Abstract
The lysine acetylation state of nonhistone proteins may be regulated through histone deacetylases (HDACs). Evidence suggests that nitric oxide (NO) synthase 3 (NOS3; endothelial NOS) is posttranslationally lysine acetylated, leading to increased NO production in the endothelium. We tested the hypothesis that NOS3 is lysine acetylated and that upregulated HDAC1-mediated deacetylation leads to reduced NO production in endothelial cells. We determined that NOS3 is basally lysine acetylated in cultured bovine aortic endothelial cells (BAECs). In BAECs, HDAC1 is expressed in the nucleus and cytosol and forms a novel protein-protein interaction with NOS3. Overexpression of HDAC1 in BAECs resulted in a significant reduction in NOS3 lysine acetylation (control = 1.0 ± 0.1 and HDAC1 = 0.59 ± 0.08 arbitrary units, P < 0.01) and significantly blunted basal nitrite production (control 287.7 ± 29.1 and HDAC1 172.4 ± 31.7 pmol·mg−1·h−1, P < 0.05) as well as attenuating endothelin-1-stimulated nitrite production (control = 481.8 ± 50.3 and HDAC1 243.1 ± 48.2 pmol·mg−1·h−1, P < 0.05). While HDAC1 knockdown with small-interfering RNA resulted in no change in NOS3 acetylation level, yet increased basal nitrite production (730.6 ± 99.1 pmol·mg−1·h−1) and further exaggerated increases in endothelin-1 stimulated nitrite production (1276.9 ± 288.2 pmol·mg−1·h−1) was observed. Moreover, overexpression or knockdown of HDAC1 resulted in no significant effect on NOS3 protein expression or NOS3 phosphorylation sites T497, S635, or S1179. Thus these data indicate that upregulated HDAC1 decreases NOS3 activity, most likely through direct lysine deacetylation of NOS3. We propose that HDAC1-mediated deacetylation of NOS3 may represent a novel target for endothelial dysfunction.
Keywords: histone deacetylase, nitric oxide synthase, endothelial cells, lysine acetylation
nitric oxide (NO) deficiency is causally linked to many diseases and is a hallmark of endothelial dysfunction. In the endothelium, NO is produced by NO synthase 3 (NOS3, endothelial NOS). NO bioavailability is regulated on many levels including transcriptional, translational, posttranslational modifications, as well as substrate, and cofactor availability (20). Derangements in these regulatory pathways lead to endothelial dysfunction in many cardiovascular diseases including atherosclerosis, diabetes, and hypertension (26).
Posttranslational modification via serine, threonine, and tyrosine phosphorylation is critically important in the regulation of NOS3-derived NO production. Phosphorylations of S635 and S1179 (positions based on bovine NOS3) have been widely studied and shown to stimulate NOS activity and NO production (36, 39), whereas phosphorylations at Y81 and S615 have also been reported to increase NO production (14, 39). Phosphorylation at S116, T495, and Y657 are negative regulatory sites with T495 being the most studied site (6, 28, 30, 39). Posttranslational modifications of NOS3 are also critical for subcellular localization and protein-protein interactions. NOS3 can undergo myristoylation (38) and palmitoylation (42), resulting in anchoring of NOS3 to caveolae in the plasma membrane. In the caveolae, NOS3 interacts with caveolin-1 (12), leading to inhibition of NOS3 (35). As well, membrane-bound NOS3 is basally S-nitrosylated in blood vessels, leading to inhibition of NOS3 activity (10, 11). However, NOS3 can be rapidly denitrosylated and activated by agonists such as vascular endothelial growth factor (10). Moreover in pathological settings, changes in the posttranslational status of NOS3 play a key role. For example, in atherosclerosis there is an increase in phosphorylation of S116 and a decrease in S1179 in the aortic vasculature, resulting in a significant decrease in NO bioavailability (28). In addition, our laboratory reported that S1179 is decreased in small mesenteric arteries in hypertensive rodents (24, 40). Thus the posttranslational modification status of NOS3 is critical for NOS3 activity.
