Abstract
Duchenne muscular dystrophy may affect cardiac muscle, producing a dystrophic cardiomyopathy in humans and the mdx mouse. We tested the hypothesis that oxidative stress participates in disrupting calcium handling and contractility in the mdx mouse with established cardiomyopathy. We found increased expression (fivefold) of the NADPH oxidase (NOX) 2 in the mdx hearts compared with wild type, along with increased superoxide production. Next, we tested the impact of NOX2 inhibition on contractility and calcium handling in isolated cardiomyocytes. Contractility was decreased in mdx myocytes compared with wild type, and this was restored toward normal by pretreating with apocynin. In addition, the amplitude of evoked intracellular Ca2+ concentration transients that was diminished in mdx myocytes was also restored with NOX2 inhibition. Total sarcoplasmic reticulum (SR) Ca2+ content was reduced in mdx hearts and normalized by apocynin treatment. Additionally, NOX2 inhibition decreased the production of spontaneous diastolic calcium release events and decreased the SR calcium leak in mdx myocytes. In addition, nitric oxide (NO) synthase 1 (NOS-1) expression was increased eightfold in mdx hearts compared with wild type. Nevertheless, cardiac NO production was reduced. To test whether this paradox implied NOS-1 uncoupling, we treated cardiac myocytes with exogenous tetrahydrobioterin, along with the NOX inhibitor VAS2870. These agents restored NO production and phospholamban phosphorylation in mdx toward normal. Together, these results demonstrate that, in mdx hearts, NOX2 inhibition improves the SR calcium handling and contractility, partially by recoupling NOS-1. These findings reveal a new layer of nitroso-redox imbalance in dystrophic cardiomyopathy.
Keywords: mdx, Duchenne, superoxide, phospholamban, ryanodine receptor, NADPH oxidase, NOS-1 uncoupling, BH4
dystrophic cardiomyopathy refers to the cardiac manifestation of three closely related diseases that include Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and X-linked dilated cardiomyopathy (13), each of which results from distinct genetic defects in dystrophin. One-third of DMD patients develop signs of cardiac dysfunction by the second decade of life, and virtually all DMD patients develop cardiac damage by the end of their life. DMD patients (10–40%) eventually die from heart failure. Cardiac involvement is more prevalent in BMD patients due to their lifespan: by age 40 yr, >90% of BMD patients display some signs of heart disease. In addition to male patients, female carriers are also at high risk (25). Nearly one-half of them have electrocardiographic abnormalities. Paradoxically, recent clinical and experimental data show that effective skeletal muscle restricted therapies for DMD actually increase cardiomyopathy and heart failure in these patients (49, 50).
Among several pathophysiological features, intracellular calcium handling is abnormal in the cardiomyocytes of the mdx mouse, a model of DMD (11, 24, 53), as well as contractility (36). In addition, the increased production of reactive oxygen species (ROS) in these animals plays a role in the dysfunction of the cardiac myocytes Ca2+ handling and mechanics (54).
Nicotinamide adenosine dinucleotide phosphate oxidase [NADPH oxidase (NOX)] is an important source of ROS in the cardiovascular system (14). The prototypic NOX is composed of a membrane-bound heterodimer consisting of a catalytic NOX2 (gp91phox) subunit and a p22phox subunit and several cytosolic regulatory subunits: p47phox, p67phox, p40, and Rac (5, 31). NOX2 has been suggested to be involved in the cardiac contractile dysfunction observed after a myocardial infarction (28) and pressure overload (17), two important pathophysiological insults that lead to heart failure. Therefore, a reduction in NOX2-derived superoxide may reverse some of the features of the adverse phenotype presented by the dystrophic cardiomyopathy. Here we tested the hypothesis that inhibition of NOX2 restores the contractile dysfunction observed in dystrophic cardiomyopathy.
MATERIALS AND METHODS
Animals.
MDX (C57BL/10ScSn-DMDmdx/J, stock no. 001801M, n = 45) and background controls mice (C57BL/10SnJ, n = 55) were purchased from Jackson Laboratories (Bar Harbor, MA). At 19 mo, mdx mice showed reduced ventricular function. Accordingly, we used animals of this age. Animals were housed in individual cages, with water and food ad libitum. All of the procedures conformed to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (1996) and were approved by the Institutional Animal Care and Use Committee of the Miller School of Medicine of the University of Miami and Universidad de Talca.
Echocardiographic analysis.
Mice were anesthetized with isoflurane (Webster Veterinary, Sterling, MA) vaporized to 4% in pure oxygen, positioned supine in a holder, and maintained at 2% isoflurane at 1.5 l/min oxygen flow. Echocardiography was carried out using Visualsonic Vevo 770 3.0.0 equipment (Toronto, Canada) with a linear transducer (frequency of 17.5 MHz and focal length of 17.5 mm). Anterior and posterior wall thickness and diastolic and systolic left ventricular (LV) dimensions were recorded from M-mode images using averaged measurements from three to five consecutive cardiac cycles. Long-axis imaging was obtained using B-mode echocardiography and putting the probe on the anterior chest wall following the angle of the normal heart axis. Short axis was obtained with the probe in the perpendicular position used for long axis. Short axis was changed from B mode to M mode when papillary muscles were clearly seen. Images were analyzed using Vevo 770 3.0.0 software. Measurements were performed at least three times in each mouse, and the average of measurements was used. Systolic function was evaluated using B mode in the long axis to estimate the ejection fraction and using M mode in the short axis to estimate the fractional shortening (FS). FS was calculated from the end-diastolic (EDD) and end-systolic (ESD) diameters using: FS = 100% × (EDD − ESD)/EDD.
Measurement of sarcomere length and [Ca2+]i.
Adult mouse myocytes were isolated as previously described (15). The myocytes were incubated 15 min with 1 μM fura 2-AM (Invitrogen). After loading, the myocytes were transferred to a Lucite chamber on the stage of an inverted microscope (NIKON TE 200) and superfused with Tyrode containing 1.8 mM Ca2+. Myocytes were field-stimulated, and sarcomere length was monitored in real time using an IonOptix iCCD camera and specialized data-acquisition software (IonWizard SarcLen Acquisition System, IonOptix). Twitch amplitude was computed as the difference between diastolic and peak systolic sarcomere lengths. Percentage of sarcomere shortening was expressed as the ratio of absolute twitch amplitude to diastolic sarcomere length. Changes in the average sarcomere length were determined by fast Fourier transform of the Z-line density trace to the frequency domain using the acquisition software noted above. Intracellular Ca2+ concentration [Ca2+]i was determined by alternately exciting with a xenon lamp at wavelengths of 360 and 380 nm (IonOptix). The emission fluorescence was reflected through a barrier filter (510 ± 15 nm) to a photomultiplier tube. The ratio of the photon live count at 360 nm (isosbestic point) compared with 380 nm represents the fura 2 fluorescence ratio.
Measurement of diastolic sarcoplasmic reticulum Ca2+ leak.
