Abstract
A case of a disseminated algal infection is reported in a young rough-coated collie dog with progressive neurologic deficits, blindness, and hemorrhagic diarrhea. Prototheca zopfii organisms were cultured from feces, urine, and blood. At necropsy, granulomas containing typical organisms were identified within the proximal colon, heart, kidneys, and eyes.
Résumé
Protothécose chez un chien. Un cas d’infection algoïde est signalé chez un jeune chien Collie à poil court avec des troubles neurologiques progressifs, de la cécité et de la diarrhée hémorragique. Des organismes de type Prototheca zopfii ont été cultivés à partir des fèces, de l’urine et du sang. À la nécropsie, des granulomes contenant des organismes typiques ont été identifiés dans le côlon proximal, le cœur, les reins et les yeux.
(Traduit par Isabelle Vallières)
Case description
A 2-year-old intact female rough-coated collie dog was referred to the Ontario Veterinary College Health Sciences Centre (OVCHSC) Emergency Service for evaluation of progressive neurologic signs, uveitis, and hemorrhagic diarrhea. The dog had spent approximately the first year of life in Thailand, and had been living in Ontario for 6 mo before becoming ill. The dog was initially presented to the referring veterinarian 3 wk prior to referral for a mild left-sided head tilt. All vital parameters were within normal limits. A point-of-care partial blood cell count revealed a mild eosinophilia [1.63 × 109/L; reference interval (RI): 0.1 to 1.49 × 109/L], a mild increase in hematocrit (0.58 L/L; RI: 0.37 to 0.55 L/L), and a mild decrease in mean cell hemoglobin concentration (293 g/L; RI: 300 to 375 g/L). The patient was prescribed enrofloxacin (Baytril; Bayer, Toronto, Ontario), 6.8 mg/kg body weight (BW), PO, q24h and prednisone (Novo-Prednisone; Novopharm, Toronto, Ontario) at an unspecified dose. SNAP 4Dx test (IDEXX Laboratories, Markham, Ontario) results were negative for Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum. A slight positive response to medication was reported by the owner, but the dog was returned to the referring veterinarian 20 d later because of a now marked right-sided head tilt, ataxia, and hemorrhagic diarrhea. At that time, a complete blood (cell) count (CBC) revealed a mild monocytosis (2.64 × 109/L; RI: 0.3 to 2.0 × 109/L). The dog was administered intravenous fluids and doxycycline hyclate (Apo-Doxy; Apotex, Toronto, Ontario), 11.4 mg/kg BW, PO, q24h. The left eye had signs of uveitis and an absent direct pupillary light reflex (PLR) which 2 d later progressed to bilateral uveitis and bilaterally absent PLR. Treatment with a topical preparation containing polymyxin B sulfate, neomycin sulfate, and dexamethasone (Maxitrol Ophthalmic suspension; Alcon Canada, Mississauga, Ontario) was initiated in both eyes 3 times daily. The following day, direct fundic examination revealed bilateral retinal hemorrhages and the dog was referred to the OVCHSC for further diagnostic evaluation and treatment.
On presentation to the OVCHSC, the dog was quiet but alert. The dog was in poor body condition (2 out of 5) and was febrile (40.5°C), but other vital parameters were normal. A marked right-sided head tilt was noted. Following admission, serum electrolytes and blood gases were found to be normal. The dog was administered famotidine (Famotidine Omega; Omega Laboratories, Montreal, Quebec), 0.5 mg/kg BW, IV, q24h, and treated with intravenous fluids (Plasmalyte-A; Baxter Canada, Mississauga, Ontario) supplemented with 20 mEq/L potassium chloride (Potassium Chloride for Injection Concentrate; Hospira, Montreal, Quebec).
