Abstract
Sepsis is strongly associated with patency of the ductus arteriosus (PDA) in critically ill newborns. Inflammation and the aminoglycoside antibiotics used to treat neonatal sepsis cause smooth muscle relaxation, but their contribution to PDA is unknown. We examined whether: 1) lipopolysaccharide (LPS) or inflammatory cytokines cause relaxation of the ex vivo mouse DA; 2) the aminoglycosides gentamicin, tobramycin, or amikacin causes DA relaxation; and 3) newborn infants treated with aminoglycosides have an increased risk of symptomatic PDA (sPDA). Changes in fetal mouse DA tone were measured by pressure myography in response to LPS, TNF-α, IFN-γ, macrophage-inflammatory protein 2, IL-15, IL-13, CXC chemokine ligand 12, or three aminoglycosides. A clinical database of inborn patients of all gestations was analyzed for association between sPDA and aminoglycoside treatment. Contrary to expectation, neither LPS nor any of the inflammatory mediators caused DA relaxation. However, each of the aminoglycosides caused concentration-dependent vasodilation in term and preterm mouse DAs. Pretreatment with indomethacin and N-(G)-nitro-L-arginine methyl ester did not prevent gentamicin-induced DA relaxation. Gentamicin-exposed DAs developed less oxygen-induced constriction than unexposed DAs. Among 488,349 infants who met the study criteria, 40,472 (8.3%) had sPDA. Confounder-adjusted odds of sPDA were higher in gentamicin-exposed infants, <25 wk and >32 wk. Together, these findings suggest that factors other than inflammation contribute to PDA. Aminoglycoside-induced vasorelaxation and inhibition of oxygen-induced DA constriction support the paradox that antibiotic treatment of sepsis may contribute to DA relaxation. This association was also found in newborn infants, suggesting that antibiotic selection may be an important consideration in efforts to reduce sepsis-associated PDA.
Keywords: human, mouse, ductus arteriosus, lipopolysaccharide, endotoxin, cytokine, aminoglycoside, gentamicin
patency of the ductus arteriosus (PDA) is critical during fetal life. The DA is responsible for diverting blood flow from the systemic venous return into the descending aorta, bypassing the uninflated fetal lungs, to support the peripheral circulation and perfuse the placenta. The DA normally closes soon after birth so that systemic venous return is directed through the pulmonary circulation, where newborn respiratory gas exchange occurs. In the full-term infant, the DA closes within 12–24 h after birth. In premature infants, particularly those with lung disease, the DA may not close for days or weeks, resulting in a long-term increase in blood flow through the low-resistance pulmonary vascular bed. If the postnatal DA fails to close, either spontaneously or by pharmacologic or surgical means, infants incur increased risk for a number of threatening conditions, including congestive heart failure, pulmonary edema, progressive respiratory insufficiency, lung injury, chronic lung disease, compromised systemic blood flow, complications related to immaturity of organ systems, and iatrogenic side effects secondary to efforts to treat these conditions. When the ductus shunt is small or without significant collateral effects, the condition is labeled PDA. When the intensity or duration of left-to-right shunting through the DA is sufficient to cause any of these side effects, the condition is referred to as symptomatic PDA (sPDA).
Of the numerous risk factors associated with PDA (40), van de Bor et al. (51) reported that sepsis was second only to hyaline membrane disease as the factor most predictive of sPDA. Neonates with early-onset sepsis have increased incidence of sPDA (49). Late-onset sepsis is also associated with sPDA (50). Rojas et al. (43) found a significant increase in the odds ratio for the occurrence of chronic lung disease when sepsis occurred simultaneously with sPDA. In a follow-up study, they showed that sepsis increased the risk for late reopening of the ductus (23). Moreover, infection preceded the diagnosis of sPDA in a majority of their infants, who were also found to have increased serum levels of prostaglandins (PGs; 6-keto PGF1α) or the inflammatory mediator, TNF-α. In another study, infants with sPDA complicated by sepsis were less likely to achieve ductus closure following treatment with ibuprofen compared with infants with sPDA and no evidence of sepsis (18). Multiple investigators also report that chorioamnionitis or histological evidence of fetal inflammation contributes to the risk for sPDA (29, 35, 44, 53).
Even though sepsis appears to be a major factor in the pathway leading from PDA to sPDA, it is not clear whether the mechanisms linking sPDA with clinical sepsis are causal or only indirect relationships. There are many possibilities that might explain this relationship. First, since hypoxia relaxes the DA in vivo (32), respiratory insufficiency secondary to the inflammatory process (25) might be expected to be one of the principal causes of DA relaxation in critically ill, premature infants with sepsis. Second, sPDA is known to be associated with excessive fluid intake (4, 48), a practice that may result from the need to treat an increasing third space volume that often accompanies sepsis and systemic infections. Third, components of bacteria, such as LPS (12, 13), or cytokines released during the host inflammatory response (27, 45) might be expected to have a vasodilatory effect on the DA. Finally, there are three compounding effects that may contribute to the link between sepsis and symptomatic PDA which should be considered: 1) the release of endogenous NO or CO (3), 2) the release of vasodilatory lipid mediators such as prostaglandin E2 or prostacyclin (23), or 3) the administration of drugs such as CYP inhibitors that are known relaxants of the DA (17).
Drugs frequently used during the treatment of sepsis, such as furosemide (16) and the aminoglycosides, are known to cause smooth muscle relaxation. For example, vasodilatory effects of aminoglycosides have been demonstrated in the myocardium and peripheral blood vessels (20, 22, 24)—effects thought to be the result of antagonistic actions of these antibiotics on Ca++ flux at the cell membrane (22, 26). The purpose of this study was to establish whether the association of sepsis with sPDA might be the result of: 1) the response of the DA to LPS or certain vasoactive cytokines generated by inflammation or 2) the relaxing effect of gentamicin or other aminoglycosides on the DA. Cannulated vessel myography was used to study directly the vasomotor tone of the isolated DA. Due to the limited availability and viability of human ductus tissues, term and preterm mouse DAs were examined. Although LPS and proinflammatory cytokines failed to relax the DA, each aminoglycoside had a significant vasodilatory effect. Analysis of a large clinical database of newborn infants with well-documented risk factors revealed that aminoglycoside exposure was independently associated with increased risk of sPDA. These results offer surprising new insights into the pathogenesis of PDA and provide precautionary guidance for antibiotic use in all critically ill newborn infants.
METHODS
Animals
Experiments were conducted in accordance with National Institutes of Health Animal Care Standards and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Adult, female CD1 mice (Charles River Laboratories, Raleigh, NC) were bred with fertile males of the same strain to produce timed pregnancies. The morning of finding a vaginal plug was designated as day 1 (d1) of pregnancy. Pregnant females were anesthetized by intraperitoneal injection of 0.4 ml of 2.5% avertin (2,2,2-tribromoethanol in tert-amyl alcohol; Sigma-Aldrich, St. Louis, MO), followed by isoflurane inhalation (Baxter, Deerfield, IL) to facilitate fetal anesthesia. Dams were euthanized by cervical dislocation on d15 (“preterm”) or d19 (“term”) of gestation, and fetuses were delivered by uterine incision.