Sarcoplasmic reticulum (SR) calcium leak is measured using the method initially described by Shannon and colleagues (42). To gain maximal accuracy in the Ca2+ measurements, fura 2-loaded cardiac myocytes were excited continuously at 340/380 nm. The cells were field stimulated at 4 Hz, at 37°C. Stimulation was then switched off, and the external solution was quickly changed to a 0 Ca2+ and 0 Na+ (replaced by Li) Tyrode solution to eliminate trans-sarcolemmal Ca2+ fluxes, resulting in a new steady-state [Ca2+]i. Then ryanodine receptor (RyR) 2 channels are inhibited by 1 mM tetracaine for 60 s (in Tyrode solution, 0 Ca2+, 0 Na+), resulting in Ca2+ shifts from the cytosol into the SR. The tetracaine-induced drop in diastolic fura 2 fluorescence ratio is used as an estimate of SR Ca2+ leak, which is insensitive to changes in SR Ca2+ uptake. Then the myocytes are exposed to 0 Ca2+, 0 Na+ Tyrode solution containing 20 mM caffeine. The amplitude of caffeine-induced Ca2+ transient is being used as an estimate of total [Ca2+]i, which includes the Ca2+ leak. The load-leak relationship is constructed by grouping cells with similar total [Ca2+]i, as described (15, 16, 42).
Detection of ROS.
After perfusion with either vehicle or apocynin (100 μM, 45 min) in Krebs-Henseleit buffer, unfixed hearts were frozen in O.C.T. (Tissue-Tek). Transverse sections (20 μm thick) were cut in a cryostat and placed on glass slides. Samples were then incubated at room temperature for 30 min with dihydroethidium (3 μM, Molecular Probes). After washing with PBS, sections were fixed with 2% paraformaldehyde for 5 min at 4°C. Finally, the samples were mounted in Prolong Gold anti-fade (Invitrogen). Images were obtained using a Zeiss LSM-700 confocal microscope. The images were deconvolved, using Huygens Essential software (Scientific Volume Imaging, Hilversum, The Netherlands).
In isolated cells, ROS were detected using 2′,7′-dichlorofluorescein diacetate (DCF). Cells were incubated with DCF-AM (Molecular Probes) 5 μM for 15 min, then washed, and incubated with either vehicle (DMSO), apocynin (100 μM), or the specific NOX inhibitor VAS2870 {7-(1,3-benzoxazol-2-ylsulfanyl)-3-benzyl-3H-[1,2,3]triazolo[4,5-d]pyrimidine,1,3-Benzoxazol-2-yl-3-benzyl-3H[1,2,3] triazolo[4,5-d]pyrimidin-7-yl sulfide}, obtained from Merck (Darmstadt, Germany) at 20 μM for 15 min. After this period, the cells were washed with PBS, fixed with 2% paraformaldehyde for 5 min at 4°C, mounted in Prolong Gold anti-fade, and visualized using a Zeiss LS M-700 confocal microscope.
NOX-dependent superoxide production.
NOX-dependent superoxide production was measured in heart homogenates using lucigenin (5 μM)-enhanced chemiluminescence (β-NADPH, 300 μM; room temperature) on a microplate luminometer (Spectramax M5, Molecular Devices). Chemiluminescence readings were expressed as integrated light units, as previously described (10).
Real-time PCR.
For quantitative polymerase chain reaction (qPCR), total RNA was extracted from mice hearts using Pure-Link Micro-to-Midi Total RNA Purification System (Qiagen, Hilden, Germany) and reverse-transcribed using high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). qPCR was performed in triplicate using 20-μl reaction mixture containing 10 ng cDNA, TaqMan Universal PCR Master Mix (Roche, Basel, Switzerland), and primers/probes sets for specific genes (TaqMan Gene Expression Assays, Applied Biosystems). As an internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined in each reaction. The primers sequences used for NOX1, 2, 3, and 4 and GAPDH correspond to those described by Hill et al. (21). Reactions conditions were performed according to the manufacturer: one cycle of 50°C for 2 min, one cycle of 90°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Software from iQ5 multicolor real-time PCR detection system (Bio-Rad, Hercules, CA) was used for PCR analyses. Relative fold change was calculated by 2ΔCt method and compared with baseline values (set at 1).
S-nitrosylation.
After treating wild-type and mdx myocytes with Tyrode buffer alone or with tetrahydrobioterin (BH4) (300 μM) for 30 min, the cells were fixed in 2% paraformaldehyde, permeabilized, and incubated with a rabbit polyclonal anti-S-nitrosocysteine antibody (Sigma) overnight at 4°C. Fluorescence-conjugated secondary antibody incubation was performed at 37°C for 1 h with anti-rabbit FITC (Jackson ImmunoResearch). As control of the technique, the myocytes were treated with HgCl2 (0.02%), a compound that breaks-down S-nitrosylation bonds, using an irrelevant (IgG) as primary antibody and secondary antibody alone, as previously described (16).
Nitrate determination.
Nitrate (NO3−) plus nitrite (NO2−) concentrations in mouse cardiac homogenates were determined using the Griess reaction. Briefly, cardiac homogenates were filtered using Amicon Ultra 3K, PR03711 (Merck Millipore, Ireland) filters. The ultrafiltrate was analyzed using Griess reagents from Cayman Chemical (Ann Arbor, MI). After the reaction took place, absorbance was measured at 540 nm, as previously described (38).
[NO]i measurements.
Direct measurements of nitric oxide (NO) production in cardiac myocytes were performed on ventricular myocytes loaded with the fluorescent NO-sensitive dye 4,5-diaminofluorescein diacetate (5 μmol/l DAF-2 DA; Calbiochem/EMD Biosciences, San Diego, CA) for 20 min at 37°C. DAF-2 was excited with the 488-nm light, and emitted fluorescence was measured at 510–525 nm, using the Ionoptix system. For this, cardiac myocytes were stimulated at baseline at 1 Hz and then switched to 4 Hz, for a period of 5 min. DAF-2 fluorescence intensity (F) in each experiment was normalized to the level of fluorescence recorded before stimulation (F0). Changes in [NO] are expressed as ΔF/F0, thus representing the total percentage increase above basal level.
Western blotting.
Cardiac tissue homogenates were prepared as previously described and resolved in NuPAGE Novex gels (Invitrogen) and transferred to PVDF membranes. Membranes were incubated with antibodies against NOX2 (gp91phox) and p67phox (BD Biosciences), NOX3, p22phox (Santa Cruz Biotechnology, Santa Cruz, CA), NOX4 (Thermo Scientific, Rockford, IL), p47phox (Upstate, Lake Placid, NY), and GAPDH (Fitzgerald, Concord, MA), phospholamban (PLB), and phosphorylated PLB (Badrilla, UK).
Statistical analysis.
Data are expressed as means ± SE. For comparisons of two groups, Student's t-test was used. For comparison of three or more groups, one- or two-way-ANOVA was performed with Newman-Keuls as post hoc test. A P value of < 0.05 was considered significant.
RESULTS
LV size and function.
We assessed in vivo cardiac function using echocardiography (Table 1). At 19 mo of age, mdx mice showed decreased ejection fraction (56.7 ± 0.5%) and FS (40 ± 0.7%) compared with wild-type mice (70.8 ± 2.4 and 51.8 ± 2.1%, respectively, P = 0.0014 and 0.0017, respectively). These values are in agreement with previous descriptions for mdx mice of this age (33, 56). Consequently, we used the animals of this age for the study.
Table 1.
