure 5C-a) and showed dose dependency. In addition, there was less immunostaining for MMP-13 and type X collagen in the 2IU A2M-treated animals compared to 1IU A2M-treated animals (Figure 5D-a,b,c). Cartilage damage was associated with the change of MMP-13 in joint lavage. In the ACLT + Saline group, the MMP-13 level in joint lavage was 2450.67±789.21ng/ml, which was higher than that in the A2M (1IU/kg) (604.35±198.76ng/ml, p=0.035), A2M (2IU/kg) (464.23±110.07ng/ml, p=0.019), and Sham + Saline groups (312.52±129.13, p=0.016) (Figure 5D).
Figure 5. Supplemental intra-articular A2M attenuated PTOA pathogenesis in rat ACLT model.
Decreased India ink staining (A-a) and a smoother surface with stronger Safranin O staining (A-b) were detected in the articular cartilage of A2M-treated animals comparing to the untreated controls. (B) OARSI Histological Grading score (Mean±SD) indicated that the cartilage damage in the ACLT + Saline group was the most severe of all the groups, while cartilage in the Sham + Saline group had the least damage. Cartilage damage was less in the higher A2M dose than the lower dose. (C) Type II collagen expression was higher in articular cartilage in the A2M-treated and the Sham groups, compared with the untreated ACLT + Saline group. In contrast, MMP-13 and type X collagen staining was elevated in OA-damaged cartilage in the ACLT + Saline group, but stayed low in the A2M-treated and Sham groups, which is consistent with less OA damage in these groups. (D) Similar to the Sham group, A2M-treated groups had a lower MMP-13 concentration in synovial fluid than the ACLT+Saline group.
A2M enhances matrix gene expression in cartilage
RT-qPCR results indicated that supplemental intra-articular A2M enhanced the levels of Col2a1 and Acan mRNA, and suppressed the mRNA levels of Mmp13, Runx2, and Col10a1 in the rat ACLT model (Figure 6). Col2a1 mRNA level in the ACLT + Saline group was significantly lower than that in the ACLT + A2M (1IU/kg), ACLT + A2M(2IU/kg) and Sham+Saline groups, and there was no significant difference among the latter three groups. The Acan mRNA levels in ACLT + A2M(2IU/kg), and Sham + Saline groups were significantly higher than that in the ACLT + Saline group. In contrast, mRNA levels of Mmp3, Mmp13, Runx2, and Col10a1 in the ACLT + Saline group were the highest among the four groups. These data suggest that A2M has a chondroprotective effect in vivo by decreasing gene expression of catabolic factors and hypertrophic markers, as well as by increasing anabolic gene expression.
Figure 6. Supplemental intra-articular A2M inhibits catabolism and enhances anabolic metabolism in rat ACLT model.
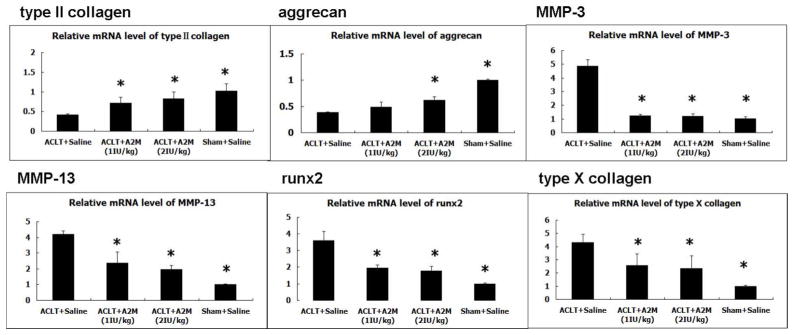
The mRNA levels of type II collagen and aggrecan were increased in the ACLT + A2M(1IU/kg) group and ACLT + A2M(2IU/kg) group compared with to the ACLT + Saline group, suggesting the positive impact of A2M on anabolic metabolism. In contrast, MMP-3, MMP-13, Runx2 and type X collagen showed the opposite pattern. These genes were expressed at a lower level in the ACLT+A2M (1IU/kg) group and ACLT+A2M (2IU/kg) group compared with the ACLT+Saline group. Mean±SEM. *, compared to the ACLT+Saline group, p<0.01.
