Abstract
Streptococcus suis was isolated postmortem from 2 lambs with a history of lameness. Identity of S. suis was confirmed by species-specific polymerase chain reaction (PCR) and by 16S rRNA gene sequencing. One isolate was untypable by serotyping and non-encapsulated, while the other isolate was serotype 33. The lambs had come from the same farm, and there was no evidence of contact between the lambs and pigs. Although the natural niche for S. suis is considered to be the pig, a wide range of host species may be affected by this pathogen.
Résumé
Isolement de Streptococcus suis chez 2 agneaux avec une anamnèse de boiterie. Streptococcus suis a été isolé post-mortem chez 2 agneaux avec une anamnèse de boiterie. L’identité de S. suis a été confirmée par une amplification en chaîne par la polymérase (ACP) et par le séquençage génétique de l’ARNr 16S. Un isolat n’a pas pu être typé par sérotypage et était non encapsulé, tandis que l’autre isolat était de sérotype 33. Les agneaux provenaient de la même ferme et il n’y avait aucune preuve de contact entre les agneaux et les porcs. Même si le créneau naturel de S. suis est considéré comme étant le porc, un vaste éventail d’espèces hôtes peuvent être affectées par cet agent pathogène.
(Traduit par Isabelle Vallières)
Streptococcus suis is a major swine pathogen responsible for important economic losses to the pig industry worldwide, causing meningitis, sudden death, septicemia, polyserositis, arthritis, endocarditis, otitis, and pneumonia (1). It is also an important human pathogen in western countries, affecting humans working with pigs or pork and the general population in some Asian countries (2). A total of 35 serotypes have been reported. The natural habitat of S. suis is the upper respiratory tract of pigs, particularly the tonsils and nasal mucosa, but also the genital and alimentary tract (1). Streptococcus suis has also been increasingly isolated from a wide range of animal species such as horses, cats, dogs, ruminants, and birds (3–6), probably affecting epidemiological aspects of this infection. This is a report of S. suis recovered from 2 lambs from the same farm that were submitted for postmortem examination.
