The oral-aboral axis of the sea urchin embryo is specified via the redox-regulated activation of nodal (Coffman et al., 2009, and references therein). Embryos cultured at high density under cover glass develop a radialized phenotype owing to inhibition of nodal expression (Coffman et al. 2004), an effect that we attributed to hypoxia. Recently Agca et al. (2009) reported that controlled hypoxia does inhibit nodal expression as well as ectoderm differentiation, but only when it is maintained until pluteus stage. However, we found that embryos developed under cover glass become radialized even when the cover glass is removed at early blastula stage (Supplemental Figure 1). We therefore asked whether other factors might contribute to this teratogenic effect.
Since embryos cultured under otherwise identical conditions develop normally, it is clear that the relevant effect of cover glass is to limit gas exchange, which in addition to hypoxia will promote acidification by preventing escape of CO2. To determine which of these factors causes radialized development, embryos were developed to hatching blastula stage under normoxic (∼10 ppm O2) or hypoxic (≤1 ppm O2) conditions in culture medium buffered to pH 8, 7.5, 7, or 6.5. While acidic pH was mildly radializing, hypoxia alone produced significant radialization, which was enhanced by acidification (Fig. 1 A, Supplemental Table 1 and Supplemental Fig. 2). Since the embryos were released from hypoxia at blastula stage, these results differ from those of Agca et al. (2009), for reasons that remain unclear. One difference that may be relevant is that we determined the level of hypoxia by measuring dissolved oxygen, whereas Agca et al. (2009) monitored atmospheric oxygen. In addition, we scored morphology at prism stage, whereas Agca et al. did so at pluteus stage, which may have allowed for recovery by regulative development. Finally, Agca et al. did not quantify the phenotypes they reported.
Figure 1.
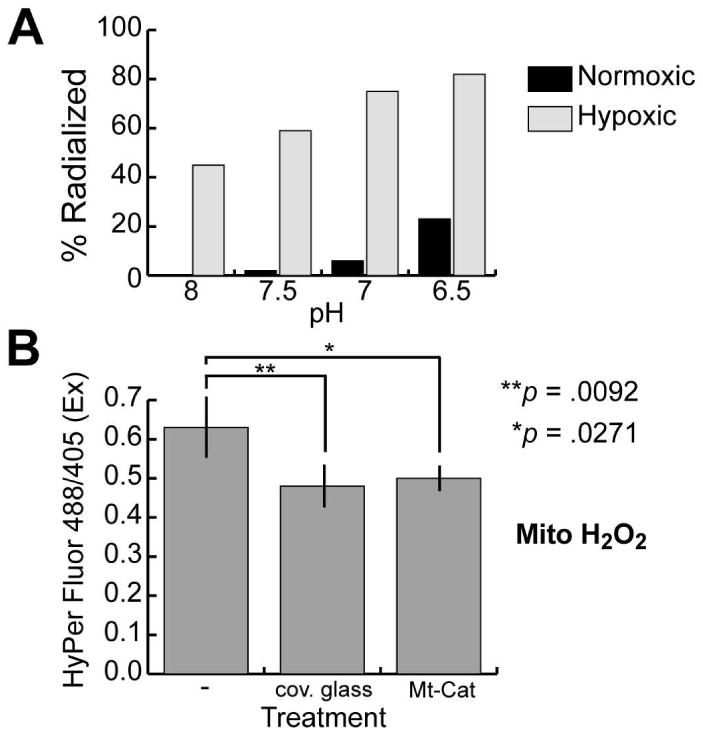
Hypoxia induces radialized development and reduces mitochondrial H2O2 levels in sea urchin embryos. (A) Differential effects of controlled hypoxia and pH on development of oral-aboral polarity. Embryos were developed under the indicated conditions (see Supplemental Table 1 for details) until hatched blastula stage, then transferred to artificial seawater and developed to prism stage under normoxic conditions. At that point the embryos were scored for morphological radialization, defined as absence of ectodermal and endodermal asymmetry, and presence of multiple radially-arrayed spicules. The graph presents the combined results from the two experiments quantified in Supplemental Table 1. (B) Relative mitochondrial H2O2 levels in embryos subjected to the indicated treatments, measured using microinjected mRNA encoding HyPer-dMito (Evrogen), a mitochondrially-targeted yellow fluorescent protein derivative whose excitation maximum shifts from of 420 nm to 500 nm upon oxidation by H2O2. The indicated ratios were calculated from the average pixel intensities obtained from projected confocal fluorescence images of pre-hatching blastulae, obtained by exciting the embryos at 405 and 488 nm and collecting fluorescence emissions at 530 nm (three to six embryos imaged per treatment). The coverslip treatments were carried out as described in Coffman et al. (2004). Preparation and microinjection of mRNA and image analyses were performed as described in Coffman et al. (2009). Error bars depict the standard deviation; ANOVA followed by Dunnett's test was used to determine the indicated significance values (JMP version 8.0; Cary, NC). A second experiment using HyPer-cyto mRNA (Evrogen) gave similar results (Supplemental Fig. 3).