An established model of neonatal polymicrobial sepsis (57) was used to examine effects on postnatal DA closure. One 6- to 8-wk-old C57BL/6J female mouse (The Jackson Laboratory, Bar Harbor, ME) was euthanized <2 wk after arrival. The abdomen was opened, and the cecum was identified. Cecal contents were expressed and weighed. Cecal contents were mixed with 5% dextrose to a final concentration of 160 mg/ml. The cecal slurry was vortexed briefly to homogenize. Aggregates were removed from the slurry preparation using a 40-μm filter, and the filtrate was drawn up into a 1-ml syringe using a 32-g needle. Newborn mouse pups were weighed and injected with a volume of 25 μl slurry to deliver ∼2.6 mg/g body wt within 15 min of preparation. Control pups were injected with an equivalent volume of 5% dextrose.
Tissue Collection
For myography studies, fetuses were submerged immediately in ice-cold, deoxygenated (95% N2, 5% CO2) Krebs buffer and secured in a supine position in a dissection dish. Krebs buffer was modified (in mM: 109 NaCl, 34 NaHCO3, 4.7 KCl, 0.9 MgSO4, 1.0 KH2PO4, 11.1 dextrose, and 2.5 CaCl2) to maintain a stable pH (7.30–7.35) and relative hypoxia in the vessel bath (dissolved oxygen content = 1.5–1.8%; measured PaO2 = 38–45 Torr). The isolated main pulmonary artery-DA-transverse aorta segment of vascular tissue was freed from any remaining tissues. At all times, care was taken to avoid excess tension or stretch of vascular tissues during dissection.
Pressure Myography
In vitro studies of DA reactivity were conducted using cannulated, pressurized vessel myography and computer-assisted videomicroscopy. Fetal mouse DAs were isolated and mounted in 4 ml chambers, as described previously (38). In other studies, the ascending aorta of d19 fetuses or third- to fourth-generation mesenteric arteries of neonatal mice on the 2nd day of life (P2) were mounted on appropriate-sized cannulae (due to technical limitations, we were unable to mount mesenteric resistance arteries from P0 or P1 mice). Fetal vessels were allowed to equilibrate for 40–60 min in nonrecirculating, deoxygenated Krebs buffer (36.5°C–37.5°C) at 6 ml/min; P2 vessels were equilibrated in nonrecirculating Krebs, bubbled with 12% oxygen, to mimic newborn conditions. Distending pressure was generated by a column of Krebs buffer. Vessels were pressurized to 20 mmHg in 5-mmHg increments (term or P2 vessels) or 6 mmHg in 2-mmHg increments (preterm vessels). Isolated vessels were then stimulated with 50 mM K+ Krebs buffer (with KCl substituted for NaCl) for 3–5 min (×2) to determine the contractile potential of each vessel. Vessels were then switched to a recirculating system (20 ml circuit volume) and allowed to re-equilibrate under pressurized-recirculating conditions for 30 min. Vessel diameter was then recorded at “baseline” conditions that represented basal myogenic tone. The response of each vessel to various drugs or change in experimental conditions was videorecorded continuously using edge-detection software (IonOptix; Milton, MA). Lumen diameter was measured at the point of maximum constriction. At the completion of each study protocol, vessels were exposed to papaverine (100 μM; Sigma-Aldrich) to determine the vessel caliber at maximal relaxation.
Drug and Cytokine Studies
Ex vivo studies.
Isolated vessels were exposed to various drugs or compounds or changes in experimental conditions. Each change in experimental parameter was maintained until a stable, new vessel diameter was established. Dilation or constriction of each vessel was compared with baseline diameter under resting, pressurized conditions.
In vivo studies.
Offspring were euthanized at 4 h of age, and the DA was surgically exposed for assessment of postnatal closure. An established visual scoring system (39, 41) was used by a blinded observer (J. Reese) to estimate the degree of PDA (categorically, from 0%, 10%, 25%, 50%, 75%, 90%, or 100% with respect to the diameter of the main pulmonary artery) in response to intraperitoneal injection of LPS, gentamicin, or vehicle.
LPS studies.
The DA response to LPS (Escherichia coli 0111:B4; Sigma Aldrich) was examined in four different study protocols. 1) The isolated, pressurized fetal DA was exposed to increasing concentrations of LPS (0.001–10 μg/ml), encompassing the effective dose range determined by previous studies. Alternatively, in some studies, the isolated DA was exposed to increasing concentrations of lipoteichoic acid (LTA; 0.001–10 μg/ml; Sigma-Aldrich), a gram-positive bacterial cell-wall toxin. 2) The isolated, pressurized fetal DA was exposed to LPS (2.5 μg/ml) for 1 h. After 1 h, 100 ng/ml TNF-α was added to the bath, and alterations in DA diameter were measured. 3) Fetal mice were euthanized, their anterior chest wall was removed, and the pericardium and investing membranes overlying the DA were removed. The open thoraces were incubated at 4°C overnight in deoxygenated Krebs buffer with the addition of 2.5 μg/ml LPS. Excised DAs were mounted for myography studies the following morning. Vessel characteristics and response to KCl (12.5–50 mM) and the thromboxane agonist U-46619 (10–100 nM) were recorded. 4) Newborn term gestation mice were injected with intraperitoneal saline or LPS (0.3–1.0 mg/kg) (55) at ∼30 min of age. After a 4-h period of observation, the pups were euthanized, and the DA was surgically exposed for assessment of postnatal closure. LPS and LTA were dissolved in water just before use.
Cytokine studies.
The isolated fetal DA was exposed to increasing doses of cytokines; effective doses were determined from published studies. Selected cytokines were chosen for study based on: 1) high levels of expression (or their receptor's expression) in the DA by microarray or PCR array analysis [CXC chemokine ligand 12 (CXCL-12), IL-13, IL-15, TNF family members] (46); 2) demonstrated roles in the DA [IL-15, macrophage-inflammatory protein 2 (MIP-2), IFN-γ, TNF-α] (27, 54); or 3) their fundamental role in the inflammatory process (IFN-γ, MIP-2, TNF-α). Cytokines (PeproTech, Rocky Hill, NJ) were studied over the following concentrations in the perfusion bath: IL-13 (0.01–100 ng/ml), IL-15 (0.10–100 ng/ml), MIP-2 (0.01–100 ng/ml), TNF-α (0.001–100 ng/ml), CXCL-12 (0.10–100 ng/ml), and IFN-γ (0.01–1,000 ng/ml). All cytokines were dissolved in aqueous buffer.
Antibiotic studies.