A CBC revealed a mature neutrophilia of 15.28 × 109/L (RI: 2.91 to 0.6 × 109/L), lymphopenia 0.38 × 109/L (RI: 0.8 to 15.1 × 109/L), and monocytosis 2.87 × 109/L (RI: 0 to 0–1.1 × 109/L). These changes were characteristic of chronic inflammation or a stress/corticosteroid-induced leukogram. A serum biochemistry profile was unremarkable, other than a high creatine kinase activity of 266 U/L (RI: 40 to 255 U/L). Prothrombin time and partial thromboplastin time were within normal limits. Urinalysis disclosed a urine specific gravity of 1.016 after fluid therapy, but was otherwise unremarkable. Thoracic radiographs revealed a single soft tissue opacity in the region of the tracheobronchial lymph nodes.
Ophthalmic examination revealed a negative dazzle reflex, menace response, and direct and indirect pupillary light reflex in both eyes. Both pupils were mydriatic in normal light and the palpebral reflex was present bilaterally. A Schirmer tear test (Intervet Canada, Kirkland, Quebec) detected low tear production in both eyes (9 mm/min OD, 10 mm/min OS; RI: 19.8 ± 5.3 mm/min; 1,2). There was no fluorescein stain uptake in either eye and intraocular pressures (Tonovet, Helsinki, Finland) were decreased (7 mmHg OD, 6 mmHg OS; RI: 10.8 +/− 3.1 mmHg; 3). Ocular anomalies noted on slit-lamp biomicroscopy (Kowa SL15Slit Lamp, Kowa Optimed, Torrance, California, USA) included bilateral mild conjunctival hyperemia and scleral blood vessel congestion and mild aqueous flare. Retinal detachments in both eyes were detected by diffuse illumination of the pupils. Indirect ophthalmoscopy (Heine; Heine Instruments Canada, Kitchener, Ontario) revealed the retinal detachment to be complete and secondary to a white subretinal transudate that mimicked the appearance of confluent granulomas. Retinal blood vessels had multifocal hemorrhages (petechiae) in the dorsal medial quadrant of both eyes.
On neurological examination the dog was somnolent with the aforementioned right head tilt and bilaterally absent PLR’s (pupils mid-range). Physiological nystagmus was absent to the right and delayed to the left, and there was no evidence of spontaneous nystagmus. The dog was ambulatory, but circling to the right. Postural reactions were mildly delayed on the right but normal on the left. Spinal reflexes were normal and no pain was elicited on spinal palpation. Based on the neurological signs a lesion was localized to the brainstem with the right side worse than the left.
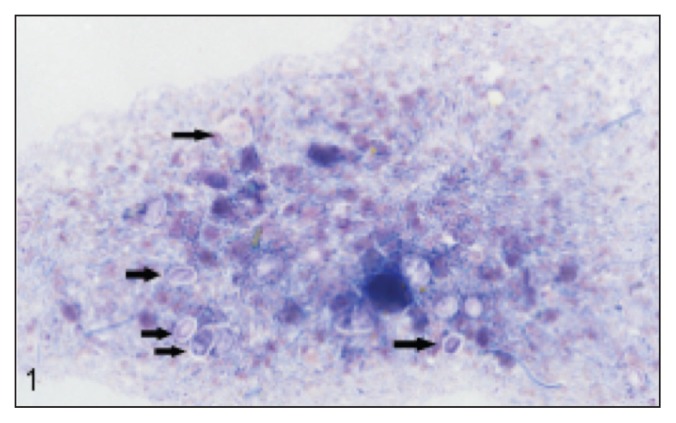
The ophthalmologic and neurological signs, together with the history of hemorrhagic diarrhea, suggested protothecosis as a potential diagnosis. This was confirmed by rectal scraping (performed using a scalpel handle) and cytology (Figure 1). Fecal, urine (collected by cystocentesis), and blood samples were submitted for culture, which yielded Prototheca zopfii. Routine antimicrobial drug susceptibility testing was performed by the Animal Health Laboratory, University of Guelph. Isolates from fecal culture were also submitted for antifungal drug susceptibility testing (Fungus Testing Laboratory, The University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA). Pending drug susceptibility tests, treatment was started with itraconazole (Sporanox; Janssen, Toronto, Ontario), 9.1 mg/kg BW, PO, q12h and nystatin (Nilstat; Pfizer Canada, Kirkland, Quebec), 22 727.3 IU/kg BW, PO, q8h. Doxycycline was continued at 11.4 mg/kg BW, PO, q12h. Maxitrol OU q6h was continued and treatment was started with an ocular lubricant (Lacrilube; Allergan Canada, Markham, Ontario) OU q6h to address diminished tear production.
