Abstract
Urocortin 2 (Ucn2) is a cardioactive peptide exhibiting beneficial effects in normal and failing heart. In cardiomyocytes, it elicits cAMP- and Ca2+-dependent positive inotropic and lusitropic effects. We tested the hypothesis that, in addition, Ucn2 activates cardiac nitric oxide (NO) signaling and elucidated the underlying signaling pathways and mechanisms. In isolated rabbit ventricular myocytes, Ucn2 caused concentration- and time-dependent increases in phosphorylation of Akt (Ser473, Thr308), endothelial NO synthase (eNOS) (Ser1177), and ERK1/2 (Thr202/Tyr204). ERK1/2 phosphorylation, but not Akt and eNOS phosphorylation, was suppressed by inhibition of MEK1/2. Increased Akt phosphorylation resulted in increased Akt kinase activity and was mediated by corticotropin-releasing factor 2 (CRF2) receptors (astressin-2B sensitive). Inhibition of phosphatidylinositol 3-kinase (PI3K) diminished both Akt as well as eNOS phosphorylation mediated by Ucn2. Inhibition of protein kinase A (PKA) reduced Ucn2-induced phosphorylation of eNOS but did not affect the increase in phosphorylation of Akt. Conversely, direct receptor-independent elevation of cAMP via forskolin increased phosphorylation of eNOS but not of Akt. Ucn2 increased intracellular NO concentration ([NO]i), [cGMP], [cAMP], and cell shortening. Inhibition of eNOS suppressed the increases in [NO]i and cell shortening. When both PI3K-Akt and cAMP-PKA signaling were inhibited, the Ucn2-induced increases in [NO]i and cell shortening were attenuated. Thus, in rabbit ventricular myocytes, Ucn2 causes activation of cAMP-PKA, PI3K-Akt, and MEK1/2-ERK1/2 signaling. The MEK1/2-ERK1/2 pathway is not required for stimulation of NO signaling in these cells. The other two pathways, cAMP-PKA and PI3K-Akt, converge on eNOS phosphorylation at Ser1177 and result in pronounced and sustained cellular NO production with subsequent stimulation of cGMP signaling.
Keywords: urocortin, cardiac myocyte, eNOS phosphorylation, nitric oxide
urocortins 1, 2, and 3 (Ucn1, 2, and 3) belong to the corticotropin-releasing factor (CRF) superfamily of peptides. The heart contains a local urocortin system. Cardiomyocytes produce urocortins and express their cognate CRF receptors (21). Urocortins exert powerful actions on cardiac function in normal as well as failing hearts, consisting of dilation of the coronary vessels, increased cardiac output, decreased peripheral resistance, and a positive chronotropic, inotropic, and lusitropic effect (1, 5, 13, 17, 37, 41, 44). However, the precise physiological and pathophysiological roles of the cardiac urocortin system are incompletely understood. Therefore, a detailed understanding of the function and regulation of the cardiac urocortin system is necessary to evaluate its roles in health and disease.
Ucn2 binds to a G protein-coupled receptor, CRFR2 (10), which is expressed in the heart (21). CRFR2 couples to Gs proteins and elevates cAMP concentration via stimulation of adenylyl cyclases. In rabbit, rat, and mouse cardiomyocytes, activation of CRFR2 by Ucn1 or Ucn2 exerts a cAMP- and Ca2+-dependent positive inotropic and lusitropic effect (5, 42, 43). The underlying mechanisms involve cAMP/PKA-dependent stimulation of L-type Ca2+ current, elevated sarcoplasmic reticulum Ca2+ content and increased intracellular Ca2+ concentration ([Ca2+]i) transients. A possible contribution of protein kinase C (PKC) and the extracellular signal-regulated kinase 1/2 (ERK1/2) to the Ucn1-induced stimulation of L-type Ca2+ current was observed in rat ventricular myocytes (36). These cellular effects on isolated cardiomyocytes may, at least in part, explain the cardiostimulatory effects of urocortins in vivo.
In addition to the cAMP-PKA pathway, urocortins may also activate mitogen-activated protein kinases (MAPK), in particular p38-MAPK, ERK1/2 (or p44/42-MAPK), and its upstream kinase MAPK kinase 1/2 or MEK1/2 (16), as well as phosphatidylinositol 3-kinase (PI3K) (4). PI3K causes activation of protein kinase B/Akt via phosphoinositide-dependent kinase (PDK)-mediated phosphorylation at Ser473 and Thr308 (6). Urocortin-dependent activation of PI3K-PDK-Akt signaling has been implicated in the protective effects of the peptide against ischemia-reperfusion injury (3, 4). PI3K-PDK-Akt signaling stimulates nitric oxide (NO) production via Akt-dependent phosphorylation of endothelial NO synthase (eNOS) at Ser1177 (12, 29). NO plays an important role in the regulation of excitation-contraction coupling in cardiomyocytes (15). Furthermore, NO signaling has been implicated in the Ucn2-induced relaxation of coronary arteries (17) and the increase in coronary blood flow (13). In endothelial cells, Ucn2 induced a large increase in eNOS-mediated NO production caused by activation of several kinase pathways (14). In cardiomyocytes, however, direct evidence for urocortin-induced stimulation of NO synthases and its role in excitation-contraction coupling is lacking. Moreover, urocortin-dependent activation of eNOS in cardiomyocytes has not been demonstrated to date and the role of this signaling cascade for the functional effects of urocortins in cardiomyocytes remains elusive.
Another important yet largely unexplored issue is whether there is functional cross talk between the various signaling cascades activated by urocortins. For example, cAMP-PKA-dependent enhancement of [Ca2+]i transients likely alters the activity of Ca2+-dependent enzymes, e.g., via Ca2+/calmodulin. eNOS activity is Ca2+/calmodulin-dependent and regulated via phosphorylation of Ser1177. In endothelial cells, several kinases may be involved in the regulation of eNOS phosphorylation at Ser1177 (14, 29), but thus far cardiac studies have been restricted largely to the PI3K-PDK-Akt pathway.
We hypothesized that, in ventricular myocytes, Ucn2 may stimulate cellular NO production and aimed at identifying the underlying signaling pathways and their possible cross talk. Using confocal NO imaging and phosphorylation studies in rabbit ventricular myocytes, we demonstrate that Ucn2 stimulates cellular NO production via activation of PI3K-PDK-Akt and cAMP-PKA signaling and that the two pathways converge on eNOS phosphorylation at Ser1177. By contrast, MEK1/2-ERK1/2 signaling, while also activated by Ucn2, is not involved in the regulation of eNOS phosphorylation at Ser1177.
METHODS
Isolation of ventricular myocytes.
Rabbits were anesthetized using thiopental (50 mg/kg) or pentobarbital (50 mg/kg) applied via the ear vein. Deep anesthesia was confirmed by loss of the eyelid and pain reflexes. The heart was quickly excised and ventricular myocytes were isolated by a standard collagenase-based Langendorff perfusion protocol (33). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by local animal welfare authorities.
Immunoblot studies.
