Abstract
Purpose
To estimate the association between the prevalence of epilepsy and potential risk factors in three Burkina Faso villages.
Methods
Three villages were selected based on local reports of high numbers of epilepsy cases and pig-rearing practices. One person aged 7 or older was selected at random from all households of selected concessions for epilepsy screening and blood sampling. Epilepsy was confirmed by a physician using the ILAE definition. The cross-sectional associations between epilepsy and selected factors and sero-response to the antigens of Taenia solium were estimated using a Bayesian hierarchical logistic regression. Prevalence odds ratios (POR) and their 95% Credible Intervals (95%BCI) were estimated.
Results
Of 888 individuals interviewed, 39 of 70 screened positive were confirmed to have epilepsy for a lifetime prevalence of 4.5% (95%CI: 3.3–6.0). The prevalence of epilepsy was associated with a positive reaction to cysticercosis Ag-ELISA serology (POR=3.1, 95% BCI= 1.0;8.3), past pork consumption (POR=9.7, 95% BCI=2.5;37.9), and being salaried or a trader compared to a farmer or housewife (POR=2.9, 95% BCI= 1.2;6.4).
Discussion
Several factors were associated with prevalent epilepsy, with Ag-ELISA suggesting the presence of neurocysticercosis. The association of epilepsy and some occupations may reflect differences in local attitudes toward epilepsy and should be further explored.
Keywords: epidemiology, epilepsy, cross-sectional study, cysticercosis, Sub-Saharan Africa
INTRODUCTION
Epilepsy is one of the most common neurological diseases in the world. In a recent meta-analysis of available data, the lifetime prevalence of epilepsy (LPE) was estimated as a median of 0.6%, 1.5% and 1.0% in developed, rural developing and urban developing countries, respectively (1). An estimated three quarters of people with epilepsy (PWE) live in developing countries and up to 94% may be untreated (2, 3).
Epilepsy has both an economic and social burden. PWE may result in reduced productivity and incur high costs in medical and traditional treatments (4, 5). The Global Burden of Disease study estimated its impact to be 0.5% of the total global burden of diseases (6). Moreover, PWE are often victims of stigmatization and marginalization from their peers (7, 8).
Among known causes of epilepsy are infections (e.g., malaria, meningitis, viral or bacterial encephalitis), tumors, severe head trauma, family history, and perinatal factors. Infections may be especially important causal agents in developing countries (9). One infection that is receiving an increased level of attention is the neglected tropical disease neurocysticercosis (NCC). Published studies of NCC suggest that an important proportion of epilepsy in some regions of the world may be attributable to NCC (10–13). NCC results when larvae of Taenia solium migrate to the central nervous system (CNS). The disease may be asymptomatic or manifest itself through a diversity of signs and symptoms such as acute symptomatic seizures and epilepsy, chronic, progressively worsening headaches or other CNS manifestations, such as hydrocephalus, depending on the location and developmental stage of the cysts (14, 15). In a recent systematic review of the literature, epilepsy (78.8%; 95%CI: 65.1%–89.7%) and chronic headaches (37.9%; 95%CI: 23.3%–53.7%) were found to be the most frequent manifestations among people with NCC seen in neurological clinics (16). In this review, seizures and epilepsy were relatively more frequent among NCC cases who had only calcified cysts as compared with those with active cysts. The purpose of this study was to estimate the association between the prevalence of lifetime and active epilepsy and associated risk factors in three rural villages of Burkina Faso. If some of those factors are modifiable, they may be targeted for control to reduce the burden of epilepsy in Burkina Faso.
MATERIAL & METHODS
Study design
The data were collected as part of a pilot cross-sectional study on the seroprevalence of cysticercosis conducted between May and November 2007 in three villages in Burkina Faso. Data were analyzed as a prevalence case-control study focused on epilepsy.
Study sites
Three study villages were selected to reflect three types of pig management practices. Batondo is located 140 kilometres from Ouagadougou, the capital city of Burkina Faso. The village is inhabited by approximately 3,000 people, and several pigs are raised, most of them left roaming. Nyonyogo, located 30 kilometres from Ouagadougou, is inhabited by approximately 1,500 people, most identifying with the Muslim faith, and where very few pigs are present. Pabré is located 20 kilometres from Ouagadougou and includes about 4,000 inhabitants. Most pigs are raised in pens or kept tethered during the rainy season but left roaming during the dry season. All villages had been reported by locals to have cases of epilepsy.
Sampling strategy
The field investigation team included a medical doctor, a veterinarian, two interviewers and one translator (for Batondo only). Before the start of the study, each village was visited and each concession -- a group of households whose members share a common ascendant and live together in proximity -- was identified and numbered. A clustered random sampling strategy was then used to select participants. In the first stage, all concessions were included in Batondo and Nyonyogo and half of the concessions were randomly selected in Pabré. In the second stage, all households in each selected concession were invited to participate. In the third stage, all members of each household were enumerated and one individual was selected randomly for epilepsy screening and interviewing. Selected individuals were asked for their written consent to participate in the study.