Figure 1.
Rectal scraping cytology, modified Wright’s stain, 600× magnification. Prototheca organisms (arrows) are round to oval, measuring 2 to 30 μm and have a granular, basophilic cytoplasm with clear cell wall. Note also the large numbers of bacteria and degenerate neutrophils in the background.
The dog became less responsive on the second day of hospitalization, but it stabilized after initiation of itraconazole and nystatin. For the next 2 d the dog’s clinical status remained stable, but no significant improvements were seen. The dog was dull, depressed, and quiet when left alone, but if stimulated, was responsive and alert. The dog continued to eat and drink well, there were no signs of nausea and no vomiting, and the diarrhea persisted but was less hemorrhagic. Vital parameters, venous electrolyte, and blood gas analysis, and hematocrit and plasma total solids remained within normal limits throughout hospitalization. The dog was discharged at the owner’s request despite recommendations for continued hospitalization. It was alert and minimally responsive, but ambulatory. Over the next 3 d the dog became non-responsive, remaining in lateral recumbency and appearing to be unaware of its surroundings. In addition, hemorrhagic diarrhea progressed to frank blood. The dog was euthanized by the referring veterinarian, and a complete necropsy was performed.
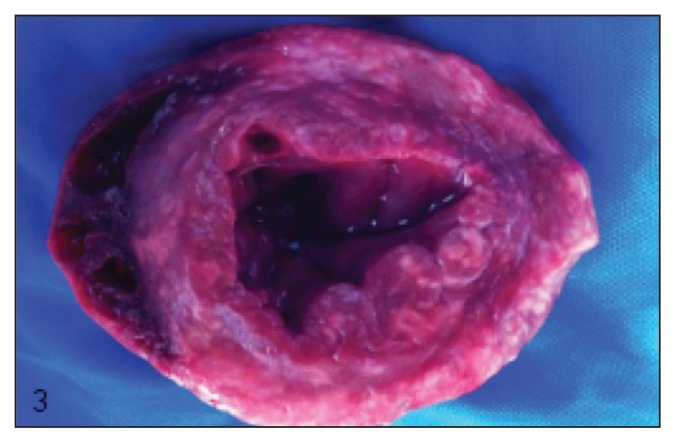
Myriad 1- to 2-mm diameter randomly distributed and typically confluent pale tan nodules were found throughout the atrial and ventricular myocardium, regionally replacing 5% to 20% of the myocardium (Figure 2). Similar nodules were present transmurally within a 10-cm segment of the proximal colon approximately 5 cm from the ileocecocolic junction, thickening up to three-fold and producing a roughened and rugose mucosa and a heavily vascularized and mottled dark purple-tan serosa (Figure 3). Lastly, similar nodules were found within the renal cortices (involving less than 1% of the renal parenchyma). Samples from the nervous tissue were too autolyzed for assessment. Histologically, these nodules were comprised centrally of macrophages surrounded by scant fibrous connective tissue, in turn surrounded by lymphocytes and plasma cells (consistent with granulomas), within the center of which were numerous 8 to 12 μm diameter round-oval algal sporangia, with thick hyaline cell walls and 1, 2 or 4 endospores (Figure 4). Within each eye, the choroid, optic nerve, retina, subretinal space, and posterior chamber contained multifocal-to-confluent nodules similar to those described in the myocardium, and extensive accumulations of neutrophils and fibrin were present within the posterior chambers; each iris and ciliary body contained increased numbers of plasma cells, macrophages, and lymphocytes. Histopathological diagnoses of multifocal granulomatous myocarditis and interstitial nephritis, transmural granulomatous colitis, bilateral multifocal pyogranulomatous posterior uveitis, and generalized lymphoplasmacytic and histiocytic anterior and posterior uveitis were made. Large numbers of intralesional sporangia in all affected organs were compared to reports of Prototheca spp., and the patterns of endosporulation and the typical “Maltese Cross” appearance were found, consistent with previous reports (Figure 4).