Ventricular myocytes were plated on culture dishes at a density of ∼5 × 105 in 5 ml M199 medium supplemented with 5 mM taurine, 0.4 mM l-glutamine, 5 mM dl-carnitine, 5 mM dl-creatine, and penicillin/streptomycin. Following attachment for 1–2 h, myocytes were treated with Ucn2 (100 nM), a maximally effective concentration, as determined in a previous study (42). Furthermore, this also allows for direct comparison with previous studies from our laboratories (40, 42, 43). Pharmacological inhibitors were applied 30 min before Ucn2 exposure. Ucn2 was applied for 30 min in these experiments. In each series of experiments, one dish remained untreated and served as control. Following incubation with Ucn2 ± inhibitors (i.e., U0126, H89, wortmannin, and LY294002), myocytes were washed with PBS and homogenized in ice-cold homogenization buffer [137 mM NaCl, 20 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 50 mM β-glycerol phosphate, 20 mM Tris·HCl (pH 7.4), 10 mM EDTA (pH 8), 1 mM EGTA (pH 7), 1 mM phenylmethylsulphonyl fluoride, 4 μg/ml aprotinin, 4 μg/ml leupeptin, 4 μg/ml pepstatin A, 1% (vol/vol) NP40, and 10% (vol/vol) glycerol]. The resulting suspension was centrifuged at 14,000 g and 4°C for 5 min. The supernatant was used for SDS-PAGE and immunoblotting.
Equal amounts of protein were loaded onto the gel and separated by SDS-PAGE using 10% Tris/SDS gels. Proteins were transferred to nitrocellulose membranes, blotted overnight at 4°C and 150 mA/cm2, fixed, and stained by PonceauS solution (Sigma). Afterwards, membranes were incubated [1 h, room temperature (RT)] in blotting buffer [170 mM NaCl, 10 mM Tris, and 0.1% (vol/vol) Tween 20 (pH 7.5), supplemented with 5% (wt/vol) dry milk]. Blots were incubated overnight at 4°C with primary antibodies: rabbit polyclonal anti-phospho-Akt (Ser473; 1:1,000; Cell Signaling), rabbit polyclonal anti-phospho-Akt (Thr308; 1:1,000; Cell Signaling), rabbit polyclonal anti-phospho-eNOS (Ser1177; 1:750; Cell Signaling), mouse monoclonal anti-phospho-p44/42-MAPK (Thr202/Tyr204) antibody (1:7,000), or mouse monoclonal anti-GAPDH (1:40,000; Bio-Trend). Membranes were washed three times with blotting buffer and then incubated (1 h, RT) with secondary antibodies (donkey anti-rabbit Ig-HRP-linked antibody, 1:3,000; or sheep anti-mouse IgG-HRP-linked antibody, 1:10,000; Amersham). Finally, membranes were washed four times in blotting buffer. Enhanced chemiluminescence was used for immunodetection. Developed immunoblots were quantified by densitometry.
Afterwards, blots were stripped by washing membranes with distilled water (4 min), 0.2 M NaOH (8 min), and distilled water (4 min) or stripping buffer [0.2 M glycine, 0.1% SDS, 1% (vol/vol) Tween-20, pH 2.2] to remove all antibodies. Blots were incubated overnight at 4°C with new primary antibodies: rabbit polyclonal anti-Akt (1:1,000; Cell Signaling), rabbit polyclonal anti-eNOS (1:1,000; Santa Cruz), or rabbit polyclonal anti-p44/42-MAPK antibody (1:1,000; Cell Signaling, Beverly, MA). All other steps were as described above.
Akt kinase activity.
Ventricular myocytes were plated on culture dishes and treated with Ucn2 ± inhibitors as described in the immunoblot study part. Following incubation with Ucn2, Ucn2 + astressin-2B, or Ucn2 + wortmannin, cells were lysed using the same homogenization buffer as for immunoblotting. The resulting suspension was centrifuged at 14,000 g for 15 min. The supernatant was used to measure Akt activity using an Akt kinase assay kit (Enzo Life Science; cat no: ADI-EKS-400A) according to the manufacturer's instructions. Absorbance at 450 nm was measured with a multimode plate reader (type Synergy Mx; BioTek, Winooski, VT). The protein assay was used to determine the concentration of protein used in the Akt assay kit. Absorbance was normalized to protein concentration.
Measuring DAF-FM fluorescence (NO) and cell shortening.
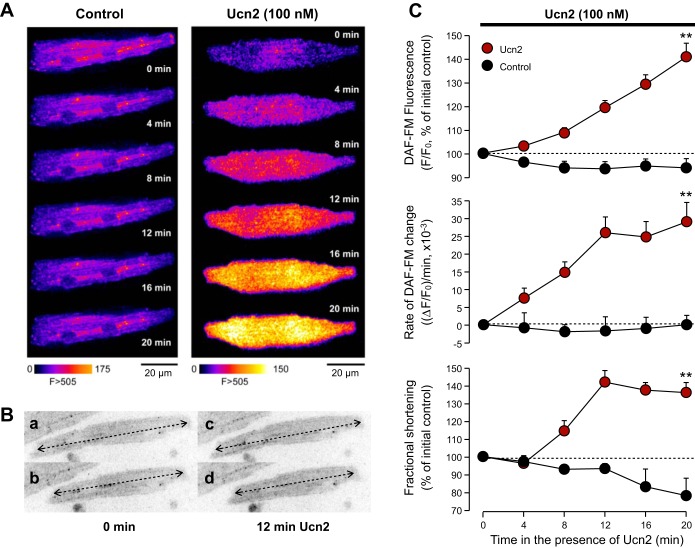
Isolated ventricular myocytes were plated on laminin-coated, glass-bottomed culture dishes. Two-dimensional (2D) confocal imaging was performed as described previously (23, 24) using a confocal imaging setup (VisiTech International) consisting of an inverted microscope (Nikon) equipped with a ×40 oil immersion objective lens (N.A. = 1.3), a Nipkow dual disc-based confocal unit, and an ICCD camera. Myocytes were loaded with the NO-sensitive dye DAF-FM by a 30-min incubation in Tyrode's solution [composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 d-glucose, and 0.1 l-arginine, pH 7.35 (NaOH)] containing 5 μM DAF-FM diacetate. Fifteen minutes were allowed for deesterification. Myocytes were placed on the stage of the microscope and field stimulated via two platinum electrodes at 0.5 Hz. DAF-FM was excited by 488-nm light from an attached argon-ion laser. Fluorescence emission was collected at wavelengths >505 nm. Images were recorded with a temporal resolution of 15 Hz. Changes of DAF-FM fluorescence are expressed as changes of background-corrected normalized fluorescence, F/F0, where F denotes DAF-FM fluorescence during the experiment and F0 the DAF-FM fluorescence at the beginning of an experiment. Fractional shortening was assessed simultaneously by measuring cell length in diastole and systole and normalizing the difference to diastolic cell length (as illustrated in Fig. 6B).
Fig. 6.
Ucn2 augments NO production. A: original fluorescence images of ventricular myocytes loaded with DAF-FM and left untreated (Control) or treated with Ucn2. DAF-FM fluorescence increases in the Ucn2-treated cell but does not change appreciably in the Ctrl cell. B: measurements of fractional shortening. Ventricular myocyte in diastole (a) and systole (b) before Ucn2 (0 min) and in diastole (c) and systole (d) in the presence of 100 nM Ucn2 (12 min). Cell length was determined in each case by positioning a line along the longitudinal axis of the myocyte and normalizing the difference of cell length in diastole minus systole to cell length in diastole. In the example shown, fractional shortening amounted to 13.2% at 0 min and to 15.8% at 12 min Ucn2. C: normalized DAF-FM fluorescence (F/F0, top), rate of change in DAF-FM fluorescence [(ΔF/F0)/min; middle], and fractional shortening (bottom) in untreated Ctrl cells (black circles; n = 8) and in cells exposed to 100 nM Ucn2 (red circles; n = 20). Dashed lines indicate initial Ctrl (100%). **P < 0.01 vs. Ctrl.
Conversion of DAF-FM fluorescence intensity into the rate of NO production.
