Abstract
Vagal nerve stimulation (VNS) has been proposed as a cardioprotective intervention. However, regional ventricular electrophysiological effects of VNS are not well characterized. The purpose of this study was to evaluate effects of right and left VNS on electrophysiological properties of the ventricles and hemodynamic parameters. In Yorkshire pigs, a 56-electrode sock was used for epicardial (n = 12) activation recovery interval (ARI) recordings and a 64-electrode catheter for endocardial (n = 9) ARI recordings at baseline and during VNS. Hemodynamic recordings were obtained using a conductance catheter. Right and left VNS decreased heart rate (84 ± 5 to 71 ± 5 beats/min and 84 ± 4 to 73 ± 5 beats/min), left ventricular pressure (89 ± 9 to 77 ± 9 mmHg and 91 ± 9 to 83 ± 9 mmHg), and dP/dtmax (1,660 ± 154 to 1,490 ± 160 mmHg/s and 1,595 ± 155 to 1,416 ± 134 mmHg/s) and prolonged ARI (327 ± 18 to 350 ± 23 ms and 327 ± 16 to 347 ± 21 ms, P < 0.05 vs. baseline for all parameters and P = not significant for right VNS vs. left VNS). No anterior-posterior-lateral regional differences in the prolongation of ARI during right or left VNS were found. However, endocardial ARI prolonged more than epicardial ARI, and apical ARI prolonged more than basal ARI during both right and left VNS. Changes in dP/dtmax showed the strongest correlation with ventricular ARI effects (R2 = 0.81, P < 0.0001) than either heart rate (R2 = 0.58, P < 0.01) or left ventricular pressure (R2 = 0.52, P < 0.05). Therefore, right and left VNS have similar effects on ventricular ARI, in contrast to sympathetic stimulation, which shows regional differences. The decrease in inotropy correlates best with ventricular electrophysiological effects.
Keywords: vagal nerve stimulation, ventricle, repolarization
the autonomic nervous system plays a significant role in the genesis and persistence of ventricular arrhythmias (54, 59). Sympathetic activation is proarrhythmic (16, 32, 53), whereas parasympathetic activation is thought to be cardioprotective (17, 31). The vagal nerve trunk provides important cardiomotor efferent fibers to the heart and also carries afferent signals from the heart. Vagal nerve stimulation (VNS) has been shown to decrease infarct size (48), reduce the ventricular fibrillation (VF) threshold (39), and decrease the incidence of ventricular arrhythmias and mortality during ischemia (13, 27, 38, 52). Furthermore, a preserved parasympathetic reflex has been reported to be protective during myocardial infarction (46). Stimulation of the right vagal nerve (RVN) has shown benefits in a series of patients with cardiomyopathy and is undergoing evaluation in clinical trials (20, 47). The mechanisms of the antiarrhythmic effects of VNS are less clear and are thought to be multifactorial, with a decrease in heart rate (HR) (15), release of nitric oxide (9), and antagonism of the sympathetic nervous system all thought to play a role (8, 30, 49).
Modulation of repolarization by sympathetic nerve stimulation has been well characterized (1, 25, 41, 55, 58). However, the effects of parasympathetic regulation of ventricular repolarization have not been extensively studied. Indeed, parasympathetic innervation of the ventricular myocardium was considered minimal for many years. Histological studies have now confirmed evidence of abundant ventricular parasympathetic innervation (24, 26, 51). Previous studies of ventricular repolarization during VNS were limited to using a small number of electrodes (35), extrastimulus pacing (33), or VF intervals (41): techniques that can alter autonomic tone (23) and do not provide detailed spatial data. Furthermore, differences between the RVN and left vagal nerve (LVN) on regional repolarization remain unclear and may be important given the presence of disease processes that can affect certain areas of the heart to different degrees. For unilateral stimulation, lack of laterality would also be significant in allowing similar effects of VNS from either side.
The aim of this study was to assess the effects of right and left VNS on cardiac function and global and regional ventricular repolarization of the endocardium and epicardium.
METHODS
Animal protocol.
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California-Los Angeles Institutional Chancellor's Animal Research Committee.
Yorkshire pigs (n = 12, 20–50 kg) were sedated with telazol (8–10 mg/kg im), intubated, and ventilated. General anesthesia was maintained with isoflurane (1–1.5%) and intravenous boluses of fentanyl (2–4 μg/kg). A median sternotomy was performed to expose the heart, and the cervical right and left vagosympathetic trunks were isolated. After the completion of surgery, the anesthesia was switched to α-chloralose (10 mg·kg−1·h−1 iv infusion). HR was monitored via electrocardiogram recordings. The right femoral artery was catheterized to monitor systemic blood pressure. Hourly arterial blood gases were obtained, and tidal volume was adjusted and/or infusions of sodium bicarbonate were given to maintain acid-base homeostasis.
Hemodynamic assessment.
Pressure-volume loops, left ventricular (LV) end-systolic pressure (LVESP), LV end-diastolic pressure (LVEDP), dP/dtmax, dP/dtmin, and the time constant of isovolumic relaxation (τ) were obtained using a 12-pole conductance pressure catheter in the LV (n = 8) connected to a MPVS Ultra Pressure Volume Loop System (Millar Instruments, Houston, TX). τ was calculated using the Weiss method (56) from the pressure-volume loop as a parameter describing the time course of the exponential decay in LV pressure during isovolumic relaxation. The following equation was used to calculate τ: P(t) = A × exp(−t/τ), where P is pressure, A is a constant, referring to the slope of the linear relationship between pressure and exp(−t/τ), and t is time.
VNS.
The RVN and LVN were stimulated separately using bipolar electrodes (Cyberonics, Houston, TX) connected to a Grass S88 Stimulator (Grass Technologies, Warwick, RI). Square stimulation pulses were delivered at 0.5–1.0 ms in duration and 10–20 Hz in frequency. To determine the threshold current, the current was increased starting from 0.2 mA by 0.2-mA intervals until either a 10–20% drop in HR (the percentage of 10% vs. 20% was picked based on the animals' baseline HR so that severe bradycardia or a significant drop in blood pressure was avoided) for both right and left VNS. After the threshold current was reached, the stimulation current was decreased by 0.1 mA to confirm that the threshold was accurate. If a stimulation frequency of 20 Hz led to complete heart block or asystole with right VNS, left VNS, or both, 10 Hz was use as the stimulation frequency for both right and left VNS. Right and left VNS was performed at 1.2 times threshold current for 15 s followed by a 15-min stabilization period to allow for activation recovery interval (ARI) and hemodynamic parameters to return to baseline. To account for any HR effects on ARI, atrial pacing at baseline HR was performed in four animals during right and left VNS. In addition, subthreshold VNS at a level just below the HR response was performed in one animal, and the ARI was analyzed.