Questionnaire interviews
Two household and one individual questionnaires were administered. One questionnaire was administered to the head of the household and listed all members with their gender and age. A second questionnaire was administered to the mother of the household to obtain a general description of hygiene and cooking practices for the household. The individual questionnaire, adapted from the epilepsy screening questionnaire designed by Preux et al. (17), was administered to the randomly selected participants. Items used for the screening of seizures are presented in the appendix. Those who screened positive were subsequently examined by the study physician using a standardized medical questionnaire. To be included in our study, a participant had to be at least 7 years of age and resident in the village for at least one year. All eligible subjects provided written consent to answer the interview questionnaire.
Definition of cases of epilepsy
Epilepsy was defined as having had at least two apparently unprovoked seizures of central nervous system origin, occurring more than 24 hours apart, and confirmed by the study physician through history and neurological evaluation. Lifetime prevalence was defined as those ever having two or more unprovoked seizures (18). Active epilepsy was defined as the occurrence of at least one epileptic seizure during the previous three years or the use of antiepileptic medications for seizures during the three last years. Seizure types were classified by the study physician according to the International League Against Epilepsy classification (19).
Selection and definition of controls
The comparison group (controls) consisted of all persons who screened negative for epilepsy. Subjects who screened positive but were not confirmed by the medical examination and those who had only one episode of seizure were excluded from the control group for the case-control analysis.
Serological test
Blood samples were left to decant at the end of each sampling day and the sera were put in freezers (−20°C) until the samples were brought to the IRSS (Institut de Recherche en Sciences de la Santé) in Bobo-Dioulasso where they were centrifuged and the sera kept at −20°C. The serum samples were tested for circulating antigens of the metacestode of T. solium using the enzyme-linked immunosorbent assay (ELISA) (20, 21). In a recent study conducted in Ecuador, Praet et al. estimated the AgELISA to have a sensitivity of 90% and a specificity of 98% in detecting current infection with T. solium metacestodes (22). The cut-off value was calculated as described by Dorny et al. (23). Based on the optical density and cut-off value, the results were classified as negative, “weak” positive and “strong” positive (24). These values were determined based on the experience of the immunologists on the research team who developed the antigen-ELISA (Ag-ELISA) test. Samples with a coefficient of variation of more than 50% were considered as missing values (n=3).
Measurement of potential risk factors
Potential risk factors included selected socio-demographic characteristics (age, gender, village of residence, occupation, lifetime school attendance), behavioral and environmental patterns (pork consumption, source of drinking water), and the results of the Ag-ELISA test for the presence of T. solium larvae.
Statistical analyses
Descriptive statistics for each variable were computed. Lifetime prevalence of epilepsy in each village was calculated by dividing the total number of confirmed cases by the total number screened (those screened positive but not seen by the physician were excluded). The 95% confidence intervals on the estimated prevalence were calculated using the “mid-p” binomial-based method (25). Positive predictive values (PPV) for the screening questionnaire were computed by dividing the number of confirmed cases of epilepsy by the number of participants who screened positive, excluding the self-reported cases. Prevalence of epilepsy across villages was compared using a Pearson chi-square test. The univariate associations between lifetime epilepsy and potential risk factors were estimated using crude prevalence odds ratios and 95% confidence intervals. A Bayesian hierarchical logistic regression was used to assess the association of covariates with the prevalence of lifetime epilepsy using prevalence odds ratios (POR) and their 95% Credible Intervals (95% BCI) while taking village clustering into consideration. This was done to account for the clustered nature of the sampling strategy. A total of 119 individuals did not provide a blood sample. A logistic regression including factors associated with seropositivity identified in a previous analysis (24) was used to impute the values of the missing Ag-ELISA results. It was assumed that, conditional on adjusting for the variables in that logistic regression, the missing serological values were missing at random. Two models were fitted, the first considering the weak positive Ag-ELISA reactions as negative and the second including those weak reactions as positive. The software used for the management and the analysis of the data were SASR 9.1 (SAS Institute Incorporated, Cary, NC, USA), Stata 9 (StataCorp, College Station, TX, USA) and WinBugsR 1.4 (Imperial College and MRC, London, UK). Open-Epi version 2.2 was used for the calculation of 95% confidence intervals (95%CI) for proportions.
Ethics
All randomly selected individuals, or parents of selected children less than 15 years old, were asked for their written consent to participate in the study. Those who did not consent to participate were excluded and replaced with another randomly selected household member. The study protocol was reviewed and approved by the ethical committee of the Center MURAZ (Ref. 02–2006/CE-CM) and by the Institutional Review Board of the University of Oklahoma Health Sciences Center (IRB# 12694).