The isolated fetal DA was studied using the following aminoglycoside protocols: 1) response to cumulative gentamicin (0.01–100 mM), tobramycin (0.01–10 mM), or amikacin (0.01–100 mM; AG Scientific, San Diego, CA); 2) pretreatment with the nonselective NO synthase inhibitor N-(G)-nitro-L-arginine methyl ester (L-NAME; 0.1 mM; Cayman Chemical, Ann Arbor, MI) and the nonselective cyclooxygenase inhibitor indomethacin (0.01 mM; Cayman Chemical) for 1 h and then exposure to increasing concentrations of gentamicin (0.01–100 mM); the response at each concentration was compared with the diameter of DAs not pretreated with L-NAME and indomethacin; or 3) pretreatment with gentamicin (3 mM) for 1 h under recirculating, deoxygenated conditions. The isolated DA was then exposed to Krebs buffer containing gentamicin that was bubbled with increasing oxygen content (0%, 2%, 5%, 12%, 21%, and 95%) under nonrecirculating conditions. DA diameter was measured at each concentration of oxygen and compared with vessels not treated with gentamicin. In a separate set of experiments, vessels were exposed to ampicillin (0.01–100 mM). L-NAME and all antibiotics were dissolved in water; indomethacin was dissolved in ethanol but did not exceed 0.1% in the final bath concentration. In some experiments, solubility limitations prevented equivalent drug exposures for all compounds. For in vivo studies, offspring of a litter that were delivered by Cesarean section were divided into three treatment groups. Newborn littermates were injected intraperitoneally with low-dose (5 mg/kg) or high-dose (50 mg/kg) gentamicin at 30 min of life and compared with untreated littermates (sham injection).
Clinical Analysis
This study comprised a descriptive review of infants from a network of 330 neonatal intensive care units (NICUs) in the United States that reported to the Pediatrix Clinical Data Warehouse. Health-care professionals providing care to these infants used a proprietary software system (BabySteps) to generate clinical admission, daily progress, and discharge notes. These data were stored in a consolidated national data set and de-identified for research purposes. The data set had no protected health information and was compliant with the Health Insurance Portability and Accountability Act of 1996 regulations.
Patients were selected from the Pediatrix Clinical Data Warehouse who met the following criteria: 1) inborn, 2) cared for at a single institution (transfers excluded), and 3) born between 1997 and 2010. sPDA was considered to be present in infants with signs of a PDA or left-to-right shunting, which lasted 3 or more days and was diagnosed after 2 days, or required ligation. Infants who died within 3 days of birth were excluded, as they would have expired before they met criteria for sPDA. Only infants that received aminoglycoside treatment before the diagnosis of sPDA were considered for inclusion. Infants with other cardiac anomalies were excluded.
Statistical Analysis
For myography studies, alterations in DA diameter were displayed as percent change in lumen diameter compared with baseline diameter at resting tone. Dose-response relationships for each compound were illustrated using best-fit curves and sigmoidal approximation (Prism 5; GraphPad Software, La Jolla, CA). For clarity, dose-response curves were shown as mean ± SE. Statistical comparisons of repeated measurements of vessel diameter were analyzed using a linear mixed-effects regression model, controlling for baseline vessel diameter. A random intercept was included in each mixed-effects model to account for the correlation arising from measuring the diameter of the same vessel at multiple concentrations. Regression models were fit using R (The R Foundation for Statistical Computing, Vienna, Austria), Stata (StataCorp, College Station, TX), and Systat (Systat Software, San Jose, CA) statistics software. Pearson's χ2 analysis was used to analyze the in vivo response to gentamicin. P < 0.05 was considered significant in all studies.
For the clinical study, multivariable logistic regression was used to estimate the association between the occurrence of significant PDA (outcome) and exposure to gentamicin. We controlled for potential confounding variables, including birth weight, gestational age, gender, presence of anomalies, type of delivery, mechanical ventilation, surfactant treatment, and antenatal steroids to evaluate the impact of exposure to aminoglycosides. The model was used in each estimated gestational-age strata to calculate stratum-specific adjusted odds ratios. The calculated and adjusted odds ratio was for the occurrence of a sPDA (dependent variable) in infants exposed to gentamicin compared with those not exposed (independent variable). We limited our model to exposure to the first 3 days, since this is the period of greatest antibiotic exposure in the neonatal population and to ensure that exposure was during the time when the PDA was closing.
RESULTS
Isolated DA Exhibits Minimal Response to a Defined Inflammatory Stimulus
With the use of a validated scoring system for estimation of PDA (39, 41), we observed impaired DA closure in a neonatal model of polymicrobial sepsis (>25% patency in eight of 11 pups from three litters vs. 0% patency in five representative vehicle-treated pups; Fig. 1A). All slurry-injected pups were viable at the time of assessment. These data suggest that the newborn mouse is a suitable model to study sepsis-associated PDA. In contrast, no detectable impairment of postnatal DA closure (by visual scoring or attempts to force blood through the lumen) was observed in newborn pups exposed to moderate (0.3 mg/kg)- or high (1 mg/kg)-dose LPS compared with their saline-injected littermates (n = 6 pups/condition; Fig. 1A). Short-term exposure of the ex vivo DA to increasing concentrations of LPS had no significant effect on vasomotor tone (n = 8 vessels from four litters; Fig. 1B). Likewise, long-term (24 h) LPS exposure did not significantly affect the ability of the DA to respond to different vasoconstrictive agents (KCl, U-46619; n = 4 vessels from two litters; Fig. 1C) compared with reference control values (P > 0.1 for each comparison) (17, 38). Exposure to LTA had no effect on the vasomotor tone of the isolated mouse DA (not shown).
Fig. 1.
Response of the ductus arteriosus (DA) to inflammatory stimuli. A: representative images of the postnatal DA at 4 h of age (arrows). The DA was closed in uninjected control mice; impaired DA closure was observed in newborn mice after cecal slurry injection but not in mice injected with 1 mg/kg LPS. AO, aorta; PA, pulmonary artery. B: exposure of the isolated, pressurized term gestation mouse DA to increasing concentrations of LPS did not alter its vasomotor tone. Data shown as mean ± SE.C: representative tracing showing that prolonged exposure (24 h) to LPS did not inhibit response of the isolated, pressurized DA to an initial stimulus with 50 mM KCl or to graduated doses of KCl (from 50 to 12.5 mM) or the thromboxane mimetic U-46619 (10–100 nM).
Cytokine Exposure Has Mixed Effects on DA Tone
By linear regression analysis, none of the studied cytokines caused relaxation of DA tone (Table 1). Instead, TNF-α, IFN-γ, CXCL-12, and IL-13 each caused vasoconstriction of 10–25% from baseline at higher concentrations (100–1,000 ng/ml). Preincubation of the isolated DA with LPS (1 h) did not affect the response to TNF-α (n = 6; data not shown), indicating that combined inflammatory stimuli did not relax the isolated DA. Neither IL-15 nor MIP-2 (CXCL-2), a murine homologue of IL-8, had significant effects on DA tone, despite exposure to concentrations of 100–1,000 ng/ml, respectively (Table 1).