Echocardiography parameters of wild-type and mdx mice
| Wild Type | mdx | |
|---|---|---|
| n | 5 | 4 |
| Age, mo | 18.8 ± 0.8 | 18.8 ± 0.4 |
| Body weight, g | 35.4 ± 0.6 | 35.0 ± 0.6 |
| Heart rate, beats/min | 497 ± 30 | 482 ± 21 |
| LVEDV, μl | 39.5 ± 2.0 | 40.4 ± 1.4 |
| LVESV, μl | 10.6 ± 1.2 | 18.7 ± 0.4* |
| LV fractional shortening, % | 51.8 ± 0.01 | 40 ± 0.02* |
| LV ejection fraction, % | 73 ± 2 | 54 ± 1* |
Values are means ± SE; n, no. of animals. LV, left ventricular; LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume.
P < 0.005 vs. wild type.
Baseline characteristics of isolated myocytes.
Next, we studied the contractile properties of isolated myocytes to confirm the observations obtained using echocardiography. The baseline features of wild-type and mdx myocytes are shown in Table 2. The degree of sarcomere shortening of mdx myocytes stimulated at 0.5 Hz was significantly lower than wild-type myocytes. Similarly, the amplitude of calcium transients at baseline was also decreased in mdx cardiomyocytes, as well as the parameters of Ca2+ removal, T50 (time to decay to the 50%), and the time constant (τ) of Ca2+ decay. These data are consistent with the reduced cardiac function observed using echocardiography.
Table 2.
Baseline parameters of sarcomere shortening and [Ca2+]i of isolated cardiomyocytes from wild-type and mdx mice, stimulated at 0.5 Hz
| Wild Type | mdx | |
|---|---|---|
| n | 42 | 25 |
| Sarcomere length diastole, μm | 1.84 ± 0.01 | 1.85 ± 0.02 |
| Sarcomere shortening (ΔL/L0) | 7.6 ± 0.55 | 3.8 ± 0.57† |
| Time to sarcomere relax 50%, s | 0.23 ± 0.01 | 0.21 ± 0.01 |
| τ Sarcomere relaxation | 0.113 ± 0.015 | 0.112 ± 0.021 |
| Amplitude [Ca2+]i | 0.72 ± 0.058 | 0.44 ± 0.11† |
| Time to decay 50%, s | 0.186 ± 0.01 | 0.215 ± 0.01* |
| τ Decay [Ca2+]i | 0.147 ± 0.004 | 0.180 ± 0.05* |
Values are means ± SE; n, no. of animals. ΔL/L0, change in length/optimal length; τ, time constant; [Ca2+]i, intracellular Ca2+ concentration.
P < 0.05 and
P < 0.001 vs. wild type.
NOX and NOX subunits expression.
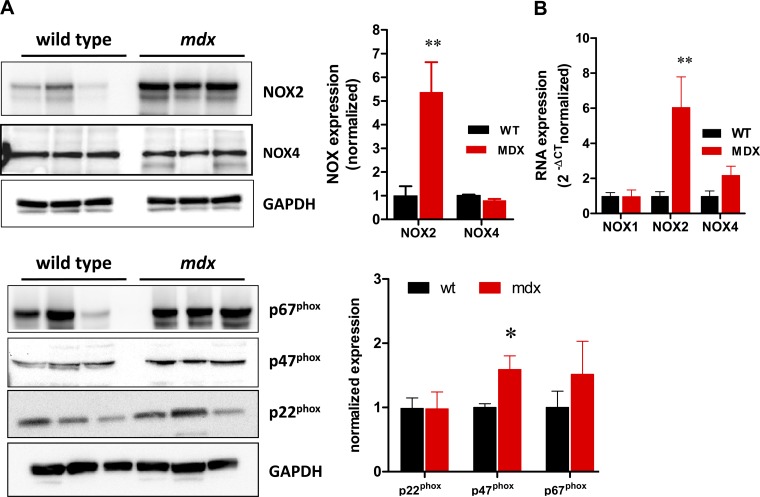
We studied the expression of NOX isoforms and different subunits of NOX as the potential source of ROS in mdx hearts (Fig. 1). Using Western blot analysis, we observed increased levels of NOX2 (fivefold, P < 0.005), while NOX4 was slightly but significantly reduced (P = 0.008). The levels of p47phox were also significantly increased (P < 0.05), although to a much lesser extent than NOX2, while p67phox and p22phox were not significantly elevated.
Fig. 1.
NADPH oxidase (NOX) isoforms and associated subunits expression. A: Western blot analysis of the isoforms of NOX and NOX2-associated subunits in wild-type (WT) and dystrophic (mdx) hearts. GAPDH is used as load control. The bar graph depicts the normalized expression of NOX and NOX2-associated subunits; n = 6 hearts for each strain. B: real-time PCR analysis for NOX1, NOX2, and NOX4 (n = 4 for WT and 5 for mdx). *P < 0.05 vs. WT. **P < 0.001 vs. WT.
In addition, we quantified mRNA of the isoforms of NOX1, 2 3, and 4 using real-time PCR (Fig. 1B). While the expression of NOX1 did not differ between wild-type and mdx mice, NOX2 was sixfold upregulated in mdx hearts (P < 0.01), confirming the results observed at the protein level by Western blotting. NOX3 was not expressed at a significant level in both strains, and NOX4 mRNA was similar in both groups.
ROS production: impact of NOX inhibition.
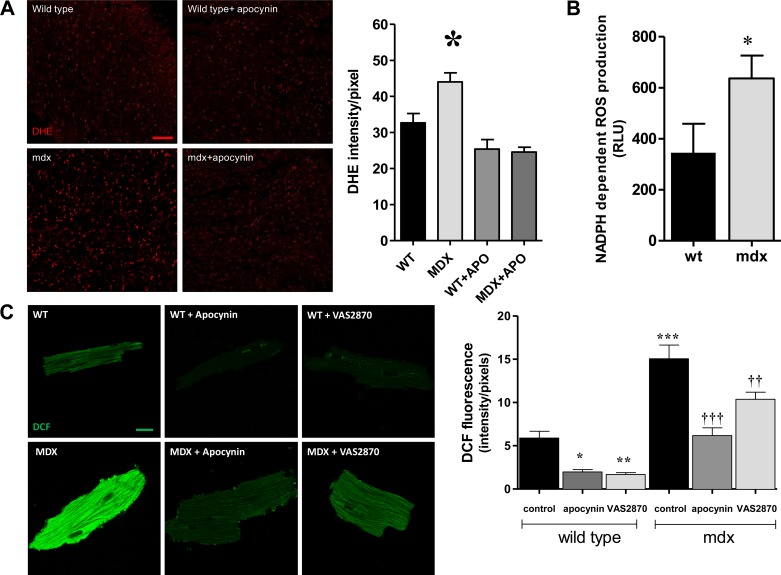
To confirm the functional upregulation of NOX, we studied superoxide production in fresh hearts sections (Fig. 2A). Using dihydroethidium as reporter of ROS, we found increased production of these species in mdx hearts compared with wild type (P < 0.001). Treatment of isolated hearts with apocynin, a NOX inhibitor (5, 40) (100 μM during 45 min), reduced the levels of ROS in mdx hearts toward levels similar to wild type. In addition, we further checked for this signal to be NOX dependent by measuring the NADPH-dependent superoxide production, using lucigenin as reporter (Fig. 2B). In this assay, mdx hearts exhibited a higher superoxide production (345 ± 113.8 relative units in wild type vs. 636.3 ± 90.1 in mdx, P < 0.05). Next, we assessed NOX-derived superoxide production in isolated myocytes using DCF as reporter (Fig. 2C). The signal for ROS was increased threefold in mdx myocytes compared with wild type (P < 0.0001). This ROS production was restored to levels similar to wild type by treatment with NOX inhibitors. As observed in whole hearts, apocynin (100 μM) diminished significantly the ROS production in mdx myocytes (P < 0.0001), as well in wild-type cells. In addition, we used the NOX-specific inhibitor VAS2870, 20 μM (47). This agent reduced significantly ROS production in both cell types (P < 0.001).