RESULTS
A total of 70 (7.9%) of 888 persons completing the screening interview for epilepsy or seizures screened positive (Figure 1). Of the 70 screened positive, 56 were examined by the physician. Of the 14 who screened positive but were not examined, six had missing information on the frequency of seizures, and one had had one occurrence of loss of consciousness. Of the remaining seven cases, six gave descriptions of symptoms that were determined by the physician as unlikely to be epilepsy. An additional four participants who had initially concealed their symptoms (therefore screened negative) subsequently informed the field team that some of their initial answers were incorrect and that they had had seizures. Those individuals were considered as screened positive and examined by the physician. Of the 60 participants examined by the physician, 39 were confirmed as PWE. The overall predictive value of the screening questionnaire for confirmed epilepsy was 67.9% (95% CI=54.9%; 79.1%). The lifetime prevalence of epilepsy was 4.5% (95% CI =3.3%; 6.0%) (Table 1). The prevalence did not differ significantly across villages (p=0.198), although it was highest in Nyonyogo (6.5%, 95% CI=3.5%; 10.7%). Thirty-four (87.2%) of the 39 PWE had active seizures, giving an overall prevalence of 3.9% (95% CI=2.8%; 5.4%) for active epilepsy. The prevalence of active epilepsy for each village is also presented in Table 1.
Figure 1.
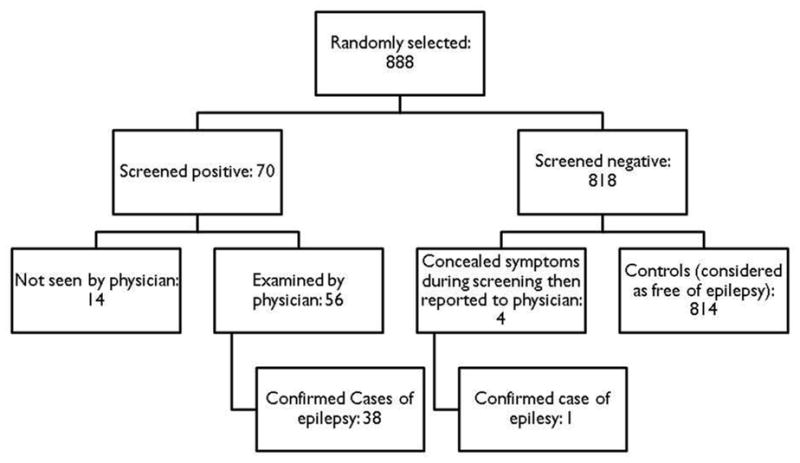
Table 1.
Number of participants interviewed, screened positive for and medically confirmed with epilepsy as well as positive predicted values of screening questionnaire and estimated prevalence of epilepsy in three villages of Burkina Faso, 2007.
| Village | Number Screened | Number screened positive for epilepsy | Medically confirmed cases (subjects screened positive)* | Medically confirmed cases (self reported)* | Positive predicted value (%) of screening questionnaire (95%CI) | Estimated lifetime prevalence of epilepsy % (95% CI)# | Number of cases with active epilepsy (%lifetime cases) ** | Estimated prevalence of active epilepsy (%)(95% CI)** |
|---|---|---|---|---|---|---|---|---|
| Batondo | 337 | 36 | 16/30 | 0/1 | 53.3 (35.6; 70.5) | 4.8 (2.9; 7.6) | 13 (81.3) | 3.9 (2.2; 6.5) |
| Pabré | 357 | 20 | 10/13 | 1/3 | 76.9 (49.1; 93.8) | 3.1 (1.7; 5.4) | 10 (90.9) | 2.9 (1.5; 5.0) |
| Nyonyogo | 187 | 14 | 12/13 | 0/0 | 92.3 (67.5; 99.6) | 6.5 (3.5; 10.7) | 11 (91.7) | 5.9 (3.1; 10.1) |
| Total | 881 | 70 | 38/56 | 1/4 | 67.9 (54.9; 79.1) | 4.5 (3.3; 6.0) | 34 (87.2) | 3.9 (2.8; 5.4) |
Denominator is the number of subjects who were examined by the physician
Active epilepsy was defined as a case with episodes of seizures that occurred within the three years preceding the medical exam or a subject on antiepileptic medications for seizures at the time of the investigation.