Table 1.
Response of the ex vivo mouse DA to vasoactive cytokines
| Marker | Regression Coefficient | P |
|---|---|---|
| CXCL-12 | −0.027 | 0.001 |
| IFN-γ | −0.014 | 0.001 |
| IL-13 | −0.022 | 0.003 |
| IL-15 | 0.002 | 0.708 |
| MIP-2 | −0.004 | 0.502 |
| TNF-α | −0.020 | 0.000 |
Regression values indicate ductus arteriosus (DA) constriction (negative values) or dilation (positive value) in response to cytokine exposure.
CXCL-12, CXC chemokine ligand 12; MIP-2, macrophage-inflammatory protein 2.
Exposure to Aminoglycoside Antibiotics Caused Significant Concentration-Dependent Dilation of the DA
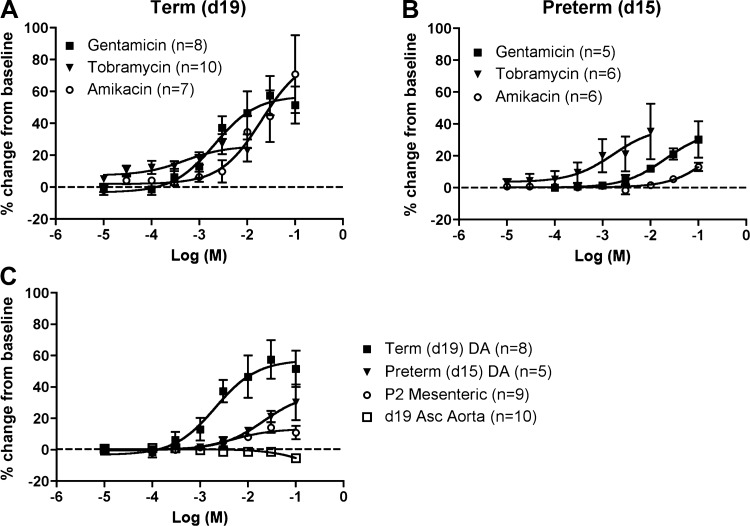
Each of the three aminoglycosides caused a significant concentration-dependent increase in the diameter of DAs from both term (Fig. 2A) and preterm (Fig. 2B) mice (P < 0.01). Tobramycin was the most potent aminoglycoside in preterm DAs but had the least effect in term DAs. The preterm DA was less affected by exposure to gentamicin than the term DA (P < 0.01; Fig. 2C). Gentamicin exposure did not affect the ascending aorta but caused a modest, concentration-dependent relaxation of mesenteric arteries of P2 mice (P < 0.01). However, gentamicin-induced relaxation of the term DA was significantly greater than the effect seen in P2 mesenteric arteries (P < 0.01; Fig. 2C). The DA and ascending aorta share common neural crest-derived progenitors of their smooth muscle coat (36). Together, these data indicate that gentamicin affects DA tone more than a conductance artery of similar size and origin (ascending aorta) or a peripheral resistance artery (mesenteric). Exposure to equimolar concentrations of ampicillin had no significant vasodilatory effects on the isolated fetal mouse DA (data not shown). Gentamicin is by far the most common aminoglycoside used in the NICU population (11), thus subsequent experiments focused on the role of gentamicin in DA relaxation.
Fig. 2.
Aminoglycoside-induced DA relaxation. A: response of the term [day 19 (d19)] DA to increasing concentrations of 3 aminoglycoside antibiotics, shown as percent change from baseline diameter. Each of the antibiotics caused a significant concentration-dependent increase in the DA diameter (P < 0.01; baseline diameter, 193 ± 18 μm, 212 ± 31 μm, and 193 ± 33 μm for gentamicin, tobramycin, and amikacin, respectively). B: response of the preterm (d15) DA to increasing concentrations of 3 aminoglycoside antibiotics, shown as percent change from baseline diameter. Each of the antibiotics caused a significant concentration-dependent increase in the DA diameter (P < 0.01; baseline diameter, 149 ± 15 μm, 151 ± 32 μm, and 167 ± 20 μm for gentamicin, tobramycin, and amikacin, respectively). C: gentamicin stimulated greater relaxation of the term than preterm DA (P < 0.01; data from A and B). Gentamicin induced less relaxation of 3rd- to 4th-generation mesenteric arteries of 2-day-old mice (P2; baseline diameter, 123 ± 6 μm) than the term DA (P < 0.01) and did not affect the ascending (Asc) aorta of term (d19) gestation mice (baseline diameter, 459 ± 14 μm). Data shown as mean ± SE. The gentamicin dose-response curve in term gestation mice (1 condition in A) was published previously as a commentary item in a general review article (40).
Gentamicin-Induced DA Dilation Is Not Mediated by PGs or NO
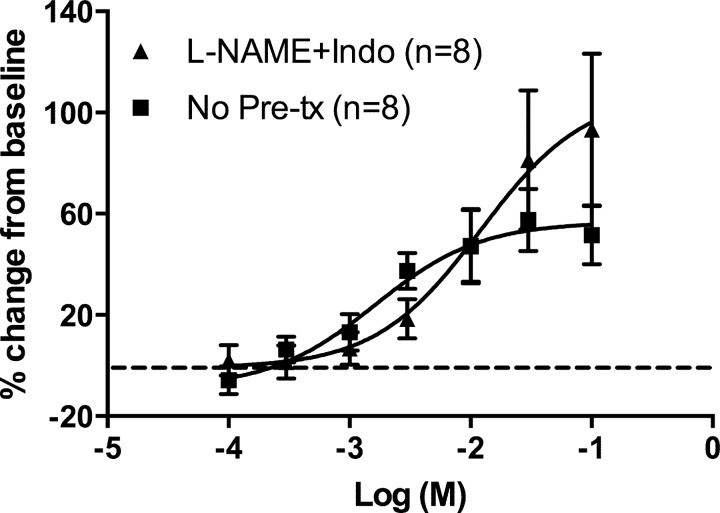
The term gestation DA (d19), either treated or not treated with L-NAME and indomethacin, had a concentration-dependent increase in DA diameter with increasing concentration of gentamicin (P < 0.001). Pretreatment with indomethacin and L-NAME had no significant effect on gentamicin-induced DA relaxation or the magnitude of increase in DA diameter (P = 0.166; Fig. 3).
Fig. 3.
Gentamicin-induced DA relaxation in the presence of indomethacin (Indo) and N-(G)-nitro-L-arginine methyl ester (L-NAME). The term DA (d19), either treated or not treated with inhibitors of prostaglandin synthesis (indomethacin) and nitric oxide synthesis (L-NAME), displayed a concentration-dependent increase in DA diameter with an increasing concentration of gentamicin [P < 0.001; baseline diameter of untreated DA, 193 ± 18 μm (data from Fig. 2A)]. Pretreatment (Pre-tx) with indomethacin and L-NAME had no significant effect on the magnitude of increase in DA diameter (P = 0.166; baseline diameter, 157 ± 27 μm). Data shown as mean ± SE.