Recently, a novel posttranslational modification of NOS3 was identified. Aspirin (acetysalicylic acid) leads to an increase in NOS3 lysine acetylation and NO production in human umbilical vein endothelial cells (HUVECs) (22). Protein acetylation is a dynamic posttranslational modification, whereby acetyl groups are added or removed from lysine residues by acetyltransferases or deacetylases, respectively (45). Deacetylation occurs via the histone deacetylases (HDACs), which were originally described to deacetylate histones (18). Recently, HDACs have been described to regulate nonhistone protein lysine deacetylation. There are four classes of HDACs based on their structure and homology to the yeast transcriptional repressors: class I HDAC 1, 2, 3, and 8; class IIa HDAC 4, 5, 7, and 9; class IIb HDAC 6 and 10; class III sirtuins; and class IV HDAC11 (see 3). Although the HDACs are classically described as being expressed in the nucleus, class I and IIb are also found in the cytosol (31, 43), suggesting that these two classes of HDACs may be more apt to deacetylate nonhistone proteins. Jung et al. (22) showed that HDAC3 antagonizes aspirin-induced NOS3 acetylation and reduces NO production in HUVECs. HDAC1 was determined to interact in a DNA binding complex with the SP1 site that is proximal to the NOS3 promoter and inhibits NOS3 transcription (15, 16). In addition, we recently found that HDAC1 expression was upregulated in a model of early life stress-induced endothelial dysfunction (19). The putative role of HDAC1 in posttranslational deacetylation of NOS3 has not been determined; thus, we were interested to test the hypothesis that HDAC1-dependent NOS3 deacetylation reduces NO production in aortic endothelial cells.
METHODS
Cell culture.
All chemicals were purchased from Sigma (St. Louis, MO) unless noted. Primary bovine aortic endothelial cells (BAECs) were purchased from Cell Applications (San Diego, CA), and COS7 (with no endogenous NOS3) were purchased from American Type Culture Collection (Manassas, VA). Both cells were grown in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY) with 1% penicillin-streptomycin and 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA). Passages 3–6 were used in the experiments. For transfection experiments, cells were grown to 90–100% confluency in 100-mm plates (immunoprecipitation experiments) or 12-well plates (nitrite experiments) and transfected at a ratio of 1 μg plasmid DNA:8 μl linear polyethyleneimine (Polysciences, Warrington, PA). Empty pcDNA3.1 was used as the vector control, and human HDAC1-Flag (9) expression plasmid was purchased from Addgene (Cambridge, MA). The cells were incubated at 37°C in a 5% CO2-95% room air incubator for 48 h with the medium changed after 24 h of transfection.
Bovine HDAC1 knockdown experiments were performed with custom bovine Mission small-interfering RNA (siRNA) of three siRNAs targeting three regions against bovine HDAC1 (NM_001037444.2) or scramble negative control. BAECs were transfected using the reverse siRNA protocol of Invitrogen with lipofectamine RNAiMax. For 12-well plates, 60 pmol of siRNA (all 3 combined simultaneously) and 1 μl of RNAiMax was placed on the BAECS for 24 h and the medium changed after 24 h of transfection.
mRNA measurements of bovine NOS1, NOS2, and NOS3.
BAECs in six-well plates were transfected with 2 μg of empty vector or HDAC1-Flag as described above. Total RNA was extracted using Tri-Reagent following the manufacturer's instructions. Relative expression of NOS1, NOS2, and NOS3 were determined by real-time, quantitative, reverse-transcriptase PCR using the QuantiFast SYBR Green RT-PCR kit (Qiagen, Valencia, CA). Bovine-specific primers were designed with Primer-Blast (45) (5′ to 3′ orientation and synthesized by Integrated DNA Technologies, Coralville, IA): NOS1F1, CCTCAAGAGCACGTTGGGAA; NOS1R1, GGACGTCTTCAGGCTTTCGT; NOS2F1, AGAGACGGGGAGATCGGAAA; NOS2R1, CATGCAGAGAACCTTGGGGT; NOS3F1, AGGGGCCCAAGTTCCCTC, NOS3R1 CAGGGCCCGTCCTGTTG; GAPDHF1, TTATGACCACTGTCCACGCC; and GAPDHR1, GATATTCTGGGCAGCCCCTC. RNA (100 ng) and 25 pmol of primers per 25 μl reaction, with an annealing temperature of 60°C, was used to determine relative mRNA expression. The data were normalized to the housekeeping gene GAPDH and standardized to the average of the vector controls, using the 2−ddCT2 method.
