Abstract
Sexual dimorphism is a well-established phenomenon, but its degree varies tremendously among species. Since the early days of Einthoven's development of the three-lead galvanometer ECG, we have known there are marked differences in QT intervals of men and women. It required over a century to appreciate the profound implications of sex-based electrophysiological differences in QT interval on the panoply of sex differences with respect to arrhythmia risk, drug sensitivity, and treatment modalities. Little is known about the fundamental mechanism responsible for sex differences in electrical substrate of the human heart, in large part due to the lack of tissue availability. Animal models are an important research tool, but species differences in the sexual dimorphism of the QT interval, the ionic currents underlying the cardiac repolarization, and effects of sex steroids make it difficult to interpolate animal to human sex differences. In addition, in some species, different strains of the same animal model yield conflicting data. Each model has its strengths, such as ease of genetic manipulation in mice or size in dogs. However, many animals do not reproduce the sexual dimorphism of QT seen in humans. To match sex linked prolongation of QT interval and arrhythmogenic phenotype, the current data suggest that the rabbit may be best suited to provide insight into sex differences in humans. In the future, emerging technologies such as induced pluripotent stem cell derived cardiac myocyte systems may offer the opportunity to study sex differences in a controlled hormonal situation in the context of a sex specific human model system.
Keywords: action potential, arrhythmia, estradiol, hormones, ion channels
differences in the electrical activity in the heart between males and females were noted nearly 100 years ago, in early ECG recordings (5). In the intervening century numerous reports have confirmed these findings, but only recently have the fundamental importance of sex differences in cardiac electrical activity been appreciated in the context of diagnosis, treatment, drug sensitivity, and impact of outcomes on human heart disease. The first ECGs were detected long before the first measurements of cardiac action potentials (APs) and the discovery of the ionic channels and fluxes that underlie the AP. Outstanding progress has been made in understanding structure-function relationships of cardiac ion channels and how changes in ion channel expression and gating alter the electrical substrate. In addition, ion channel properties are significantly modified by subunit assembly and environmental conditions (pH, ionic concentrations, and hormones), which in turn have substantial effects on electrical activity. Profound changes in the ECG have helped in the discovery of mutations in ion channels and diseases caused by subunit mutations are known as “channelopathies.” Despite this revolution in our understanding of ion channel biophysics, there is a significant gap in translating the behavior of ion channels at the level of the single myocyte to alterations in the ECG because of heterogeneities in ion channel expression among ventricular cells and among the various cell types that comprise the heart. This is particularly true when considering sex differences in the human ECG because of incomplete and controversial information on sex differences in the expression and properties of ion channels and their regulation by sex hormones.
This review examines some of the recent advances in the study of sex differences in cardiac electrophysiology, specifically focusing on new hypotheses derived from novel data. A detailed appreciation of the molecular basis for the profoundly different electrical and arrhythmogenic substrate between men and women will require an integrated systems approach that incorporates several diverse factors, including developmental differences, hormonal effects on ion channels (steroid and nonsteroidal hormones), and an understanding of differences in coupling between membrane potential (Vm) and intracellular Ca2+ (Cai), Vm→Cai, and the reverse coupling Cai→Vm.
The ECG.
The major source for information on sex differences in electrical activity of the human heart is somewhat indirect, as it largely comes from the ECG. In the absence of cardiac disease, there are several significant differences in the ECG from adult men and women. The ECG is commonly used as a simple and convenient noninvasive diagnostic tool, and the exercise ECG is the recommended and most frequently performed diagnostic evaluation of coronary artery disease. However, this test has significantly lower diagnostic accuracy in predicting heart disease in women than in men (75). Knowledge of the underlying molecular basis of sex differences in the ECG and of differences in the physiology and pathophysiology of cardiac electrical activity will profoundly improve diagnosis and treatment, particularly for women.
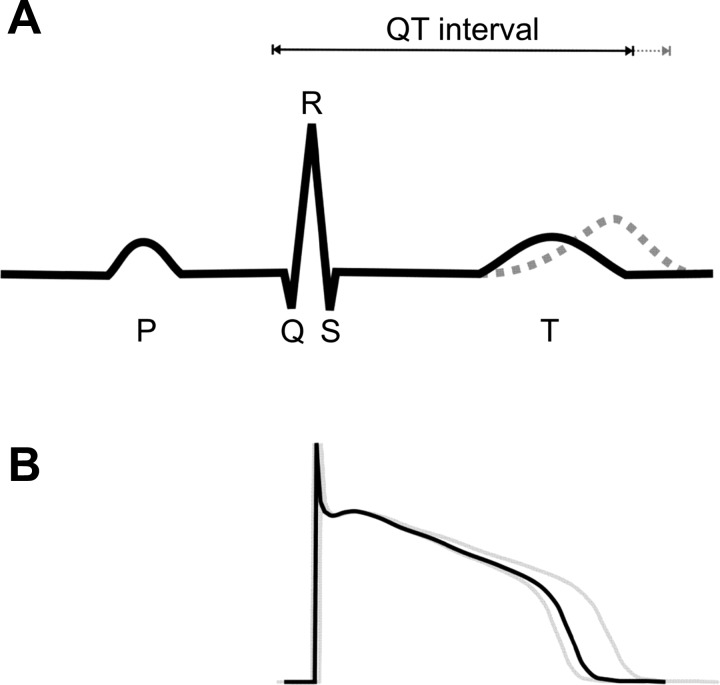
The voltage deflections of the ECG are the physical manifestation of the vectorial sum of complex interactions of hundreds of different electrogenic currents flowing in billions of cells that make up the heart (Fig. 1). The P wave reflects the electrical depolarization of atrial myocytes that is initiated in the sinoatrial node and propagates across the upper chambers. Signs of atrial repolarization are imperceptible as they are swamped by the initiation of the ventricular depolarization. The QRS complex indicates the timing of the rapid spread of depolarization throughout the ventricle. The short duration of the QRS interval reflects well-coordinated ventricular depolarization via the His-Purkinje fibers. The broader T wave is the result of the slower and more gradual timing of ventricular repolarization. The width and amplitude of the T wave is related to the degree of heterogeneity of ventricular repolarization (3), although the exact cellular basis for dispersion of the T wave remains controversial (63). It may reflect changes in the transmural dispersion of repolarization, i.e., the endocardial, midmyocardial, and epicardial regions (3) or may be due to the direction of repolarization from apex to base, independent of the depolarization sequence (14, 34), and appears to be modulated by sympathetic activity (52).