Pretreatment with Gentamicin Inhibits Oxygen-Induced DA Constriction
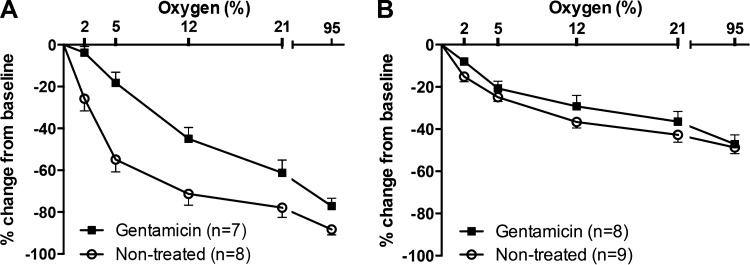
The mouse DA is exquisitely sensitive to oxygen tension in vivo and in vitro (38). Here, we observed that the diameter of the term gestation DA (d19) decreased with increasing oxygen concentration, regardless of whether the vessel was pretreated with gentamicin (P < 0.01). However, at all concentrations of oxygen tested (0%, 2%, 5%, 12%, 21%, and 95%), the diameter of the term DA was significantly greater in the vessels exposed to gentamicin (P = 0.008; Fig. 4A). In untreated vessels, complete closure of the term DA lumen was frequently observed under 21–95% oxygen conditions but did not occur in gentamicin-treated DAs at any oxygen concentration. Oxygen exposure induced less constriction of the preterm than term DA (P < 0.01; Fig. 4B); complete closure of the preterm DA lumen was not observed under any oxygen conditions. Preterm DAs exposed to gentamicin experienced less oxygen-induced constriction than untreated preterm DAs (P < 0.05; Fig. 4B).
Fig. 4.
Inhibition of oxygen-induced DA constriction by gentamicin. The DA diameter of term (A) and preterm (B) mice decreased with increasing oxygen concentration in the presence or absence of gentamicin (P < 0.01). A: at given concentrations of oxygen, the term (d19) DA diameter was significantly greater in the group treated with gentamicin (P = 0.008). B: as described previously (38), the preterm (d15) mouse DA was less responsive to oxygen than the term DA (P < 0.01). The diameter of gentamicin-exposed preterm DAs was greater than nontreated preterm DAs (P < 0.05). Data shown as mean ± SE.
Gentamicin Exposure Inhibits Postnatal DA Closure in Vivo
Postnatal closure of the DA lumen is typically accomplished in the first few hours of life in mice (14, 39). With the use of a visual scoring system, we observed complete DA closure in the majority of untreated pups at 4 h of age. In contrast, newborn mice injected with 5 mg/kg gentamicin more frequently had an unclosed DA at 4 h of age (Fig. 5). This dose is similar to loading doses that are used in neonates (21, 37). A 10-fold-higher gentamicin dose was used to examine drug-induced changes in DA status, although neonates would not encounter this dosing regimen. A larger gentamicin dose was associated with greater degrees of postnatal PDA (P = 0.002; Fig. 5).
Fig. 5.
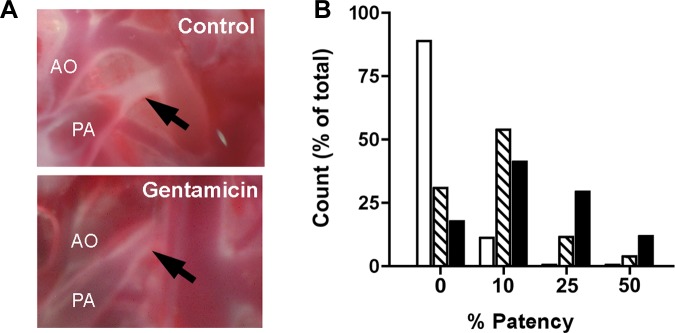
Gentamicin-induced DA relaxation in vivo. A: newborn littermates were injected with low- or high-dose gentamicin (5 mg/kg or 50 mg/kg, respectively). Representative images show complete DA closure at 4 h of age in untreated control mice and ∼10% DA patency (the most frequent outcome) in littermates exposed to a low dose of gentamicin (arrows). B: frequency distribution of patent ductus arteriosus (PDA) in newborn mice exposed to low-dose (5 mg/kg; hatched bars) or high-dose (50 mg/kg; solid bars) gentamicin (shown as %DA patency). The majority of untreated mice (open bars) had complete DA closure by 4 h of age, whereas gentamicin exposure was associated with PDA (P = 0.002).
Aminoglycoside Exposure Increases the Risk for PDA in Term and Preterm Infants
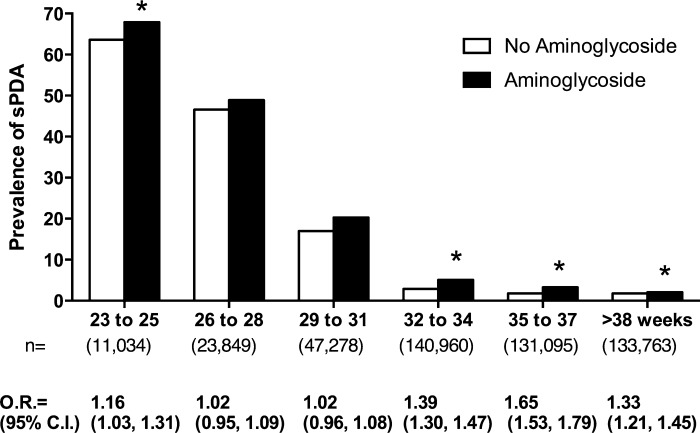
In a large clinical data set collected from a multicenter network of NICUs over a 13-yr period, there were 488,349 patients that met study criteria; 40,472 (8.3%) had a sPDA, and 447,877 did not. The type of data deposited into our clinical database did not allow us to distinguish reliably between true sepsis and suspected sepsis. Based on the information available, early-onset infection (d0, d1, or d2) was rare n = 636 (1.6%) in the PDA group and 6,624 (1.5%) in the control group. The presence of a positive blood culture was not an independent risk factor for a PDA. Therefore, we used eligibility criteria based on the definitions that the clinicians used to report their patients' data to the central data repository. As anticipated, infants with PDA were smaller, more immature, more often had other anomalies, and were more often delivered by Cesarean section. They were more often treated with mechanical ventilation, surfactant, and aminoglycosides than infants without sPDA (Table 2). The adjusted odds of sPDA were higher in subjects exposed to gentamicin compared with subjects not exposed to gentamicin over all gestational ages. When adjusted odds ratios were estimated, stratified on gestational age, a significant gentamicin-associated, increased risk of sPDA was found in all gestational age groups except 26–28 wk and 29–31 wk (Fig. 6).