Nuclear and cytosolic extracts.
BAECs were fractionated in nuclear and cytosolic compartments using Cayman Chemical's (Ann Arbor, MI) nuclear extraction kit. Ten micrograms of nuclear and cytosolic fractions were separated by SDS-PAGE on 8% polyacrylamide gels and Western blot analyses performed as previously described (21).
Immunoprecipitation.
Immunoprecipitations were completed with 500 μg of protein (21), loaded with anti-acetyl-lysine-conjugated, anti-NOS3-conjugated, or IgG control protein A/G beads (Santa Cruz Biotechnology) overnight as previously described (21). Western blot analyses of immunoprecipitated proteins and lysates were performed as previously described (21).
Antibodies.
The following antibodies were used: anti-acetyl-lysine (No. 9441, Cell Signaling, Danvers, MA), anti-acetyl-lysine (No. 9681, Cell Signaling), anti-NOS3 (BD Biosciences, San Jose, CA), anti-actin (Sigma), anti-DYKDDDDK (Flag, No. 8146, Cell Signaling), anti-phospho-T495 NOS3 (No. 9574, Cell Signaling), anti-phospho-S1179 NOS3 (No. 9571, Cell Signaling), and anti-phospho-S635 NOS3 (EMD Millipore, Billerica, MA).
Nitrite analyses.
Nitrite experiments were preformed as previously described (21). BAECs were treated with vehicle (water) or 100 nM endothelin-1 (American Peptide, Sunnyvale, CA, dissolved in water) or vehicle (0.1% DMSO) or 3 μM ionomycin (Cayman Chemical) for 1 h. Nitrite was measured by high-performance liquid chromatography on the ENO-20 (Eicom, San Diego, CA).
Statistical analysis.
All experiments were replicated in triplicate, at least 3 independent times on different days, N = 3 or otherwise noted. Unpaired, two-tailed Student's T-test and two-factor ANOVA (for treatment and time) with Bonferroni post hoc test were used. P < 0.05 was considered statistically significant.
RESULTS
HDAC1 is expressed in BAECs and interacts with NOS3.
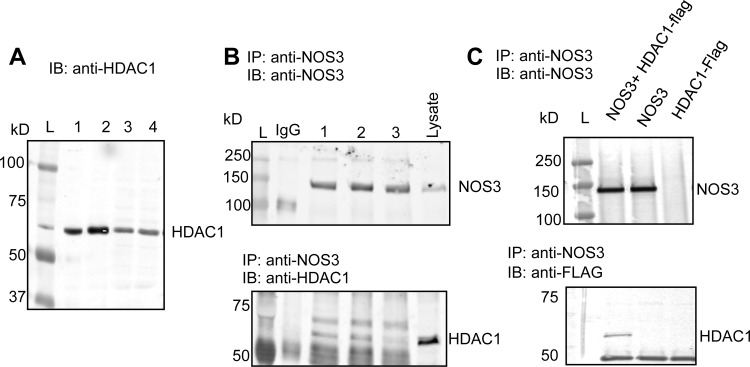
A single band with molecular mass of 62 kDa, which corresponds to the predicted molecular mass for HDAC1, was specifically detected in the BAEC nuclear and cytosolic fractions (Fig. 1A), indicating that HDAC1 is endogenously expressed in BAECs. To determine if HDAC1 forms a protein-protein interaction with NOS3, we performed immunoprecipitation pull-down assays in BAECs and a reconstituted system in COS7 cells. HDAC1 and NOS3 endogenously interact in BAECs (Fig. 1B), as well as in a reconstituted system with overexpression of HDAC1-Flag and NOS3 in COS7 cells, verifying these two proteins form a protein-protein interaction (Fig. 1C).
Fig. 1.