Fig. 1.
A: stylized ECG showing the major deflections. The T wave can change shape, duration, and timing, the latter resulting in long QT. B: human ventricular action potentials. The well-synchronized rapid upstroke leads to the short QRS duration. In contrast, the action potentials have a wide variety of durations, leading to the broad T wave.
The major quantitative components of the ECG are the RR interval (a measure of the time between successive ventricular depolarizations and thus heart rate), the QT interval (typically measured from the initial Q-wave deflection to the end of the T wave), and the width and shape of the T wave. The QT interval is in part a function of heart rate (or RR interval), and so several formulae have been used to calculate a corrected QT interval (QTc) that is a QT interval independent of heart rate.
Several sex differences are well recognized in the human ECG. Most notably, women have longer QTc than men (5, 26, 54), which increases the likelihood of polymorphic ventricular tachycardia, known as Torsade de Pointes (TdP). TdP is an arrhythmia that occurs in the absence of structural deformities and can lead to ventricular fibrillation (VF) and sudden cardiac death. In addition to QTc differences, the timing, dispersion, and morphology of the T wave is sex dependent (8, 54, 62, 77, 92). Women have shorter resting RR intervals, reflecting a higher resting heart rate. The higher heart rate likely reflects an intrinsic difference, as women have higher vagal tone and lower heart rate variability than men (6, 18). The QT-to-RR ratio is steeper in women than in men (37, 78), with the result that at slower heart rates, sex differences are more pronounced and the QRS complex shorter in women than men (80).
The underlying molecular mechanisms for these fundamental sex differences in the electrical profile of the healthy human are largely unknown. Sex differences in ECGs cannot be explained by sex differences in body weight, left ventricular mass, or height (61). Sex differences in QTc interval are not present at birth (78), but postpubertal women have longer baseline QTc than men (26, 54). This change may be due to shortening of the male QT interval (66, 79). The transition of electrical properties postpuberty (at ∼14 yr) may be due to the surge of sex steroids. Although women have the potentially proarrhythmic longer QTc than men, women are actually less likely than men to suffer sudden cardiac death in their reproductive years (35). Furthermore, the QTc interval in women is prolonged during pregnancy, presumably as a protective event (40). Thus, for sex differences in arrhythmia susceptibility, QT prolongation alone is not a satisfactory indicator.
There are few, yet conflicting, reports on the effect of the menstrual cycle on QTc. Some studies have reported changes in QTc with menstrual cycle (23, 58), and others have reported no changes (11, 31). A few studies linked the phase of the menstrual cycle to arrhythmia vulnerability (57, 70) and to the ability of drugs to prolong QTC (69). These findings are consistent with several studies that show no linear correlation between numerical change in QTc and the risk of total mortality or sudden cardiac death (26, 29, 68). In other words, the absolute QTc interval is not an accurate predictor of arrhythmia risk. This should not be surprising because the QTc interval is not particularly sensitive to changes in the profile of ionic currents that can cause profound changes in Cai handling and in the arrhythmogenic substrate.
Sex differences in electrical diseases: the long QT syndromes.
Although QTC prolongation in premenopausal women is not associated with a higher incidence of sudden cardiac death than in men, it does predispose women to greater problems with diseases associated with further QT prolongation. Sex-based differences in cardiac electrical activity, long noted but little understood, have gained new notoriety and interest with the identification of channelopathies that cause long QT syndromes (LQTS). LQTS is a family of disparate conditions that can be congenital or drug induced, which result in QT prolongation and increases the risk to TdP, VF, and sudden death (68, 74, 86, 87) and where postpubertal women are at higher risk for both congenital and acquired forms of LQT and TdP than men (16, 46, 51, 89, 95). The diagnosis of LQTS is sex specific and defined as QTc > 440 ms for adult males and > 460 ms for adult females (12).
LQTS is considered to be an electrical disease, as it occurs in structurally normal hearts and arises from a diverse range of mutations or drugs that alter ionic current during the AP with a common outcome of AP and QTc prolongation. Genetic LQTS is the result of mutations in the main or ancillary subunits of cardiac ion channels causing an increase or decrease of their associated currents, referred to as a “loss” or “gain” of function mutations. Although mutations in 13 genes have been associated with LQTS, mutations in three ion channels account for most of the LQTS (56). Women are at higher risk than men of TdP with LQT type 1 and type 2 (LQT1 and LQT2) (46), but both sexes are equally vulnerable to LQT3 (95). Sex differences in human electrophysiological data are summarized in Table 1.
Table 1.