Case descriptions
Lamb 1
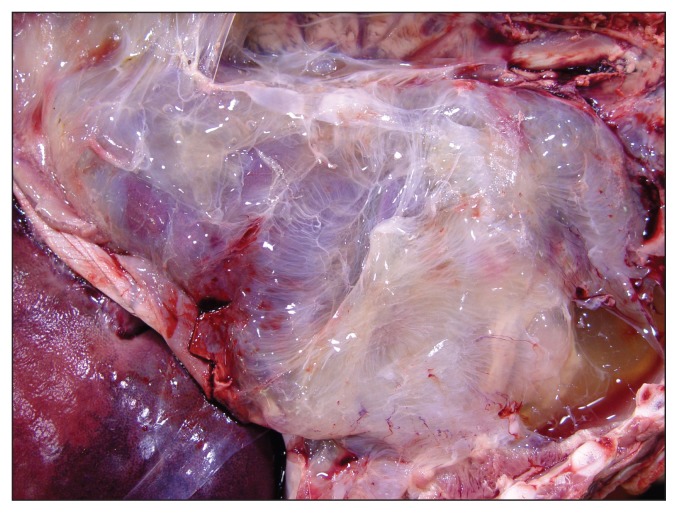
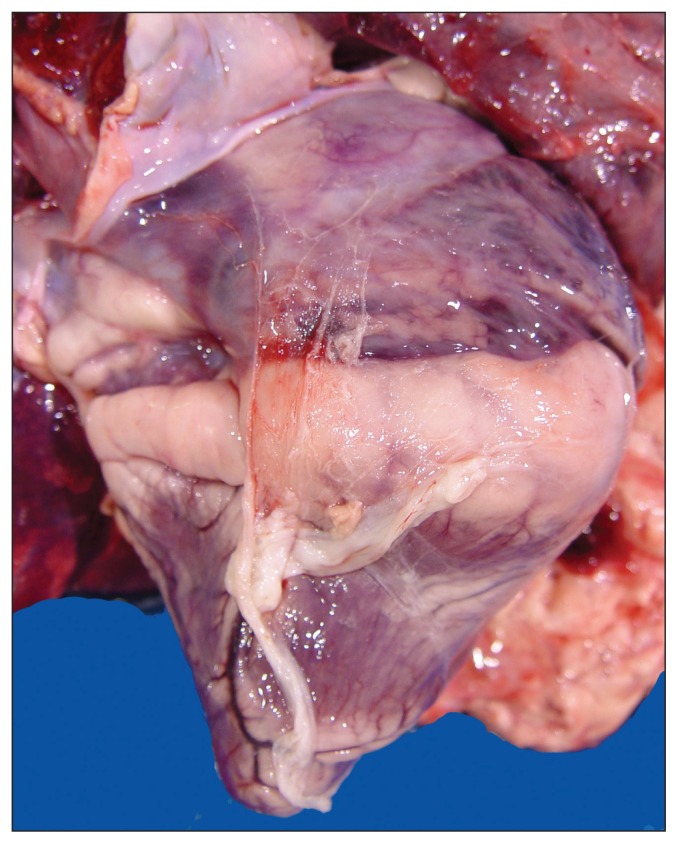
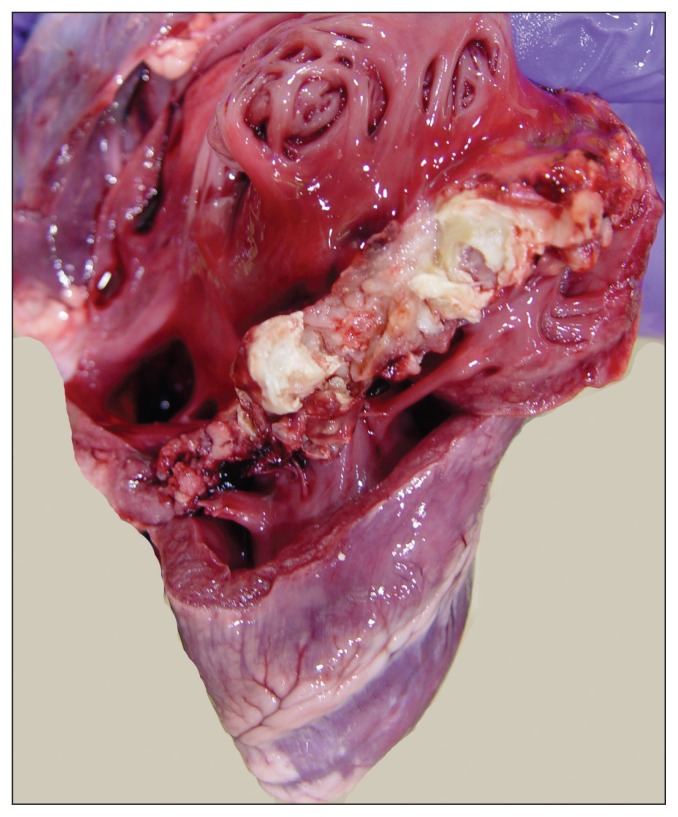
A 2-month-old, 34.5-kg lamb with a history of lameness was presented for postmortem examination. The carcass was moderately autolytic and appeared to be in reasonably good body condition with abundant fat stores. Mild subcutaneous edema was present in the thorax, neck, and hips. The laryngeal and tracheal mucosae were mildly hyperemic and the major bronchi were filled with foamy fluid. Approximately 500 mL of blood-tinged fluid were present in the thoracic cavity and the entire pleural surface was conspicuously covered with a thick layer of fibrin and fibrous adhesions (Figure 1). These adhesions were firmly attached to the visceral and parietal pleura, diaphragm, and pericardium. The pericardial sac was slightly thickened and contained 200 mL of pale straw-colored fluid admixed with loose fibrin tags (Figure 2). Irregular areas of pale discoloration were present in the right atrium and right ventricle, and a thick layer of caseous pale-yellow exudate covered the entire tricuspid valve (Figure 3). Other heart valves were unremarkable. Approximately 1.0 L of blood-tinged fluid containing loose strands of fibrin was present in the abdominal cavity and the entire peritoneal surface was also covered with fibrous adhesions. The liver was moderately congested and on cut surface the hepatic parenchyma exhibited a subtle reticular pattern. Externally, the right shoulder appeared swollen and the scapula-humeral joint had severe synovial effusion with yellow to brown caseous exudate covering the articular cartilage. The affected synovial membrane was swollen and edematous but the articular cartilage was unremarkable. Selected tissues were fixed in formalin for microscopic examination.