Figure 2.
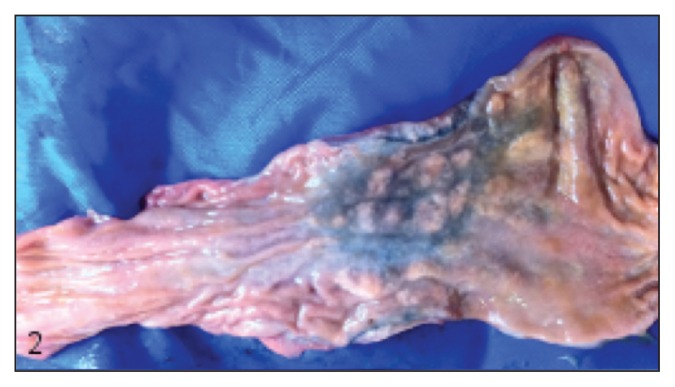
Heart, cut section of apex. At all levels of the myocardium there are numerous poorly defined pale tan-white nodules.
Figure 3.
Colon and rectum, mucosal surface. There is segmental dark discoloration of the colonic wall, throughout which are multiple poorly defined white-tan nodules. Some green discoloration is present as a result of both local hemorrhage and autolysis.
Figure 4.
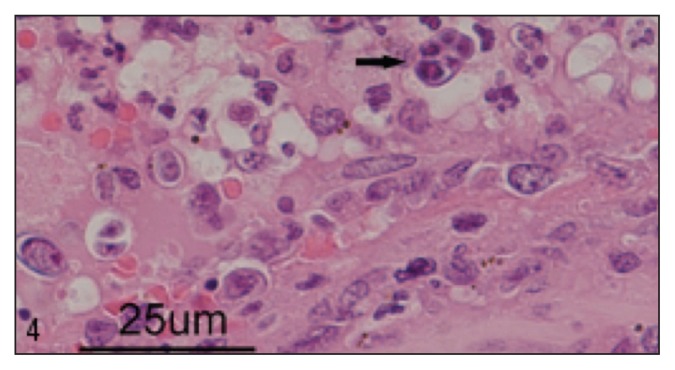
Posterior chamber of the eye, H&E stain, 600× magnification. Within the posterior uvea and anterior chamber are numerous 8 to 12 μm diameter round-oval algal sporangia, with thick hyaline cell walls and 1, 2 or 4 endospores; 1 of these sporangia has a typical “Maltese cross” appearance for Prototheca spp. These sporangia are surrounded by large numbers of neutrophils.
Antimicrobial susceptibility results became available postmortem. Kirby-Bauer zones of inhibition for antibacterial drugs with reported activity against Prototheca spp. were the following: doxycycline–0 mm; amikacin–14 mm; gentamicin 15–mm; and kanamycin 15–mm. Minimum lethal concentrations for antifungal drugs were: amphotericin B — 0.5 μg/mL; nystatin– 8 μg/mL; ketoconazole–4 μg/mL; fluconazole– > 4 μg/mL; itraconazole–0.25 μg/mL; posaconazole–0.25 μg/mL; and voriconazole–0.5 μg/mL.