Sex differences in electrophysiological characteristics of lonq QT syndromes
| Mutated Gene | Protein Product | Affected Current | LQT Penetrance, % | Overall Frequency, % | Sex Differences in Human EP Studies | |
|---|---|---|---|---|---|---|
| LQT1 | KCNQ1 | KvLQT1 | IKs | 25–100 (59) | 30–50 (59) | Males younger at first cardiac event (17, 46, 73) |
| Jervell and Lange-Nielsen Syndrome; Romano Ward Syndrome | Kv | Prepubertal males at higher risk of cardiac event (95) | ||||
| Post pubertal females have longer baseline QTc than males (17) | ||||||
| Location of mutation in channel is related to likelihood of cardiac event in females but not males (17) | ||||||
| LQT2 | HERG | Kv11.2, | IKr | 50–70 (59) | 25–40 (59) | Females more likely to have cardiac events than males (46, 55, 95) |
| Romano Ward Syndrome | KCNH2 | Females have longer baseline QTc than males (55) | ||||
| Females more likely than males to have events postpuberty (55, 95) | ||||||
| Location of mutation in channel is related to likelihood of cardiac event in males but not in females (55) | ||||||
| LQT3 | SCN5A | Nav1.5 | INa | 79 (59) | 5–10 (59) | Males have longer baseline QTc than females (46) |
| Romano Ward Syndrome | ||||||
| LQT4 | ANK2, ANKB | Ankyrin B | INa, ICa | 1–2 | Not available | |
| LQT5 | KCNE1 | MinK | IKs, IKr | 1–2 (59) | Not available | |
| Jervell and Lange-Nielsen syndrome; Romano Ward Syndrome | ||||||
| LQT6 | KCNE2 | MiRP1 | IKr | 1 (59) | Not available | |
| Romano Ward Syndrome | ||||||
| LQT7 | KCNJ2 | Kir2.1 | IK1 | 71 (83) | Rare | Not available |
| Anderson Tawil Syndrome | ||||||
| LQT8 | CACNA1C | Cav1.2 | ICa | Rare | Not available | |
| Timothy Syndrome | ||||||
| LQT9 | CAV3 | Caveolin-3 | INa | Rare | Not available | |
| LQT10 | SCN4B | B-subunit | INa | Rare | Not available | |
| LQT11 | AKAP9 | A kinase anchor protein | IKs | Rare | Not available | |
| LQT12 | SNTA1 | syntrophin | INa | Rare | Not available | |
| LQT13 | GIRK4 | Kir3.4 | IKAch | Rare | Not available |
See main text for definitioins of abbreviations.
LQT1 is the result of mutations in KCNQ1 ion channel. In the heart, KCNQ1 combines with KCNE1 to produce IKs, the slow delayed rectifier current. IKs activates relatively slowly and accelerates AP repolarization during adrenergic stimulation. Mutations in this channel reduce the net K+ efflux that prolongs the AP during exercise compared with normal individuals. The incidence of LQT1 events is highest in prepubertal males (17), despite the fact that there is no difference between the QT interval between boys and girls with LQT1 at ∼<14 yr old. Following puberty, the male QT interval is reduced and the female QTc is significantly longer than the adult male. Adult women are more at risk to TdP (46) or at the same risk (17) as adult males. The penetrance of LQT1 in genetically affected individuals is only 55% (59). Recently, exciting evidence points to the importance sex differences in the effects of basic biophysical structure-function properties of the LQT1 mutations. LQT1 mutations are distributed throughout the LQT1 gene, and the distribution of the mutations in the different locations was reported to be similar for both men and women (17). For men, the location of the mutation did not matter, but women with mutations in the cytoplasmic loop region were much more likely to have an aborted cardiac event or sudden cardiac death than for women with mutations outside this region (17). These cytoplasmic regions are the site of interaction of α-channel subunit with regulatory β-subunits. Ancillary subunits change the level of expression but can also profoundly affect kinetics and drug sensitivity (7). Thus these new data strongly suggest that sex-dependent ancillary subunit modification of ion channel function may play a significant role in the sex differences of arrhythmogenic substrate and pharmacological sensitivity.
LQT2 is caused by mutations in the human ether-à-go-go-related gene (hERG) KCNH2 channel, which encodes for the rapid component of the delayed rectifier current (IKr). Similar to IKs, this delayed rectifier current turns on with a 100–200 ms delay after the AP upstroke to drive the rapid AP downstroke. Reduction in IKr density through reduced surface expression or alteration in gating of hERG can delay repolarization. Penetrance of LQT in genetically affected individuals is 70% (59). Women with LQT2 are more likely than men to suffer a life-threatening cardiac event (55, 95). Men with LQT2 arising from a pore loop mutation were more likely to suffer an event than men with no pore loop mutations. In contrast, women had no dependence on the location of the mutation (55). This disparity in mutation location again points to sex differences being important at the level of ion channel structure-function.
LQT3 arises from mutations in the Na+ channel, which is responsible for the rapid initiation of excitation in the ventricle and underlies the QRS complex in the ECG. There are no reported sex differences in the cardiac events of LQT3, either pre- or postpubertally (95). Penetrance of LQT3 in genetically affected individuals is 79% (59).
There is substantial overlap of QTc among genetically affected individuals compared with family members without the mutation (59), see Fig. 2. The penetrance of LQT1–3 genes ranges from 55–70% (59), which points to the importance of studying the mutated channel in human myocytes and in considering other factors beyond the primary defect, such as sex differences, hormonal status, etc.
Fig. 2.
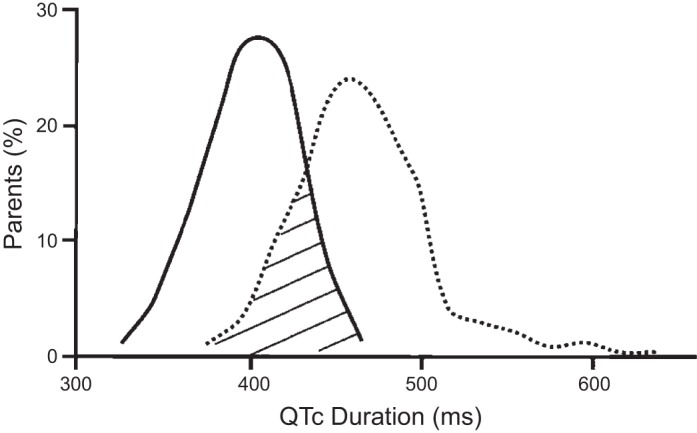
There is significant overlap in QT durations between affected (dotted line) and unaffected (solid line) individuals. There is an increase in the mean QT interval for individuals carrying long QT mutations. However, there is substantial overlap between groups, which suggests that QT interval is modulated by multiple factors. QTc, corrected QT. Graphical representation based on data from Napolitano et al. (59).