NO does not dissociate from DAF-FM once it has reacted with the dye, i.e., NO binds irreversibly to DAF-FM. The detected NO-sensitive DAF-FM fluorescence, therefore, primarily represents the cumulative amount of NO produced within the cardiomyocytes. To more accurately present the time-dependent NO production in our cells, we performed a conversion of the DAF-FM fluorescence into the rate of the DAF-FM fluorescence change (45). To estimate the rate of NO production, the difference of the normalized DAF-FM fluorescence (ΔF/F0) between two time points was divided by the time interval.
cGMP and cAMP measurements.
cGMP and cAMP concentrations in isolated rabbit ventricular myocytes were determined by means of radioimmunoassay as described previously for endothelial cells (35). Ventricular myocytes were plated on culture dishes coated with laminin at a density of 3–5 × 105 per 5 ml Tyrode's solution (containing 0.1 mM l-arginine) per dish and left to adhere for 1 h at RT. For each experiment, six different preparations were carried out: 1 and 2) an untreated control {±10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylate cyclase (sGC)}; 3 and 4) Ucn2 (100 nM) treatment for 20 min (±10 μM ODQ); and 5 and 6) spermine/NO (SNO; 100 μM) treatment for 10 min (±10 μM ODQ). During the experiment, myocytes were electrically stimulated at 0.5 Hz. Experiments were stopped by removal of the Tyrode's solution followed by the addition of 0.01 N HCl (0.3 ml). After 30 min, the lysate was collected and stored at −20°C.
For determination of cGMP levels, 100-μl aliquots of the cell lysates were incubated at 4°C overnight with 200 μl of 50 mM sodium acetate buffer, pH 4.85, containing 125I-labeled 2′-O-succinyl guanosine-3′,5′-cyclic monophosphate-l-tyrosine methyl ester [10,000–20,000 counts/min, prepared as described previously (34)], rabbit polyclonal anti-cGMP antibody (1:2,000, generated in our own laboratory), and γ-globulins from bovine blood (5 mg/ml). Following ethanol precipitation, samples were centrifuged (30 min at 5,000 g) and radioactivity of the pellets was measured with a gamma spectrometer. For quantitating cAMP, an identical protocol was used with the exception that the immunoreaction was performed in 50 mM imidazole buffer (pH 7.0) and 125I-labeled 2′-O-succinyl adenosine-3′,5′ cyclic monophosphate-l-tyrosine methyl ester, and rabbit polyclonal anti-cAMP antiserum was used as radioactive antigen and antibody, respectively.
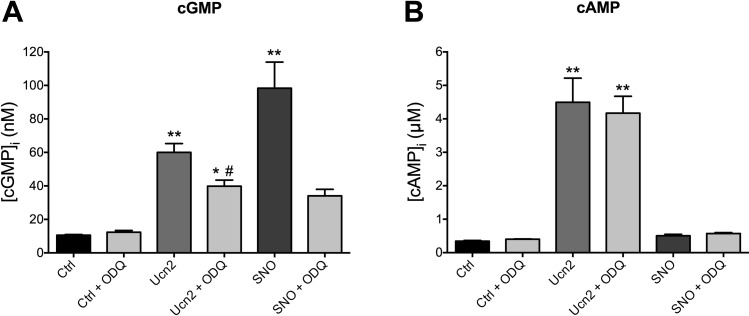
Intracellular cGMP and cAMP concentrations were calculated assuming a volume of 30 pl for a single rabbit ventricular myocyte (32). Then, the volume of 5 × 105 ventricular myocytes amounts to 15 μl and lysis in 0.3 ml 0.01 N HCl represents a dilution of 1:20. Hence, cGMP and cAMP concentrations as determined in the RIA were multiplied by 20 to yield the final intracellular concentration of the cyclic nucleotides (as presented in Fig. 7).
Fig. 7.
Ucn2 increases intracellular cGMP and cAMP concentrations ([cGMP]i and [cAMP]i). A: intracellular cGMP concentration increases in the presence of Ucn2 (100 nM) as well as of spermine/NO (SNO; 100 μM) but does not change in myocytes treated with an inhibitor (ODQ; 10 μM) of soluble guanylyl cyclase (sGC). ODQ reduces the Ucn2-induced increase in [cGMP]i. B: Ucn2 (100 nM) also increases intracellular cAMP concentration. This effect is neither inhibited by ODQ nor mimicked by SNO; n = 8 for A and B. *P < 0.05 and **P < 0.01 vs. Ctrl; #P < 0.05 vs. Ucn2.
Drugs.
Ucn2 (mouse), ODQ, and 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126) were obtained from Sigma-Aldrich. Forskolin, N-{2-[(p-bromocinnamyl)amino]ethyl}-5-isoquinolinesulfonamide (H89), NG-nitro-l-arginine methyl ester (l-NAME), l-N5-(1-iminoethyl)ornithine (l-NIO), 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002), and wortmannin were purchased from Calbiochem, and SNO was obtained from Invitrogen, and astressin-2B was from Tocris.
Statistics.
Data are presented as means ± SE. Differences between data sets were evaluated by ANOVA, nonparametric Wilcoxon signed rank test, or Student's t-test and considered significant at P < 0.05.
RESULTS
Ucn2 increases phosphorylation of Akt, eNOS, and ERK1/2 in a time-dependent manner.
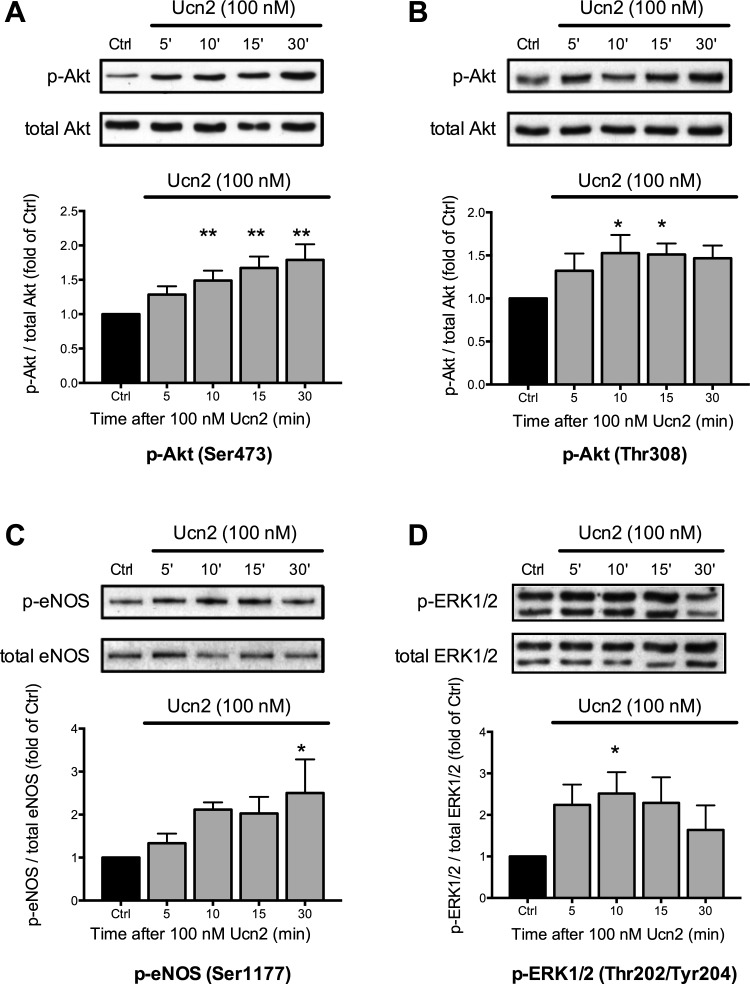
eNOS-dependent NO production may be stimulated by phosphorylation of eNOS through Akt (8). Akt is activated through phosphorylation at Ser473 and Thr308 (39). Thus we studied whether Ucn2 increased phosphorylation of Akt at Ser473 and Thr308. Rabbit ventricular myocytes were left untreated [control (Ctrl)] or exposed to a maximally effective concentration of Ucn2 (100 nM) (42) for 5, 10, 15, and 30 min (Fig. 1). Ucn2 increased phosphorylation of Akt at Ser473 (Fig. 1A) and at Thr308 (Fig. 1B) in a time-dependent manner. Phosphorylation was quantified as the ratio of phosphorylated to total Akt and normalized to the untreated control. Following Ucn2 exposure, phosphorylation at Ser473 increased progressively and was maximal after 30 min (Fig. 1A, ∼80% above Ctrl). Phosphorylation at Thr308 was also increased by Ucn2 and displayed a somewhat earlier and broader maximum between 10–30 min (Fig. 1B, ∼50% above Ctrl).