A: expression of histone deacetylases (HDAC1) in both the nuclear (lanes 1 and 2) and cytosolic fractions (lanes 3 and 4) of bovine aortic endothelial cells (BAECs). B: HDAC1 and nitric oxide synthase 3 (NOS3) interact basally in BAECs shown by anti-NOS3 immunoprecipitation (IP) with immunoblot (IB) for HDAC1 from total BAEC lysate, N = 3. C: NOS3 and HDAC1 interact in COS7 cell-reconstituted system with overexpressed NOS3 and/or HDAC1-Flag tag. L, molecular mass ladder.
HDAC1 overexpression reduces NOS3 acetylation and decreases nitrite production in BAECs.
BAECs express high levels of NOS3 mRNA [average cycle threshold (CT) = 22 ± 0.1]. HDAC1 overexpression resulted in a significant 60% reduction in NOS3 mRNA (relative expression to the vector group, 1.0 ± 0.1 vs. 0.4 ± 0.04 arbitrary units; P < 0.01). Comparatively, we found very little NOS1 or NOS2 mRNA in either the vector or HDAC1 overexpressing cells (average CT = 30 ± 0.2).
Overexpression of HDAC1 was confirmed by Western blot analysis with anti-HDAC1 (Fig. 2A). Basally, NOS3 was found to be lysine acetylated in BAECs (Fig. 2B) as determined by immunoprecipitation analysis. Overexpression of HDAC1 resulted in a significant reduction of NOS3 acetylation (P < 0.001, Fig. 2, B and C); however, increased HDAC1 did not significantly affect NOS3 phosphorylation sites T497, S635, S1179 (based on bovine sequence), or total NOS3 protein expression (Fig. 2, D and E).
Fig. 2.
A: HDAC1-transfected cells expressed more HDAC1 protein than untransfected or vector-transfected BAECs. B: IP of anti-acetylated lysine with IB of anti-NOS3 revealed that overexpression of HDAC1 (lanes 4–6) decreased NOS3 acetylation (Ace-NOS3) with quantification by densitometry displayed in C. N = 3; *P < 0.05. D: overexpression of HDAC1 in BAECs did not significantly affect total NOS3 expression or NOS3 phosphorylation status at sites T497, S635, S1179 with quantification by densitometry displayed in E. N = 3; P > 0.05.
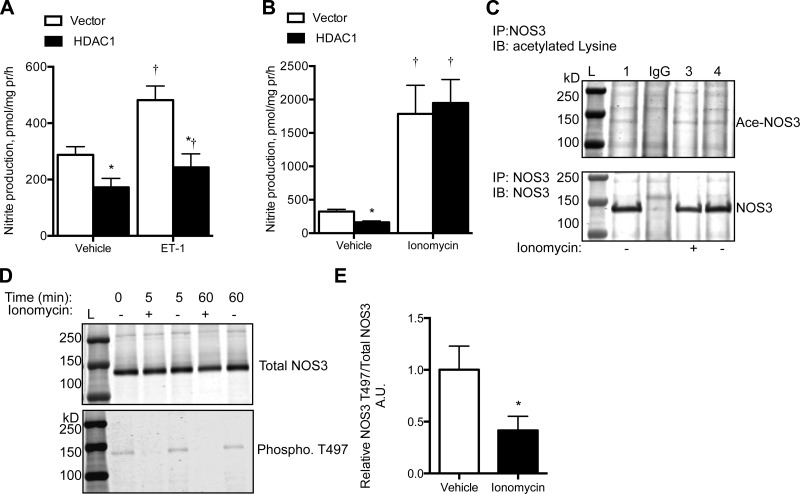
Under basal conditions, BAECs overexpressing HDAC1 had significantly reduced nitrite production compared with that of control cells (P < 0.05, Fig. 3A). To stimulate NOS3 activity, acute endothelin-1 (ET-1) and ionomycin treatment were analyzed. In control cells, acute ET-1 treatment led to a significant increase in nitrite production (P < 0.05, Fig. 3A). However, BAEC-overexpressing HDAC1 had an attenuated response to ET-1, producing significantly less nitrite than control BAECs (Fig. 3A). Ionomycin resulted in a significant increase in BAEC nitrite production, about five times higher than vehicle-treated cells; however, ionomycin-dependent nitrite production was similar between the control and HDAC1 overexpressing BAECs (P = 0.78, Fig. 3B). Ionomycin treatment did not significantly affect NOS3 acetylation level (Fig. 3C); however, it significantly reduced T497 phosphorylation (Fig. 3, D and E).