Although congenital LQTS remains a rare disease, drug-induced LQTS remains a major public health problem resulting from drugs that interact with cardiac ion channels. Healthy individuals can develop acquired or drug-induced LQTS in response to a wide variety of drugs where women are at much greater risk than men of suffering drug-induced LQTS and developing the potentially fatal TdP (22, 41, 45, 50, 88). Drug-induced TdP can arise from diverse drugs (67, 68) (e.g., antiarrhythmics, antidepressants, antihistamines, antipsychotics, antiemetics). Currently, this susceptibility is attributed to drug interactions with the hERG channel, in combination with the longer female AP, reduced repolarization reserve compared with that in males and the effects of sex steroids (primarily estrogen and testosterone). Although these broad concepts may be correct, Na+ channel mutation prolongs the AP, but LQT3-related TdP is relatively insensitive to sex differences, suggesting that factors other than repolarization reserve may contribute to overall sex differences in TdP risk in LQT1 and 2. Postpubertal women are at higher risk for both congenital and acquired forms of TdP than men (16, 46, 51, 89, 95).
Sex differences in ventricular AP.
The ECG is the vectorial sum of current flow across the membranes of single cells (∼109 cells) that comprise the human heart. The question is what combination of cellular ionic channel changes underlies sex differences in the ECG. Direct extrapolation from the ECG to the individual cellular currents is not possible. Clearly, the information that can be gathered from ECG signals is limited, but there is a tight correlation between the QT interval and ventricular AP durations (APDs). Hence, the longer QTc duration in women should be correlated with longer APDs in females compared with males. The channel proteins and the ionic currents of the human ventricular AP can be generally classified as depolarizing and repolarizing currents where an increase in depolarizing current and a decrease in repolarizing current result in APD prolongation. The main depolarizing currents are the rapidly activating and inactivating voltage-gated Na+ current (INa) that produces the AP upstroke and the voltage-gated L-type Ca2+ current (ICa,L), which provides the Ca2+ influx that triggers further Ca2+ release from the sarcoplasmic reticulum to initiate contractions and helps maintain the AP plateau phase (Fig. 1B). The entry of Ca2+ during each beat is balanced by Ca2+ efflux via the electrogenic Na+/Ca2+ exchanger (NCX), which is a depolarizing current, (INCX) in the forward mode where the influx of three Na+ ions drives the efflux of one Ca2+. The main repolarizing currents are the rapid and slow components of delayed rectifying K+ outward currents (IKr and IKs), which turn on the AP downstroke. IKr is a major determinant of APD and QT-interval. The voltage-dependent transient outward K+ current (Ito) is a rapidly activating and inactivating current that is primarily expressed in the epicardium. Paradoxically, Ito prolongs rather than shortens APD because it contributes to a very early repolarization, immediately after the AP upstroke that delays the AP time course. Human ventricular APs, like most nonrodent APs, have a high plateau phase, which is the result of battling depolarizing (ICa,L) and repolarizing (IK1 mediated a K+ channel responsible for the resting Vm) currents.
One study addressed sex differences in human APs through measurements on midmyocardial left ventricular myocytes from explanted hearts in end-stage heart failure (85). Despite concerns regarding the health of these hearts, APs from female myocytes were significantly longer than in males, consistent with longer QT intervals in women. There was also a trend toward larger ICa,L and smaller Ito in female compared with male myocytes, which did not reach statistical significance because of cell-to-cell variability.
The lack of voltage clamp data, the gold standard for determining functional ion channels, from freshly isolated human myocytes means that inferences have to be made from relative expression of mRNA and protein. These studies have so far proved inconclusive, with some demonstrating sex differences in channel expression, and others not, depending on the region of the heart studied (2, 25).
The paucity of human data and the fact that most studies on human tissue are not sufficiently powered to detect sex-dependent differences mean that alternative analysis is required. Computer modeling, based on the combination of disparate data sets, has helped to test potential hypotheses (15, 27, 93). The lack of primary data from healthy, age-matched human tissue has the result that most of what is commonly accepted about our understanding of sex-dependent mechanisms in the heart is inferred from animal studies. The degree of correlation between electrophysiological behavior in the animal and human heart is dependent on the ion channel under investigation, as well as species.
Animal models for sex differences in the heart.
The majority of our understanding of the molecular basis of sex differences in the cellular cardiac AP comes from animal studies. A wide range of animal models have been used to this furnish information, as freshly isolated human cardiac myocytes from healthy men and women are rarely available for electrophysiological analysis. This electrophysiological work on the cellular basis of sex differences has been performed on isolated ventricular myocytes from various animal models, and it is not always clear how good a correlation there is between these species and the human heart. At many levels, including the heart, there are marked species and strain differences in the nature and the degree of sexual dimorphism. Consequently, animal experiments have produced conflicting results, leading to a need to carefully consider the merits of animal models of human sex differences.
Mouse models of human diseases have received a lot of traction, because mice can be molecularly engineered to study the effects of specific human genetic abnormalities. However, marked differences between human and murine electrophysiology (72) make it difficult to gain new insights regarding the molecular basis of human sex differences. For instance, mouse ventricular AP are >10 times shorter than humans, lack a plateau phase, and express a different combination of ion channels (72). In addition, mice have considerably faster heart rates (>600 beats/min) and an ECG that is substantially different from the human ECG. Human data indicate that sex differences are particularly noticeable at lower heart rates, so perhaps unsurprisingly, data on sex differences in mice are difficult to interpret. Some report no sex differences in mouse ECGs (10, 49), others report changes (9), and another report detected sex differences in arrhythmia risk but only in the presence of drugs or anesthesia (20) or in distinct phases of the estrus cycle (71).