Fourteen subjects screened positive but not examined by the physician were excluded from the analysis (six from Batondo, seven from Pabré, and one from Nyonyogo)
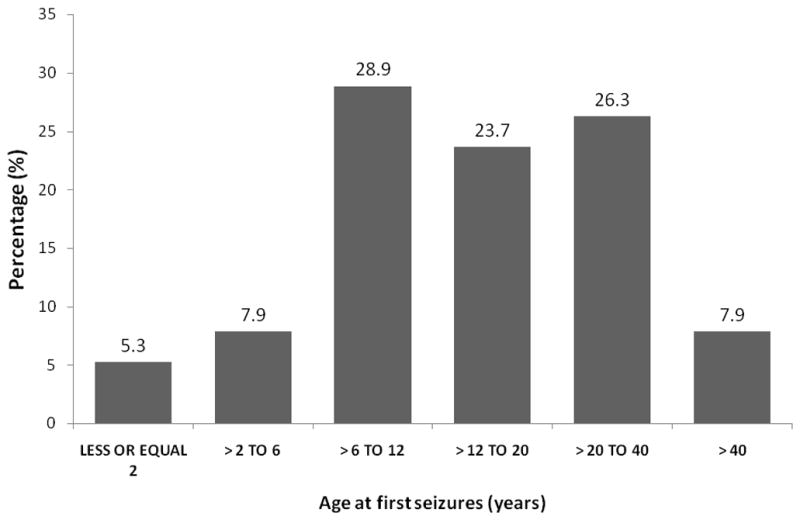
Many PWE reported different types of seizures during their lifetime. Nineteen participants (48.7%) reported two or more types of seizures. Overall, 29 (74.4%), 21 (53.9%) and three (7.7%) reported having experienced generalized seizures, simple partial seizures and partial seizures secondarily generalized at least once, respectively. One case reported having had complex partial seizures. Half of the cases with epilepsy had their first seizure between the age of 12 and 40 (figure 2). Five PWE (12.8%) had a strong positive reaction to Ag-ELISA for T. solium larvae, with four of them from Batondo and one from Pabré.
Figure 2.
The control group included 814 persons. In the univariate analyses, compared to the controls, PWE had higher proportions of participants who were salaried or traders, were Ag-ELISA positive for T. solium, reported having stopped eating pork, and reported a previous tapeworm infection (Table 2).
Table 2.
Distribution of cases of lifetime epilepsy and participants who screened negative for epilepsy (controls) by selected potential risk factors in three villages of Burkina Faso, 2007
| Variable | Category | Confirmed Epilepsy (%) | Controls (%) | Crude prevalence odds ratio (95% CI) |
|---|---|---|---|---|
| Number | 39 | 814 | ||
| Gender | Female | 18 (46.2) | 430 (52.8) | Reference |
| Male | 21 (53.8) | 384 (47.2) | 1.3 (0.7; 2.5) | |
| Age range | 7–17 years | 10 (25.6) | 221 (27.1) | Reference |
| 18–27 years | 11 (28.2) | 201 (24.7) | 1.2 (0.5; 2.9) | |
| 28–41 years | 12 (30.8) | 186 (22.9) | 1.4 (0.6; 3.4) | |
| 42 years or older | 6 (15.4) | 206 (25.3) | 0.6 (0.2; 1.8) | |
| Village | Nyonyogo | 12 (30.8) | 173 (21.3) | Reference |
| Batondo | 16 (41.0) | 306 (37.6) | 0.8 (0.3; 1.6) | |
| Pabré | 11 (28.2) | 335 (41.1) | 0.5 (0.2; 1.1) | |
| Ever attended school | No | 23 (59.0) | 510 (62.7)$ | Reference |
| Yes | 16 (41.0) | 303 (37.3) | 1.2 (0.6; 2.3) | |
| Occupation | Farmer or housewife | 27 (69.2) | 603 (74.0) | Reference |
| Pupil | 3 (7.7) | 124 (15.3) | 0.5 (0.2; 1.8) | |
| Salaried worker/trader | 9 (23.1) | 87 (10.7) | 2.3 (1.1; 5.1) | |
| Pork consumption& | Never | 12 (30.8) | 255 (31.3) | Reference |
| Before but not now | 6 (15.4) | 19 (2.3) | 6.7 (2.3; 19.9) | |
| Now/well cooked pork | 14 (35.9) | 443 (54.4) | 0.7 (0.3; 1.5) | |
| Now/undercooked pork | 7 (17.9) | 95 (11.7) | 1.6 (0.6; 4.1) | |
| Primary source of water | Public pumps | 20 (51.3) | 388 (47.7) | 1.1 (0.6; 2.2) |
| Traditional wells (with or without curbstones) | 19 (48.7) | 423 (52.0) | Reference | |
| Other | 0 | 3 (0.3) | Undefined | |
| Hygiene | Do not use toilet | 34 (87.2) | 643 (79.0) | Reference |
| Use toilet | 5 (12.8) | 171 (21.0) | 0.6 (0.2; 1.4) | |
| History of tapeworm infection | No | 30 (76.9) | 727 (89.5) | Reference |
| Yes | 9 (23.1) | 85 (10.4) | 2.6 (1.2; 5.6) | |
| Do not know | 0 | 2 (0.1) | Undefined | |
| Ag-ELISA for Taenia solium larvae | Negative | 30 (76.9) | 659 (81) | Reference |
| Positive | 5 (12.8) | 28 (3.4) | 3.9 (1.4; 10.9) | |
| Weak reaction | 1 (2.6) | 11 (1.3) | 2.0 (0.2; 16.0) | |
|
| ||||
| Missing | 3 (7.7) | 116 (14.3) | # NA | |
Data missing for 2 controls
One missing data
NA: not applicable
Table 3 reports the estimates of the PORs obtained from the Bayesian hierarchical logistic regression. The prevalence odds of epilepsy was higher among those with positive Ag-ELISA assays (POR=3.1 (95%BCI: 1.0; 8.3)) than in those with negative Ag-ELISA assays, in participants who reported having stopped eating pork compared to those who reported never eating pork (POR=9.7 (95% BCI: 2.5; 37.9)), and in participants who were salaried workers or traders as compared to pupils and housewives (POR=2.9 (95% CI 1.2; 6.4)). Previous tapeworm infection was no longer associated with epilepsy in the multivariable model. Excluding participants from Nyonyogo from the multivariable model yielded similar results but larger credible intervals due to the reduced sample size. No other socio-demographic, behavioral, or environmental characteristics showed notable associations with the lifetime prevalence of epilepsy.