ARI recordings.
A 56-electrode nylon sock was placed around the heart (n = 12), and a 64-electrode basket catheter (n = 9) was placed into LV via the left carotid artery. Unipolar electrograms (EGMs) were obtained using a Prucka CardioLab System (GE Healthcare, Fairfield, CT) and a custom-made, 128-channel multiplexor. EGMs were band-pass filtered between 0.05 and 500 Hz. A minimum of 10 EGMs was analyzed from each electrode before and during stimulation. An electrofield electroanatomic mapping system (NavX, St. Jude Medical, St. Paul, MN) was used to assess the basket catheter position. At the end of the experiment, direct incision of the LV was performed to confirm endocardial electrode locations. ARIs were measured and calculated using customized software (ScalDyn, University of Utah, Salt Lake City, UT) as previously described (55). ARI has been shown to correlate well with action potential duration (APD) and allows for multiple simultaneous measurements. Furthermore, changes in ARI at a given site during an intervention correlate well with changes in APD measured from microelectrodes (21, 37). This method allows for measurements of local APD without extrastimulus delivery or induction of VF, methods that can alter autonomic tone. Global dispersion of repolarization (DOR) was calculated using the variance of all mean ARIs recorded in a specific region. EGMs with flattened T waves, fractionation, or noise were excluded.
Regional ARI analysis.
For purposes of this report, anterior refers to the ventral aspect and posterior refers to the dorsal aspect of the animal. Mean ARI in the following regions was analyzed: LV anterior, lateral, and posterior, right ventricular (RV) anterior, lateral, and posterior, RV outflow tract, and LV base and apex. The median number of electrodes in each region was four (range: 3–7). For the assessment of transmural (endocardial vs. epicardial) differences in ARI, electrodes directly across from each other on the anterior, lateral, and posterior aspects of the mid-LV were used.
Three-dimensional sock ARI data were projected onto two-dimensional polar maps using publicly available software (Map3D, University of Utah; http://www.sci.utah.edu/cibc/software/107-map3d.html).
Statistical analysis.
For comparison of continuous variables, a Wilcoxon rank-sum test or Wilcoxon signed-rank test was used. For regional analysis, means and variances in ARI were compared using parametric repeated-measures ANOVA. The P value for a particular pairwise, mean, standardized ARI comparison was considered significant only if the corresponding overall F-statistic was significant. Dispersion in ARI was defined as the variance in the mean ARI recorded from all the electrodes over a given region. To account for baseline differences, the percent change in ARI was also compared. Given the range of values for DOR, the log mean difference was used for statistical analysis of regional differences. Data are presented as means ± SE. SAS 9.1 was used for statistical analysis. P values of <0.05 were considered statistically significant. Correlations between hemodynamics parameters and ARI changes were performed using the Pearson correlation test.
RESULTS
The right VNS current was 4.0 ± 0.8 mA, and the left VNS current was 4.2 ± 0.8 mA (P = 0.6 for right vs. left VNS current). Three animals developed complete heart block with right VNS, and four animals developed complete heart block with left VNS.
Hemodynamic responses to stimulation.
The effects of right and left VNS on hemodynamic parameters are shown in Table 1. Both right and left VNS significantly decreased dP/dtmax and LVESP and increased dP/dtmin and τ. There were no statistically significant differences between right and left VNS on hemodynamic parameters, and, therefore, there was no laterality on the hemodynamic effects of right versus left VNS (Table 1). The increase in dP/dtmin and τ by both right and left VNS suggested a worsening of diastolic function by VNS. The PR interval increased with right VNS from 114 ± 4 to 137 ± 5 ms and increased with left VNS from 112 ± 4 to 140 ± 4 ms. There was no difference in PR interval prolongation between right versus left VNS (P = 0.4), suggesting that both the RVN and LVN provided innervation to the atrioventricular (AV) node.
Table 1.
Responses to RVN and LVN stimulation compared with BL
| RVN |
LVN |
|||
|---|---|---|---|---|
| BL | During stimulation | BL | During stimulation | |
| Heart rate, beats/min | 84 ± 5 | 71 ± 5* | 84 ± 4 | 73 ± 5* |
| Systolic blood pressure, mmHg | 120 ± 7 | 110 ± 8* | 119 ± 8 | 111 ± 8* |
| Diastolic blood pressure, mmHg | 83 ± 8 | 73 ± 8* | 83 ± 9 | 74 ± 9* |
| End-systolic pressure, mmHg | 89 ± 9 | 77 ± 9* | 91 ± 9 | 83 ± 9* |
| End-diastolic pressure, mmHg | 4 ± 1 | 6 ± 1 | 6 ± 2 | 6 ± 2 |
| dP/dtmax, mmHg/s | 1,660 ± 154 | 1,490 ± 160* | 1,595 ± 155 | 1,416 ± 135* |
| dP/dtmin, mmHg/s | −1,511 ± 211 | −1,090 ± 208* | −1,520 ± 213 | −1,161 ± 231* |
| Time constant of isovolumic relaxation, ms | 41 ± 4 | 50 ± 7* | 41 ± 3 | 50 ± 5* |
| PR interval, ms | 114 ± 4 | 137 ± 5* | 112 ± 4 | 140 ± 4* |
RVN, right vagal nerve; LVN, left vagal nerve.
P < 0.05 vs. baseline (BL).
Effects of right and left VNS on epicardial ARI and DOR.