Reports on sex differences in ionic channels in mice are inconsistent and summarized in Table 2. The basis for these inconsistencies is unclear, but strain differences most likely account for these divergent findings. In other mammals, sex differences in cellular properties may be due to regional heterogeneities of ion channel expression in the heart (76).
Table 2.
A selection of conflicting data from sex differences in individual ionic currents in adult animal models
| Current | F < M | No Change | F > M |
|---|---|---|---|
| INa | Mouse (82) | Late INa (47) | |
| ICa | Guinea pig (32) | mouse (82) | Dog (91) |
| Rat (39) | Rabbit LV base (76) | ||
| Rat (30) | Human failing mid (trend to F > M) (85) | ||
| Rabbit LV apex (76) | |||
| Ito | Dog (RV epicardium) (19) | Mouse (82) | |
| Dog, endo (91) | Rabbit (44) | ||
| Mouse (septum) (10) | Rat (39) | ||
| Mouse (90) | Mouse RV(10) | ||
| Mouse RV (71) | LV apex (10) | ||
| Failing Human (trend to F < M) (85) | Dog epi (91) | ||
| Dog mid (91) | |||
| (85) | |||
| IKur | Mouse (82) | Mouse RV (10) | |
| Mouse septum (10) | Mouse LV apex (10) | ||
| Mouse estrus (71) | |||
| IKss | Mouse septum (10) | Mouse RV (10) | |
| Mouse, estrus (71) | Mouse LV apex (10) mouse (82) | ||
| IKr | Rabbit (44) | Dog (91) | |
| Guinea pig Day 4 estrus cycle (32) | Guinea pig day 0 estrous cycle (32) | ||
| IKs | Rabbit LV (96) | Dog, LV mid (91) | LV dog, epi (91) |
| LV dog, endo (91) | |||
| IK1 | Outward IK1 rabbit (44) | Mouse RV (10) | |
| Inward IK1 guinea pig (32) | Mouse LV apex (10) Mouse septum (10) | ||
| Mouse (82) | |||
| Rat (39) | |||
| Dog (91) | |||
| Failing human (85) |
See main text for definitions of abbreviations.
The dog, with a heart size and AP characteristics similar to humans, has been used extensively as a model for heart failure and sudden cardiac death. However, sex differences have not been observed in the QTc interval or other ECG parameters (24, 28, 53, 81). Despite a lack of sex differences in QTc, significant differences were reported in individual current densities, and APDs (20%) are longer in female dogs than in males in Purkinje fibers and midmyocardium (1, 91). In addition, the dispersion of INa (4) and the density of ICa,L (30–40%) are greater in females, whereas the density of Ito is lower (40%) in the endocardium and IKs is greater (60%) in the epicardium and endocardium but not the midmyocardium (91). The interpretation of these data may be limited because of strain differences, intrinsic regional differences in current densities between the base and apex of the heart, and insufficient controls regarding the estrus cycle of the females. Still, substantial differences in the electrical substrate can occur with no apparent changes in QTc (91).
The lagomorph or, more precisely, the New Zealand white rabbit is a frequently used strain of animals. It exhibits similar sex differences in LQT2-related arrhythmias as in humans, for both adult and prepubertal rabbits and approximately the same combination of ionic currents underlie rabbit and human APs. At rapid pacing rates, there are no sex differences in the rabbit ECG, but sex differences are revealed at longer cycle lengths (21, 33, 44, 96). The rabbit exhibits significant sex differences in response to drugs (48) and has longer ventricular APDs in females than in males (65, 84, 96). An important difference between humans and rabbits is that rabbits are inducible ovulators and they lack a menstrual cycle that is often considered to be a valuable advantage in studies of female hearts with constant levels of sex steroids. For these reasons, sex differences have been extensively studied in rabbits as the most appropriate animal model to study sex differences in cardiac electrical activity.
The marked difference in the propensity to TdP in women is readily observed in adult female compared with male rabbits (76). Moreover, the TdP risk is reversed in prepubertal rabbits (43), in agreement with subsequent analysis of human data (42). Optical mapping of APs and Ca2+ transients revealed that TdP was initiated by early afterdepolarizations (EADs) at the base but not the apex of the ventricles in female rabbits with drug-induced LQT2 (76). EADs that triggered TdP were elicited by large secondary release of Ca2+ from the sarcoplasmic reticulum (60, 76). Ventricular myocytes isolated from the base had significantly greater ICa,L and INCX densities than cells from the apex. In ventricular myocytes isolated from the base, both currents decreased within 2 days to equal the currents measured at the apex, but incubation with estrogen (0.3 nM) produced a genomic upregulation of mRNA, proteins (Cav1.2α and NCX1), and current density in 24–48 h. Female myocytes from the apex, the endocardium and the midmyocardium, and male myocytes did not respond to incubation with estrogen incubation (13, 94). Estrogen was found to upregulate ICa,L by a classical genomic mechanism mediated by the binding of estrogen receptor α-isoform to the promoter region of the gene that encodes for the L-type Ca2+ channel. Chip-on-chip assays identified eight biologically active estrogen receptor α-isoform response elements in the promoter region of the human gene (94). It should be noted that numerous studies reported acute effects of estrogen in cardiac ionic currents, but one should be cautious in interpreting data based on nonphysiological concentrations of sex steroids and how the steroid was delivered (38). Some sex differences appear to manifest themselves at a localized level with gradients of channel density being sex dependent. Channel density gradients can play a central role in establishing the arrhythmogenicity of the substrate. Experiments that detail the location of the myocytes note that there are significant differences in ion channels in some regions but not others. For example, ICa,L is larger in the base than the apex of adult female rabbit hearts, but there was no difference in male hearts (76). This is reversed in the prepubertal rabbit, where expression of ICa,L is higher in the male base than in the female base (76). There is also an epi-endo gradient in the female rabbit that is not observed in the male rabbit (64).