Table 3.
Multivariable-adjusted prevalence odds ratios (POR) of associations between lifetime epilepsy status and selected covariates with and without imputation of the missing data for the AgELISA test
| Without imputation of missing AgELISA data (95% CI) | After imputation of missing AgELISA data (95% BCI) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Weak AgELISA as negative | Weak AgELISA as positive | Weak AgELISA as negative | Weak AgELISA as positive | |||
| Variable | Category | Reference | ||||
| AgELISA | Positive | Negative | 3.0 (1.0; 9.5) | 2.8 (1.0; 7.6) | 3.9 (1.1; 12.4) | 3.1 (1.0; 8.3) |
| Pork consumption | Eats well-cooked pork | Never ate pork | 1.6 (0.6; 4.4) | 1.8 (0.6; 4.8) | 2.3 (0.7; 8.3) | 2.4 (0.7; 8.7) |
| Eats undercooked pork | 0.6 (0.2; 1.4) | 0.6 (0.3; 1.5) | 1.0 (0.3; 3.3) | 1.1 (0.4; 3.4) | ||
| Ate pork in the past, not now | 9.9 (3.1; 31.5) | 10.8 (3.5; 33.5) | 9.2 (2.3; 36.5) | 9.7 (2.5; 37.9) | ||
| Occupation | Pupil | Farmers and Housewives | 0.7 (0.2; 2.4) | 0.7 (0.2; 2.4) | 0.6 (0.1; 1.7) | 0.6 (0.1; 1.8) |
| Salaried and traders | 3.1 (1.4; 7.3) | 3.1 (1.4; 7.3) | 2.9 (1.2; 6.5) | 2.9 (1.2; 6.4) | ||
DISCUSSION
The estimated lifetime prevalence of epilepsy in the three investigated villages (45 per 1,000) was higher than the mean LPE (15.4 per 1,000) but within its 5th – 95th percentile range in rural areas of developing countries (4.8; 49.6) (1). Our study was not designed to represent the prevalence of epilepsy in the entire country. Indeed, the three villages were selected based on information from local residents indicating the presence of cases of epilepsy. It is therefore possible that the studied villages would have a higher prevalence of epilepsy than in the country as a whole. Our estimate is similar, however, to results from door-to-door surveys conducted in one village in Cameroon and another in rural areas of Ivory Coast where the prevalence of epilepsy was estimated as 4.9% and 7.6%, respectively (26, 27). A study including 11 villages in Tanzania found the prevalence to vary between 0.6% and 3.7%, with epilepsy defined as having had at least two unprovoked seizures (28). These estimates are higher than the results reported by Debouverie and Kaboré in 1993 (1.1%; 95% CI= 0.9%; 1.2%) from a door-to-door survey of 18 villages selected randomly in the provinces of Passoré and Yatenga in Burkina Faso (29). Both a screening questionnaire and medical examinations were used to identify cases of epilepsy in the study of Debouverie and Kaboré. The random sampling strategy of those villages in contrast to our approach based on local information may explain the difference in prevalence as may the use of different measurement tools (questionnaire adapted from ILAE versus one adapted from WHO) (30). Finally, there may be a true difference in prevalence of epilepsy between the investigated areas and over the nearly 20 year difference between the studies. The largest survey of epilepsy in Africa was conducted in Ethiopia between 1986 and 1988 among 60,820 participants (31). The authors reported a prevalence of 5.2 per 1000 for active epilepsy defined as a history of “at least two unprovoked seizures of which at least one should have occurred during the last five years” or being under antiepileptic treatment at the time of the investigation (31). In addition to differences in sampling strategies, as mentioned above, the Ethiopian study screened for partial seizures using the following description: “sudden jerky movements in part of the body”. Our screening questionnaire included items to detect partial seizures with smelling, visual, auditory, sensitive as well as motor symptoms (see Appendix). Six of the 34 participants (18%) with seizures within the last three years had partial seizures with no motor symptoms, nor loss of consciousness. This could have led to an underestimation of the prevalence of epilepsy in the Ethiopian study.