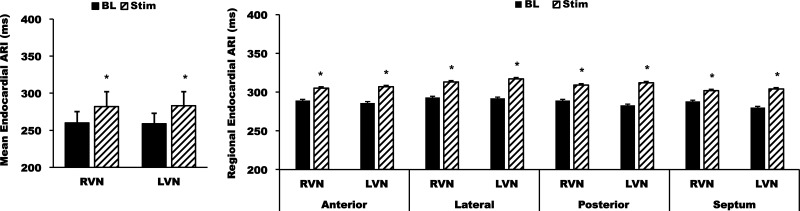
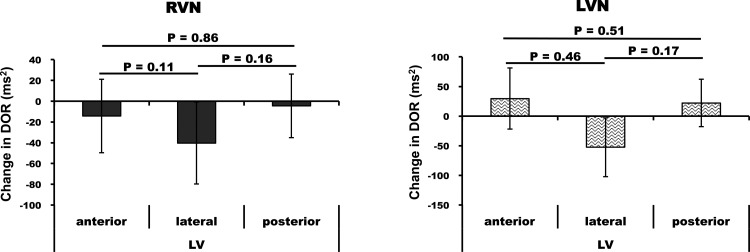
Right and left VNS prolonged global epicardial ARI from 327 ± 18 to 350 ± 23 ms (7 ± 2%, P < 0.05 vs. baseline) and from 327 ± 16 to 347 ± 21 ms (6 ± 2%, P < 0.05 vs. baseline), respectively, with no significant differences between right or left VNS (P = 0.4). Epicardial DOR was increased by both right and left VNS from 354 ± 53 to 512 ± 121 ms2 and from 338 ± 57 to 476 ± 110 ms2, respectively (P < 0.05 vs. baseline for both conditions; Fig. 1). With respect to anterior, posterior, and lateral changes on the RV and LV, each region demonstrated ARI prolongation (P < 0.05) compared with baseline (Fig. 2) without statistically significant differences in prolongation between these regions. Regional changes in DOR at baseline and during right and left VNS are shown in Table 2. These changes from baseline were not statistically significant. With regard to regional comparisons, no significant anterior, lateral, or posterior differences in DOR during right or left VNS were observed on the epicardium (Fig. 3). Detailed regional DOR comparisons are provided in Tables 3–6.
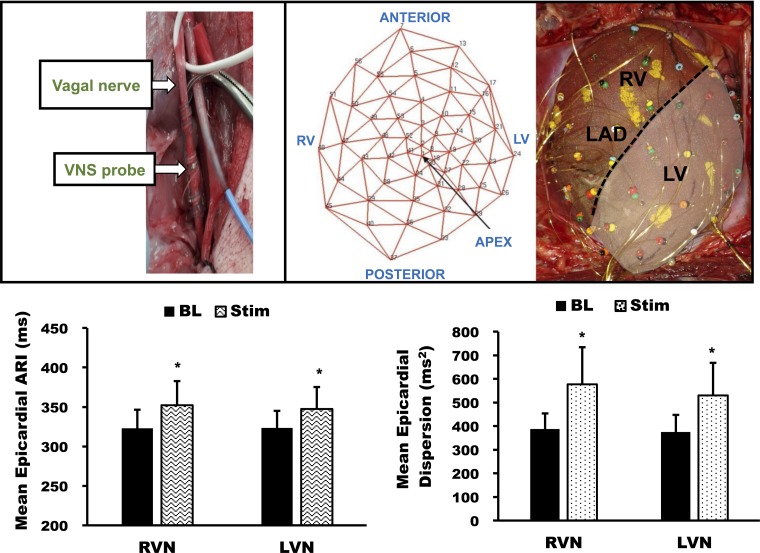
Fig. 1.
Global epicardial activation recovery interval (ARI) responses to right vagal nerve (RVN) stimulation (RVNS) and left vagal nerve (LVN) stimulation (LVNS). Top: images showing the isolation of the vagal nerves, bipolar electrodes used for vagal nerve stimulation (VNS probe), and the sock electrode on the heart. Locations of the electrodes used to create ARI polar maps are marked. RV, right ventricle; LV, left ventricle; LAD, left anterior descending artery; BL, baseline; Stim, during stimulation. Bottom: mean epicardial ARI (left) and dispersion of repolarization (DOR; right) showing significant differences during stimulation (Stim) compared with baseline (BL) but no significant differences between RVNS and LVNS. Data are means ± SE. *P < 0.01 vs. BL.
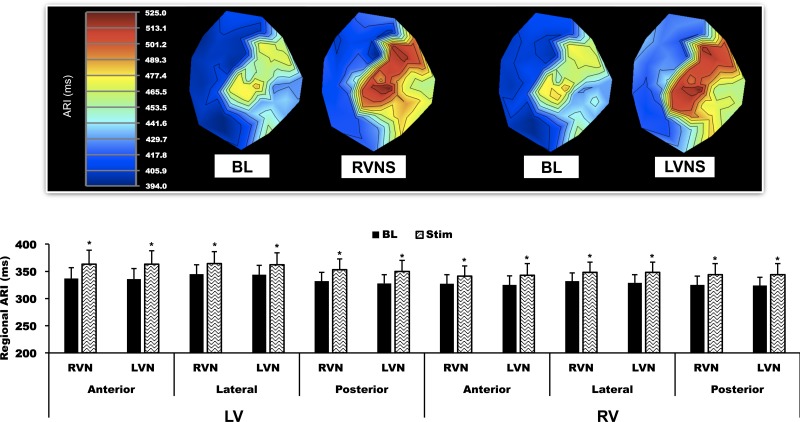
Fig. 2.
Regional epicardial ARI responses to RVNS and LVNS. Top: polar maps obtained from one animal at baseline and during RVNS and LVNS. No significant anterior, lateral, or posterior regional differences in responses were found. Bottom: quantified data for all animals. Data are means ± SE. *P < 0.01 vs. BL.
Table 2.
Regional dispersion of repolarization in response to RVN and LVN stimulation
| RVN |
LVN |
|||||
|---|---|---|---|---|---|---|
| BL, ms2 | During stimulation, ms2 | P value vs. BL | BL, ms2 | During stimulation, ms2 | P value vs. BL | |
| Epicardial regional dispersion of repolarization | ||||||
| LV | ||||||
| Anterior | 158 ± 38 | 148 ± 35 | 0.82 | 131 ± 39 | 113 ± 42 | 0.50 |
| Lateral | 131 ± 44 | 143 ± 52 | 0.72 | 122 ± 42 | 176 ± 52 | 0.45 |
| Posterior | 111 ± 49 | 173 ± 79 | 0.30 | 94 ± 44 | 109 ± 69 | 0.90 |
| RV | ||||||
| Anterior | 110 ± 31 | 160 ± 68 | 0.10 | 104 ± 35 | 145 ± 41 | 0.14 |
| Lateral | 125 ± 51 | 183 ± 94 | 0.90 | 120 ± 56 | 220 ± 107 | 0.05 |
| Posterior | 156 ± 67 | 206 ± 87 | 0.92 | 143 ± 57 | 227 ± 96 | 0.22 |
| Endocardial regional dispersion of repolarization | ||||||
| LV | ||||||
| Anterior | 52 ± 32 | 41 ± 13 | 0.56 | 37 ± 20 | 66 ± 46 | 0.60 |
| Lateral | 88 ± 42 | 33 ± 17 | 0.10 | 103 ± 52 | 50 ± 20 | 0.60 |
| Posterior | 84 ± 40 | 88 ± 41 | 0.74 | 34 ± 16 | 60 ± 34 | 0.20 |
LV, left ventricle; RV, right ventricle.