Fig. 2.
NOX-derived reactive oxygen species (ROS) production. A: fresh sections of hearts stained with dihydroethidium (DHE) to detect the production of ROS. The bar graph depicts the quantification of the fluorescence intensity. WT under control conditions (n = 16 sections from 6 hearts), mdx in control conditions (n = 10 sections from 6 hearts), WT treated with 100 μM apocynin (APO) (n = 17 sections from 4 hearts), and mdx treated with APO (n = 12 sections from 3 hearts) are shown. *P < 0.001 vs. all of the other groups. Scale bar indicates 100 μm. B: NADPH-dependent ROS production assessed by lucigenin chemiluminescence in WT and mdx cardiac homogenates (n = 5 each strain). RLU, relative light units. *P = 0.038. C: NOX-derived ROS production in myocytes. Freshly isolated cardiac myocytes from WT and mdx myocytes were loaded with 2′,7′-dichlorofluorescein diacetate (DCF) and treated with vehicle (DMSO) or the NOX inhibitors APO (100 μM) or VAS2870 (20 μM) for 20 min and then fixed and visualized under confocal microscopy. For each group, n = 60 cells from 4 WT hearts and 3 mdx hearts. Scale bar indicates 10 μm. *P < 0.05, **P < 0.001, and ***P < 0.0001 vs. WT control. ††P < 0.001 and †††P < 0.0001 vs. mdx control.
Shortening-frequency relationship: impact of NOX inhibition.
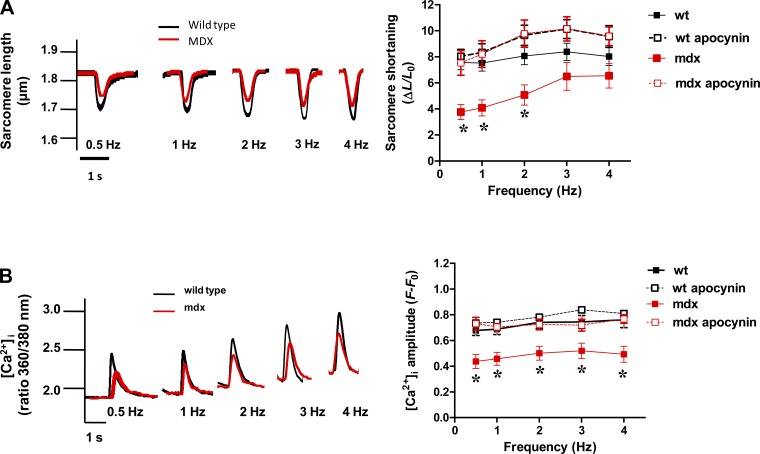
We studied the potential impact of NOX2 inhibition on the reduced contractility of mdx cardiomyocytes (as seen in Table 1). Myocytes were stimulated with a train of frequencies of 0.5–1–2–3–4 Hz, for 1 min at each frequency. Wild-type myocytes presented a flat response of sarcomere shortening to increasing frequencies, but the twitch amplitude at each frequency was higher than for mdx myocytes (P < 0.05, Fig. 3A). Treatment of cardiomyocytes for 45 min with apocynin (100 μM) restored the amplitude of sarcomere shortening in mdx myocytes to a similar extent to wild-type myocytes. In parallel, we analyzed the amplitude of the evoked [Ca2+]i transients in these cardiomyocytes submitted to the increasing frequencies of stimulation (Fig. 3B). The amplitude of these transients was significantly smaller in mdx myocytes at every frequency of stimulation (P < 0.05), but was restored to the level of wild-type myocytes when treated with apocynin.
Fig. 3.
Sarcomere shortening-frequency relationship. A: contractility was evaluated in cardiac myocytes isolated from controls (WT) and mdx mice. A, left: representative traces of twitches from cardiomyocytes electrically paced between 0.5 and 4 Hz. Right: the response (sarcomere shortening) to the train of stimulation (0.5–4 Hz) from WT (n = 31, from 7 hearts), WT plus APO (n = 23 from 6 hearts), mdx (n = 25 from 6 hearts), and mdx myocytes with APO (n = 15, from 4 hearts). ΔL/L0, change in length/optimal length. *P < 0.0001 vs. WT, 2-way ANOVA. B: intracellular Ca2+. Left: representative traces of the amplitude of evoked intracellular Ca2+ concentration ([Ca2+]i) transients in isolated cardiomyocytes. Right: the response of WT (n = 35 from 7 hearts), mdx (n = 21, from 6 hearts), WT treated with APO (n = 12 from 3 hearts), and mdx treated with APO myocytes (n = 10 from 3 hearts) to the train of stimulation (0.5–4 Hz). *P < 0.05 vs. all of the other groups (two-way ANOVA).
Calcium re-uptake.
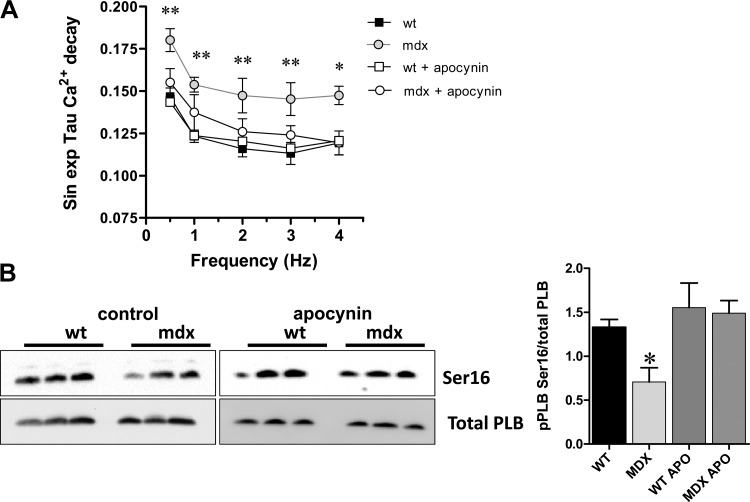
We studied this point analyzing the rate of intracellular Ca2+ decay, as a measurement of SERCA activity (Fig. 4). The values for the τ constant of [Ca2+]i decay, an index of Ca2+ re-uptake, as function of frequency of stimulation, were significantly higher in mdx myocytes at all of the rates of stimulation studied (Fig. 4A), indicating slower re-uptake rates (P < 0.05 vs. wild type). NOX inhibition with apocynin restored the rate of intracellular Ca2+ decay in mdx close to normal. Since this acute pharmacological inhibition of NOX is unlikely to have an impact on SERCA2 protein levels in mdx hearts, we analyzed other possible molecular underpinnings that may explain the reduced cardiac contractility and [Ca2+]i transients observed in mdx hearts. We evaluated the levels of phosphorylated PLB, a major regulator of cardiac contractility (Fig. 4B). We analyzed PLB phosphorylation at serine 16 (Ser16). Under control conditions, mdx hearts showed reduced levels of PLB phosphorylation at Ser16 (P < 0.05 vs. wild type for both sites). Treatment with apocynin (100 μM, 45 min) restored the phosphorylation levels of PLB Ser16.