Discussion
The results of this study suggest that A2M is a powerful inhibitor for many cartilage catabolic factors that can attenuate PTOA cartilage degeneration. A2M, a major protease inhibitor, is produced by the liver, resulting in serum concentrations of 2.2 to 2.3 mg/ml. We have shown that A2M is also produced by chondrocytes and synoviocytes, although SF levels are lower than those in serum (Fig. 1 and 2). We found that higher levels of A2M are present in the sera compared to SF of normal and OA human subjects. This difference is thought to be due to the large molecular weight of A2M, which prevents it from migrating into the SF (31) (32).
Since A2M inhibits all classes of endoproteases (20, 21), it could be used to slow the development of PTOA by neutralizing cartilage catabolic factors. Studies have shown that A2M inhibits activities of ADAMTS-4,-5,-7, -12 (20, 21), MMP-13 activity (34) Thus, the protease/A2M balance may play an important role in mediating cartilage destruction by catabolic factors. We found that the concentrations of MMP-13 were 2.8-fold higher in human OA SF samples when compared with serum, but A2M levels were 7-fold lower in human OA SF samples than in serum (Fig. 3A). MMP-3 and IL-1 beta concentrations are also higher in SF of OA subjects compared to serum by a factor of approximately 10 (35) (36) (37).
We have also shown that exogenous A2M decreases these cartilage catabolic cytokines and enzymes in vitro (Fig. 3B–C). Our FMT in vivo data confirm that peak levels of the cartilage catabolic enzymes, cathepsins B, L, S, and plasmin, can be detected at day 2 after joint injury in a mouse model (Fig. 4). Since elevated levels of catabolic enzymes in SF appear to induce chondrocyte death and cartilage matrix degeneration within one week of injury (8) (12) (13, 14) (15), early intervention may be critical for preventing or minimizing the development of PTOA. Our in vivo results in a rat ACL transection (ACLT) model suggest that this is true. Early supplemental intra-articular injection of A2M reduced the level of MMP-13 in SF and attenuated the loss of cartilage proteoglycans and collagen erosion (Fig. 5 and 6). Therefore, A2M, a negative regulator of catabolic cytokines and enzymes, is likely a therapeutic candidate (20, 21). The level of A2M in normal SF is 0.126mg/mL. One inhibitor unit is equal to 0.048mg of A2M and will increase by 38% the A2M concentration assuming the rat joint contains 1 mL SF. Future studies will focus on optimizing the dosing strategy.
Recent studies have demonstrated that A2M binds a range of cytokines, such as IL1β and TNFα and also enters into cells to regulate cellular responses to other growth factors and cytokines (38) (39) (40). Although the exact mechanism by which supplemental intra-articular A2M attenuates cartilage degeneration is not clear, it is very likely that A2M acts by binding cytokines in addition to directly neutralizing enzyme activities (41) (42) (20). The relative contributions of these mechanisms will be addressed in future studies.
A limited number of studies have attempted to indirectly quantify active A2M by measuring conversion of total A2M to inactive A2M. In one study, 90% of A2M is active in plasma, however, neutrophils and free radicals can inactivate A2M. Total SF A2M is less than serum (41). During joint inflammation or sepsis, A2M becomes inactive, presumably by complexing to proteinases(41) (43). This would suggest that from a therapeutic perspective, adequate supplemental A2M would be needed to quench catabolic enzymes. We did not directly analyze SF for inactive versus active A2M since currently available reagents only recognize total A2M.