Fig. 3.
Overexpression of HDAC1 in BAECs reduced nitrite production under basal and endothelin-1 (ET-1; 100 nM, 1 h)-stimulated states (A), whereas ionomycin (3 μM, 1 h)-stimulated nitrite production was not significantly affected by overexpression of HDAC1 (B). Ionomycin did not significantly affect NOS3 acetylation (C), but ionomycin significantly reduced NOS3 T497 phosphorylation (D) after 5 or 60 min of treatment (E). N = 3 to 4. *P < 0.05 compared with vector + vehicle; †P < 0.05 compared with respective vehicle treatment. AU, arbitrary units.
HDAC1 knockdown increases NO production in BAECs.
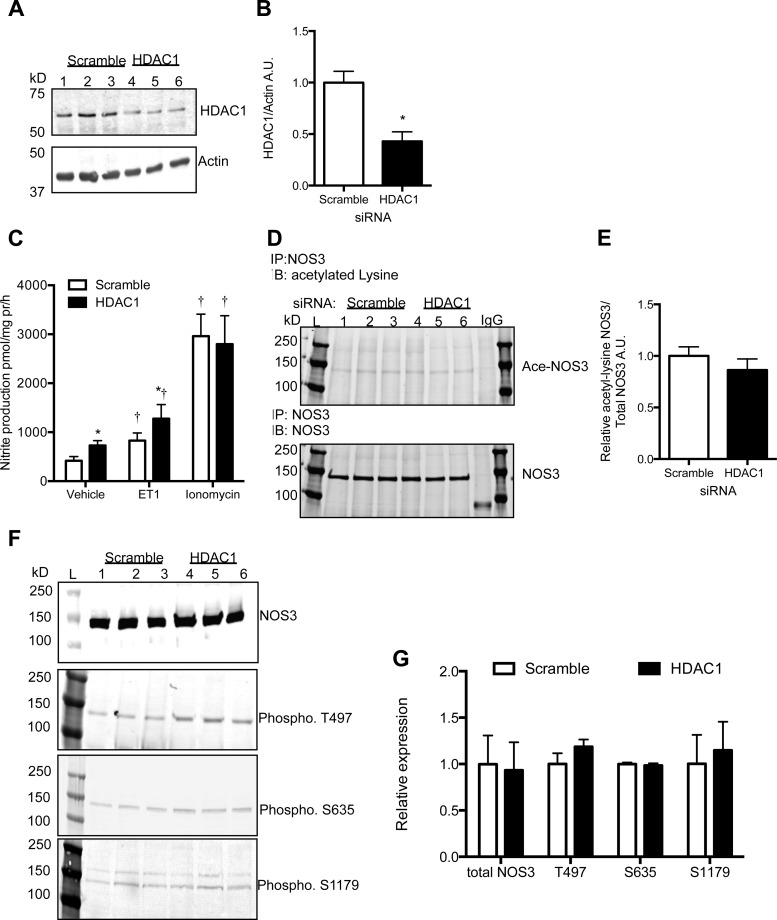
Bovine-specific HDAC1 siRNA resulted in a 50% reduction in HDAC1 protein expression (Fig. 4, A and B). BAECs with reduced HDAC1 produced significantly more nitrite than scramble siRNA-transfected BAECs (Fig. 4C). Acute endothelin-1 treatment also resulted in significantly more nitrite produced by HDAC1 knockdown BAECs compared with scramble (Fig. 4C); however, acute ionomycin treatment significantly increased nitrite production five times higher in both scramble control and HDAC1 knockdown BAECs (Fig. 4C). HDAC1 knockdown did not significantly affect NOS3 acetylation level (Fig. 4, D and E, P = 0.19), NOS3 phosphorylation, or total NOS3 protein expression (Fig. 4, F and G).
Fig. 4.