Fig. 1.
Urocortin 2 (Ucn2) elicits a time-dependent increase in phosphorylation of Akt, endothelial nitric oxide synthase (eNOS), and ERK1/2. Original immunoblots (top) and average data (bottom) of Ucn2-induced changes in phosphorylation of Akt at Ser473 (A; n = 11) and at Thr308 (B; n = 8), of eNOS at Ser1177 (C; n = 8), and of ERK1/2 at Thr202/Tyr204 (D; n = 9). Phosphorylation of Akt, eNOS, and ERK increased in a time-dependent manner. Average data of the ratio of phosphorylated to total Akt, eNOS, and ERK1/2 were normalized to the untreated control (Ctrl). *P < 0.05 and **P < 0.01 vs. Ctrl.
Akt stimulates eNOS by phosphorylation at Ser1177 (8). We thus assessed whether Ucn2 increased eNOS phosphorylation at Ser1177 (Fig. 1C). Ucn2 increased phosphorylation of eNOS in a time-dependent manner. Phosphorylation increased progressively with a maximum after 10–30 min (Fig. 1C, ∼100% above Ctrl). These results are consistent with the notion that, in rabbit ventricular myocytes, Ucn2 induces eNOS phosphorylation via Akt.
MEK1/2-ERK1/2 signaling has also been implicated in the functional and protective effects of Ucn1 in rat cardiomyocytes (3, 5, 36). Thus we studied whether Ucn2 stimulates MEK1/2-ERK1/2 signaling in rabbit ventricular myocytes using MEK1/2-mediated phosphorylation of ERK1/2 at Thr202/Tyr204 as readout. As shown in Fig. 1D, Ucn2 also increased ERK1/2 phosphorylation at Thr202/Tyr204 by a factor of ∼2.5. The time course of ERK1/2 phosphorylation was different from the time course of Akt and eNOS phosphorylation though. Maximal phosphorylation of ERK1/2 occurred already after 5–15 min, and, afterwards, ERK1/2 phosphorylation started to decline (Fig. 1D).
Previously, it was shown that Ucn2 mediates positive inotropic and lusitropic effects in ventricular myocytes via activation of the cAMP-PKA pathway (42, 43). This demonstrates, in conjunction with the current results (Fig. 1), that Ucn2 activates at least three distinct signaling pathways in rabbit ventricular myocytes: Akt, MEK1/2-ERK1/2, and cAMP-PKA signaling. The following experiments, therefore, were designed to study the putative role and potential cross talk of these different signaling pathways in Ucn2-mediated phosphorylation of eNOS and stimulation of eNOS-mediated NO production in ventricular myocytes.
Ucn2 increases phosphorylation of Akt in a concentration-dependent manner and increases Akt kinase activity via activation of CRFR2.
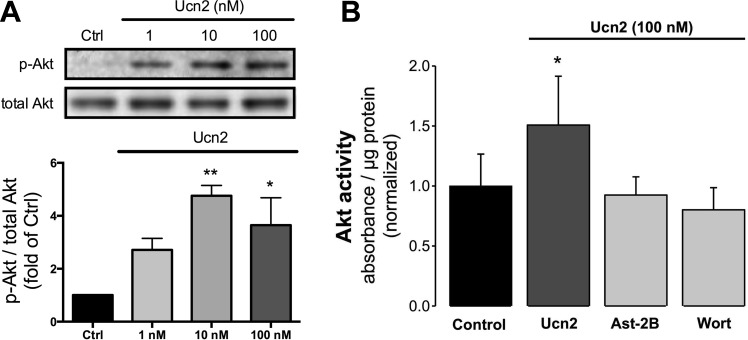
First, we studied the concentration dependence of Ucn2-induced phosphorylation of Akt. Myocytes were treated with 1, 10, and 100 nM Ucn2 for 30 min. Ucn2 induced a concentration-dependent increase in Akt phosphorylation at Ser473 that was maximal at 10–100 nM Ucn2 (Fig. 2A). This concentration dependence is consistent with our previous study in rabbit ventricular myocytes (42). Based on these data, we used a Ucn2 concentration of 100 nM for the remainder of the study to achieve maximal stimulation of Ucn2-induced signaling pathways and to allow for better comparison with previous studies of Ucn actions on adult cardiac myocytes, e.g., Refs. 36, 40, 42, 43.
Fig. 2.
Ucn2 elicits a concentration-dependent increase in phosphorylation of Akt and increases Akt kinase activity via activation of corticotropin-releasing factor 2 receptors (CRFR2). A: original immunoblots (top) and average data (bottom) of changes in phosphorylation of Akt at Ser473 induced by 1, 10, and 100 nM Ucn2 (n = 3). Average data of the ratio of phosphorylated to total Akt were normalized to the untreated Ctrl. B: Ucn2-induced increase in Akt activity. Average data of Ucn2 (100 nM)-induced changes in Akt kinase activity (n = 5) in the absence (Ucn2) and presence of astressin-2B (Ast-2B; 1 μM) or wortmannin (Wort; 0.3 μM). Absorbance at 450 nm was normalized to protein concentration. *P < 0.05 and **P < 0.01 vs. Ctrl.
Next, we tested whether Ucn2-induced phosphorylation of Akt also increased Akt kinase activity. As shown in Fig. 2B, Ucn2 (100 nM) induced a ∼50% increase in Akt kinase activity, which was blocked by astressin-2B (Ast-2B; 1 μM), a selective antagonist of CRFR2, and by wortmannin (Wort; 0.3 μM), an inhibitor of PI3K. These data indicate 1) that increased phosphorylation of Akt by Ucn2 results in an increase in Akt kinase activity, and 2) that Ucn2 acts via activation of CRFR2, which, in turn, stimulates PI3K signaling (see also below).
Effects of MEK1/2 inhibition on Ucn2-induced phosphorylation of Akt and eNOS.
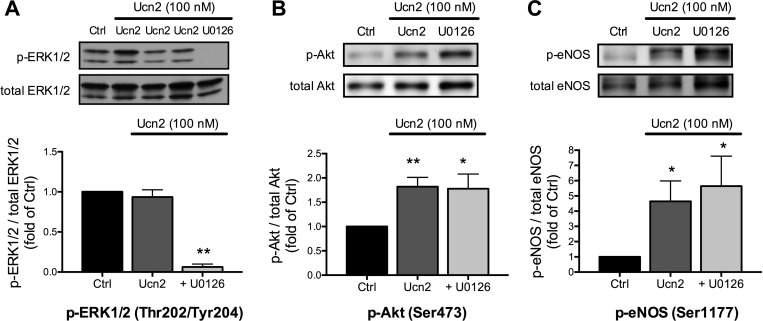
Next, we investigated the potential role of MEK1/2-ERK1/2 signaling for the Ucn2-induced phosphorylation of Akt and eNOS. To block MEK1/2-ERK1/2 signaling, we used U0126, an inhibitor of MEK1/2 (9). In the presence of U0126 (10 μM), Ucn2-mediated ERK1/2 phosphorylation was almost completely abolished (Fig. 3A), confirming the efficacy of the inhibitor. Inhibition of ERK1/2 phosphorylation by U0126, however, did not affect basal (n = 4, data not shown) or Ucn2-induced phosphorylation of Akt at Ser473 (Fig. 3B) nor Ucn2-induced phosphorylation of eNOS at Ser1177 (Fig. 3C). These results suggest that MEK1/2-ERK1/2 signaling is not involved in either Akt or eNOS phosphorylation stimulated by Ucn2.