A potential limitation to this study is that surgical ACLT may not be as traumatic as an ACL injury sustained during physical activity. Bone bruises and chondral lesions frequently occur in the latter, and these concomitant injuries may also play a role in the development of PTOA. Nonetheless, the animal ACLT model has been frequently used to study OA, and mimics human OA both macroscopically and biochemically (11, 49). Minimizing local joint inflammation until ACL reconstruction is performed may be an important preventative measure against the long-term development of PTOA. Another limitation in our study was the use of specimens from non-age-matched patients. Obtaining age-matched controls without OA is challenging for studies of human OA. Therefore, A2M analyses were performed using “relatively normal” cartilage and OA cartilage from the same patient. We recognize that the regions in which cartilage appears normal in the OA joint may not be entirely normal and this cartilage is subject to OA SF(50). However, it provides us with a reasonable benchmark for comparison, since it is tissue with minimal damage and minimizes biologic variability.
In summary, up-regulation of cartilage catabolic cytokines and enzymes is thought to be a key mechanism of cartilage damage. Thus, inhibition of these molecules will likely slow or prevent the progression of disease. Our novel data indicate that A2M is a master inhibitor of many types of cartilage-degrading enzymes, which acts not only by blocking activity, but also by decreasing gene expression and protein levels in the joint. The innate levels of A2M in SF may not be sufficient to reduce the activities of catabolic enzymes present after joint injury. In this study, supplemental intra-articular injection of A2M attenuated cartilage degeneration in the rat ACLT model, suggesting a potential novel therapy for PTOA.
Acknowledgments
The project was supported by Grant R01AR059142 from NIH/NIAMS, R01CA166089 from NIH/NIGMS, and P20RR024484 from NIH/NCRR, NSFC 81071495, 81171676 and 81201435, SXNSF 2011011042, and the Arthritis National Research Foundation (ANRF). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors gratefully acknowledge Dr. Shi Ke for help with the imaging.
Footnotes
Authors’ contributions: Shaowei Wang participated in the study design, wrote the manuscript, performed most of the experiments and analyzed data. Jingming Zhou, Jing Zhang, and Kai Li performed some of the experiments and analyzed data. Mary Goldring provided the C28 cell line and provided advice on its use. Richard Terek, Braden C. Fleming, Xiaochun Wei and Michael G. Ehrlich provided the human samples. Xiaochun Wei, Jingming Zhou, Jing Zhang, Kai Li, Qian Chen, Richard Terek, Braden C. Fleming, Mary Goldring, Michael G. Ehrlich, and Ge Zhang participated in the interpretation of the data and/or revised the manuscript critically. Lei Wei conceived of the study, participated in its design and data analysis, and revised the manuscript carefully and critically. All authors have read and approved the final manuscript.
Disclosures: We have nothing to disclose.
References
- 1.Petersson IF, Boegard T, Saxne T, Silman AJ, Svensson B. Radiographic osteoarthritis of the knee classified by the Ahlback and Kellgren & Lawrence systems for the tibiofemoral joint in people aged 35–54 years with chronic knee pain. Annals of the Rheumatic Diseases. 1997;56(8):493–6. doi: 10.1136/ard.56.8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fithian DC, Paxton EW, Stone ML, Luetzow WF, Csintalan RP, Phelan D, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. American Journal of Sports Medicine. 2005;33(3):335–46. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 3.Fink C, Hoser C, Benedetto KP. Development of arthrosis after rupture of the anterior cruciate ligament. A comparison of surgical and conservative therapy. Unfallchirurg. 1994;97(7):357–61. [PubMed] [Google Scholar]
- 4.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. American Journal of Sports Medicine. 1994;22(5):632–44. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 5.Jomha NM, Borton DC, Clingeleffer AJ, Pinczewski LA. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clinical Orthopaedics & Related Research. 1999;(358):188–93. [PubMed] [Google Scholar]