HDAC1 small-interfering RNA (siRNA) knockdown in BAECs resulted in a 50% reduction in HDAC1 protein expression (A) compared with scramble siRNA control BAECs (B). C: HDAC1 knockdown resulted in an increase in nitrite production under basal and ET-1 (100 nM, 1 h)-stimulated states, whereas ionomycin (3 μM, 1 h)-stimulated nitrite production was not significantly affected by HDAC1 knockdown. D: NOS3 acetylation was similar between scramble and HDAC1 siRNA cells as quantified in E. F: NOS3 phosphorylation levels were not significantly different from scramble controls. G: densitometry of the phosphorylation sites of NOS3. N = 3–5. *P < 0.05 compared with scramble + vehicle; †P < 0.05 compared with respective vehicle treatment.
DISCUSSION
The novel finding of this study demonstrates that upregulated HDAC1 deacetylates NOS3, directly resulting in decreased endothelial NO production. Thus endogenous lysine acetylation of NOS3 is a novel posttranslational modification, and deacetylation by HDAC1 reduces NO production in endothelial cells. Furthermore, HDAC1 knockdown increased endothelial NO production. We propose that HDAC1-specific inhibitors may be relevant targets for cardiovascular disease, specifically endothelial dysfunction.
Lysine acetylation is a reversible posttranslational modification (41). Recently, ∼1,700 proteins were determined to be lysine acetylated and proposed to regulate many biological processes (7). For example, in endothelial cells, hypoxia leads to an upregulation of HDAC6, resulting in deacetylation of cortactin, a critical mediator in angiogenesis through endothelial cell sprouting and migration (23). In our study, we identified NOS3 as a substrate for lysine acetylation and further determined that HDAC1, a class I HDAC, deacetylates NOS3, resulting in a significant decrease in NO production without changes to total NOS3 expression or NOS3 phosphorylation status. These data indicate that endothelial cell production of NO is regulated by direct lysine acetylation of NOS3, which is inhibited by HDAC1. Moreover, we determined that HDAC1 inhibits endothelial NO production under stimulated states as well. ET-1 activates endothelial NO production (4, 17), and HDAC1 overexpression blunted ET-1-dependent NO production in our study. Increasing intracellular calcium is a prominent NOS3 stimulus (29, 32), and in our study we found a significant fivefold increase in endothelial NO production with ionomycin treatment; however, HDAC1 overexpression did not significantly affect NO production. These findings indicate that HDAC1 regulates NOS3 lysine acetylation and endothelial NO production under basal and agonist-stimulated states, however, interestingly, not when intracellular calcium is maximally increased with ionomycin (13), suggesting that the acetylation/deacetylation of NOS3 may regulate the sensitivity of NOS3 activity to cofactors/protein activators or subcellular localization or desensitize NOS3 activity to inactivators without modulating the posttranslational phosphorylation of NOS3. Ionomycin leads to a significant reduction in phosphorylation of NOS3 T497, as previously shown (30) and confirmed in this study to activate NO production. We speculate that under these conditions when intracellular calcium is greatly increased, phosphorylation regulation is dominant in regulating NOS3 activity. However, when calcium levels are lower, acetylation/deacetylation of NOS3 may be more dominant. This allows for more “fine tuned” regulatory steps for NOS3 activation under different stimuli (such as acute increases in ET-1 or shear stress) to mediate differing degrees of NO production. This type of regulation also provides redundancy to maintain critical NOS3 activation. Accordingly, we would predict that HDAC1 inhibition may not be beneficial in all cases. Further experimentation is necessary to test these hypotheses.