Figure 1.
Thoracic cavity showing chronic pleuritis. Note the entire pleural surface is covered by a thick layer of fibrous adhesions which extend into the pericardium and diaphragm.
Figure 2.
Heart showing fibrinous pericarditis. Note fibrin covering the epicardium and also a dilated right atrium.
Figure 3.
Right heart showing vegetative valvular endocarditis. Note large mass of exudate covering the tricuspid valve.
Lamb 2
A 2-month-old, 37.5-kg lamb with a history of progressive lameness and skeletal deformity involving the hind limbs was presented for postmortem examination. This lamb originated from the same farm as lamb 1 and according to the farmer had never been treated or vaccinated. On gross examination the carcass was autolytic and in fair body condition with abundant visceral fat but with moderately reduced muscular mass. Bronchial and tracheal mucosae were congested but without evidence of inflammation. The pericardial sac was moderately distended with 10 to 15 mL of blood-tinged fluid but with no fibrin on the pericardial surface. Externally, the right hip was notably swollen and dissection of this joint revealed abundant fibrinous adhesions extending from the coxofemoral joint to the articular capsule and adjacent soft tissues. Internally, there was synovial effusion and abundant fibrinous exudate in the synovial cavity.
Histopathology
Microscopic examination of the affected tricuspid valve revealed abundant fibroblasts and collagen fibers extending deep into the myocardial tissue. Attached to this valve was a large amorphous mass composed of organized fibrin-containing lavish cell debris, neutrophils, and large colonies of Gram-positive bacteria. The articular capsule of the right-scapulohumeral joint was thickened and infiltrated by abundant fibroblasts, macrophages, and neutrophils. Coronal sections of the brain were macroscopically unremarkable.
Bacteriology
Samples of liver, lung, and heart of lamb 1 and liver, lung, kidney, and exudate from the right shoulder of lamb 2 were submitted for culture on Columbia agar with 5% sheep blood and MacConkey agar. There was no growth from the liver and lung or kidney of the lambs after 48 h aerobic incubation at 35°C. The heart swab of lamb 1 and the joint of lamb 2 after 24 h aerobic incubation yielded moderate numbers of pure growth of small moist, translucent-to-white colonies with a zone of incomplete (green) hemolysis. There was no growth on MacConkey agar. Colonies were catalase-negative and a Gram stain revealed Gram-positive cocci. Both isolates gave a positive amylase test on starch agar and were identified by API 20 Strep (BioMérieux, St. Laurent, Quebec) as Streptococcus suis group 1 (90.1% probability) with API Profile Number 4771433. The Biolog Micro Station System (Biolog, Hayward, California, USA) listed S. suis as the first choice species identification for both isolates. Matrix Assisted Laser Desorption Ionization Time of Flight mass spectrometry (MALDI-TOF) (microflex LT; Bruker Daltonics, Billeria, Massachusetts, USA) identified the lamb 1 isolate as S. suis with an organism best match score value of 1.726. MALDI-TOF gave no species identification for the lamb 2 isolate, but listed S. suis as the first choice. Identification of the 2 isolates as S. suis was confirmed by a species-specific PCR (7) and by sequencing of 16S rRNA genes (99% of identity) (8). Serotyping was carried out using the coagglutination test with reference antisera (3). The S. suis isolate from lamb 1 was non-typable and the S. suis isolate from lamb 2 was serotype 33. The hydrophobicity of the non-typable strain was tested as described (9) and results showed a high value (87%), which strongly suggests the strain was non-encapsulated.