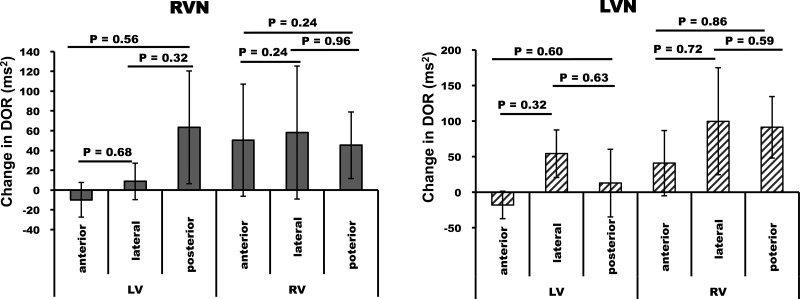
Fig. 3.
Epicardial differences in the change in DOR from BL for anterior, lateral, posterior regions of the LV and RV for RVNS and LVNS. Note that no regional differences in DOR were found. Data are means ± SE.
Table 3.
Comparison of the differences in the change of DOR from BL between regions during right VNS
| Region | Versus Region | Log Mean Difference | SE of the Difference | P Value |
|---|---|---|---|---|
| LV endocardium | ||||
| Anterior | Lateral | 0.98 | 0.61 | 0.11 |
| Anterior | Posterior | 0.11 | 0.61 | 0.86 |
| Anterior | Septum | 0.34 | 0.61 | 0.58 |
| Lateral | Posterior | −0.87 | 0.61 | 0.16 |
| Lateral | Septum | −0.64 | 0.61 | 0.30 |
| Posterior | Septum | 0.23 | 0.61 | 0.70 |
| LV epicardium | ||||
| Anterior | Lateral | 0.20 | 0.49 | 0.68 |
| Anterior | Posterior | −0.28 | 0.49 | 0.56 |
| Lateral | Posterior | −0.49 | 0.49 | 0.32 |
| RV epicardium | ||||
| Anterior | Lateral | 0.58 | 0.49 | 0.24 |
| Anterior | Posterior | 0.61 | 0.49 | 0.24 |
| Anterior | RVOT | −0.02 | 0.50 | 0.98 |
| Lateral | Posterior | 0.03 | 0.49 | 0.96 |
| Lateral | RVOT | −0.60 | 0.50 | 0.24 |
| Posterior | RVOT | −0.63 | 0.50 | 0.22 |
DOR, dispersion of repolarization; RVOT, RV outflow tract. The log of the mean difference was used given the range of dispersion observed between animals.
Table 6.
Epicardial versus endocardial regional DOR comparisons from baseline during LVN stimulation
| Region | Versus Region | Log Mean Difference | SE of the Difference | P Value |
|---|---|---|---|---|
| Endocardial LV anterior | Epicardial LV anterior | 0.43 | 0.52 | 0.41 |
| Endocardial LV anterior | Epicardial RV anterior | −0.26 | 0.52 | 0.61 |
| Epicardial LV anterior | Epicardial RV anterior | −0.69 | 0.46 | 0.14 |
| Endocardial LV lateral | Epicardial LV lateral | −0.46 | 0.52 | 0.38 |
| Endocardial LV lateral | Epicardial RV lateral | −0.86 | 0.52 | 0.10 |
| Epicardial LV lateral | Endocardial RV lateral | −0.40 | 0.46 | 0.39 |
| Endocardial LV posterior | Epicardial LV posterior | 0.59 | 0.55 | 0.29 |
| Endocardial LV posterior | Epicardial RV posterior | 0.21 | 0.55 | 0.70 |
| Epicardial LV posterior | Epicardial RV posterior | −0.37 | 0.46 | 0.42 |
Table 4.
Epicardial versus endocardial regional DOR comparisons from BL during RVN stimulation
| Region | Versus Region | Log Mean Difference | SE of the Difference | P Value |
|---|---|---|---|---|
| Endocardial LV anterior | Epicardial LV anterior | 0.17 | 0.56 | 0.76 |
| Endocardial LV anterior | Epicardial RV anterior | −0.32 | 0.56 | 0.56 |
| Epicardial LV anterior | Epicardial RV anterior | −0.50 | 0.49 | 0.31 |
| Endocardial LV lateral | Epicardial LV lateral | −0.60 | 0.56 | 0.28 |
| Endocardial LV lateral | Epicardial RV lateral | −0.72 | 0.56 | 0.20 |
| Epicardial LV lateral | Endocardial RV lateral | −0.11 | 0.49 | 0.82 |
| Endocardial LV posterior | Epicardial LV posterior | −0.22 | 0.56 | 0.69 |
| Endocardial LV posterior | Epicardial RV posterior | 0.18 | 0.56 | 0.76 |
| Epicardial LV posterior | Epicardial RV posterior | 0.40 | 0.49 | 0.42 |
Table 5.
Comparison of the differences in the change of DOR from BL between regions during LVN stimulation
| Region | Versus Region | Log Mean Difference | SE of the Difference | P Value |
|---|---|---|---|---|
| LV endocardium | ||||
| Anterior | Lateral | 0.43 | 0.58 | 0.46 |
| Anterior | Posterior | −0.40 | 0.60 | 0.51 |
| Anterior | Septum | 0.61 | 0.58 | 0.30 |
| Lateral | Posterior | −0.83 | 0.60 | 0.17 |
| Lateral | Septum | 0.18 | 0.58 | 0.76 |
| Posterior | Septum | 1.00 | 0.60 | 0.099 |
| LV epicardium | ||||
| Anterior | Lateral | −0.46 | 0.46 | 0.32 |
| Anterior | Posterior | −0.24 | 0.46 | 0.60 |
| Lateral | Posterior | 0.22 | 0.46 | 0.63 |
| RV epicardium | ||||
| Anterior | Lateral | −0.17 | 0.46 | 0.72 |
| Anterior | Posterior | 0.08 | 0.46 | 0.86 |
| Anterior | RVOT | 0.00 | 0.49 | 0.99 |
| Lateral | Posterior | 0.25 | 0.46 | 0.59 |
| Lateral | RVOT | 0.17 | 0.49 | 0.73 |
| Posterior | RVOT | −0.08 | 0.49 | 0.86 |
The log of the mean difference was used given the range of dispersion observed between animals.