Fig. 4.
Ca2+ re-uptake kinetics. A: the graph depicts the values of time constant (τ) of [Ca2+]i decay, a measurement of Ca2+ re-uptake, as function of frequency of stimulation. Mdx myocytes display higher values of τ at all frequencies studies. *P < 0.05 and **P < 0.01, mdx vs. WT. B: phospholamban (PLB) phosphorylation. Western blot analysis of heart homogenates from WT and mdx mice, under control conditions or treated with 100 μM APO during 45 min is shown. Left, top: Western blots for phosphorylated PLB in serine 16 (Ser16). Bottom: total levels of PLB (Total PLB) for each heart. Right: the bar graphs depict the quantification of the densitometry for each phosphorylation (n = 3 hearts for each group). *P < 0.05 vs. WT control (ANOVA).
SR Ca2+ stores.
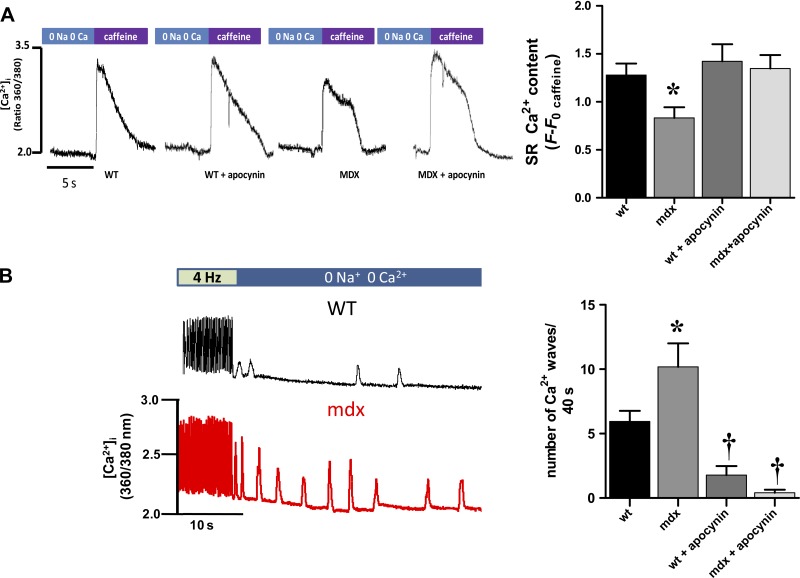
Since the amplitude of the Ca2+ transients was reduced in mdx cardiomyocytes, we evaluated the intra-SR Ca2+ content at the end of the train of stimulation described above (Fig. 5A). After reaching 4 Hz, the intra-SR stores were opened with a rapid application of caffeine (20 mM), and the total SR Ca2+ was analyzed. As suspected, the content of SR Ca2+ was reduced in dystrophic myocytes (P < 0.05 vs. wild type). The SR Ca2+ levels in mdx myocytes were restored on treatment with the NOX inhibitor.
Fig. 5.
Sarcoplasmic reticulum (SR) Ca2+ stores and arrhythmogenic Ca2+ release in mdx myocytes: impact of NOX inhibition. A: assessment of intra-SR Ca2+ stores. Representative traces of calcium signal (fura 2) from myocytes paced at 4 Hz and challenged with caffeine (20 mM) are shown. The bar graph on the right depicts total SR Ca2+ content in WT myocytes (n = 12 cells from 4 hearts), mdx (n = 12, from 4 hearts), WT treated with APO (n = 6 from 3 hearts), and mdx with APO (n = 6, from 3 hearts). *P < 0.05 vs. all the other groups. B, left: the protocol to assess the sensitivity of the ryanodine receptor (RyR). The myocytes are paced at 4 Hz for 10 s, then stimulation is stopped, and the bathing Tyrode solution is rapidly changed for one free of Na+ and Ca2+, to rule out influences of sarcolemmal channels (Na+ current, Ca2+ current) and the sodium/calcium exchanger. Under this condition, the Ca2+ release evoked is the consequence of activated RyR2 channels. The bar graph on the right indicates the number of events in 40 s in each group: WT control (n = 18 cells from 3 hearts), mdx control (n = 11 cells from 3 hearts), WT treated with APO (n = 9 cells from 3 hearts), and mdx with APO (n = 5 from 3 hearts). *P < 0.01 vs. all other groups and †P < 0.05 vs. WT in control conditions.
Spontaneous Ca2+ release.
It has been recently described that, in mdx myocytes, the RyR is hypersensitive and “leaky” (12, 39), generating spontaneous Ca2+ release. To assess this feature, we used a protocol superfusing the cells with a 0 Na+-0 Ca2+ free Tyrode buffer to abolish transsarcolemmal Ca2+ fluxes (42) (Fig. 5B). After a train of stimuli, pacing is stopped, and the bath medium is rapidly changed for a solution free of Na+ and Ca2+ (0 Na-0 Ca), during 40 s, to rule out influences of sarcolemmal channels (Na+ current, Ca2+ current) and the sodium/calcium exchanger. Under this condition, the Ca2+ release evoked is the consequence of activated RyR2 channels in diastole. Spontaneous Ca2+ release events were observed both in wild-type and mdx myocytes. Nevertheless, the number of waves observed in 40 s was significantly higher in mdx myocytes (5.9 ± 0.8 vs. 10.2 ± 1.8 events/40 s, P < 0.001, Fig. 5B). Pretreatment with apocynin reduced the number of events in both control (1.8 ± 0.7, events/40 s, P < 0.05 vs. wild-type control) and mdx cells (0.4 ± 0.25 events/40 s, P < 0.001 vs. wild-type and mdx control). The results obtained with this protocol, along with the data obtained from caffeine application, allow comparing the relation between the number of Ca2+ events and the total SR Ca2+ content. Since the SR Ca2+ content is reduced in mdx myocytes, this suggests that the increased RyR2 activity is not due to luminal SR Ca2+, but an intrinsic property of the channel.
SR calcium leak: impact of NOX inhibition.