Increased HDAC activity is associated with numerous pathologies including cancer and cardiovascular diseases. Two HDAC inhibitors are approved by the Food and Drug Administration for the treatment of cutaneous T-cell lymphoma (27). More evidence is emerging that deranged HDAC activity is also linked to cardio-renal pathologies, including heart failure (34), hypertension (5), and diabetes (1, 8), and in sickle cell disease, specifically, HDAC1 and HDAC2 are implicated (2). HDAC inhibitors show broad anti-inflammatory effects through inhibition of proinflammatory cytokines (33), cardiac hypertrophy (5, 25), and renal fibrosis (1, 37). Advandi et al. (1) showed that in vivo HDAC inhibition with Vorinostat (a pan HDAC inhibitor) in a model of diabetes resulted in reduced glomerular NOS3 expression and diminished renal injury through inhibition of nitrosative and oxidative stress (1). Moreover, we recently reported that mice exposed to maternal separation with early weaning (a model for early life stress, anxiety, and depression) exhibit endothelial dysfunction, which is reversed with pan-HDAC inhibition (19). Thus the underlying etiology of the vascular pathology may have great impact on the specificity of HDAC inhibition (selective isoform vs. broad class specific).
Previously, HDAC3 (class I HDAC) overexpression was reported to not affect NOS3 expression in HEK293 or HUVECs (22). Here, we show that HDAC1 overexpression decreased NOS3 mRNA expression, but after 48 h there was no detectable difference in NOS3 protein expression. This suggests that chronic increases in endothelial HDAC1 may also reduce NOS3 protein expression, leading to further endothelial dysfunction. Recently, in an ischemia-reperfusion injury model in HUVECs, there was global increase in HDAC activity and a significant decrease in NOS3 expression proposed to be due to decreased NOS3 transcription via HDAC1 inhibition of the NOS3 promoter (44). HDAC1 interacts with the SP1 site, just proximal to the NOS3 promoter, and inhibits NOS3 transcription in endothelial cells (15). Furthermore, treatment of HUVECs with the pan-HDAC inhibitor trichostatin A (75 and 150 nmol/l for 6 h) prevented the injury-mediated NOS3 downregulation (44). Thus, in the cell injury model, deranged HDAC activity results in epigenetic modifications and changes in NOS3 expression. Taken together, these in vitro and in vivo studies suggest that pan-HDAC inhibition may have opposing effects on transcriptional regulation of NOS3 expression, leading to changes in NO production. We speculate that targeting specific HDACs, such as the case of HDAC1 in our study, may have different outcomes than using pan- or class-HDAC inhibitors with respect to NOS3 expression and NO production.
On the contrary to HDAC1 overexpression, HDAC1 knockdown with siRNA led to a significant increase in basal and ET-1-stimulated nitrite production. However, this did not significantly affect NOS3 acetylation as determined by immunoprecipitation. Further experiments are required to confirm these results with mass spectrometry analysis of NOS3 lysine acetylation sites that are HDAC1 sensitive. We speculate that HDAC1 knockdown may influence acetylation/deacetylation of NOS3 specific-interacting proteins and/or availability of cofactors such that HDAC1 is involved in a complex regulatory scheme of NOS3.
Perspectives.
HDAC inhibitors are proposed to have enormous therapeutic potential for many diseases. HDAC inhibition may be a novel treatment for endothelial dysfunction, a pathology associated with numerous cardiovascular diseases. For instance, patients with pulmonary arterial hypertension have significantly higher lung HDAC1 levels, increased HDAC1 expression in remodeled pulmonary vessels, and HDAC inhibition reduced established hypoxia-induced pulmonary hypertension in a rat model of pulmonary arterial hypertension (47). We hypothesize that hyperactive HDAC1 activity in the endothelium leads to a significant decrease in acetylated NOS3, resulting in decreased NO production and endothelial dysfunction. Studies in our laboratory are underway to test this hypothesis in chronic models of endothelial dysfunction. Finally, more studies determining the outcomes of broad versus selective or specific HDAC isoform inhibition are needed to determine the best therapeutic approach to treating HDAC-mediated cardiovascular diseases.
GRANTS
This study was supported by American Heart Association Postdoctoral Fellowship 11POST7240008 (to K. A. Hyndman) and National Heart, Lung, and Blood Institute F32 Individual Postdoctoral Fellowship HL-116145 (to D. H. Ho) and Grants P01-HL69999 and HL-95499 (to J. S. Pollock).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.A.H., D.H.H., and J.S.P. conception and design of research; K.A.H., D.H.H., and M.F.S. performed experiments; K.A.H., D.H.H., M.F.S., and J.S.P. analyzed data; K.A.H., D.H.H., M.F.S., and J.S.P. interpreted results of experiments; K.A.H. prepared figures; K.A.H. drafted manuscript; K.A.H., D.H.H., M.F.S., and J.S.P. edited and revised manuscript; K.A.H., D.H.H., M.F.S., and J.S.P. approved final version of manuscript.