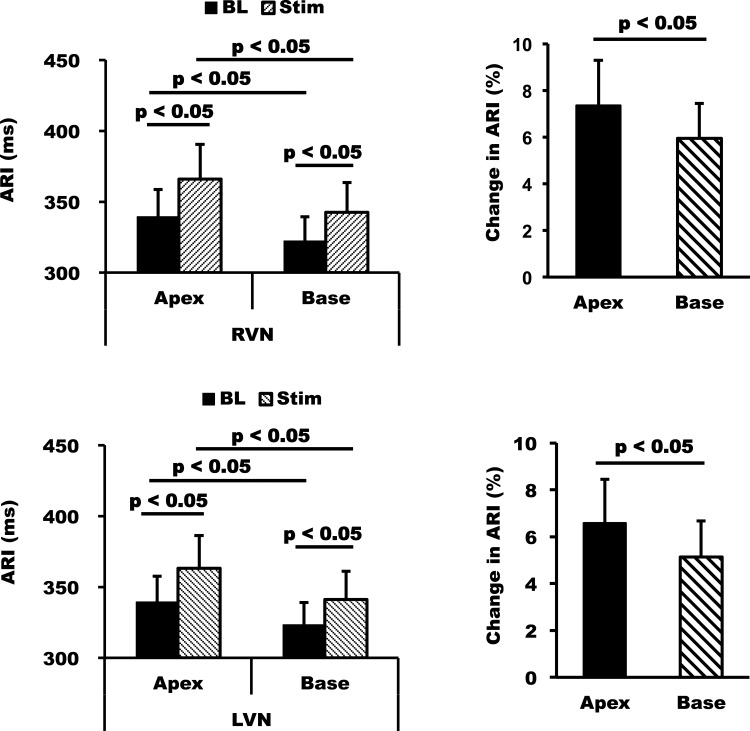
When apicobasal differences were compared, the apex showed a slightly greater prolongation of ARI than the base with both right and left VNS from 339 ± 19 to 366 ± 25 ms and from 339 ± 18 to 363 ± 23 ms, respectively (P < 0.05 vs. baseline for both right and left VNS; Fig. 4). ARI at the base increased from 322 ± 17 to 342 ± 21 ms with right VNS and from 323 ± 16 to 341 ± 20 ms with left VNS. The direction of repolarization, however, was maintained during stimulation as the base of heart had a shorter ARI compared with the apex at baseline before both right and left VNS (P < 0.05 for baseline apex vs. base mean ARI; Fig. 4).
Fig. 4.
Apicobasal differences in response to RVNS and LVNS. RVNS and LVNS had greater effects on the apex than the base without changing the direction of repolarization, as the base of the heart had a shorter ARI at baseline. The effects of RVNS versus LVNS on the apex and base were similar, again showing no laterality in response. Data are means ± SE.
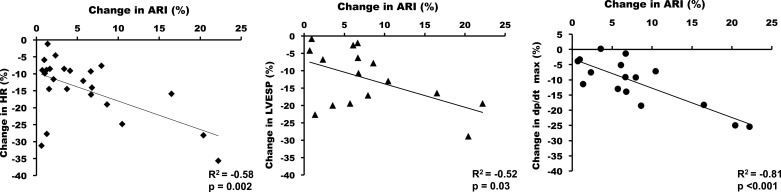
Among the hemodynamic parameters, the effects of VNS on HR, dP/dtmax, and LVESP had the highest correlation with the increase in ventricular ARI (R2 = −0.58 for HR, P = 0.002; R2 = 0.81, P < 0.0001 for dP/dtmax; and R2 = −0.52, P = 0.04 for LVESP). Of note, the parameter that correlated most strongly with ARI effects was dP/dtmax (Fig. 5).
Fig. 5.
Correlation between effects of VNS on ARI and hemodynamic parameters. The decrease in dP/dtmax, and not heart rate (HR) or LV end-systolic pressure (LVESP), had the highest correlation with the increase in ARI, also further illustrating the ventricular myocardial effects of VNS. Plots show combined RVNS and LVNS data.
Effects of right and left VNS on endocardial ARI and DOR.
On the endocardium, right and left VNS prolonged ARI from 281 ± 16 to 298 ± 17 ms (P < 0.001) and from 276 ± 18 to 297 ± 19 ms (P = 0.04) versus baseline, respectively. DOR of the LV endocardium changed from 84 ± 22 to 127 ± 28 ms2 with right VNS and from 115 ± 35 to 139 ± 49 ms2 with left VNS, although these differences from baseline were not statistically significant (P = 0.2 for right VNS and P = 0.4 for left VNS). Similar to the epicardium, no regional differences in the increase in endocardial ARI across the anterior, lateral, and posterior regions of the LV were found during right or left VNS or between right and left VNS (Fig. 6). Similar to the epicardium, no significant anterior, lateral, or posterior differences in DOR during right or left VNS were observed on the endocardium (Fig. 7). Detailed regional DOR comparisons are shown in Tables 3–6.
Fig. 6.
Global and regional mean endocardial ARIs in response to RVNS and LVNS. Note that all endocardial regions (anterior, lateral, posterior, and septal LV) demonstrated a similar increase in ARI with no significant differences in the magnitude of the response for each region. No differences between RVNS versus LVNS were found. Data are means ± SE. *P < 0.01 vs. BL.
Fig. 7.
Endocardial comparisons in the change in DOR from BL for anterior, lateral, and posterior regions of the LV and RV for RVNS and LVNS. Note that no statistically significant regional differences in the change of DOR from BL were found. Data are means ± SE.
Transmural differences in ARI.
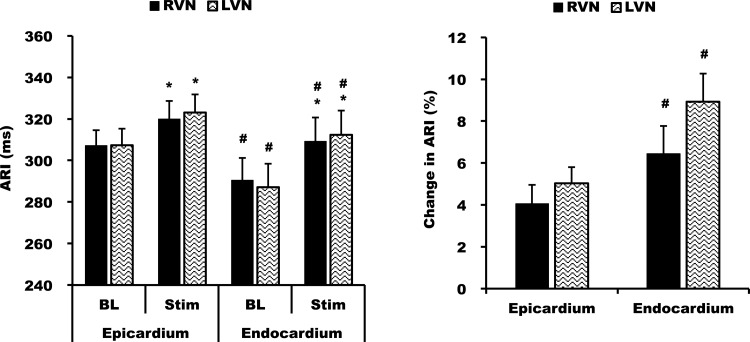
During right VNS, mean LV endocardial ARI in the midregion of the LV increased from 281 ± 16 to 298 ± 17 ms (6 ± 2%, P = 0.04). LV epicardial ARI increased from 313 ± 11 to 324 ± 13 ms (4 ± 1%, P < 0.01). During left VNS, LV endocardial ARI prolonged from 276 ± 18 to 297 ± 19 ms (8 ± 2%, P < 0.01). LV epicardial ARI prolonged from 312 ± 12 to 325 ± 12 ms (4 ± 1%, P < 0.01). Therefore, the endocardium showed a greater prolongation of ARI than the epicardium during both right and left VNS (P < 0.01 both conditions; Fig. 8). The greater increase in ARI on the endocardium was consistent across each region.