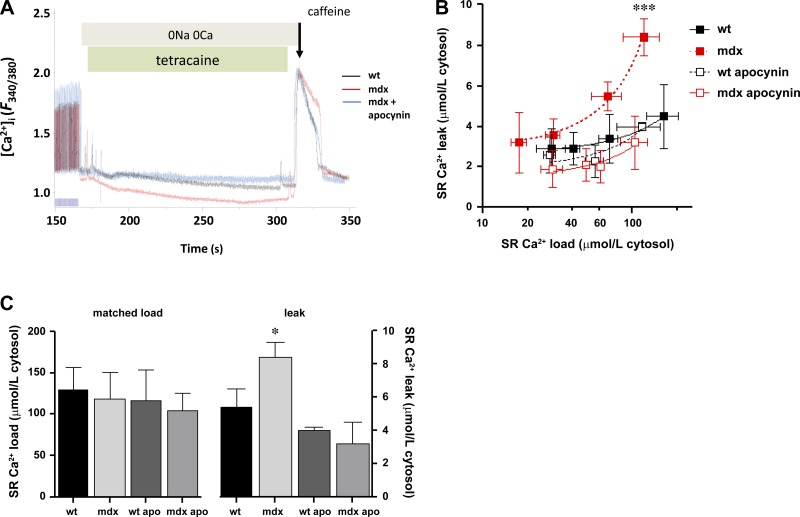
The observed reduction in SR Ca2+ content and spontaneous calcium release may have been a consequence of increased SR Ca2+ leak, as our laboratory has observed in other pathological situations of altered cardiac nitroso-redox homeostasis (15, 16). To test this hypothesis, we assessed Ca2+ leak as previously reported, using the method described by Shannon and colleagues (42). It basically consist of superfusing the cells with a Na+- and Ca2+-free Tyrode buffer to abolish transsarcolemmal Ca2+ fluxes and in the presence of tetracaine, a RyR2 blocker. After a train of stimuli, pacing was stopped, and the bath medium was rapidly changed for a solution free of Na+ and Ca2+ (as in the previous experiment), plus the addition of tetracaine during 40 s. Under this condition, the Ca2+ release evoked is the consequence of activated RyR2 channels. Then tetracaine is stopped, and, after a brief washout, a pulse of caffeine was applied to release the total intra-SR Ca2+ (Fig. 6A). SR Ca2+ leak is a function dependent on intra-SR Ca2+, in an exponential fashion (42–44). Nevertheless, this function is clearly exacerbated in mdx myocytes (Fig. 6B). The results obtained with this protocol, along with the data obtained from caffeine application, allow comparing the relationship between Ca2+ leak and the total SR Ca2+ content. This can be computed in two ways: first, by fitting the best curve to an exponential function. The curve from mdx myocytes is shifted to the left and upwards compared with wild type (P = 0.0002, Fig. 6B). Treatment of mdx myocytes with apocynin significantly reduced SR Ca2+ leak toward normal. Second, by comparing the amount of SR Ca2+ leak at similar (matched) SR Ca2+ loads (Fig. 6C), mdx myocytes exhibit increased SR Ca2+ leak (P < 0.005, one-way ANOVA), which is restored to normal on treatment with apocynin.
Fig. 6.
SR Ca2+ leak: impact of NOX inhibition. A: typical trace of the protocol used to evaluate SR calcium leak using tetracaine. The tetracaine-induced drop in diastolic fura 2 fluorescence (F) ratio is used as an estimate of SR Ca2+ leak, which is insensitive to changes in SR Ca2+ uptake. B: load-leak function. N = 7–8 cells in each group, from 5 WT and 4 mdx hearts. ***P = 0.0002. C: comparison of the amount of SR calcium leak at similar (matched) SR calcium loads. *P = 0.0243, mdx vs. WT and mdx treated with APO.
NOS-1 uncoupling.
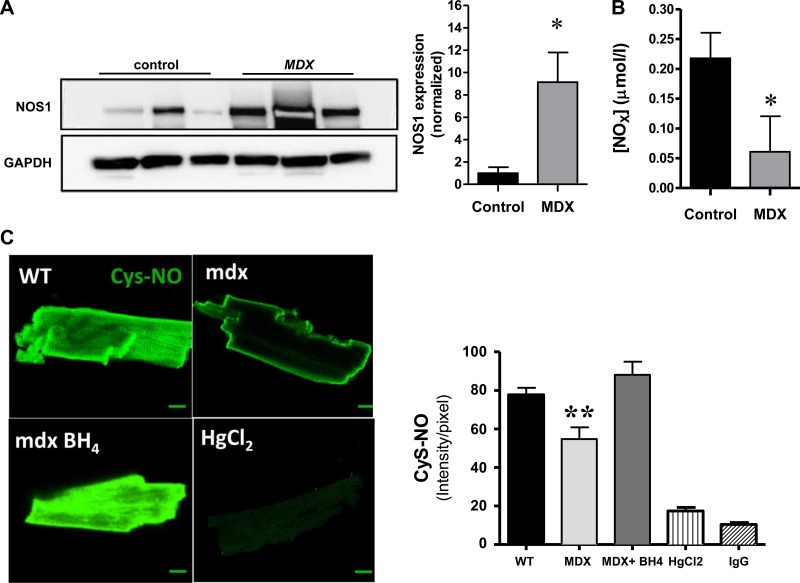
Given that mdx hearts exhibited decreased PLB phosphorylation, which was recovered by NOX inhibition treatment, we tested whether this effect was due to reduced levels of NOS-1, since it has been described that NOS-1 regulates the basal levels of PLB phosphorylation at Ser16, in the mouse (52, 57). For this, we evaluated the levels of NOS-1 by Western blotting (Fig. 7A). Contrary to what we expected, NOS-1 levels were increased in mdx hearts, but the content of nitrate, a metabolite of NO, was reduced in these hearts. With these results, one possibility is that NOS-1 is found in an uncoupled state: this is dissociated from the activity to convert l-arginine to NO and citrulline and, instead, diverted toward the production of superoxide. This phenomenon has been described as a result of reduced levels of the NOS cofactor BH4, which becomes oxidized in pathological states, to dihydrobioterin (1, 30). To test this possibility, we treated mdx myocytes with exogenous BH4 in an attempt to recouple NOS-1 (Fig. 7B). For this, we assessed in myocytes the levels of S-nitrosylation (that depend on NOS-1 activity) using an ant-nitrosocysteine antibody, as previously described (3, 16). Application of BH4 (300 μM, 45 min) restored the levels of S-nitrosocysteine in mdx myocytes to a level similar to that of wild-type cells.
Fig. 7.
Nitric oxide synthase (NOS)-1 uncoupling. A: Western blot analysis for NOS1 in WT and mdx cardiac homogenates. *P < 0.05. B: nitrate levels in cardiac tissue from WT and mdx cardiac homogenates. *P < 0.05. C: S-nitrosothiol levels in cardiac myocytes. Cardiomyocytes from WT (n = 85) and mdx myocytes (n = 43) were assessed for S-nitrosothiol levels by immunostaining for S-nitrocysteine residues. Tetrahydrobioterin (BH4) supplementation (300 μM, 30 min) increased the S-nitrosylation levels in mdx myocytes (n = 55). As control, WT myocytes were treated with HgCl2 (n = 7) a redox agent that abolishes S-nitrosylation. In addition, cells were treated with an irrelevant IgG as control for the antibody specificity. **P < 0.005, mdx vs. WT and mdx + BH4.