Ratiometric imaging was used to measure the effect of limiting gas exchange on intracellular H2O2 and pH (Fig. 1 B, Supplemental Fig. 3). Both mitochondrial and cytoplasmic H2O2 were reduced by this treatment, with a decrease in mitochondrial H2O2 comparable in magnitude to that produced by a mitochondrially-targeted catalase (Mt-Cat) shown previously to both inhibit nodal activation and entrain oral-aboral polarity (Coffman et al. 2009) (Fig. 1B). This decrease in H2O2, a major reactive oxygen species (ROS), contradicts the untested assumption of Agca et al. (2009) that hypoxia elevates ROS. As would be expected, limiting gas exchange also lowers intracellular pH, but less so than the decrease produced by pH buffering that did not induce radialization (Supplemental Fig. 3), suggesting that the relevant pH effects are extracellular.
We conclude that hypoxia is the major cause of the radialization caused by development under cover glass. This is probably attributable at least in part to decreased H2O2 levels, as the window of vulnerability corresponds to the initial H2O2-dependent activation phase of nodal expression (Supplemental Figure 1; Coffman et al., 2009). Extracellular acidification appears to play an ancillary role, perhaps weakening ligand-receptor interactions and thus by dampening the feedback amplification of nodal expression.
Supplementary Material
Supplemental Figure 1. Experimental determination of the developmental window of vulnerability to cover glass induced radialization. Approximately 1000 eggs were arrayed onto protamine sulfate-treated recessed glass bottoms of MatTek dishes, fertilized, and then covered for the indicated intervals (black bars) with a glass coverslip (see Coffman et al., 2004 for detailed protocol). The embryos were then developed to prism stage (∼48 hrs post fertilization) and scored for radialization, as described in the legend to Figure 1. The numbers in parentheses indicate the number of experiments that were carried out for each treatment. Fifty embryos were scored per experiment, and the numbers combined for the reported percentages. Untreated controls developed normally.
Supplemental Figure 2. Examples of five day old larvae produced by embryos developed in bicarbonate-buffered artificial seawater (pH 8) under normoxic or hypoxic conditions until hatching (see Supplemental Table 1).
Supplemental Figure 3. Ratiometric measurements of relative intracellular H202 and pH in confocally imaged embryos subjected to the indicated treatments after preloading with (A) HyPer-cyto (Evrogen) mRNA or (B) carboxy-SNARF-1 AM acetate (Invitrogen), a ratiometric pH indicator whose fluorescence emission maximum shifts from ∼640 to ∼580 nm with acidification. Ten embryos were imaged per treatment. The data for (A) were obtained as described in Fig. IB. The data for (B) were obtained by exciting the embryos at 488 nm and collecting fluorescence at 560-615 and 636-754 nm. Statistics are as described for Fig. 1, except the Tukey-Kramer HSD test was used instead of Dunnett's for the comparisons depicted in panel (B).
Supplemental Table 1: Effects of hypoxia and seawater acidification on the development of oral-aboral polarity
Acknowledgments
Funding: NIH R01- ES016722 and R25-ES016254
References
- Agca C, Klein WH, Venuti JM. Reduced O2 and elevated ROS in sea urchin embryos leads to defects in ectoderm differentiation. Dev Dyn. 2009;238(7):1777–1787. doi: 10.1002/dvdy.22001. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Coluccio A, Planchart A, Robertson AJ. Oral-aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H2O2. Dev Biol. 2009;330:123–130. doi: 10.1016/j.ydbio.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman JA, McCarthy JJ, Dickey-Sims C, Robertson AJ. Oral-aboral axis specification in the sea urchin embryo II. Mitochondrial distribution and redox state contribute to establishing polarity in Strongylocentrotus purpuratus. Dev Biol. 2004;273(1):160–171. doi: 10.1016/j.ydbio.2004.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Experimental determination of the developmental window of vulnerability to cover glass induced radialization. Approximately 1000 eggs were arrayed onto protamine sulfate-treated recessed glass bottoms of MatTek dishes, fertilized, and then covered for the indicated intervals (black bars) with a glass coverslip (see Coffman et al., 2004 for detailed protocol). The embryos were then developed to prism stage (∼48 hrs post fertilization) and scored for radialization, as described in the legend to Figure 1. The numbers in parentheses indicate the number of experiments that were carried out for each treatment. Fifty embryos were scored per experiment, and the numbers combined for the reported percentages. Untreated controls developed normally.
Supplemental Figure 2. Examples of five day old larvae produced by embryos developed in bicarbonate-buffered artificial seawater (pH 8) under normoxic or hypoxic conditions until hatching (see Supplemental Table 1).
Supplemental Figure 3. Ratiometric measurements of relative intracellular H202 and pH in confocally imaged embryos subjected to the indicated treatments after preloading with (A) HyPer-cyto (Evrogen) mRNA or (B) carboxy-SNARF-1 AM acetate (Invitrogen), a ratiometric pH indicator whose fluorescence emission maximum shifts from ∼640 to ∼580 nm with acidification. Ten embryos were imaged per treatment. The data for (A) were obtained as described in Fig. IB. The data for (B) were obtained by exciting the embryos at 488 nm and collecting fluorescence at 560-615 and 636-754 nm. Statistics are as described for Fig. 1, except the Tukey-Kramer HSD test was used instead of Dunnett's for the comparisons depicted in panel (B).
Supplemental Table 1: Effects of hypoxia and seawater acidification on the development of oral-aboral polarity