Discussion
The genus Prototheca entails species of achlorophyllous, unicellular, saprophytic, aerobic algae closely related to Chlorella spp. (4). These algae are ubiquitous in the environment and may be isolated from fresh and marine water, soil, mud, tree sap, and sewage (5). Prototheca reproduce asexually by internal cleavage (endosporulation), resulting in the formation of 2 to 20 small endospores within the sporangium, which expand until they are released when the sporangium ruptures (6). Five species of Prototheca are currently recognized, including P. blaschkeae, P. stagnora, P. ulmea, P. wickerhami, and P. zopfii (7,8); a sixth species, P. cutis, was recently proposed based on genetically distinct isolates from a human patient with skin disease (9). Of these, P. wickerhami and P. zopfii are recognized as pathogenic to humans, cattle, and dogs. Human cases have largely been reported from North America and Asia (10).
In human patients, protothecosis has 3 manifestations: i) dermatitis (comprising more than half of reported human cases), ii) olecranon bursitis, and iii) disseminated or systemic infection. Most lesions of the skin and olecranon bursa result from local traumatic inoculation. Human systemic infections are almost uniformly associated with immune compromise, occurring most commonly with anticancer therapy, organ transplantation, or clinical AIDS, and most commonly affect the skin and subcutis, alimentary tract, peritoneum, blood, and spleen (11).
Canine cases of protothecosis are uncommon but are increasingly recognized worldwide. A recent review of canine protothecosis (10) identified 31 canine cases in the primary literature, largely arising from the United States. To our knowledge, this is the first case report of protothecosis in a dog from Canada (though with origins in Thailand). An electronic search of medical records at the Ontario Veterinary College back to 1985 yielded 1 other case, diagnosed in 1999, which had no travel history. Human protothecosis appears to be a rare diagnosis in Canada, with a single case report in 1981 (12). More frequently in Canada, Prototheca spp. are isolated and implicated as a cause of mastitis in dairy cattle. A recent study identified 18 herds in Canada with Prototheca mastitis, and in comparison to control herds, implicated human interventions as principle risk factors in its incidence (13).
In contrast to the human disease, canine protothecosis typically involves a broadly disseminated infection, particularly involving the colon, nervous system, and eyes, as well as the heart, kidneys, skeletal muscle, and liver (10). Frequent involvement of the colon makes colitis (with or without hematochezia) a common presenting complaint; other common presenting complaints include neurologic disease, blindness, and less frequently, polyuria and polydipsia. The necropsy findings herein present a typical distribution in the canine patient. Unfortunately, profound autolytic and freezing artifact precluded the evaluation of most of the central nervous system; however, the clinical presentation and extensive involvement of the optic nerves allow us to presume broader involvement of the brain in this case.
Little is known regarding the pathogenesis of canine protothecosis. In human cases the predominance of uncomplicated cutaneous lesions suggests that direct traumatic inoculation is most likely; however, the paucity of cutaneous lesions in canine patients with disseminated disease suggests this is less likely. Most sources suggest the colon as the most likely principle site of infection, resulting in chronic granulomatous colitis and eventual dissemination of the alga to other sites of predilection. Immune dysfunction is often posited as a contributor to this pathogenesis in order to account for its sporadic occurrence, as well as to explain the possible predisposition of collies (14) and boxers (10). One study attempted to examine the immune response to natural Prototheca spp. infection in a dog, but did not definitively confirm or establish a mechanism for this dysfunction (15).
Protothecosis carries a grave prognosis in the canine patient. Stenner et al (10) identified only 2 cases of canine protothecosis that survived the infection out of 31 cases reviewed. In contrast, the same review found only 2 human cases in which death was attributable to protothecosis. Stenner et al (10) also described 17 cases of protothecosis in dogs from Australia, including 6 cases that underwent treatment. Two dogs that were presented with colitis without any indication of disseminated infection were treated with amphotericin B, with survival times noted as 12 and 17 mo and no indication of dissemination of infection (specific causes of death were not indicated). One dog presenting with colitis and later developing nervous and ocular disease was treated with amphotericin B and itraconazole, and survived to the time of publication. Three dogs were treated with ketaconazole; 2 of these animals failed to improve with therapy and were euthanatized, and 1 was euthanatized before adequate time had elapsed to assess therapeutic efficacy.