Fig. 8.
Epicardial and endocardial differences in ARI during RVNS and LVNS. Both RVNS and LVNS increased endocardial ARI more than epicardial ARI. *P < 0.01 vs. BL; #P < 0.05 vs. the epicardium.
Effect of HR on ventricular ARI by VNS.
Atrial pacing was performed during VNS at the same HR as baseline in four animals. Global ARI prolonged from 362 ± 30 to 389 ± 32 ms by right VNS alone. During pacing, ARI still prolonged to 370 ± 30 ms (mean ± SE). Left VNS also demonstrated a prolongation of ARI from 357 ± 23 to 379 ± 30 ms and remained prolonged during atrial pacing (367 ± 24 ms).
The PR interval before right VNS was 123 ± 4 ms, increased to 133 ± 8 ms during VNS, and further increased to 194 ± 10 ms (means ± SE), likely due faster pacing during VNS leading to a greater decremental conduction in the AV node. Before left VNS, the PR interval was 128 ± 8 ms, which increased to 142 ± 13 ms during VNS and further increased to 198 ± 10 ms (means ± SE) with atrial pacing during VNS.
Subthreshold VNS in one animal showed that despite a lack of change in HR, right VNS prolonged ARI from 423 ± 4 to 444 ± 5 ms and left VNS prolonged ARI from 424 ± 4 to 431 ± 4 ms.
DISCUSSION
Major findings.
The major findings of the present study are as follows:
1. Both right and left VNS increased ventricular ARI. This increase was strongly correlated with dP/dtmax. HR and LVESP showed moderate correlations. Parameters of diastolic function were not improved with VNS.
2. During both right and left VNS, epicardial ARI at the apex prolonged more than ARI at the base of the heart. VNS prolonged ARI to a similar degree on the anterior, posterior, and lateral walls of the LV and RV.
3. The endocardium showed a greater prolongation in ARI compared with the epicardium. These effects were similar between right and left VNS.
4. There were no differences between right and left VNS on hemodynamic responses or regional repolarization of the epicardium or endocardium. Therefore, no significant laterality to functional innervation of these nerves was observed.
Global and regional increases in ARI due to right and left VNS.
A histological study (14) has shown that there may be small differences in the parasympathetic innervation of the RV and LV; however, few studies have analyzed functional regional differences in detail. Martin et al. (35) demonstrated that left VNS showed a slightly greater prolongation of the LV epicardial posterior wall (mean difference of 1.2 ms) compared with right VNS in a canine model. Furthermore, they found that VNS did not prolong APD on the anterior epicardial RV (35). In our study, no significant regional differences between right and left VNS on ARI of the LV or RV across anterior, posterior, and lateral regions were noted. A clear increase in endocardial ARI compared with epicardial ARI was observed with both right and left VNS, and both increased ARI at the apex more than the base. Furthermore, a significant prolongation in ARI from baseline with both right and left VNS on the anterior RV epicardium was seen. The difference in the results of this study may be due to a more detailed assessment of repolarization, with multiple electrodes in each region, the fact that ARI may be a more accurate surrogate of APD, and interspecies differences in innervation between canine and porcine models.
From a physiological and anatomic perspective, the lack of anterior, lateral, and posterior regional differences and laterality of the effects of right and left VNS on repolarization may be due to the type of nerve fibers (preganglionic rather than postganglionic fibers) in the vagosympathetic trunk and their obligatory synapse within the ganglia of the intrinsic cardiac nervous system (3, 44). Postganglionic fibers to the myocardium arise from these intrinsic cardiac ganglia (43). The intrinsic cardiac nervous system is known to regulate hemodynamic effects of VNS via a complex, integrated neural network. Therefore, elimination of a single ganglion may reduce but does not eliminate chronotropic or dromotropic responses (43). The fact that both right and left VNS have similar effects suggests that the neurons of the intrinsic cardiac ganglia mitigate and “smooth out” any electrophysiological laterality between the two sides, distributing the postganglionic effects to all regions in a more homogenous manner. These results are further confirmed by a microdialysis study (2) that demonstrated that the level of LV epicardial ACh release is similar with right versus left VNS. This is unlike sympathetic ganglia, where the left stellate ganglion's postganglionic fibers provide greater functional innervation to the posterior walls of the ventricles and the right stellate ganglion provides greater innervation to the anterior walls of the ventricles (29, 55, 58). These results support the value of unilateral VNS for cardiac therapeutic purposes as the net effects are likely to be distributed uniformly to the ventricles.
Apicobasal differences in ARI.
In the present study, significant apicobasal differences in response to VNS were noted, with the apex demonstrating a greater prolongation of ARI than the base. Right or left VNS, however, did not lead to a reversal in the direction of repolarization as the base had a shorter ARI at baseline. Mantravadi et al., using an optical mapping study in a decentralized Langendorff model, showed that bilateral VNS prolonged APD more at the apex than the base, consistent with this study. Mantravadi and colleagues (32a), however, reported that the repolarization at the apex was shorter than the base at baseline. This difference with our study may be due to the type of ex vivo preparation versus the location of the optical mapping performed, as certain regions of the anterior base of the LV have been reported to have a longer APD than the apex or the posterior base of the heart (6, 50). In the present study, the base overall had a shorter ARI at baseline, consistent with rabbit myocyte, canine, porcine, and human mapping studies showing a shorter APD, functional refractory period, and ARI at the base under control conditions (10, 11, 40, 42, 55). The shorter ARI at baseline in vivo could be due to the distribution of K+ channels such as a greater concentration of slowly activating delayed rectifier K+ current (IKs) channels at the base as well as a greater concentration of sympathetic fibers in this region (11, 26). The apicobasal differences during VNS were surprising and cannot be attributed to HR alone, as bradycardia would have affected all regions. Furthermore, they cannot be solely attributed release of other cotransmitters that may prolong APD, such as vasoactive intestinal peptide, as this would have lead to a greater prolongation of ARI at the base rather than the apex, similar to differences observed on the endocardium versus the epicardium. Therefore, the apicobasal differences may be due to reflex sympathetic activation (via activation of IKs, which is more prominent at the base, leading to shorter APD) or the distribution of ACh-dependent K+ channels, which maybe more densely distributed at the base, leading to a greater shortening of ARI at the base compared with the apex.