Table 2.
Patient demographics
| Symptomatic PDA | No PDA | P | |
|---|---|---|---|
| n (%) | 40,472 (8.3%) | 447,877 (91.7%) | |
| EGA, median (10–90th) | 29 (25–36) | 35 (31–40) | <0.001 |
| APGAR1, median (10–90th) | 6 (2–8) | 8 (4–9) | <0.001 |
| APGAR5, median (10–90th) | 8 (6–9) | 9 (7–9) | <0.001 |
| Birth weight, median (10–90th) | 1.18 (0.65–2.77) | 2.44 (1.46–3.66) | <0.001 |
| Major anomalies | 7,013 (17.3%) | 35,137 (7.8%) | <0.001 |
| Antenatal steroids | 26,111 (64.5%) | 131,503 (29.4%) | <0.001 |
| Male | 21,264 (52.5%) | 249,265 (55.7%) | <0.001 |
| Cesarean section | 28,433 (70.3%) | 242,507 (54.1%) | <0.001 |
| Ventilator | 24,843 (61.4%) | 57,568 (12.9%) | <0.001 |
| Surfactant | 26,530 (65.6%) | 62,534 (14.0%) | <0.001 |
| Aminoglycoside | 32,351 (79.9) | 283,838 (63.4) | <0.001 |
PDA, DA patency; EGA, estimated gestational age.
Fig. 6.
Aminoglycoside-associated PDA in the neonatal intensive care unit (NICU), stratified by gestational age. The effect of aminoglycoside antibiotics on the prevalence of symptomatic PDA (sPDA) was determined by analysis of a large clinical database. Among 488,349 infants who were inborn and cared for in the NICU of a single institution (transfer patients excluded) between 1997 and 2010, 8.3% (40,472) had sPDA. With the use of multivariate-regression modeling, which included the factors most commonly associated with an increased risk of PDA, an adjusted odds ratio (O.R.) was calculated for the effect of aminoglycoside exposure on sPDA. x-Axis values represent gestational age at birth in completed weeks and the total number of patients in each age range. C.I., confidence interval. *P < 0.01.
DISCUSSION
This project was designed to address the hypothesis that the association of sPDA with sepsis in premature infants was due to the response of the ductus to LPS or certain vasoactive cytokines generated by inflammation. The rationale for this hypothesis is that sepsis is frequently accompanied by hypotension and altered vascular tone and that these factors are known for their involvement in inflammation-mediated smooth muscle relaxation (30, 31, 52). LPS-induced relaxation of the ex vivo DA has previously been observed in term and preterm fetal lambs (12, 13). Thus the results of our experiments were unexpected. Not only did LPS not cause the ductus to relax or constrict in vitro or in vivo, but also, none of the proinflammatory cytokines induced significant relaxation. Also surprising, four (IFN-γ, CXCL-12, TNF-α, and IL-13) of the six cytokines caused significant vasoconstriction of the ex vivo DA. Interestingly, patients that are genetically predisposed to low IFN-γ levels are at an increased risk for PDA (7). These findings support a suggested role for inflammatory mediators in permanent closure and remodeling of the postnatal DA (54) rather than the vasodilatory role that we envisioned.
With the lack of evidence that inflammatory mediators play a direct role in the association between sepsis and sPDA in our studies, we considered other potential links between these two conditions. Gentamicin is an aminoglycoside antibiotic that is the mainstay of sepsis treatment in premature infants and is one of the most commonly prescribed medications in the NICU (11). Since gentamicin has known vasodilatory properties, we examined whether the DA shares this response. Our results demonstrate that gentamicin and two other aminoglycosides used in the neonatal population—amikacin and tobramycin— cause relaxation of the ex vivo murine ductus. These results were highly significant, concentration dependent, and occurred in both the term and preterm DA. Gentamicin effects may be restricted to specific vascular beds, since P2 mesenteric arteries, but not the ascending aorta, also displayed a vasodilatory response, albeit to a lesser degree than in the DA. More importantly, we found that gentamicin injection soon after birth impaired the postnatal DA closure process in neonatal mice. The gentamicin concentrations that caused DA relaxation in vitro and in vivo were higher than serum levels typically experienced by human neonates. However, myography studies frequently require doses beyond the therapeutic range to demonstrate drug effects, including gentamicin (22, 42, 56). Moreover, newborn infants experience prolonged drug exposure times that cannot be studied by short-term in vitro treatment protocols. Nevertheless, our combined in vitro and in vivo findings support a causative role for gentamicin in relaxation of the postnatal DA in mice.
Other investigators have shown that gentamicin and other aminoglycosides induce a variety of adverse responses, including negative inotropic effects on rat ventricular myocytes (5), relaxation of isolated guinea pig left atria to increasing concentrations of gentamicin (20), relaxation of rat aortic rings (42), relaxation of preconstricted canine cerebral arterial rings, increased coronary blood flow, reduced force of contraction, decreased rate of automaticity, decreased sinus rate, and increased atrioventricular conduction time in canine hearts (24). Several mechanisms are thought to mediate the vasodepressor response to gentamicin and other aminoglycosides. A decrease in Ca++ influx (5) and inhibition of PLC interfering with intracellular Ca++ accumulation (22) have been proposed. In addition, gentamicin has been shown to inhibit mitochondrial metabolism (28), an action that could disrupt the role of H2O2 as the putative oxygen sensor in regulation of the DA (1). Indeed, we found that gentamicin significantly inhibited the effects of oxygen, one of the most potent and clinically relevant factors for postnatal DA closure. Gentamicin had more profound effects on the oxygen response at term than preterm gestation, although underdeveloped mechanisms for oxygen sensing in the immature ductus may partially account for the diminished effect of gentamicin in preterm DAs. Aminoglycosides have also been shown to inhibit vasoconstriction induced by endothelin, an effect reversed by a selective PKC inhibitor (56), which may warrant further consideration in future studies.
Gentamicin is also well known for its involvement in neuromuscular blockade, a phenomenon in which aminoglycosides cause paralysis, either alone or in connection with nondepolarizing agents, such as pancuronium. Gentamicin causes dose-dependent, neuromuscular blockade of the isolated phrenic nerve-diaphragm preparation of the rat (19, 33). These drugs interfere with the movement of Ca++ through calcium channels and inhibit the release of acetylcholine at the neuromuscular junction (34). Given that the ductus is richly innervated with both cholinergic and adrenergic nerve fibers in various species (2, 6, 8, 47), it is not unreasonable to speculate that the ductus tone may be inhibited, in part, by a mechanism similar to neuromuscular blockade.