The concept of reduced repolarization reserve in females compared with males has been used to explain sex differences in arrhythmia risk in LQTS. The reduced repolarization reserve of female hearts was attributed to lower repolarizing K+ currents. This difference was thought to be primarily due to testosterone-mediated increases in IKr and IK1, resulting in shorter APDs and QTc intervals in male hearts. As females have longer baseline QTc in physiological conditions, one would expect the female heart to be more susceptible to even longer QTc intervals in the presence of LQTS mutations associated with loss of function of repolarizing K+ channels. The greater expression of ICa,L observed at the base of female rabbit hearts is consistent with a reduced repolarization reserve, resulting in prolonged APD and QTc intervals by two mechanisms: 1) larger ICa,L leads to a more positive plateau potential, which requires greater repolarizing K+ currents to bring the voltage back to baseline, and 2) larger ICa,L enhances intracellular free Ca2+ during the plateau phase and activates the forward mode of the NCX, resulting in depolarization of the AP plateau that causes a further AP prolongation, particularly in bradycardia (36). However, as discussed above, QT prolongation is not a satisfactory predictor of arrhythmia risk as attested by clinical and experimental animal studies. In a rabbit model of LQT2, with thorough blockade of IKr and IK1, adult male rabbit ventricles attained APDs of well over 2 s. However, except for occasional EADs, there was no arrhythmia (76). In contrast, heterogeneous ICa,L expression, higher at the base than apex, accounts for the greater arrhythmia risk by promoting EADs and ectopic activity at the base of the heart (13, 76, 94) and by enhancing dispersion of repolarization and the amplitude of T waves to help sustain arrhythmias (36). These findings challenge the significance of the concept of repolarization reserve, advancing Ca2+ homeostasis as an underlying mechanism that can predict arrhythmia risk.
Future directions.
Although sex differences in the human heart are clearly apparent at the level of the ECG, determining the molecular basis for these differences has proved difficult. Animal models have played a vital role in investigating the molecular bases for sex differences in ventricular repolarization and arrhythmogenesis. Despite some limitations, the rabbit appears to generally parallel the overall electrophysiological sex differences reported in the human. However, other animal models may be equally or more suitable for a specific condition or ion channel. What is clear is that sex differences in the electrical substrate are not the result of a simple change in expression of a single or even a few ionic currents. There are likely to be multiple changes in several ion channels, as well as their regulatory subunits. In addition, these changes are subtle and are not readily detectable by microarray techniques, and care must be taken to test for ion channel distribution across regions of the heart. The recent evidence pointing to sex differences at the level of fundamental structure-function relationships in ion channel biophysics adds another exciting new direction.
The paucity of data on sex differences in cellular electrophysiology from freshly isolated human preparations has severely hampered determination of the key components contributing to sex differences in human myocytes. The recent development of human cardiac myocytes derived from induced pluripotent stem cells offers the potential to have a significant impact on this field, as sex differences, in normal hearts as well as in the presence of known human channelopathies, can be studied in the context of male and female human myocytes and well-defined hormonal treatment, as they are grown in culture. Furthermore, cardiac myocytes derived from induced pluripotent stem cells are plentiful and are sufficiently robust to withstand the long protocols required to determine the effects of sex steroids on ion channel biophysics; a key component of sex differences in the electrical substrate.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grants HL-093074 (to G. Salama) and HL-093631 (to G. Bett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.S. and G.C.L.B. conception and design of research; G.S. and G.C.L.B. drafted manuscript; G.S. and G.C.L.B. edited and revised manuscript; G.S. and G.C.L.B. approved final version of manuscript; G.C.L.B. prepared figures.