Transmural differences in ARI.
Overall, both right and left VNS caused small increases in DOR on the epicardium. This may be due to the bradycardia caused by VNS (28) or to the more sparse density of parasympathetic nerve fibers on the epicardium (51). In fact, functional parasympathetic innervation of the endocardium was greater than the epicardium, consistent with a histological study (51) showing that the endocardium has a greater density of parasympathetic fibers. Furthermore, parasympathetic fibers are thought to run from the endocardium to the epicardium, as supported by the observation that denervation of the epicardium does not reduce the endocardial ventricular response to VNS (34).
Hemodynamic correlates of VNS.
Cervical VNS has been shown to result in a negative chronotropic and inotropic response (4, 18, 22, 36, 57), although specific branches of the thoracic vagus nerve intermingle with sympathetic fibers and can have varied localized chronotropic and inotropic effects (5). Previous studies (12, 19) have suggested that the RVN may provide greater innervation to the sinoatrial (SA) node, whereas the LVN may provide greater innervation to the AV node in decentralized hearts. However, Armour et al. (4, 5) showed that both right and left VNS affect the SA node in a canine model with intact VNS. In the present study, we aimed to keep a similar sinus rate between right and left VNS to eliminate differences between right and left VNS that maybe due to HR alone. However, we did not observe a difference on PR interval and, therefore, AV node conduction between right versus left VNS. With regard to LV pressure, both right and left cervical VNS decrease LV pressure to a similar degree in a decentralized rabbit Langendorff model (7). Analogously, our study showed a significant decrease in LVESP and dP/dtmax. However, dP/dtmax showed the strongest correlation with repolarization effects. This finding is potentially significant in that when assessing ventricular effects of stimulation, particularly in clinical trials, HR may not be the best marker of the appropriate level of VNS.
Effects of VNS on lusitropy and diastolic function are more controversial. Xenopolous et al. (57) demonstrated no changes in LVESP or τ (57), whereas Henning et al. (22) showed that VNS decreased LVESP and increased τ in a canine model. In this porcine model, both right and left VNS decreased LVESP without affecting LVEDP, increased dP/dtmin, and increased τ, suggesting that VNS does not improve, and may in fact worsen, diastolic function.
Limitations.
General anesthetics can suppress nerve activity; however, we were able to reliably record a cardiomotor response during VNS. In addition, the drug concentrations were maintained at a constant level in this study. Furthermore, to reduce effects of inhaled anesthetics, an α-chloralose infusion was used during ARI recordings and VNS. For global and regional analyses, ARIs were not corrected for HR, as any HR effects on DOR are physiologically important. Atrial and ventricular pacing were not performed in all animals given the effect of pacing on altering autonomic tone. Finally, HR would not affect comparisons of regional differences within right and left VNS, and we were able to achieve a similar mean HR response during both right and left VNS. β-Blockers were not given during these experiments. Therefore, the effects of VNS in the setting of sympathetic blockade cannot be assessed from these experiments. Finally, VNS is currently being performed in various clinical trials in different ways without a clear standard; therefore, the results of this study may not completely duplicate what may be seen in clinical studies.
Conclusions.
In the present study, the detailed assessment of regional ARI and hemodynamic parameters showed a lack of laterality between the effects of right versus left VNS. In addition, the functional effects of VNS were greater at the apex than the base and greater on the endocardium than the epicardium, likely reflecting the functional distribution of parasympathetic innervation. The ventricular electrophysiological effects of VNS correlate best with the decrease in ventricular inotorpy and, specifically, dP/dtmax. Our results have significant implications given the advent of neuromodulation therapies using unilateral VNS for ventricular arrhythmias and cardiomyopathy.
GRANTS
This work was supported by American Heart Association National Fellow to Faculty Transition Award 11FTF755004 (to M. Vaseghi) and National Heart, Lung, and Blood Institute Grant R01-HL-084261 (to K. Shivkumar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.Y., E.L.S., P.S.R., J.D.H., N.M., and M.V. performed experiments; K.Y., P.S.R., J.D.H., N.M., and M.V. analyzed data; K.Y., E.L.S., A.M., K.S., and M.V. interpreted results of experiments; K.Y., E.L.S., and M.V. prepared figures; K.Y. and M.V. drafted manuscript; K.Y., E.L.S., P.S.R., J.D.H., N.M., A.M., K.S., and M.V. approved final version of manuscript; E.L.S., P.S.R., J.D.H., N.M., A.M., K.S., and M.V. edited and revised manuscript; K.S. and M.V. conception and design of research.