Once it was recognized that gentamicin had a relaxant effect on the ex vivo and in vivo mouse DA, we performed a multivariate analysis of a large clinical database to determine whether aminoglycoside exposure was related to sPDA in infants. This analysis primarily reflects exposure to gentamicin, because the number of infants who received tobramycin or amikacin was much smaller than the number of patients who received gentamicin and who met entry criteria. After controlling for the factors most commonly associated with PDA, the adjusted odds ratio for exposure to gentamicin showed a significant association between gentamicin treatment and sPDA. The magnitude of increased risk for sPDA among gentamicin-exposed neonates is similar to the odds ratios for other neonatal variables associated with PDA (10). The increased risk of gentamicin-associated PDA was limited to infants with gestational ages <26 wk and ≥32 wk. There is no obvious explanation for the increased risk seen at both ends of the gestational-age spectrum. However, it is important to note that taking aminoglycoside exposure as the common element, there were only 9,623 infants with PDA of gestational age 25 wk or less compared with 250,699 infants with PDA at 32 wk gestation or greater (Fig. 6). The preponderance of risk of sPDA among the more mature infants parallels the ex vivo mouse ductus, which had greater relaxation in the term DA (19 days) than in the preterm DA (15 days). The relaxation response of the mouse DA is also greater in the term DA than in the preterm DA, following exposure to either furosemide (16) or to cimetidine (17). The more prominent effect of gentamicin on oxygen-induced constriction in the term rather than preterm mouse DA likely reflects developmentally regulated oxygen sensing, which also matures with advancing gestation in infants. The vulnerability of more mature newborn infants to gentamicin-associated sPDA helps validate a biological basis for these findings in the mouse DA.
This study demonstrates that aminoglycosides relax DA vascular smooth muscle and decrease the constrictive effect of oxygen. These findings are paradigm shifting and raise questions regarding the possibility that treatment of susceptible newborn infants with aminoglycosides, particularly the commonly administered antibiotic gentamicin, will contribute to sPDA in immature infants that are already at risk for this disorder. Infants treated with piperacillin-tazobactam (a synthetic penicillin derivative combined with a beta-lactamase inhibitor), instead of ampicillin and gentamicin, have a decreased trend in the need for medical or surgical treatment of sPDA (9). Until other studies clarify the relationship between antibiotics and PDA, our results support recent arguments (15) that antibiotic exposure, particularly aminoglycosides, in the NICU population should be of short duration or limited to infants with a defined bacterial infection where aminoglycoside use is clearly warranted.
GRANTS
Support for this work was provided by National Institutes of Health Grants GM-106143 (to J. L. Wynn), HD-44741 (to B. C. Paria), and HL-77395, HL-96967, and HL-109199 (to J. Reese).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. The material contained in this manuscript is original work performed by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.L.W., B.C.P., M.A.R., and J.R. conception and design of research; M.M.V., J.A.G., N.J.E., S.D.P., N.B., and J.L.W. performed experiments; M.M.V., R.B.C., J.A.G., N.J.E., S.D.P., N.B., J.L.W., J.C.S., R.H.C., and M.A.R. analyzed data; M.M.V., R.B.C., E.L.S., B.C.P., R.H.C., and M.A.R. interpreted results of experiments; M.M.V., R.B.C., E.L.S., N.J.E., S.D.P., J.C.S., and R.H.C. prepared figures; M.M.V. and R.B.C. drafted manuscript; R.B.C., E.L.S., J.L.W., B.C.P., R.H.C., and J.R. edited and revised manuscript; M.M.V., R.B.C., E.L.S., J.A.G., N.J.E., S.D.P., N.B., J.L.W., B.C.P., J.C.S., R.H.C., M.A.R., and J.R. approved final version of manuscript.
REFERENCES
- 1.Archer SL, Wu XC, Thebaud B, Moudgil R, Hashimoto K, Michelakis ED. O2 sensing in the human ductus arteriosus: redox-sensitive K+ channels are regulated by mitochondria-derived hydrogen peroxide. Biol Chem 385: 205–216, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aronson S, Gennser G, Owman C, Sjoberg NO. Innervation and contractile response of the human ductus arteriosus. Eur J Pharmacol 11: 178–186, 1970 [DOI] [PubMed] [Google Scholar]
- 3.Baragatti B, Brizzi F, Barogi S, Laubach VE, Sodini D, Shesely EG, Regan RF, Coceani F. Interactions between NO, CO and an endothelium-derived hyperpolarizing factor (EDHF) in maintaining patency of the ductus arteriosus in the mouse. Br J Pharmacol 151: 54–62, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell EF, Warburton D, Stonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med 302: 598–604, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Belus A, White E. Effects of antibiotics on the contractility and Ca2+ transients of rat cardiac myocytes. Eur J Pharmacol 412: 121–126, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bodach E, Coceani F, Dumbrille A, Okpako DT, Olley PM. The response of the isolated ductus arteriosus to transmural stimulation and drugs. Br J Pharmacol 71: 419–427, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokodi G, Derzbach L, Bányász I, Tulassay T, Vásárhelyi B. Association of interferon gamma T+874A and interleukin 12 p40 promoter CTCTAA/GC polymorphism with the need for respiratory support and perinatal complications in low birthweight neonates. Arch Dis Child Fetal Neonatal Ed 92: F25–F29, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassels DE, Moore RY. Sympathetic innervation of the ductus arteriosus in relation to patency. Chest 63: 727–731, 1973 [DOI] [PubMed] [Google Scholar]
- 9.Chong E, Reynolds J, Shaw J, Forur L, Delmore P, Uner H, Bloom BT, Gordon P. Results of a two-center, before and after study of piperacillin-tazobactam versus ampicillin and gentamicin as empiric therapy for suspected sepsis at birth in neonates ≤ 1500 g. J Perinatol 33: 529–532, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI. Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr 151: 629–634, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 117: 1979–1987, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Coceani F, Ackerley C, Seidlitz E, Kelsey L. Function of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the ductus arteriosus from foetal lamb: differential development and change by oxygen and endotoxin. Br J Pharmacol 132: 241–251, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coceani F, Kelsey L, Seidlitz E, Marks GS, McLaughlin BE, Vreman HJ, Stevenson DK, Rabinovitch M, Ackerley C. Carbon monoxide formation in the ductus arteriosus in the lamb: implications for the regulation of muscle tone. Br J Pharmacol 120: 599–608, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coceani F, Liu Y, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, Yanagisawa M. Endothelin A receptor is necessary for O(2) constriction but not closure of ductus arteriosus. Am J Physiol Heart Circ Physiol 277: H1521–H1531, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Cotten CM, Benjamin DK, Jr, Smith PB, Stoll BJ, Spitzer AR, Clark RH. Empirical antibiotic therapy for suspected early-onset bacterial sepsis. Pediatrics 130: e1052–e1053; author reply e1055–e1057, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Cotton R, Suarez S, Reese J. Unexpected extra-renal effects of loop diuretics in the preterm neonate. Acta Paediatr 101: 835–845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotton RB, Shah LP, Poole SD, Ehinger NJ, Brown N, Shelton EL, Slaughter JC, Baldwin HS, Paria BC, Reese J. Cimetidine-associated patent ductus arteriosus is mediated via a cytochrome P450 mechanism independent of H2 receptor antagonism. J Mol Cell Cardiol 59: 86–94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dani C, Bertini G, Corsini I, Elia S, Vangi V, Pratesi S, Rubaltelli FF. The fate of ductus arteriosus in infants at 23–27 weeks of gestation: from spontaneous closure to ibuprofen resistance. Acta Paediatr 97: 1176–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 19.de Rosayro M, Healy TE. Tobramycin and neuromuscular transmission in the rat isolated phrenic nerve-diaphragm preparation. Br J Anaesth 50: 251–254, 1978 [DOI] [PubMed] [Google Scholar]