REFERENCES
- 1.Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin JP, Pollard CE. Evidence for gender differences in electrophysiological properties of canine Purkinje fibres. Br J Pharmacol 142: 1255–1264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosi CM, Yamada KA, Nerbonne JM, Efimov IR. Gender differences in electrophysiological gene expression in failing and nonfailing human hearts. PloS One 8: e54635, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol 30 Suppl: 168–175, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Barajas-Martinez H, Haufe V, Chamberland C, Roy MJ, Fecteau MH, Cordeiro JM, Dumaine R. Larger dispersion of INa in female dog ventricle as a mechanism for gender-specific incidence of cardiac arrhythmias. Cardiovasc Res 81: 82–89, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 7: 353–370, 1920 [Google Scholar]
- 6.Beckers F, Verheyden B, Aubert AE. Aging and nonlinear heart rate control in a healthy population. Am J Physiol Heart Circ Physiol 290: H2560–H2570, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bett GC, Rasmusson RL. Modification of K+ channel-drug interactions by ancillary subunits. J Physiol 586: 929–950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, Bertran G, Arini P, Biagetti MO, Quinteiro RA. Sex-dependent electrocardiographic pattern of cardiac repolarization. Am Heart J 140: 430–436, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Brouillette J, Trepanier-Boulay V, Fiset C. Effect of androgen deficiency on mouse ventricular repolarization. J Physiol 546: 403–413, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke JH, Goldberger JJ, Ehlert FA, Kruse JT, Parker MA, Kadish AH. Gender differences in heart rate before and after autonomic blockade: evidence against an intrinsic gender effect. Am J Med 100: 537–543, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation 114: 2534–2570, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Yang X, Alber S, Shusterman V, Salama G. Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen. J Physiol 589: 1061–1080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol 543: 615–631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieniawa J, Baszak J, Olchowik G, Widomska J. Modeling gender effects on electrical activity of single ventricular myocytes. Comput Biol Med 43: 1063–1072, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Coker SJ. Drugs for men and women—how important is gender as a risk factor for TdP? Pharmacol Ther 119: 186–194, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Costa J, Lopes CM, Barsheshet A, Moss AJ, Migdalovich D, Ouellet G, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Combined assessment of sex- and mutation-specific information for risk stratification in type 1 long QT syndrome. Heart Rhythm 9: 892–898, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 53: 678–687, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Perez GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 106: 2004–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Drici MD, Baker L, Plan P, Barhanin J, Romey G, Salama G. Mice display sex differences in halothane-induced polymorphic ventricular tachycardia. Circulation 106: 497–503, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 94: 1471–1474, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Ebert SN, Liu XK, Woosley RL. Female gender as a risk factor for drug-induced cardiac arrhythmias: evaluation of clinical and experimental evidence. J Womens Health 7: 547–557, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Endres S, Mayuga KA, Cristofaro A, Taneja T, Goldberger JJ, Kadish AH. Menstrual cycle and ST height. Ann Noninvasive Electrocardiol 9: 121–126, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulop L, Banyasz T, Szabo G, Toth IB, Biro T, Lorincz I, Balogh A, Peto K, Miko I, Nanasi PP. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol (Oxf) 188: 163–171, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gaborit N, Varro A, Le Bouter S, Szuts V, Escande D, Nattel S, Demolombe S. Gender-related differences in ion-channel and transporter subunit expression in nondiseased human hearts. J Mol Cell Cardiol 49: 639–646, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RJ, Bengtson J, Chen ZY, Anderson KM, Locati E, Levy D. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience). Am J Cardiol 67: 55–58, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez R, Gomis-Tena J, Corrias A, Ferrero JM, Rodriguez B, Saiz J. Sex and age related differences in drug induced QT prolongation by dofetilide under reduced repolarization reserve in simulated ventricular cells. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2010: 3245–3248, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hanton G, Rabemampianina Y. The electrocardiogram of the Beagle dog: reference values and effect of sex, genetic strain, body position and heart rate. Laboratory animals 40: 123–136, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation 103: 2004–2013, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Howlett SE. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol Heart Circ Physiol 298: H659–H670, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Hulot JS, Demolis JL, Riviere R, Strabach S, Christin-Maitre S, Funck-Brentano C. Influence of endogenous oestrogens on QT interval duration. Eur Heart J 24: 1663–1667, 2003 [DOI] [PubMed] [Google Scholar]
- 32.James AF, Arberry LA, Hancox JC. Gender-related differences in ventricular myocyte repolarization in the guinea pig. Basic Res Cardiol 99: 183–192, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Johansson M, Carlsson L. Female gender does not influence the magnitude of ibutilide-induced repolarization delay and incidence of torsades de pointes in an in vivo rabbit model of the acquired long QT syndrome. J Cardiovasc Pharmacol Ther 6: 247–254, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Kanai A, Salama G. Optical mapping reveals that repolarization spreads anisotropically and is guided by fiber orientation in guinea pig hearts. Circ Res 77: 784–802, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J 136: 205–212, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Kim JJ, Nemec J, Papp R, Strongin R, Abramson JJ, Salama G. Bradycardia alters Ca2+ dynamics enhancing dispersion of repolarization and arrhythmia risk. Am J Physiol Heart Circ Physiol 304: H848–H860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kligfield P, Lax KG, Okin PM. QT interval-heart rate relation during exercise in normal men and women: definition by linear regression analysis. J Am Coll Cardiol 28: 1547–1555, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Kurokawa J, Tamagawa M, Harada N, Honda S, Bai CX, Nakaya H, Furukawa T. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol 586: 2961–2973, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leblanc N, Chartier D, Gosselin H, Rouleau JL. Age and gender differences in excitation-contraction coupling of the rat ventricle. J Physiol 511: 533–548, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lechmanova M, Kittnar O, Mlcek M, Slavicek J, Dohnalova A, Havranek S, Kolarik J, Parizek A. QT dispersion and T-loop morphology in late pregnancy and after delivery. Physiol Res 51: 121–129, 2002 [PubMed] [Google Scholar]
- 41.Lehmann MH, Hardy S, Archibald D, MacNeil DJ. JTc prolongation with d,l-sotalol in women versus men. Am J Cardiol 83: 354–359, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Liu JF, Jons C, Moss AJ, McNitt S, Peterson DR, Qi M, Zareba W, Robinson JL, Barsheshet A, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin J, Vincent M, Zhang L, Goldenberg I. Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long QT syndrome. J Am Coll Cardiol 57: 941–950, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T, Choi BR, Drici MD, Salama G. Sex modulates the arrhythmogenic substrate in prepubertal rabbit hearts with Long QT 2. J Cardiovasc Electrophysiol 16: 516–524, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M, Woosley RL. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther 285: 672–679, 1998 [PubMed] [Google Scholar]