The later onset of some seizures (after the age of 12 years) suggests the involvement of environmental factors in their causation, although it may also reflect a survivor effect if the mortality rate among those with onset of seizures at the youngest ages is higher than in later ages. The prevalence of epilepsy was higher among participants with positive results to the Ag-ELISA test for T. solium compared to subjects with negative results. This finding is suggestive of a high prevalence of cysticercosis among PWE and corroborates the results of a recent meta-analysis of eleven studies in Sub-Saharan Africa where a random-effects pooled odds ratio estimate of 3.4 (95% CI: 2.7; 4.3) was found between epilepsy and seropositivity to tests to detect T. solium antibodies or antigens (32). It should be emphasized that the present study investigated only the co-occurrence of infection with T. solium and epilepsy and this co-occurrence does not suffice to determine that NCC was the cause of the epilepsy. Cases of cysticercosis have also been identified in developed countries, mainly in immigrant communities, with however some reported autochthonous cases (33, 34, 35, 36).
Having eaten pork in the past compared to having never eaten pork was also positively associated with epilepsy. This may be explained by a local belief that PWE should stop consuming pork in order to avoid exacerbating the seizures. Thus, the local communities are somewhat aware of a link between pork and epilepsy, even though it is not an entirely accurate one. An observation of a phenomenon over time may lead people to think of or find an association between this phenomenon and another one, without necessarily having a scientific or rational explanation (“old wives’ tale”). This observation was not found anywhere else in the literature on epilepsy.
Prevalence of epilepsy was higher in traders and salaried persons compared to farmers and housewives. This observation may be an artifact and explained by a higher survival rate of PWE in salaried and traders. This group may also better understand the questions being asked in the screening questionnaire and hence be more likely to answer the questions accurately. Indeed, a larger proportion of traders and salaried persons who screened positive were confirmed as having epilepsy.
Our results suggest that the high prevalence of epilepsy in some of the investigated areas may be due, in part, to NCC, even though imaging would be required to confirm the diagnosis of NCC. Although the prevalence of epilepsy was approximately the same in the three study sites, some observations suggest that the etiologies may be different across villages. Indeed, no strong positive serology for T. solium was observed among PWE in Nyonyogo where pig-raising is minimal or nonexistent and where the seroprevalence was very low. Similar results were reported in a case-control study of epilepsy in the Gambia where the majority of the population self-identifies as being of Muslim faith and has little exposure to pigs (37). These results reflect the variability of underlying causes of epilepsy within and across countries and suggest the need for a comprehensive (clinical, serological and radiological) investigation of cases in order to have a better understanding of causal factors and appropriate control measures.
Our study had some limitations. First, our inclusion criteria may have resulted in either underestimation or overestimation of the true prevalence of epilepsy in the investigated area. Indeed, we purposefully included villages known to have cases of epilepsy, which could have led to an overestimate. On the other hand, we did not include subjects younger than seven years, which likely resulted in an underestimate of prevalence. Moreover, people suffering from epilepsy are still subject to stigmatization in many African communities. This was the case in the villages where the investigation took place as indicated by a qualitative survey including some PWE and other members of the local community. One confirmed case first denied having epileptic symptoms when being screened and then came later to declare his disease to the study physician. The true prevalence of the disease might have been underestimated if additional PWE did not declare their symptoms during the interviews.
Epilepsy is a disease with a large set of different signs and symptoms, often obvious but sometimes with minor signs that are difficult to detect (e.g., complex partial seizures, absence). Manifestations associated with these types of seizures may not be reported during the interview because they are not perceived as seizures by the individual or their friends and family. Only clinical methods were used for the diagnosis of epilepsy and its classification. As Preux and Druet-Cabanet (9) noted, many types of seizures other than generalized tonic-clonic are difficult to identify without appropriate diagnostic equipment such as an electroencephalogram (EEG). Finally, the study was also limited by the types of variables captured. Indeed, the variables of interest did not include genetic factors (family history of epilepsy) and participants’ status for other infections reported to be associated with epilepsy (e.g. toxocarosis and echinococcosis) (9, 11, 38, 39).
This preliminary study supports the hypothesis of an association between T. solium infection and epilepsy. If these results are confirmed by brain imaging, some cases of epilepsy in this area may be prevented by the implementation of better pig management and improved sanitation.