- 20.Descotes J, Evreux JC. Cardiac depressant effects of some recent aminoglycoside antibiotics. J Antimicrob Chemother 7: 197–200, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Fjalstad JW, Laukli E, van den Anker JN, Klingenberg C. High-dose gentamicin in newborn infants: is it safe? Eur J Pediatr 173: 489–495, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Gergawy M, Vollrath B, Cook D. The mechanism by which aminoglycoside antibiotics cause vasodilation of canine cerebral arteries. Br J Pharmacol 125: 1150–1157, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 128: 470–478, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Gotanda K, Yanagisawa T, Satoh K, Taira N. Are the cardiovascular effects of gentamicin similar to those of calcium antagonists? Jpn J Pharmacol 47: 217–227, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol 39: 247–256, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Humes HD, Sastrasinh M, Weinberg JM. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity. J Clin Invest 73: 134–147, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki S, Minamisawa S, Yokoyama U, Akaike T, Quan H, Nagashima Y, Nishimaki S, Ishikawa Y, Yokota S. Interleukin-15 inhibits smooth muscle cell proliferation and hyaluronan production in rat ductus arteriosus. Pediatr Res 62: 392–398, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Jensen-Smith HC, Hallworth R, Nichols MG. Gentamicin rapidly inhibits mitochondrial metabolism in high-frequency cochlear outer hair cells. PloS One 7: e38471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim ES, Kim EK, Choi CW, Kim HS, Kim BI, Choi JH, Park JS, Moon KC. Intrauterine inflammation as a risk factor for persistent ductus arteriosus patency after cyclooxygenase inhibition in extremely low birth weight infants. J Pediatr 157: 745–750.e1, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med 36: 2019–2029, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Moss AJ, Emmanouilides GC, Adams FH, Chuang K. Response of ductus arteriosus and pulmonary and systemic arterial pressure to changes in oxygen environment in newborn infants. Pediatrics 33: 937–944, 1964 [PubMed] [Google Scholar]
- 33.Paradelis AG, Triantaphyllidis C, Giala MM. Neuromuscular blocking activity of aminoglycoside antibiotics. Methods Find Exp Clin Pharmacol 2: 45–51, 1980 [PubMed] [Google Scholar]
- 34.Paradelis AG, Triantaphyllidis CJ, Mironidou M, Crassaris LG, Karachalios DN, Giala MM. Interaction of aminoglycoside antibiotics and calcium channel blockers at the neuromuscular junctions. Methods Find Exp Clin Pharmacol 10: 687–690, 1988 [PubMed] [Google Scholar]
- 35.Perrone S, Toti P, Toti MS, Badii S, Becucci E, Gatti MG, Marzocchi B, Picardi A, Buonocore G. Perinatal outcome and placental histological characteristics: a single-center study. J Matern Fetal Neonatal Med 25 Suppl 1: 110–113, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Pfaltzgraff ER, Shelton EL, Galindo CL, Nelms BL, Hooper CW, Poole SD, Labosky PA, Bader DM, Reese J. Embryonic domains of the aorta derived from diverse origins exhibit distinct properties that converge into a common phenotype in the adult. J Mol Cell Cardiol 69: 88–96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao SC, Srinivasjois R, Hagan R, Ahmed M. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 9: CD005091, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Reese J, O'Mara PW, Poole SD, Brown N, Tolentino C, Eckman DM, Aschner JL. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat 88: 89–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA 97: 9759–9764, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reese J, Veldman A, Shah L, Vucovich M, Cotton RB. Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol 34: 222–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, Reese J. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol 287: R652–R660, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Richter J, Zhou J, Pavlovic D, Scheibe R, Bac VH, Blumenthal J, Hung O, Murphy MF, Whynot S, Lehmann C. Vancomycin and to lesser extent tobramycin have vasomodulatory effects in experimental endotoxemia in the rat. Clin Hemorheol Microcirc 46: 37–49, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr 126: 605–610, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Seliga-Siwecka JP, Kornacka MK. Neonatal outcome of preterm infants born to mothers with abnormal genital tract colonisation and chorioamnionitis: a cohort study. Early Hum Dev 89: 271–275, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Shea-Donohue T, Notari L, Sun R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil 24: 802–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelton EL, Ector G, Galindo CL, Hooper C, Brown N, Wilkerson I, Pfaltzgraff ER, Paria BC, Cotton RB, Stoller JZ, Reese J. Transcriptional profiling reveals ductus arteriosus-specific genes that regulate vascular tone. Physiol Genomics 46: 457–466, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva DG, Ikeda M. Ultrastructural and acetylcholinesterase studies on the innervation of the ductus arteriosus, pulmonary trunk and aorta of the fetal lamb. J Ultrastruct Res 34: 358–374, 1971 [DOI] [PubMed] [Google Scholar]
- 48.Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, Tucker R, Vohr BR. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 28: 123–128, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, Fanaroff AA, Lemons JA, Donovan EF, Oh W, Stevenson DK, Ehrenkranz RA, Papile LA, Verter J, Wright LL. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 129: 72–80, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110: 285–291, 2002 [DOI] [PubMed] [Google Scholar]
- 51.van de Bor M, Verloove-Vanhorick SP, Brand R, Ruys JH. Patent ductus arteriosus in a cohort of 1338 preterm infants: a collaborative study. Paediatr Perinat Epidemiol 2: 328–336, 1988 [DOI] [PubMed] [Google Scholar]
- 52.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol 288: H1016–H1021, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Visconti LF, Morhy SS, Deutsch AD, Tavares GM, Wilberg TJ, Rossi Fde S. Clinical and echocardiographic characteristics associated with the evolution of the ductus arteriosus in the neonate with birth weight lower than 1,500g. Einstein (Sao Paula) 11: 317–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waleh N, Seidner S, McCurnin D, Yoder B, Liu BM, Roman C, Mauray F, Clyman RI. The role of monocyte-derived cells and inflammation in baboon ductus arteriosus remodeling. Pediatr Res 57: 254–262, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med 11: 343–353, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wickman G, Nessim MA, Cook DA, Vollrath B. The polycationic aminoglycosides modulate the vasoconstrictive effects of endothelin: relevance to cerebral vasospasm. Br J Pharmacol 133: 5–12, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer LL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28: 675–683, 2007 [DOI] [PubMed] [Google Scholar]