- 45.Liu XK, Wang W, Ebert SN, Franz MR, Katchman A, Woosley RL. Female gender is a risk factor for torsades de pointes in an in vitro animal model. J Cardiovasc Pharmacol 34: 287–294, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, Andrews M, Timothy K, Hall WJ. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation 97: 2237–2244, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Lowe JS, Stroud DM, Yang T, Hall L, Atack TC, Roden DM. Increased late sodium current contributes to long QT-related arrhythmia susceptibility in female mice. Cardiovasc Res 95: 300–307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu HR, Remeysen P, Somers K, Saels A, De Clerck F. Female gender is a risk factor for drug-induced long QT and cardiac arrhythmias in an in vivo rabbit model. J Cardiovasc Electrophysiol 12: 538–545, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol Genomics 15: 84–91, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 270: 2590–2597, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res 100: e72–e80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsunaga T, Mitsui T, Harada T, Inokuma M, Murano H, Shibutani Y. QT corrected for heart rate and relation between QT and RR intervals in beagle dogs. J Pharmacol Toxicol Methods 38: 201–209, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation 80: 1301–1308, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm 8: 1537–1543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet 372: 750–763, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Myerburg RJ, Cox MM, Interian A, Jr, Mitrani R, Girgis I, Dylewski J, Castellanos A. Cycling of inducibility of paroxysmal supraventricular tachycardia in women and its implications for timing of electrophysiologic procedures. Am J Cardiol 83: 1049–1054, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, Saikawa T. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol 29: 607–613, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, Bottelli G, Cerrone M, Leonardi S. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA 294: 2975–2980, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Nemec J, Kim JJ, Gabris B, Salama G. Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2. Heart Rhythm 7: 1686–1694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okin PM, Kligfield P. Gender-specific criteria and performance of the exercise electrocardiogram. Circulation 92: 1209–1216, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Ono T, Saitoh H, Yi G, Hnatkova K, Kobayashi Y, Atarashi H, Katoh T, Takano T, Malik M. Clinical implication of T-wave morphology analysis as a new repolarization descriptor. Circ J 69: 666–670, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Patel C, Burke JF, Patel H, Gupta P, Kowey PR, Antzelevitch C, Yan GX. Is there a significant transmural gradient in repolarization time in the intact heart? Cellular basis of the T wave: a century of controversy. Circulation Arrhythmelectrophysiol 2: 80–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pham TV, Robinson RB, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovasc Res 53: 752–762, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Pham TV, Sosunov EA, Gainullin RZ, Danilo P, Jr, Rosen MR. Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by I(K)-blocking drugs. Circulation 103: 2207–2212, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 8: 690–695, 1992 [PubMed] [Google Scholar]
- 67.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 58: 32–45, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 285: 1322–1326, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Rosano GM, Leonardo F, Sarrel PM, Beale CM, De Luca F, Collins P. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet 347: 786–788, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res 105: 343–352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salama G, London B. Mouse models of long QT syndrome. J Physiol 578: 43–53, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation 112: 2517–2529, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 47: S4–S20, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Sims C, Reisenweber S, Viswanathan PC, Choi BR, Walker WH, Salama G. Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ Res 102: e86–e100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stramba-Badiale M, Locati EH, Martinelli A, Courville J, Schwartz PJ. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24-h Holter recordings. Eur Heart J 18: 1000–1006, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Stramba-Badiale M, Spagnolo D, Bosi G, Schwartz PJ. Are gender differences in QTc present at birth? MISNES Investigators Multicenter Italian Study on Neonatal Electrocardiography and Sudden Infant Death Syndrome. Am J Cardiol 75: 1277–1278, 1995 [PubMed] [Google Scholar]
- 79.Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol 40: 1870–1876, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Taneja T, Mahnert BW, Passman R, Goldberger J, Kadish A. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol 24: 16–21, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Tattersall ML, Dymond M, Hammond T, Valentin JP. Correction of QT values to allow for increases in heart rate in conscious Beagle dogs in toxicology assessment. J Pharmacol Toxicol Methods 53: 11–19, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Trepanier-Boulay V, St Michel C, Tremblay A, Fiset C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ Res 89: 437–444, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110: 381–388, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valverde ER, Biagetti MO, Bertran GR, Arini PD, Bidoggia H, Quinteiro RA. Developmental changes of cardiac repolarization in rabbits: implications for the role of sex hormones. Cardiovasc Res 57: 625–631, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Verkerk AO, Wilders R, Veldkamp MW, de Geringel W, Kirkels JH, Tan HL. Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes. Int Heart J 46: 1105–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Vincent GM. Long QT syndrome. Cardiol Clin 18: 309–325, 2000 [DOI] [PubMed] [Google Scholar]
- 87.Vincent GM. The molecular genetics of the long QT syndrome: genes causing fainting and sudden death. Annu Rev Med 49: 263–274, 1998 [DOI] [PubMed] [Google Scholar]
- 88.Wolbrette D. Gender differences in the proarrhythmic potential of QT-prolonging drugs. Curr Womens Health Rep 2: 105–109, 2002 [PubMed] [Google Scholar]
- 89.Wolbrette D, Naccarelli G, Curtis A, Lehmann M, Kadish A. Gender differences in arrhythmias. Clin Cardiol 25: 49–56, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovasc Res 53: 763–769, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Xiao L, Zhang L, Han W, Wang Z, Nattel S. Sex-based transmural differences in cardiac repolarization and ionic-current properties in canine left ventricles. Am J Physiol Heart Circ Physiol 291: H570–H580, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Yang H, Elko P, Fromm BS, Baga JJ, Pires LA, Schuger CD, Steinman RT, Lehmann MH. Maximal ascending and descending slopes of the T wave in men and women. J Electrocardiol 30: 267–276, 1997 [DOI] [PubMed] [Google Scholar]
- 93.Yang PC, Clancy CE. In silico prediction of sex-based differences in human susceptibility to cardiac ventricular tachyarrhythmias. Front Physiol 3: 360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang X, Chen G, Papp R, Defranco DB, Zeng F, Salama G. Oestrogen upregulates L-type Ca(2)(+) channels via oestrogen-receptor- by a regional genomic mechanism in female rabbit hearts. J Physiol 590: 493–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol 42: 103–109, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Ai X, Oster RA, Bers DM, Pogwizd SM. Sex differences in repolarization and slow delayed rectifier potassium current and their regulation by sympathetic stimulation in rabbits. Pflügers Arch 465: 805–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]