- 6.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. American Journal of Sports Medicine. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 7.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis & Rheumatism. 2004;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. Journal of Orthopaedic Research. 2011;29(6):802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KS, Choi HM, Lee Y-A, Choi IA, Lee S-H, Hong S-J, et al. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatology International. 31(4):543–7. doi: 10.1007/s00296-010-1592-1. [DOI] [PubMed] [Google Scholar]
- 10.Kanbe K, Takemura T, Takeuchi K, Chen Q, Takagishi K, Inoue K. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. Journal of Bone & Joint Surgery - British Volume. 2004;86(2):296–300. doi: 10.1302/0301-620x.86b2.14474. [DOI] [PubMed] [Google Scholar]
- 11.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. Journal of Orthopaedic Research. 2010;28(7):900–6. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DM, Noble PC, Bocell JR, Jr, Ahuero JS, Poteet BA, Birdsall HH. Effect of early full weight-bearing after joint injury on inflammation and cartilage degradation. Journal of Bone & Joint Surgery - American Volume. 2006;88(10):2201–9. doi: 10.2106/JBJS.E.00812. [DOI] [PubMed] [Google Scholar]
- 13.Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. Journal of Orthopaedic Research. 29(4):501–10. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrelli J, Jr, Tinsley K, Ricci WM, Burns M, Karl IE, Hotchkiss R. Induction of chondrocyte apoptosis following impact load. Journal of Orthopaedic Trauma. 2003;17(9):635–41. doi: 10.1097/00005131-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Tochigi Y, Buckwalter JA, Martin JA, Hillis SL, Zhang P, Vaseenon T, et al. Distribution and progression of chondrocyte damage in a whole-organ model of human ankle intra-articular fracture. Journal of Bone & Joint Surgery - American Volume. 93(6):533–9. doi: 10.2106/JBJS.I.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fangyuan Wei JZ, Wei Xiaochun, Zhang Juntao, Fleming Braden C, Terek Richard, Pei Ming, Chen Qian, Liu Tao, Wei Lei. Activation of Indian Hedgehog Promotes Chondrocyte Hypertrophy and Upregulation of MMP-13 in Human Osteoarthritic Cartilage. Osteoarthritis and Cartilage. 2012 doi: 10.1016/j.joca.2012.03.010. PubMed - as supplied by publisher. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis & Rheumatism. 2009;60(10):2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis & Rheumatism. 62(8):2382–91. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jay GD, Elsaid KA, Kelly KA, Anderson SC, Zhang L, Teeple E, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis & Rheumatism. 64(4):1162–71. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, et al. Alpha2-macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. Journal of Biological Chemistry. 2004;279(17):17554–61. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 21.Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis & Cartilage. 2008;16(11):1413–20. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis & Cartilage. 2012;20(7):755–63. doi: 10.1016/j.joca.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. American Journal of Sports Medicine. 2003;31(1):83–6. doi: 10.1177/03635465030310012601. [DOI] [PubMed] [Google Scholar]
- 24.van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. Journal of Orthopaedic Research. 1992;10(1):58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
- 25.Claassen H, Schicht M, Brandt J, Reuse K, Schadlich R, Goldring MB, et al. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors--Useful cells in study of cartilage metabolism. Annals of Anatomy. 193(1):23–9. doi: 10.1016/j.aanat.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nature Medicine. 2009;15(12):1421–5. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 27.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nature Medicine. 2003;9(1):123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 28.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. European Radiology. 2003;13(1):195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 29.Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Annals of the Rheumatic Diseases. 1972;31(6):457–64. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis & Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Woolley DE, Roberts DR, Evanson JM. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976;261(5558):325–7. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]