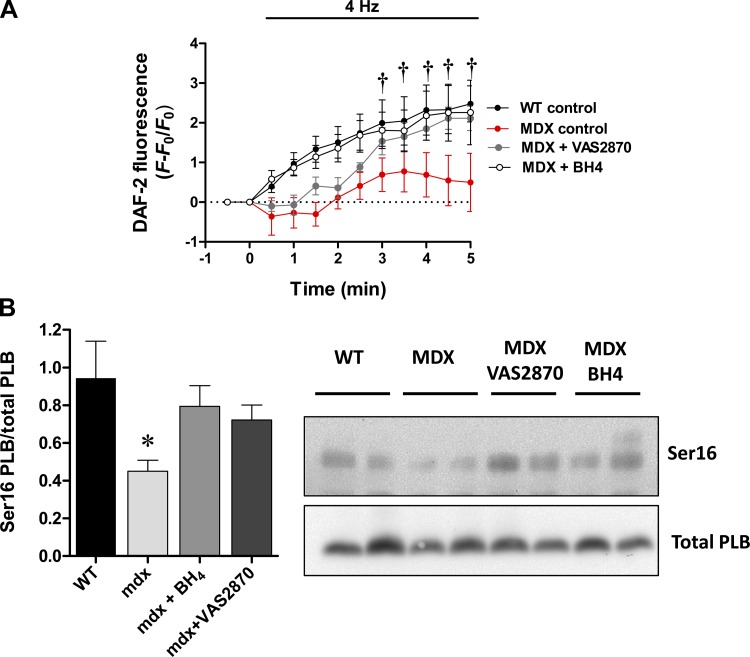
Next, we evaluated the possibility to recouple NOS-1 using the NOX inhibitor VAS2870, along with BH4, in cardiomyocytes stimulated at 4 Hz. For this, mdx and wild-type myocytes were loaded with NO-sensitive probe DAF-DA, to monitor in real time NO production (Fig. 8A). In this setting, mdx myocytes showed an impaired NO production compared with wild-type cells (P < 0.005). After treating mdx myocytes with BH4 (300 μM, 15 min), these cells displayed a NO signal very similar to that of wild-type myocytes. In addition, NO signal was also recovered in mdx myocytes pretreated with the NOX inhibitor VAS2870 (20 μM, 20 min). These results suggest that, in mdx cardiomyocytes, NOS-1 is found in an uncoupled state, due to a NOX-dependent oxidative stress and, in this case, most likely NOX-2. To further test this idea, we treated isolated mdx and wild-type hearts with BH4 and VAS2870 (Fig. 8B), in a similar way to isolated myocytes, to evaluate the levels of PLB phosphorylation that depend on NOS-1 activity. These treatments restored the levels of PLB phosphorylation in Ser16 to a level similar to wild-type hearts and similarly to the results previously obtained using apocyinin.
Fig. 8.
Real-time nitric oxide (NO) production and PLB phosphorylation. A: isolated cardiomyocytes were loaded with the NO-sensitive probe 4,5-diaminofluorescein (DAF)-diacetate (DA) and stimulated at 1 Hz (baseline). Then the frequency stimulation is switched to 4 Hz, and the signal for DAF-NO is registered for 5 min. The graph depicts the average signal ± SE for WT (n = 4), mdx (n = 5), and mdx myocytes treated either with BH4 (300 μM, 15 min, n = 6) or the NOX inhibitor VAS2870 (20 μM, 20 min, n = 5). †P < 0.05 vs. mdx control. B: levels of PLB phosphorylation at Ser16 in isolated hearts from control mice, mdx mice, and mdx treated with BH4 (100 μM, 15 min), or mdx treated with VAS2870 (20 μM, 20 min); n = 5 hearts each group. The graph (left) depicts average ± SE. *P < 0.05, mdx vs. all other groups. Right: representative Western blot for phosphorylated PLB (top) and total PLB (bottom).
These results suggest that oxidative stress may cause oxidation of BH4, an essential cofactor for NOS activity, causing NOS-1 uncoupling. This uncoupling diverts NOS from NO production to superoxide and impairs PLB phosphorylation, compromising cardiac relaxation and SR calcium load and reducing contractility.
DISCUSSION
Here we have shown that, in mdx hearts with overt cardiomyopathy, ROS production derived from the increased expression of NOX2 contributes to the altered contractility and calcium handling observed in this model of Duchenne dystrophy. This is probably an effect of ROS at the level of the calcium-handling machinery and other proteins. In our hands, we observed that NOX-derived superoxide affected negatively the intra-SR calcium stores (reduced PLB phosphorylation), increase in SR diastolic Ca2+ leak, and increased the incidence of arrhythmogenic Ca2+ release in mdx cardiomyocytes. The appearance of these spontaneous Ca2+ events is of importance. This is probably an effect at the level of the RyR2, which is extremely redox sensitive (15, 48). Recently, Prosser and colleagues (32), in an elegant study, demonstrated these observations. Using a protocol that consisted of stretching cardiac cells, they reported a substantial increase of Ca2+ waves and oscillating cells in mdx myocytes compared with control, associated with increased ROS production (32). Here, we expand those observations, associating this increase in ROS production to NOX upregulation directly, with an impact in cardiac contractility. Indeed, this increase in NOX-derived ROS production, which, under physiological conditions (32) favors the calcium-induced calcium release process, becomes deregulated in dystrophic cardiomyopathy. Here we show that this hyperactivity of the RyR2 also decreases the content of Ca2+ in the SR. Oxidation of cysteines in RyR2 induces increased responsiveness to activating calcium (29). The hyperactivity of RyR2 in muscular dystrophy has been recently described in terms of increased excitation-contraction coupling gain at low extracellular calcium levels (12, 51). In addition, Fauconnier et al. (12) showed that spontaneous Ca2+ waves and increased Ca2+ sparks frequency in isolated myocytes from mdx myocytes was associated with the in vivo increased occurrence of premature ventricular contractions. Indeed, electrocardiographic abnormalities are present in DMD patients (4).
The present results suggest the involvement of the NOX2 system in the hyperactivity of RyR2 in dystrophic myocytes, resulting in increased diastolic Ca2+ leak, decreased SR Ca2+ content, and inducing arrhythmogenic spontaneous Ca2+ release, consistent with the observations of Kyrychenko et al. (27) that showed similar Ca2+ leak and increased RyR2 oxidation. Donoso and colleagues (8) recently showed that, in a model of ischemia-reperfusion, NOX activation results in an increased RyR2 sensitivity to activating Ca2+, associated with S-glutathionylation of the channel. Interestingly, this effect was fully prevented by treatment with apocynin and VAS2870 (8). Our findings are consistent with previous evaluation of NOXs in the progression of cardiomyopathy. Both NOX2 and p47phox contribute to the process of LV remodeling after myocardial infarction in mice (7, 28). NOX2 also plays a central role in the contractile dysfunction observed in a model of pressure overload (17). NOX2 upregulation is probably triggered by the activation of the rennin-angiotensin system. In addition, angiotensin is the classical activator of NOX in the cardiovascular system (31). Also, we cannot discard the role of fibroblasts and inflammatory cell in this process, since they also may express NOX2, particularly macrophages (2).
Limitations of apocynin.
Besides its property to inhibit NOX2, apocynin is not selective, being able to inhibit the other NOX isoforms. In addition, it has been described that apocynin also possesses antioxidant capacity (20). For these reasons, we used the more specific NOX inhibitor VAS2870 (9, 55). The use of VAS2870 supported the findings with apocynin, since both agents were able to restore NO production and PLB phosphorylation in mdx mice.
Our results regarding PLB are of interest. PLB phosphorylation governs the activity of SERCA2, which is downregulated in the mdx hearts. This pathway is downregulated in patients suffering Duchenne dystrophy, according to a recent transcriptome study (26). Our observation of decreased levels of Ser16 phosphorylation of PLB agrees with that of Williams and Allen (53). Whether this decreased phosphorylation was due to increased activity or expression of protein phosphatases or increased levels/activity of phosphodiesterases and the role of NOX2-derived ROS remain to be determined. A likely explanation for this observation is related to NOS-1 uncoupling. The activity of NOS-1 is required for basal PLB phosphorylation in the mouse heart (52, 57), with NOS-1 uncoupling resulting in impaired ventricular relaxation and diastolic dysfunction (46). This NOS-1 uncoupling in mdx cardiomyocytes is probably the result of the NOX2-derived oxidative stress, which ultimately leads to BH4 oxidation. This interaction between increased ROS and decreased reactive nitrogen species production is another example of nitroso-redox imbalance, which is emerging as a common theme in organ dysfunctions (18, 19, 37).