Arguments may be made that the poor prognosis associated with protothecosis could be related to the late stage of diagnosis in the progression of disease and that early detection of infection might permit earlier and more effective treatment before extensive dissemination. However, early differential diagnosis of protothecosis is complicated by the numerous other causes of chronic diarrhea and by the very small likelihood of Prototheca infection. Unfortunately, Prototheca frequently is not considered as a differential diagnosis until seen by referral specialists, or until the development of secondary lesions of dissemination to the brain or eye. Even with the development of granulomatous endophthalmitis there are numerous more likely differential diagnoses in the dog, including Blastomyces dermatitidis and Cryptococcus neoformans, more rarely Coccidiodes immitis or Histoplasma capsulatum infection (16). It is usually the development of ocular and/or nervous dysfunction in the face of chronic colitis that directs the clinician toward a diagnosis of disseminated protothecosis. A better understanding of the factors that contribute to the development of this disease might provide early indicators of Prototheca spp. infection.
Another issue that may influence the prognosis of this infection in canine patients is a limited database of information regarding antimicrobial susceptibility and interpretation of susceptibility tests. Lass-Florl and Mayr (11) found that there are few studies on the susceptibility of Prototheca spp. and no official guidelines for the interpretation of these tests in human patients, and that test results are difficult to reproduce and often do not correlate to clinical outcome. In general, human clinical isolates of Prototheca spp. have been susceptible in vitro to amphotericin B, fluconazole, itraconazole, and voriconazole, tetracycline, gentamicin, and amikacin, but have been resistant to 5-flucytosine and griseofulvin. Resistance and susceptibility to miconazole, clotrimazole, and polymyxin B vary between species and reports (11). Liposomal amphotericin B appears to have the greatest activity against this alga, and ketoconazole, itraconazole, and fluconazole are also commonly used in treatment of human patients. In this case, amikacin, gentamicin, and kanamycin had a demonstrated antimicrobial effect on culture isolates, and amphotericin B, itracozole, posaconazole, and voriconazole appeared to have optimal minimum lethal concentrations. Studies in the canine patient have been more limited. One study demonstrated profound heterogeneity in susceptibility of 3 canine urinary tract isolates to common antifungals; however, decision points for susceptibility and resistance are not provided (17). Surgical resection of localized infections, with ancillary antimicrobial therapy for deep infections, is considered most useful when applicable in human patients; however, veterinary patients more commonly present with broadly systemic disease, limiting the utility of surgery to those cases in which disease appears to be localized.
The idiosyncracies of canine protothecosis, including methods of transmission, the tendency towards broad systemic dissemination, predilection for specific organs, and possible differential susceptibility of boxers (10) and collies (14) provide many questions to be answered on this disease. Additionally, further systemic investigation is needed to provide better diagnostic tools for early detection of this pathogen as well as improved, evidence-based means to treat infection when it arises. However, the low numbers of cases that occur worldwide, the somewhat obscure nature of this pathogen, and the limited means to repeatedly reproduce a representative disease in vivo hamper large systematic examination of meaningful numbers of cases in a controlled fashion. Considering that many human cases arise in North America, it is important to consider protothecosis as a rare but important differential diagnosis for cases of hemorrhagic colitis with onset of multisystemic disease in the canine patient.