Fig. 3.
Effects of MEK1/2 inhibition by U0126 on Ucn2-induced phosphorylation of ERK1/2, Akt, and eNOS. Myocytes were treated for 30 min with Ucn2 (100 nM) in the absence (Ucn2) and presence of 10 μM U0126 (+U0126). In A, Ucn2 was applied for 30, 60, and 120 min (Ucn2, from left to right). Original immunoblots (top) and average data (bottom) of the ratio of phosphorylated to total protein normalized to the untreated Ctrl from 7–8 independent experiments are shown. *P < 0.05 and **P < 0.01 vs. Ctrl.
Inhibition of PI3K reduces Ucn2-mediated phosphorylation of Akt and eNOS.
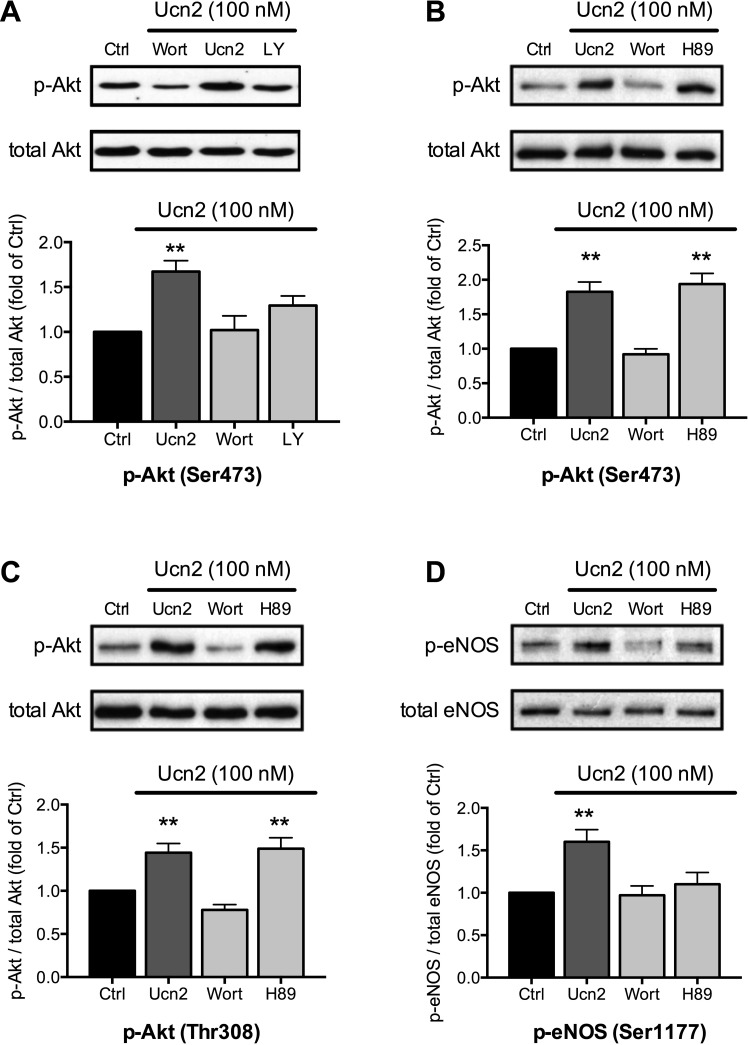
Akt may be phosphorylated at Ser473 and Thr308 by PI3K-PDK signaling (6), which may be stimulated by CRFR2-mediated activation of Gβγ proteins. To determine whether PI3K-PDK signaling underlies the Ucn2-induced phosphorylation of Akt, we used wortmannin and LY294002, two structurally different inhibitors of PI3K (Fig. 4A). Wortmannin (0.3 μM) tended to reduce basal phosphorylation of Akt at Ser473 (to 63 ± 23% of Ctrl, n = 4, P = NS, data not shown). It completely prevented the Ucn2-induced phosphorylation of Akt at Ser473 and LY294002 (LY, 10 μM) reduced it by ∼60%. Furthermore, wortmannin also suppressed Ucn2-induced phosphorylation of Akt at Thr308 (Fig. 4C) and phosphorylation of eNOS at Ser1177 (Fig. 4D). Thus, in rabbit ventricular myocytes, Ucn2 activates PI3K-PDK signaling to phosphorylate Akt (at Ser473 and Thr308), which increases Akt kinase activity (Fig. 2A) to phosphorylate eNOS (at Ser1177).
Fig. 4.
Effects of wortmannin, LY294002 and H89 on Ucn2-induced phosphorylation of Akt and eNOS. Original immunoblots (top) and average data (bottom) of Ucn2 (100 nM)-induced changes in phosphorylation of Akt at Ser473 (A; n = 9; and B; n = 16) and at Thr308 (C; n = 15) and of eNOS at Ser1177 (D; n = 14) in the absence and presence of wortmannin (0.3 μM), LY294002 (LY; 10 μM), and H-89 (5 μM). Average data of the ratio of phosphorylated to total Akt or eNOS were normalized to the untreated Ctrl. **P < 0.01 vs. Ctrl.
Inhibition of PKA reduces Ucn2-mediated phosphorylation of eNOS but not of Akt.
Besides the PI3K-PDK-Akt-dependent eNOS phosphorylation, a cAMP-PKA-Akt-dependent phosphorylation of eNOS has been described in coronary microvessels (46). Since Ucn2 also stimulates cAMP-PKA signaling in ventricular myocytes (42, 43), we sought to determine a putative contribution of this pathway to the Ucn2-induced phosphorylation of Akt and eNOS in rabbit ventricular myocytes. For this purpose, PKA activity was inhibited by H89 (5 μM). In the presence of H89, basal phosphorylation of Akt at Ser473 (n = 4, data not shown) as well as Ucn2-mediated phosphorylation of Akt at Ser473 and Thr308 remained unchanged (Fig. 4, B and C). By contrast, H89 almost completely abolished the Ucn2-induced increase in eNOS phosphorylation at Ser1177 (Fig. 4D). This suggested that Ucn2 mediates phosphorylation of eNOS at Ser1177 in cardiac myocytes by at least two separate mechanisms: activation of PI3K-PDK-Akt and cAMP-PKA signaling.
Direct stimulation of cAMP-PKA signaling increases phosphorylation of eNOS but not of Akt.