The effects of NOX inhibition and BH4 supplementation may also have an impact on myofilament sensitivity to activating Ca2+. Indeed, mdx myocytes present decreased myofilament sensitivity (54). This may explain the fact that apocynin treatment improved contractility in both wild-type and mdx myocytes, beyond the increase in cytosolic Ca2+. Additionally, NOS-1 and BH4 may play a role in this effect, since we previously described that NOS-1-deficient mice present decreased myofilament sensitivity that is restored with NO donors (10), and BH4 causes increases in myofilament sensitivity (23). Nevertheless, this point needs further investigation.
Besides the evidence presented here of altered Ca2+ handling originated at the SR level, other sources of Ca2+ may play a role in the pathophysiology of dystrophic cardiomyopathy. For instance, abnormal Ca2+ influx through transient receptor potential 1 (TRPC1) and TRPC6 channels is described in mdx and mdx/utrophin-deficient mice (53, 41). In addition, pannexins and connexin hemichannels are also a potential route for Ca2+ influx, particularly connexin-43 hemichannels, which are being shown to be redox sensitive (6, 34, 35).
Therapies aimed to treat muscular dystrophy should target skeletal and cardiac muscle, since both suffer the lack of dystrophin, and, for example, improving only skeletal muscle function may uncover or aggravate cardiac function in Duchenne patients (49). Current therapies for dystrophic cardiomyopathy include angiotensin I-converting enzyme inhibitors and β-blockers (22). NOX2 inhibition appears to be an interesting target in both types of muscle in muscular dystrophy, as increased levels of NOX have been reported in the skeletal muscle of mdx mice (45, 54).
In conclusion, we have described that NOX inhibition restores contractile function in dystrophic cardiomyopathy. This effect is associated with improved intracellular calcium handling that involves two mechanisms: the RyR activity and PLB phosphorylation. In addition, increased ROS resulting from NOX2 leads to NOS-1 uncoupling and decreased NO production. Together, these findings provide insights into new ways to modulate these biochemical derangements pharmacologically and, as such, have therapeutic implications.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants RO1-HL-65455 and HL-094849–01 (J. M. Hare) and Proyecto Fondecyt 1120595, Chile (D. R. Gonzalez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.R.G. and J.M.H. conception and design of research; D.R.G., A.V.T., G.L., V.M., Y.C., and R.A.D. performed experiments; D.R.G., A.V.T., G.L., V.M., and R.A.D. analyzed data; D.R.G., G.L., and R.A.D. interpreted results of experiments; D.R.G., A.V.T., and R.A.D. prepared figures; D.R.G. drafted manuscript; D.R.G., R.A.D., and J.M.H. edited and revised manuscript; D.R.G., A.V.T., G.L., V.M., Y.C., R.A.D., and J.M.H. approved final version of manuscript.
REFERENCES
- 1.Alkaitis MS, Crabtree MJ. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep 9: 200–210, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 109: 4314–4319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beynon RP, Ray SG. Cardiac involvement in muscular dystrophies. QJM 101: 337–344, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003 [DOI] [PubMed] [Google Scholar]
- 6.D'hondt C, Iyyathurai J, Vinken M, Rogiers V, Leybaert L, Himpens B, Bultynck G. Regulation of connexin- and pannexin-based channels by post-translational modifications. Biol Cell 105: 373–398, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100: 894–903, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Donoso P, Finkelstein JP, Montecinos L, Said M, Sanchez G, Vittone L, Bull R. Stimulation of NOX2 in isolated hearts reversibly sensitizes RyR2 channels to activation by cytoplasmic calcium. J Mol Cell Cardiol 68: 38–46, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulce RA, Yiginer O, Gonzalez DR, Goss G, Feng N, Zheng M, Hare JM. Hydralazine and organic nitrates restore impaired excitation-contraction coupling by reducing calcium leak associated with nitroso-redox imbalance. J Biol Chem 288: 6522–6533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanchaouy M, Polakova E, Jung C, Ogrodnik J, Shirokova N, Niggli E. Pathways of abnormal stress-induced Ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium 46: 114–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 107: 1559–1564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology 99: 1–19, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A 104: 20612–20617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285: 28938–28945, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol 47: 817–826, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hare JM. Oxidative stress and apoptosis in heart failure progression. Circ Res 89: 198–200, 2001 [PubMed] [Google Scholar]
- 19.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med 351: 2112–2114, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hill AJ, Drever N, Yin H, Tamayo E, Saade G, Bytautiene E. The role of NADPH oxidase in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol 210: 466.e1–5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, Neish SR, Smith EO, Towbin JA. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation 112: 2799–2804, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC, Jr, Solaro RJ. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol 56: 44–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res 77: 766–773, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kaspar RW, Allen HD, Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract 21: 241–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal 5: ra56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyrychenko S, Polakova E, Kang C, Pocsai K, Ullrich ND, Niggli E, Shirokova N. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc Res 97: 666–675, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J 74: 1263–1277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moens AL, Kietadisorn R, Lin JY, Kass D. Targeting endothelial and myocardial dysfunction with tetrahydrobiopterin. J Mol Cell Cardiol 51: 559–563, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 71: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord 14: 491–496, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Retamal MA. Connexin and pannexin hemichannels are regulated by redox potential. Front Physiol 5: 80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Sinovas A, Sanchez JA, Fernandez-Sanz C, Ruiz-Meana M, Garcia-Dorado D. Connexin and pannexin as modulators of myocardial injury. Biochim Biophys Acta 1818: 1962–1970, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Sapp JL, Bobet J, Howlett SE. Contractile properties of myocardium are altered in dystrophin-deficient mdx mice. J Neurol Sci 142: 17–24, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation 112: 3415–3422, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Saraiva RM, Minhas KM, Zheng M, Pitz E, Treuer A, Gonzalez D, Schuleri KH, Vandegaer KM, Barouch LA, Hare JM. Reduced neuronal nitric oxide synthase expression contributes to cardiac oxidative stress and nitroso-redox imbalance in ob/ob mice. Nitric Oxide 16: 331–338, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG, Wehrens XH. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci U S A 107: 13165–13170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120: 254–291, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, Cingolani OH, Kass DA. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circ Res 114: 823–832, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93: 592–594, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Shannon TR, Wang F, Bers DM. Regulation of cardiac sarcoplasmic reticulum Ca release by luminal [Ca] and altered gating assessed with a mathematical model. Biophys J 89: 4096–4110, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N. Reciprocal amplification of ROS and Ca(2+) signals in stressed mdx dystrophic skeletal muscle fibers. Pflügers Arch 458: 915–928, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 121: 519–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ten FH, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Townsend D, Yasuda S, Chamberlain J, Metzger JM. Cardiac consequences to skeletal muscle-centric therapeutics for Duchenne muscular dystrophy. Trends Cardiovasc Med 19: 50–55, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 16: 832–835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol 297: H1992–H2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol 294: C1566–C1575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol 292: H846–H855, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 293: H1969–H1977, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol 164: 866–883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, ten HM, Schneider JE, Stuckey DJ, Sebag-Montefiore L, Bia BL, Radda GK, Davies KE, Neubauer S, Clarke K. Abnormal cardiac morphology, function and energy metabolism in the dystrophic mdx mouse: an MRI and MRS study. J Mol Cell Cardiol 45: 754–760, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 102: 242–249, 2008 [DOI] [PubMed] [Google Scholar]