Supplementary Material
Acknowledgments
This study was funded by the National Institute for Neurological Disorders and Stroke (NINDS) and the Fogarty International Center (FIC) of the National Institutes of Health (“Epidemiology and Burden of NCC in Burkina Faso” (R21 NS055353) under the Brain Disorders in the Developing World: Research Across the Lifespan (BRAIN) program. We would like to thank Dr. Adèle Kam from IRSS for her substantial help in the analyses of the blood samples. We are also thankful to Henri Somé for all his help in the database structuring and data entry. The field team including Mmes Alida Da and Chantal, Millogo, Mr Léopold Bado as well as Dr Adama Sow who were instrumental to the success of this work. Finally, we are grateful to all the inhabitants from the three villages for their wonderful welcome and participation.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.NGUGI AK, BOTTOMLEY C, KLEINSCHMIDT I, SANDER JW, NEWTON CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;5:883–90. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PAL DK, CARPIO A, SANDER J. Neurocysticercosis and epilepsy in developing countries. J Neurol Neurosurg Psychiatry. 2000;68:137–43. doi: 10.1136/jnnp.68.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MEYER AC, DUA T, MA J, SAXENA S, BIRBECK G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–6. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MILLOGO A, RATSIMBAZAFY V, NUBUKPO P, BARRO S, ZONGO I, PREUX PM. Epilepsy and traditional medicine in Bobo-Dioulasso (Burkina Faso) Acta Neurol Scand. 2004;109:250–4. doi: 10.1111/j.1600-0404.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 5.NSENGIYUMVA G, DRUET-CABANAC M, NZISABIRA L, PREUX PM, VERGNENEGRE A. Economic evaluation of epilepsy in Kiremba (Burundi):a case-control study. Epilepsia. 2004;45:673–7. doi: 10.1111/j.0013-9580.2004.36303.x. [DOI] [PubMed] [Google Scholar]
- 6.LEONARDI M, USTUN T. The Global Burden of Epilepsy. Epilepsia. 2002;43:21–5. doi: 10.1046/j.1528-1157.43.s.6.11.x. [DOI] [PubMed] [Google Scholar]
- 7.JILEK-AALL L, JILEK M, KAAYA J. Psychosocial study of epilepsy in Africa. Soc Sci Med. 1997;45:783–95. doi: 10.1016/s0277-9536(96)00414-5. [DOI] [PubMed] [Google Scholar]
- 8.BAKER GA. The psychosocial burden of epilepsy. Epilepsia. 2002;43:26–30. doi: 10.1046/j.1528-1157.43.s.6.12.x. [DOI] [PubMed] [Google Scholar]
- 9.PREUX PM, DRUET-CABANAC M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 10.DEL BRUTTO OH, SANTIBÁÑEZ R, IDROVO L. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–7. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 11.NICOLETTI A, BARTOLONI A, REGGIO A, et al. Epilepsy, cysticercosis, and toxocariasis: a population-based case-control study in rural Bolivia. Neurology. 2002;58:1256–61. doi: 10.1212/wnl.58.8.1256. [DOI] [PubMed] [Google Scholar]
- 12.FOYACA-SIBAT H, COWAN LD, CARABIN H, et al. Accuracy of serological testing for the diagnosis of prevalent neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa. PLoS Negl Trop Dis. 2009;3:e562. doi: 10.1371/journal.pntd.0000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WINKLER AS, BLOCHER J, AUER H, GOTWALD T, MATUJA W, SCHMUTZHARD E. Epilepsy and neurocysticercosis in rural Tanzania: an imaging study. Epilepsia. 2009;50:987–93. doi: 10.1111/j.1528-1167.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 14.DEGEORGIO CM, MEDINA MT, DURON R. Neurocysticercosis. Epilepsy Curr. 2004;4:107–11. doi: 10.1111/j.1535-7597.2004.43008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHITE CA, GARCIA HH. Recent developments in the epidemiology, diagnosis, treatment, and prevention of neurocysticercosis. Curr Infect Dis Rep. 1999;1:434–40. doi: 10.1007/s11908-999-0055-x. [DOI] [PubMed] [Google Scholar]
- 16.CARABIN H, NDIMUBANZI PC, BUDKE C, et al. Clinical manifestations associated with neurocysticercosis: A systematic review. PLoS Negl Trop Dis. 2011;5:e1152. doi: 10.1371/journal.pntd.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PREUX PM, DRUET-CABANAC M, DEBROCK C, TAPIE P, DUMAS M. Comité de recherche sur l’épilepsiede l’Institut d’épidémiologie neurologique et de neurologie tropicale de Limoges. [Questionnaire for the investigation of epilepsy in tropical countries] Afr J Neurol Sci. 2003:22. [Google Scholar]