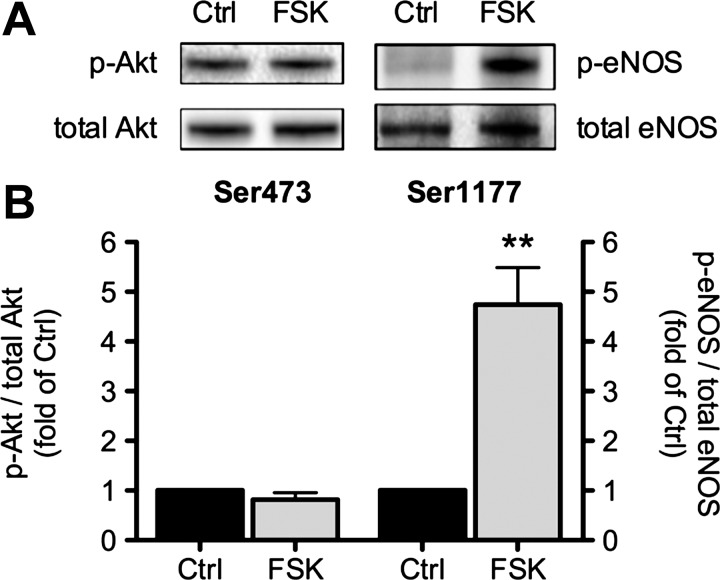
To determine whether cAMP-PKA signaling stimulates eNOS directly, as suggested by the previous set of experiments, ventricular myocytes were challenged with forskolin, a direct activator of adenylyl cyclases, to elicit a receptor-independent, selective elevation of [cAMP]i with subsequent stimulation of PKA. Cells were treated with forskolin for 30 min to allow for direct comparison with Ucn2. A maximally effective concentration of forskolin (10 μM) was unable to increase phosphorylation of Akt at Ser473 (Fig. 5, left). By contrast, forskolin caused a large increase in phosphorylation of eNOS at Ser1177 (Fig. 5, right). These results demonstrate that, in rabbit ventricular myocytes, direct stimulation of cAMP-PKA signaling causes phosphorylation of eNOS at Ser1177 but not of Akt.
Fig. 5.
Forskolin increases phosphorylation of eNOS but not of Akt. A: original immunoblots (top) and B, average data (bottom) of forskolin (10 μM)-induced changes in phosphorylation of Akt at Ser473 (left; n = 9) and of eNOS at Ser1177 (right; n = 9). Average data of the ratio of phosphorylated to total Akt and eNOS were normalized to the untreated Ctrl. **P < 0.01 vs. Ctrl.
Ucn2 stimulates cellular NO production.
Phosphorylation of eNOS at Ser1177 causes stimulation of enzyme activity (8), which should lead to an increase in cellular NO production. Thus we tested whether Ucn2 mediates an enhancement in NO production in rabbit ventricular myocytes. [NO]i was measured directly using the NO-sensitive dye DAF-FM and confocal microscopy (Fig. 6A). Under control conditions, i.e., in the absence of Ucn2, DAF-FM fluorescence remained unchanged (Fig. 6A, left). By contrast, in the presence of Ucn2 (Fig. 6A, right), DAF-FM fluorescence increased progressively indicating stimulation of cellular NO production by Ucn2. To quantify the NO increase, the DAF-FM fluorescence in the presence of Ucn2 (F) was normalized to the level of fluorescence recorded before Ucn2 exposure (F0) (Fig. 6C, top). Within 20 min, Ucn2 increased normalized DAF-FM fluorescence by 41 ± 6% (P < 0.01 vs. Ctrl). The rate of change of DAF-FM fluorescence [(ΔF/F0)/min], a measure for NO production, is shown in Fig. 6C, middle. It revealed a significant Ucn2-induced increase to 0.025 ± 0.004 (ΔF/F0)/min after 12 min, which remained stable over the following 8 min. The plateau indicates that cellular NOS activity was increased by Ucn2 and reached a constant rate of NO production. The increase in NO production was accompanied by an increase in fractional shortening of 36 ± 6% with a similar time course (Fig. 6C, bottom). Fractional shortening was determined as illustrated in Fig. 6B. In untreated control cells, on the other hand, neither the normalized DAF-FM fluorescence (Fig. 6C, top) nor the rate of NO production (Fig. 6C, middle) changed significantly over the 20-min observation period. Furthermore, during the same time interval a small decrease in fractional shortening of 12 ± 4% was observed (Fig. 6C, bottom). These results are consistent with the time-dependent eNOS phosphorylation induced by Ucn2 (see Fig. 1C). They indicate that Ucn2 increases cellular NO production in rabbit ventricular myocytes, presumably via Akt- and PKA-dependent phosphorylation of eNOS, and that the increase in cellular NO production is accompanied by a positive inotropic effect.
Ucn2 increases cGMP and cAMP concentration.
As increased NO production should augment [cGMP]i via stimulation of sGC, we tested for increased [cGMP]i following Ucn2 exposure (Fig. 7A). Ucn2 increased [cGMP]i to 567% of control. A NO donor, SNO (100 μM), increased [cGMP]i to 930% of control, in line with the augmented increase in DAF-FM fluorescence (compare Fig. 8C). Ucn2- and SNO-induced increases in [cGMP]i were reduced by ODQ (10 μM), an inhibitor of sGC. ODQ inhibited the Ucn2-induced NO increase only partially. A possible explanation might be that, in addition, Ucn2 could have stimulated particulate GC, e.g., via stimulation of ANP and/or BNP release as previously demonstrated in neonatal cardiomyocytes (19). Taken together, these data demonstrate that Ucn2 increased [cGMP]i in part via NO-mediated stimulation of sGC.
Fig. 8.
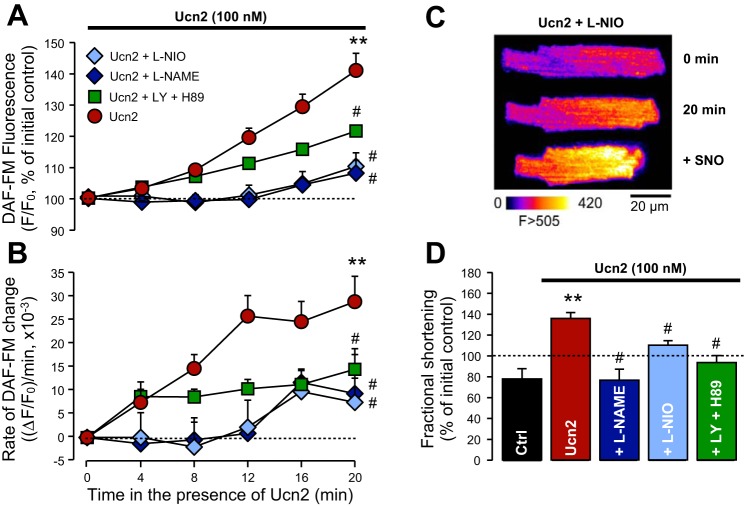
eNOS as well as phosphatidylinositol 3-kinase (PI3K) plus PKA inhibition attenuate the urocortin 2-induced increases in NO production and fractional shortening. A: DAF-FM fluorescence changes during Ucn2 application (100 nM, red circles; n = 20; same cells as in Fig. 6) in the absence and presence of NG-nitro-l-arginine methyl ester (l-NAME; 1 mM, blue diamonds, n = 4), l-N5-(1-iminoethyl)ornithine (l-NIO; 10 μM, light blue diamonds, n = 8), and LY294002 plus H89 (10 and 1 μM, respectively, green squares, n = 10). B: rate of change of DAF-FM fluorescence. Groups and symbols as in A. C: original images illustrate an experiment in the presence of l-NIO. After 20 min of Ucn2, l-NIO-treated cells were challenged with the NO donor SNO (100 μM). Ucn2 caused a small increase, whereas SNO elicited a large increase in DAF-FM fluorescence. D: alterations in fractional shortening in untreated Ctrl cells (after 20 min) and in cells exposed for 20 min to Ucn2 (n = 20) in the absence (Ucn2) and presence of l-NAME (n = 4), l-NIO (n = 8), and LY/H89 (n = 10). Dashed lines indicate initial Ctrl (100%). All inhibitors attenuated the Ucn2-induced increase in DAF-FM fluorescence, the rate of DAF-FM fluorescence change and fractional shortening. **P < 0.01 vs. Ctrl; #P < 0.05 vs. Ucn2.