Discussion
Polyserositis and endocarditis in sheep are frequently encountered in veterinary diagnostic laboratories, but isolation of S. suis from ovine exudates was entirely unexpected since this is not a typical ovine pathogen (10,11). There is 1 report of S suis endocarditis in an experimental sheep that had been implanted with a cardiac device (12). This experimental sheep, in addition to endocarditis, had meningitis, a lesion that was not detected in either of the 2 lambs reported here. The chronicity of pleural, valvular, and synovial fibrosis was remarkable in both lambs suggesting that initial infection with S. suis must have occurred soon after birth. Another interesting finding that is difficult to explain was the good body condition in which these 2 lambs were presented for postmortem examination. Chronic lesions, as severe as those seen in these lambs, are generally accompanied by emaciation characterized by serous atrophy of fat and loss of muscle mass, a metabolic condition known as septic cannibalism (13). It may be speculated that the systemic inflammatory response caused by S. suis in these lambs was kept in balance by the compensatory anti-inflammatory response, thus preventing the development of severe sepsis and septic shock (14). This view is also supported by the fact that neither disseminated intravascular coagulation (DIC) nor vasculitis, 2 lesions often found in severe sepsis and septic shock, was detected in these lambs (14). Finally, there was no gross or microscopic evidence of embolic pneumonia which would have been expected in an animal having a large vegetative mass in the tricuspid valve (10).
The typable strain involved in 1 of the cases was a serotype 33. Interestingly, the reference strain of serotype 33 was originally isolated from the joints of a diseased lamb. Recently, Tien et al (15) suggested that the reference strain of serotype 33 should be removed from the S. suis species, although its taxonomic designation and determinative phenotypic characteristics have yet to be addressed. However, 17 strains of S. suis serotype 33 have been isolated from diseased pigs in the last 4 years in Canada (16). One of these strains was highly virulent and caused septic shock in pigs with poor response to antibiotic treatment (16). The strain isolated in the present study was classified as S. suis by 16S rRNA. It is perhaps premature to definitively eliminate this serotype from the S. suis species and research with more field strains should be done. At the reference laboratory for serotyping, the 35 serotypes are still considered for diagnosis.
The second strain was a poorly encapsulated non-typable strain. Gottschalk et al (16) recently reported that between 14% and 33% of isolates recovered from pigs are non-typable. Of these, 89% of isolates were considered poorly or non-encapsulated. It is also difficult to ascertain if this isolate was already non-encapsulated when causing the disease or if it lost the capsular polysaccharide during isolation and culture. It has been previously reported that 34% of isolates belonging to serotype 2 or 1/2 and recovered from cases of endocarditis in Japan were non-encapsulated due to deletions and insertions in genes of the capsular polysaccharide locus (17). It was concluded that, although the capsule is considered to be an important virulence factor, loss of capsular production might be beneficial to S. suis in the course of some infections. In fact, non-encapsulated strains were shown to possess not only higher adherence to mammalian cells but also the capacity to form biofilms (9). Consequently, the potential virulence of this specific isolate cannot be ruled out.