- 18.SENANAYAKE N, ROMAN GC. Epidemiology of epilepsy in developing countries. Bull World Health Organ. 1993;71:247–58. [PMC free article] [PubMed] [Google Scholar]
- 19.ENGEL J. Report of the ILAE Classification Core Group. Epilepsia. 2006;47:1558–68. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 20.BRANDT JRA, GEERTS S, DE DEKEN R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–7. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 21.VAN KERCKHOVEN I, VAN STEENKISTE W, CLAES M, GEERTS S. Improved detection of circulating antigen in catlle infected with Taenia saginata metacestodes. Vet Parasitol. 1999;76:269–74. doi: 10.1016/s0304-4017(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 22.PRAET N, RODRIGUEZ-HIDALGO R, SPEYBROECK N, et al. Infection with versus Exposure to Taenia solium: what do serological test results tell us? Am J Trop Med Hyg. 2010;83:413–5. doi: 10.4269/ajtmh.2010.10-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DORNY P, PHIRI IK, VERCRUYSSE J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–76. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 24.CARABIN H, MILLOGO A, PRAET N, et al. Seroprevalence to the antigens of Taenia solium cysticercosis among residents of three villages in Burkina Faso: a cross-sectional study. PLoS Negl Trop Dis. 2009;3:e555. doi: 10.1371/journal.pntd.0000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NEWCOMBE RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statist Med. 1998;17:857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.KOUASSI B, KOFFI JK, DIARRA JA. Prevalence of epilepsy in rural Ivory Coast: a pilot study. Pub Med Afr. 1998;89:25–30. [Google Scholar]
- 27.NJAMNSHI AK, SINI V, DJIENTCHEU V. Risk factors associated with epilepsy in a rural area in Cameroon: a preliminary study. Afr J Neurol Sci. 2007:26. Available at URL: http://www.ajns.paans.org/article.php3?id_article=228.
- 28.RWIZA HT, KILONZO GP, MATUJA WBP. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: A community-based study. Epilepsia. 1992;33:1051–6. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 29.DEBOUVERIE M, KABORE J. Epidémiologie de l’épilepsie au Burkina Faso. In: Dumas M, Giordano C, Gentillini F, editors. Neurologie Tropicale. Paris: John Libbey Eurotex; 1993. pp. 57–61. [Google Scholar]
- 30.SCHOENBERG BS. Clinical neuroepidemiology in developing countries: neurology with few neurologists. Neuroepidemiology. 1982;1:143–53. [Google Scholar]
- 31.TEKLE-HAIMANOT R, et al. Clinical and encephalographic characteristics of epilepsy in rural Ethiopia: a community-based study. Epilepsy Res. 1990;7:230–9. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 32.QUET F, GUERCHET M, PION SDS, NGOUNGOU EB, NICOLETTI A, PREUX PM. Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia. 2010;51:830–7. doi: 10.1111/j.1528-1167.2009.02401.x. [DOI] [PubMed] [Google Scholar]
- 33.SCHANTZ PM, MOORE AC, MUÑOZ JL, et al. Neurocysticercosis in an orthodox Jewish community in New York City. N Engl J Med. 1992;327:692–5. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- 34.OVERBAUSCH D, OOSTERHUIS JW, KORTBEEK LM, GARCIA-ALBEA E. Neurocysticercosis in Europe. In: Craig P, Pawłowski Z, editors. Cestode zoonoses: echinococcosis and cysticercosis- A global emergent problem. Amsterdam: IOS Press; 2002. pp. 33–40. [Google Scholar]
- 35.ONG S, TALAN DA, MORAN GJ, et al. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis. 2002;8:608–13. doi: 10.3201/eid0806.010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DEGIORGIO C, PIETSCH-ESCUETA S, TSANG V, et al. Sero-prevalence of Taenia solium cysticercosis and Taenia solium taeniasis in California, USA. Acta Neurol Scand. 2005;111:84–8. doi: 10.1111/j.1600-0404.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- 37.SECKA A, GRIMM F, VICTOR B, MARCOTTY T, DE DEKEN R, NYAN O, HERERA O, VAN MARCK E, GEERTS S. Epilepsy is not caused by cysticercosis in the Gambia. Trop Med Int Health. 2010;15:476–9. doi: 10.1111/j.1365-3156.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 38.ARPINO C, GATTINARA GC, PIERGILI D, CURATOLO P. Toxocara infection and epilepsy in children: a case-control study. Epilepsia. 1990;31:33–6. doi: 10.1111/j.1528-1157.1990.tb05356.x. [DOI] [PubMed] [Google Scholar]
- 39.GARCIA HH, MODI M. Helminthic parasites and seizures. Epilepsia. 2008;49:25–32. doi: 10.1111/j.1528-1167.2008.01753.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