REFERENCES
- 1.Ajijola OA, Vaseghi M, Zhou W, Yamakawa K, Benharash P, Hadaya J, Lux RL, Mahajan A, Shivkumar K. Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am J Physiol Heart Circ Physiol 304: H579–H588, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama T, Yamazaki T. Effects of right and left vagal stimulation on left ventricular acetylcholine levels in the cat. Acta Physiol Scand 172: 11–16, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford Univ. Press, 2004, p. 118–152 [Google Scholar]
- 4.Armour JA, Randall WC. Rebound cardiovascular responses following stimulation of canine vagosympathetic complexes or cardiopulmonary nerves. Can J Physiol Pharmacol 63: 1122–1132, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Armour JA, Randall WC, Sinha S. Localized myocardial responses to stimulation of small cardiac branches of the vagus. Am J Physiol 228: 141–148, 1975 [DOI] [PubMed] [Google Scholar]
- 6.Autenrieth G, Surawicz B, Kuo CS. Sequence of repolarization on the ventricular surface in the dog. Am Heart J 89: 463–469, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci 124: 69–80, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone–epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol 95: 80–92, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 583: 695–704, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess MJ, Green LS, Millar K, Wyatt R, Abildskov JA. The sequence of normal ventricular recovery. Am Heart J 84: 660–669, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilideclass III agents. Cardiovasc Res 43: 135–147, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Cohn AE, Lewis T. The predominant influence of the left vagus nerve upon conduction between the auricles and ventricles in the dog. J Exp Med 18: 739–747, 1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corr PB, Gillis RA. Role of the vagus nerves in the cardiovascular changes induced by coronary occlusion. Circulation 49: 86–97, 1974 [DOI] [PubMed] [Google Scholar]
- 14.Crick SJ, Anderson RH, Ho SY, Sheppard MN. Localisation and quantitation of autonomic innervation in the porcine heart II: endocardium, myocardium and epicardium. J Anat 195: 359–373, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du XJ, Cox HS, Dart AM, Esler MD. Depression of efferent parasympathetic control of heart rate in rats with myocardial infarction: effect of losartan. J Cardiovasc Pharmacol 31: 937–944, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Euler DE, Nattel S, Spear JF, Moore EN, Scanlon PJ. Effect of sympathetic tone on ventricular arrhythmias during circumflex coronary occlusion. Am J Physiol Heart Circ Physiol 249: H1045–H1050, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Facchini M, De Ferrari GM, Bonazzi O, Weiss T, Schwartz PJ. Effect of reflex vagal activation on frequency of ventricular premature complexes. Am J Cardiol 68: 349–354, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Hageman GR, Randall WC, Armour JA. Direct and reflex cardiac bradydysrhythmias from small vagal nerve stiumaltions. Am Heart J 89: 338–348, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Hamlin RL, Smith CR. Effects of vagal stimulation on S-A and A-V nodes. Am J Physiol 215: 560–568, 1968 [DOI] [PubMed] [Google Scholar]
- 20.Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, Mann DL. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J 163: 954–962 e951, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 81: 281–288, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Henning RJ, Levy MN. Effects of autonomic nerve stimulation, asynchrony, and load on dP/dtmax and on dP/dtmin. Am J Physiol Heart Circ Physiol 260: H1290–H1298, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Herre JM, Thames MD. Responses of sympathetic nerves to programmed ventricular stimulation. J Am Coll Cardiol 9: 147–153, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res 62: 112–121, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Janse MJ, Schwartz PJ, Wilms-Schopman F, Peters RJ, Durrer D. Effects of unilateral stellate ganglion stimulation and ablation on electrophysiologic changes induced by acute myocardial ischemia in dogs. Circulation 72: 585–595, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18: 32–39, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation 47: 291–298, 1973 [DOI] [PubMed] [Google Scholar]
- 28.Kim JJ, Nemec J, Papp R, Strongin R, Abramson JJ, Salama G. Bradycardia alters Ca2+ dynamics enhancing dispersion of repolarization and arrhythmia risk. Am J Physiol Heart Circ Physiol 304: H848–H860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kralios FA, Martin L, Burgess MJ, Millar K. Local ventricular repolarization changes due to sympathetic nerve-branch stimulation. Am J Physiol 228: 1621–1626, 1975 [DOI] [PubMed] [Google Scholar]
- 30.Levy MN, Blattberg B. Effect of vagal stimulation on the overflow of norepinephrine into the coronary sinus during cardiac sympathetic nerve stimulation in the dog. Circ Res 38: 81–84, 1976 [DOI] [PubMed] [Google Scholar]
- 31.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Martins JB. Autonomic control of ventricular tachycardia: sympathetic neural influence on spontaneous tachycardia 24 hours after coronary occlusion. Circulation 72: 933–942, 1985 [DOI] [PubMed] [Google Scholar]
- 32a.Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res 100: e72–e80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res 46: 100–110, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Martins JB, Zipes DP. Epicardial phenol interrupts refractory period responses to sympathetic but not vagal stimulation in canine left ventricular epicardium and endocardium. Circ Res 47: 33–40, 1980 [DOI] [PubMed] [Google Scholar]
- 35.Martins JB, Zipes DP, Lund DD. Distribution of local repolarization changes produced by efferent vagal stimulation in the canine ventricles. J Am Coll Cardiol 2: 1191–1199, 1983 [DOI] [PubMed] [Google Scholar]
- 36.Massari VJ, Dickerson LW, Gray AL, Lauenstein JM, Blinder KJ, Newsome JT, Rodak DJ, Fleming TJ, Gatti PJ, Gillis RA. Neural control of left ventricular contractility in the dog heart: synaptic interactions of negative inotropic vagal preganglionic neurons in the nucleus ambiguus with tyrosine hydroxylase immunoreactive terminals. Brain Res 802: 205–220, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72: 1372–1379, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, Goldstein RE, Epstein SE. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation 49: 943–947, 1974 [DOI] [PubMed] [Google Scholar]
- 39.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 73: 750–760, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 4: 341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Opthof T, Dekker LR, Coronel R, Vermeulen JT, van Capelle FJ, Janse MJ. Interaction of sympathetic and parasympathetic nervous system on ventricular refractoriness assessed by local fibrillation intervals in the canine heart. Cardiovasc Res 27: 753–759, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci USA 103: 6309–6314, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285: R1066–R1075, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Randall WC, Ardell JL, Wurster RD, Milosavljevic M. Vagal postganglionic innervation of the canine sinoatrial node. J Auton Nerv Syst 20: 13–23, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Randall WC, Armour JA. Regional vagosympathetic control of the heart. Am J Physiol 227: 444–452, 1974 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation 69: 790–800, 1984 [DOI] [PubMed] [Google Scholar]
- 47.Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L, Odero A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 10: 884–891, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH, Chattipakorn N. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm 10: 1700–1707, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Stramba-Badiale M, Vanoli E, De Ferrari GM, Cerati D, Foreman RD, Schwartz PJ. Sympathetic-parasympathetic interaction and accentuated antagonism in conscious dogs. Am J Physiol Heart Circ Physiol 260: H335–H340, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Toyoshima H, Lux RL, Wyatt RF, Burgess M, Abildskov JA. Sequences of early and late phases of repolarization on dog ventricular epicardium. J Electrocardiol 14: 143–152, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Ulphani JS, Cain JH, Inderyas F, Gordon D, Gikas PV, Shade G, Mayor D, Arora R, Kadish AH, Goldberger JJ. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm 7: 1113–1119, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 68: 1471–1481, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Vaseghi M, Shivkumar K. Neuraxial modulation for ventricular arrhythmias: a new hope. Heart Rhythm 9: 1888–1889, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50: 404–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaseghi M, Yamakawa K, Sinha A, So EL, Zhou W, Ajijola OA, Lux RL, Laks M, Shivkumar K, Mahajan A. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am J Physiol Heart Circ Physiol 305: H1020–H1030, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 58: 751–760, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xenopoulos NP, Applegate RJ. The effect of vagal stimulation on left ventricular systolic and diastolic performance. Am J Physiol Heart Circ Physiol 266: H2167–H2173, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res 18: 416–428, 1966 [DOI] [PubMed] [Google Scholar]
- 59.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 3: 108–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]