- 32.Salvesen G, Enghild JJ. alpha-Macroglobulins: detection and characterization. Methods in Enzymology. 1993;223:121–41. doi: 10.1016/0076-6879(93)23041-k. [DOI] [PubMed] [Google Scholar]
- 33.Demirag B, Sarisozen B, Durak K, Bilgen OF, Ozturk C. The effect of alpha-2 macroglobulin on the healing of ruptured anterior cruciate ligament in rabbits. Connective Tissue Research. 2004;45(1):23–7. doi: 10.1080/03008200490278115. [DOI] [PubMed] [Google Scholar]
- 34.Demirag B, Sarisozen B, Ozer O, Kaplan T, Ozturk C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. Journal of Bone & Joint Surgery - American Volume. 2005;87(11):2401–10. doi: 10.2106/JBJS.D.01952. [DOI] [PubMed] [Google Scholar]
- 35.Tchetverikov I, Ronday HK, Van El B, Kiers GH, Verzijl N, TeKoppele JM, et al. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(7):881–3. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Research & Therapy. 12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darabos N, Hundric-Haspl Z, Haspl M, Markotic A, Darabos A, Moser C. Correlation between synovial fluid and serum IL-1beta levels after ACL surgery-preliminary report. International Orthopaedics. 2009;33(2):413–8. doi: 10.1007/s00264-008-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaMarre J, Wollenberg GK, Gonias SL, Hayes MA. Cytokine binding and clearance properties of proteinase-activated alpha 2-macroglobulins. Laboratory Investigation. 1991;65(1):3–14. [PubMed] [Google Scholar]
- 39.Wollenberg GK, LaMarre J, Rosendal S, Gonias SL, Hayes MA. Binding of tumor necrosis factor alpha to activated forms of human plasma alpha 2 macroglobulin. American Journal of Pathology. 1991;138(2):265–72. [PMC free article] [PubMed] [Google Scholar]
- 40.Borth W, Scheer B, Urbansky A, Luger TA, Sottrup-Jensen L. Binding of IL-1 beta to alpha-macroglobulins and release by thioredoxin. Journal of Immunology. 1990;145(11):3747–54. [PubMed] [Google Scholar]
- 41.Abbink JJ, Kamp AM, Nieuwenhuys EJ, Nuijens JH, Swaak AJ, Hack CE. Predominant role of neutrophils in the inactivation of alpha 2-macroglobulin in arthritic joints. Arthritis & Rheumatism. 1991;34(9):1139–50. doi: 10.1002/art.1780340910. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Yang M, Yang D, Cavey G, Davidson P, Gibson G. Molecular interactions of MMP-13 C-terminal domain with chondrocyte proteins. Connective Tissue Research. 51(3):230–9. doi: 10.3109/03008200903288902. [DOI] [PubMed] [Google Scholar]
- 43.Abbink JJ, Nuijens JH, Eerenberg AJ, Huijbregts CC, Strack van Schijndel RJ, Thijs LG, et al. Quantification of functional and inactivated alpha 2-macroglobulin in sepsis. Thrombosis & Haemostasis. 1991;65(1):32–9. [PubMed] [Google Scholar]
- 44.Kraus VB, Nevitt M, Sandell LJ. Summary of the OA biomarkers workshop 2009--biochemical biomarkers: biology, validation, and clinical studies. Osteoarthritis & Cartilage. 18(6):742–5. doi: 10.1016/j.joca.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Nelson AE, Renner JB, Shi XA, Shreffler JH, Schwartz TA, Jordan JM. Cross-sectional comparison of extended anteroposterior and posteroanterior fixed flexion positioning to assess radiographic osteoarthritis at the knee: the Johnston County Osteoarthritis Project. Arthritis care & research. 62(9):1342–5. doi: 10.1002/acr.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oksendahl HL, Gomez N, Thomas CS, Badger GD, Hulstyn MJ, Fadale PD, et al. Digital radiographic assessment of tibiofemoral joint space width: a variance component analysis. The Journal of Knee Surgery. 2009;22(3):205–12. doi: 10.1055/s-0030-1247750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bear DM, Szczodry M, Kramer S, Coyle CH, Smolinski P, Chu CR. Optical coherence tomography detection of subclinical traumatic cartilage injury. Journal of Orthopaedic Trauma. 24(9):577–82. doi: 10.1097/BOT.0b013e3181f17a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintana DJ, Garnero P, Huebner JL, Charni-Ben Tabassi N, Kraus VB. PIIANP and HELIXII diurnal variation. Osteoarthritis & Cartilage. 2008;16(10):1192–5. doi: 10.1016/j.joca.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis & Rheumatism. 1991;34(12):1560–7. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 50.Rout RMS, Hollander A, Davidson R, Clark I, Murray D, Gill H, Hulley P, Price A. Increased type I collagen in undamaged cartilage of anteromedial osteoarthritis of the knee. Journal of Bone and Joint Surgery - British. 2011;93-B(SUPP_I):30. [Google Scholar]