Acknowledgment
The authors thank Durda Slavic of the Animal Health Laboratory for facilitating culture and antimicrobial susceptibility testing. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rubin LF, Lynch RK, Stockman WS. Clinical estimation of lacrimal function in dogs. J Am Vet Med Assoc. 1965;147:946–947. [PubMed] [Google Scholar]
- 2.Gelatt KN, Peiffer RL, Jr, Erickson JL, Gum GG. Evaluation of tear formation in the dog, using a modification of the schirmer tear test. J Am Vet Med Assoc. 1975;166:368–370. [PubMed] [Google Scholar]
- 3.Knollinger AM, La Croix NC, Barrett PM, Miller PE. Evaluation of a rebound tonometer for measuring intraocular pressure in dogs and horses. J Am Vet Med Assoc. 2005;227:244–248. doi: 10.2460/javma.2005.227.244. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth SR. Canine protothecosis. Vet Clin North Am Small Anim Pract. 2000;30:1091–1101. doi: 10.1016/s0195-5616(00)05008-7. [DOI] [PubMed] [Google Scholar]
- 5.Blogg JR, Sykes JE. Sudden blindness associated with protothecosis in a dog. Aust Vet J. 1995;72:147–149. doi: 10.1111/j.1751-0813.1995.tb15038.x. [DOI] [PubMed] [Google Scholar]
- 6.Sudman MS, Kaplan W. Identification of the prototheca species by immunofluorescence. Appl Microbiol. 1973;25:981–990. doi: 10.1128/am.25.6.981-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno R, Urano N, Wada S, Kimura S. Optimization of heterotrophic culture conditions for n-alkane utilization and phylogenetic position based on the 18S rDNA sequence of a thermotolerant prototheca zopfii strain. J Biosci Bioeng. 2002;94:160–165. doi: 10.1263/jbb.94.160. [DOI] [PubMed] [Google Scholar]
- 8.Roesler U, Moller A, Hensel A, Baumann D, Truyen U. Diversity within the current algal species Prototheca zopfii: A proposal for two Prototheca zopfii genotypes and description of a novel species, Prototheca blaschkeae sp. nov. Int J Syst Evol Microbiol. 2006;56:1419–1425. doi: 10.1099/ijs.0.63892-0. [DOI] [PubMed] [Google Scholar]
- 9.Satoh K, Nagayama H, Makimura K. Prototheca cutis sp. nov., a newly discovered pathogen of protothecosis isolated from inflamed human skin. Int J Syst Evol Microbiol. 2010;60:1236–1240. doi: 10.1099/ijs.0.016402-0. [DOI] [PubMed] [Google Scholar]
- 10.Stenner VJ, Mackay B, King T, et al. Protothecosis in 17 Australian dogs and a review of the canine literature. Med Mycol. 2007;45:249–266. doi: 10.1080/13693780601187158. [DOI] [PubMed] [Google Scholar]
- 11.Lass-Florl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230–242. doi: 10.1128/CMR.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapica L. First case of human protothecosis in Canada: Laboratory aspects. Mycopathologia. 1981;73:43–48. doi: 10.1007/BF00443013. [DOI] [PubMed] [Google Scholar]
- 13.Pieper L, Godkin A, Roesler U, et al. Herd characteristics and cow-level factors associated with Prototheca mastitis on dairy farms in Ontario, Canada. J Dairy Sci. 2012;95:5635–5644. doi: 10.3168/jds.2011-5106. [DOI] [PubMed] [Google Scholar]
- 14.Tyler DE, Lorenz MD, Blue JL, Munnell JF, Chandler FW. Disseminated protothecosis with central nervous system involvement in a dog. J Am Vet Med Assoc. 1980;176:987–993. [PubMed] [Google Scholar]
- 15.Perez J, Ginel PJ, Lucena R, Hervas J, Mozos E. Canine cutaneous protothecosis: An immunohistochemical analysis of the inflammatory cellular infiltrate. J Comp Pathol. 1997;117:83–89. doi: 10.1016/s0021-9975(97)80068-0. [DOI] [PubMed] [Google Scholar]
- 16.Wilcock B. Eye and ear. In: Maxie MG, editor. Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, Pennsylvania: Elsevier Saunders; 2007. pp. 501–503. [Google Scholar]
- 17.Pressler BM, Gookin JL, Sykes JE, Wolf AM, Vaden SL. Urinary tract manifestations of protothecosis in dogs. J Vet Intern Med. 2005;19:115–119. doi: 10.1892/0891-6640(2005)19<115:utmopi>2.0.co;2. [DOI] [PubMed] [Google Scholar]