As this and a previous study (42) in rabbit ventricular myocytes suggested, Ucn2 should also increase [cAMP]i. Direct measurement of [cAMP]i (Fig. 7B) indeed revealed a large increase in this cyclic nucleotide following Ucn2 exposure. The Ucn2-induced increase in [cAMP]i was neither inhibited by ODQ nor mimicked by SNO.
Ucn2-induced stimulation of cellular NO production is attenuated by inhibition of eNOS or inhibition of PKA plus PI3K.
To determine whether the Ucn2-induced NO increase was mediated by eNOS, two different inhibitors, l-NAME and l-NIO, were used. In the presence of either l-NAME (1 mM) or l-NIO (10 μM), the Ucn2-induced increase in DAF-FM fluorescence was significantly attenuated (Fig. 8A). To exclude the possibility that the reduced Ucn2-induced [NO]i increase was caused by a lack of responsiveness of DAF-FM, in l-NIO-treated cells, a NO donor, SNO (100 μM), was added after 20 min of exposure to Ucn2. As shown in the original example in Fig. 8C, SNO elicited a large increase in DAF-FM fluorescence. On average, over 6 min, SNO elevated DAF-FM fluorescence to 60 ± 14% above the baseline at 20 min (not shown). The inhibition of the Ucn2-induced NO increase by l-NAME and l-NIO was associated with a significantly reduced rate of NO production [Fig. 8B; 0.009 ± 0.01 (ΔF/F0)/min and 0.0075 ± 0.005 (ΔF/F0)/min for l-NAME and l-NIO, respectively, after 20-min exposure to Ucn2]. In addition, the positive inotropic effect of Ucn2 was suppressed in the presence of the NOS inhibitors (Fig. 8D).
Since both PI3K-PDK-Akt and cAMP-PKA signaling are able to cause phosphorylation of eNOS at Ser1177, we tested whether inhibition of these two pathways could attenuate Ucn2-induced stimulation of cellular NO production. LY294002 (10 μM) and H-89 (1 μM) were used to inhibit PI3K and PKA, respectively. Under basal conditions, the two inhibitors, applied either alone or in combination (n = 3–4, data not shown), caused a small reduction of DAF-FM fluorescence of ∼1–10% and a reduction of fractional shortening of ∼15–25% (within 14 min), similar to what was observed in untreated control myocytes (compare Fig. 6C). In the presence of LY294002 plus H-89, the Ucn2-induced increase in DAF-FM fluorescence was reduced by ∼50% (Fig. 8A). The rate of NO production was likewise significantly decreased and reached a plateau already after 4 min [Fig. 8B, ∼0.010 (ΔF/F0)/min], revealing that NOS activity was significantly blunted. Moreover, also the positive inotropic effect of Ucn2 was suppressed by inhibition of PI3K and PKA (Fig. 8D). These results suggest that PI3K-PDK-Akt as well as cAMP-PKA signaling are important mediators of the Ucn2-induced increase in eNOS activity and cellular NO production.
DISCUSSION
Ucn2 exerts powerful actions on cardiac function consisting of dilation of coronary vessels, enhanced cardiac output, and a positive inotropic and lusitropic effect in normal as well as in failing hearts (1, 7, 13, 31, 41, 44), but the cellular mechanisms underlying these effects are incompletely understood. Previous studies on ventricular myocytes from rabbit, rat, and mouse heart have unraveled some of the underlying cellular mechanisms, including Ucn2-induced activation of cAMP-PKA and Ca2+/calmodulin-CaMKII signaling (42, 43) and Ucn1-induced activation of PKC-ERK1/2 signaling (36). The present study extends these findings and shows, for the first time in cardiac myocytes, that Ucn2 also stimulates eNOS-mediated NO and cGMP signaling. Moreover, it provides a detailed molecular view on how stimulation of NO signaling in cardiomyocytes is brought about: two separate pathways activated by Ucn2, i.e., cAMP-PKA and PI3K-PDK-Akt signaling, converge on eNOS to increase phosphorylation of this enzyme at Ser1177 with consequent stimulation of eNOS activity and intracellular NO production. MEK1/2-ERK1/2 signaling, while also activated by Ucn2, is not involved in cardiomyocyte NO signaling.
MEK1/2-ERK1/2 signaling is activated by Ucn2 but is not involved in stimulation of NO signaling in ventricular myocytes.
Previous studies have shown that urocortins can activate MEK1/2-ERK1/2 signaling. In rat cardiomyocytes, Ucn1-induced activation of MEK1/2-ERK1/2 signaling has been implicated in stimulation of the l-type Ca2+ current (36) and the positive inotropic effect (5). In neonatal cardiac myocytes and ex vivo Langendorff hearts, MEK1/2-ERK1/2 signaling activated by Ucn1 or Ucn2 has been shown to be protective in models of ischemia and reperfusion injury (2, 3). Finally, in endothelial cells Ucn1 induced phosphorylation of ERK1/2, which, together with PI3K-Akt and p38-MAPK, was involved in phosphorylation of eNOS at Ser1177 (14). Here, we have shown that Ucn2 activates MEK1/2-ERK1/2 signaling in rabbit ventricular myocytes. Activation of this MAPK pathway by Ucn2 was transient with phosphorylation of ERK1/2 being maximal after 10 min and returning to baseline values after 30 min of exposure to Ucn2. This time course resembled the one previously seen in neonatal cardiac myocytes (2). It was clearly different from the time course of Ucn2-induced Akt and eNOS phosphorylation. Together with the fact that inhibition of MEK1/2-ERK1/2 signaling by U0126 did not affect Akt or eNOS phosphorylation, it indicates that MEK1/2-ERK1/2 signaling is not involved in Ucn2-induced NO signaling in cardiac myocytes. This observation is at variance with results from porcine aortic endothelial cells (14) and suggests differential signaling of urocortins in cardiac myocytes vs. endothelial cells. Thus, while being activated by Ucn2, MEK1/2-ERK1/2 signaling does not contribute to eNOS-mediated NO signaling in cardiac myocytes.
Ucn2 activates PI3K-PDK-Akt and cAMP-PKA signaling separately to mediate phosphorylation of eNOS at Ser1177.
The activity of Akt is regulated by PI3K-PDK-dependent phosphorylation of Ser473 and Thr308 (39). Phosphorylation of both amino acids is regulated independently and required for full activation of the kinase (6). This study demonstrates the time-dependent Ucn2 induced phosphorylation of Akt at Ser473 as well as at Thr308 in rabbit ventricular myocytes (Fig. 1). Phosphorylation at both sites was suppressed by PI3K inhibitors wortmannin and LY294002 (Fig. 4), indicating that Ucn2 activated PI3K-PDK signaling to phosphorylate Akt. Furthermore, phosphorylation of Akt resulted in an increase in Akt kinase activity (Fig. 2).
Previous studies have demonstrated that Ucn2 activates cAMP-PKA signaling in cardiac myocytes, including rabbit ventricular myocytes (2, 5, 18, 42–44). Here we investigated whether there is cross talk between PI3K-PDK-Akt and cAMP-PKA signaling in rabbit ventricular myocytes. Such cross talk has been reported previously in cardiac myocytes. In rat ventricular myocytes, PI3K (which did not exert any effects on its own) confined β2-adrenergic receptor (AR) signaling to the sarcolemma (20). Inhibition of PI3K during concurrent β2-AR activation resulted in enhanced phosphorylation of phospholamban and contraction, effects presumably mediated by a phosphatase preventing phosphorylation of nonsarcolemmal proteins involved in sarcoplasmic reticulum Ca2+ handling and contraction (20). β1-AR signaling also activated PI3K, which limited the cAMP-PKA-dependent positive inotropy, an effect mediated via inhibition of L-type Ca2+ current (26). In neonatal rat cardiomyocytes, activation of β-ARs caused PKA-dependent phosphorylation of Akt at both Thr308 and Ser473 to modulate insulin receptor signaling pathways (28). None of these studies, however, investigated the effects of PI3K-PDK-Akt and cAMP-PKA signaling on eNOS stimulation.