REFERENCES
- 1.Advani A, Huang Q, Thai K, Advani SL, White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA, Gilbert RE. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol 178: 2205–2214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradner JE, Mak R, Tanguturi SK, Mazitschek R, Haggarty SJ, Ross K, Chang CY, Bosco J, West N, Morse E, Lin K, Shen JP, Kwiatkowski NP, Gheldof N, Dekker J, DeAngelo DJ, Carr SA, Schreiber SL, Golub TR, Ebert BL. Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease. Proc Natl Acad Sci USA 107: 12617–12622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush EW, McKinsey TA. Targeting histone deacetylases for heart failure. Expert Opin Ther Targets 13: 767–784, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension 35: 1237–1241, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 56: 437–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Christensen DP, Dahllof M, Lundh M, Rasmussen DN, Nielsen MD, Billestrup N, Grunnet LG, Mandrup-Poulsen T. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med 17: 378–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emiliani S, Fischle W, Van Lint C, Al-Abed Y, Verdin E. Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci USA 95: 2795–2800, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280: 19888–19894, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281: 151–157, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 271: 22810–22814, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem 280: 35943–35952, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gan Y, Shen YH, Utama B, Wang J, Coselli J, Wang XL. Dual effects of histone deacetylase inhibition by trichostatin A on endothelial nitric oxide synthase expression in endothelial cells. Biochem Biophys Res Commun 340: 29–34, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gan Y, Shen YH, Wang J, Wang X, Utama B, Wang XL. Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. J Biol Chem 280: 16467–16475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension 37: 516–523, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene 363: 15–23, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ho DH, Pollock JS. Histone deacetylase inhibition attenuates early life stress-induced endothelial dysfunction (Abstract). Hypertension 60: A57, 2012 [Google Scholar]
- 20.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20: 295–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, Cole MP, Kumar A, Dericco JS, Jeon BH, Irani K. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res 107: 877–887, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaluza D, Kroll J, Gesierich S, Yao TP, Boon RA, Hergenreider E, Tjwa M, Rossig L, Seto E, Augustin HG, Zeiher AM, Dimmeler S, Urbich C. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J 30: 4142–4156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase-dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab 302: E481–E495, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 3: 166–179, 2011 [PMC free article] [PubMed] [Google Scholar]
- 28.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie 92: 1186–1198, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Koyama T, Kimura C, Park SJ, Oike M, Ito Y. Functional implications of Ca2+ mobilizing properties for nitric oxide production in aortic endothelium. Life Sci 72: 511–520, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of l-arginine metabolism to efficient nitric oxide production. J Biol Chem 278: 44719–44726, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Longworth MS, Laimins LA. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene 25: 4495–4500, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Malek AM, Jiang L, Lee I, Sessa WC, Izumo S, Alper SL. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun 254: 231–242, 1999 [DOI] [PubMed] [Google Scholar]
- 33.McKinsey TA. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med 17: 434–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 52: 303–319, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 277: 42344–42351, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H, Zhuang S. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem 112: 2138–2148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock JS, Klinghofer V, Forstermann U, Murad F. Endothelial nitric oxide synthase is myristylated. FEBS Lett 309: 402–404, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol 210: 271–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 43: 1080–1085, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Scott I. Regulation of cellular homoeostasis by reversible lysine acetylation. Essays Biochem 52: 13–22, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem 271: 6518–6522, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Takami Y, Nakayama T. N-terminal region, C-terminal region, nuclear export signal, and deacetylation activity of histone deacetylase-3 are essential for the viability of the DT40 chicken B cell line. J Biol Chem 275: 16191–16201, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Yang D, Xie P, Liu Z. Ischemia/reperfusion-induced MKP-3 impairs endothelial NO formation via inactivation of ERK1/2 pathway. PloS One 7: e42076, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell 31: 449–461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13: 134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 126: 455–467, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]