In the present report, there was no proven contact between the lambs and pigs. The owners confirmed that no pigs had been on the property for at least 15 y. Although the natural niche for S. suis is considered to be the pig, a wide range of host species may be affected by this pathogen. The specific niche for S. suis serotype 33 should be further studied. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Gottschalk M. Streptococcocis. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 10th ed. Ames, Iowa: Blackwell Publishing; 2012. pp. 841–855. [Google Scholar]
- 2.Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010;5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- 3.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest. 1990;2:249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka Y, Sugimoto C, Nakazawa M, Morozumi T, Kashiwazaki M. The epidemiological studies of Streptococcus suis infections in Japan from 1987 to 1991. J Vet Med Sci. 1993;55:623–626. doi: 10.1292/jvms.55.623. [DOI] [PubMed] [Google Scholar]
- 5.Devriese LA, Haesesbrouck F, De Herdt P, et al. Short communication. Streptococcus suis infections in birds. Avian Pathol. 1994;23:721–724. doi: 10.1080/03079459408419040. [DOI] [PubMed] [Google Scholar]
- 6.Muckle A, Giles J, Lund L, Stewart T, Gottschalk M. Cross Canada Disease Report. Isolation of Streptococcus suis from the urine of a clinically ill dog. Can Vet J. 2010;51:773–774. [PMC free article] [PubMed] [Google Scholar]
- 7.Gottschalk M, Lacoutoure S, Bonifait L, Grenier D. Distribution of Streptococcus suis capsular types isolated from diseased pigs in 2008 and 2009 in Canada. 21st Proceedings of the International Pig Veterinary Society Congress; Vancouver, Canada. 2010. [Google Scholar]
- 8.Brousseau R, Hill J, Préfontaine G, Goh S-H, Harel J, Hemmingsen S. Streptococcus suis serotypes characterized by analysis of Chaperonin 60 gene sequences. Appl Environ Microbiol. 2001;67:4828–4833. doi: 10.1128/AEM.67.10.4828-4833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifait L, Gottschalk M, Grenier D. Research Letter. Cell surface characteristics of nontypeable isolates of Streptococcus suis. FEMS Microbiol Lett. 2010;311:160–166. doi: 10.1111/j.1574-6968.2010.02086.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopez A. The respiratory system, pleurae, and mediastinum. In: Zachary JF, McGavin MD, editors. Pathologic Basis of Veterinary Diseases. 5th ed. Philadelphia, Pennsylvania: Elsevier; 2012. pp. 516–518. [Google Scholar]
- 11.Thomson K. Bones and joints. In: Maxie MG, editor. Pathology of Domestic Animals. 5th ed. Edinburgh, UK: Elsevier; 2007. [Google Scholar]
- 12.Wilson JW, Griffith JW. Endocarditis and meningitis caused by Streptococcus suis after cardiac surgery in a sheep. Contemp Top Lab Anim Sci. 2000;3:43–46. [PubMed] [Google Scholar]
- 13.Michie HR. Metabolism of sepsis and multiple organ failure. World J Surg. 1996;20:460–464. doi: 10.1007/s002689900072. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DH, Chan DL, Pinheiro D, Armitage-Chan E, Garden OA. The immunopathology of sepsis: Pathogen recognition, systemic inflammation, the compensatory anti-inflammatory response, and regulatory T cells. J Vet Intern Med. 2012;26:457–482. doi: 10.1111/j.1939-1676.2012.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tien LHT, Nishibori T, Nishitani Y, Nomoto R, Osawa R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA-DNA homology and sodA and recN phylogenies. Vet Microbial. 2013;162:842–849. doi: 10.1016/j.vetmic.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Gottshalk M, Lacoutoure S, Bonifait L, Roy D, Fittipaldi N, Grenier D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Québec, Canada. Vet Microbiol. 2013;162:819–825. doi: 10.1016/j.vetmic.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Lakkitjaroen N, Takamatsu N, Okura M, Sato M, Osaki M, Sekizaki T. Loss of capsule among Streptococcus suis isolates from porcine endocarditis and its biological significance. J Med Microbiol. 2011;60:1669–1676. doi: 10.1099/jmm.0.034686-0. [DOI] [PubMed] [Google Scholar]