The results of the current study are summarized in Fig. 9. They indicate that Ucn2-CRFR2 signaling, similar to β-AR signaling, activates the PI3K-PDK-Akt (through Gβγ) and the cAMP-PKA pathway (through Gαs). Both pathways cause phosphorylation of eNOS at Ser1177. Via cAMP-PKA signaling Ucn2 also increases intracellular [Ca2+]i transients in rabbit ventricular myocytes (42), and this is expected to stimulate eNOS activity via Ca2+/calmodulin (Ca/CaM) binding to the enzyme. A recent study in coronary microvessels also reported that PKA increased cellular NO production (46). However, in that study PKA acted indirectly via increased phosphorylation of Akt, similar to what was observed in neonatal cardiomyocytes (28). We did not find any evidence for such a mechanism in rabbit ventricular myocytes as Ucn2-dependent phosphorylation of Akt was insensitive to PKA inhibition (Fig. 4) and direct stimulation of cAMP-PKA signaling (by forskolin) did not increase Akt phosphorylation (Fig. 5). Therefore, unlike β-AR signaling in neonatal cardiomyocytes and forskolin-induced activation of PKA in coronary microvessels, which both may cause PKA-dependent phosphorylation of Akt, Ucn2 signaling in ventricular myocytes involves separate activation of the PI3K-PDK-Akt and cAMP-PKA pathways. Both pathways do not appear to interact directly but converge on eNOS phosphorylation at Ser1177.
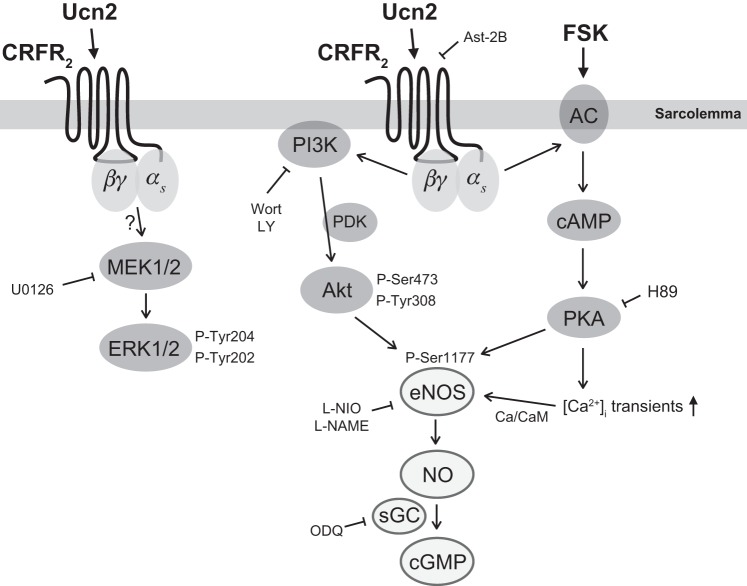
Fig. 9.
Scheme illustrating the signaling pathways activated by Ucn2 in rabbit ventricular myocytes to induce increased production of NO via eNOS phosphorylation at Ser1177. NO, in turn, stimulates sGC to increase cGMP concentration. Arrows indicate activation, phosphorylation or increased production. Enzyme inhibitors and receptor antagonists used in this study and their respective targets are shown with blunt arrows. The CRFR2-PKA-dependent increase in [Ca2+]i transients was demonstrated in a previous study in these cells (42). PDK, phosphoinositide-dependent kinase; FSK, forskolin; AC, adenylyl cyclase.
The phosphorylation at Ser1177 by Akt and PKA (and other kinases) represents a major mechanism for the activation of eNOS (8, 12, 29). The constitutively expressed eNOS is a main source for NO production in cardiac myocytes, and NO plays an important role in the regulation of cardiac excitation-contraction coupling (15, 27). It was shown previously that Ucn2 leads to phosphorylation of eNOS at Ser1177 in endothelial cells (14). The authors concluded that “urocortin II caused a cAMP-dependent and Ca2+-related phosphorylation of ERK, Akt and p38 leading to eNOS activation” (14). The present study revealed, for the first time, Ucn2-dependent phosphorylation of eNOS at Ser1177 in ventricular myocytes. In line with endothelial cells, we found involvement of cAMP-PKA and Akt signaling in Ucn2-dependent phosphorylation of eNOS. At variance with the studies on endothelial cells, however, there was no cross talk between cAMP-PKA and Akt signaling in cardiac myocytes but rather separate activation of both pathways by Ucn2. Furthermore, ERK was not involved in phosphorylation of eNOS in cardiac myocytes nor in the phosphorylation of Akt. Thus there appear to be important differences between endothelial cells and cardiac myocytes with regard to the signaling pathways activated by Ucn2 and how they interact with each other.
Ucn2 stimulates eNOS-mediated NO production and increases [cGMP]i in ventricular myocytes.
The present study shows, for the first time in ventricular myocytes, that Ucn2 augments cellular NO production via stimulation of eNOS (through phosphorylation at Ser1177), as evident from its suppression by eNOS inhibitors. The time courses of eNOS phosphorylation and stimulation of cellular NO production were very similar: both started to increase after ∼5 min and were maximal after ∼15–30 min. Furthermore, both eNOS phosphorylation and cellular NO production were attenuated by inhibition of PI3K-PDK-Akt and cAMP-PKA signaling, indicating that the two pathways are activated by Ucn2 and are major mediators of this Ucn2 effect in cardiac myocytes. The Ucn2-induced increase in eNOS-mediated NO signaling stimulated sGC to increase [cGMP]i (Figs. 7 and 9). Interestingly, the Ucn2-induced increase in [cGMP] was inhibited only partially by ODQ, suggesting that mechanisms other than sGC contributed to it. Such mechanisms may include activation of particulate GC through ANP or BNP, as previously it was demonstrated in neonatal cardiac myocytes that Ucn2 stimulates both ANP and BNP release (19). Whatever the cause of the additional cGMP increase, however, the results clearly revealed a new signaling pathway induced by Ucn2 in adult ventricular myocytes: Ucn2 stimulates eNOS-NO-sGC-cGMP signaling. Activation of this pathway has widespread implications for regulation of cardiac function by Ucn2, as it may affect various cellular effects of Ucn2 including modulation of excitation-contraction coupling.
Ucn2 increases contractility: possible role of eNOS-mediated NO increase.
Fractional shortening of ventricular myocytes was increased by Ucn2, as observed in previous studies, e.g., Refs. 5, 42, 43. It is mediated by an increase of the Ca2+ transient caused by activation of cAMP-PKA, CaMKII, and PKC-ERK signaling (5, 42, 43). In the current study, the increase in fractional shortening occurred with a similar time course as the Ucn2-induced phosphorylation of eNOS and stimulation of NO production. Furthermore, when the Ucn2-induced NO increase was attenuated, by inhibition of eNOS or PI3K-PDK-Akt and cAMP-PKA signaling, the increase in fractional shortening was reduced likewise. This suggests that NO signaling and the positive inotropic effect of Ucn2 are linked. In the heart, NO can either increase or decrease contractility, depending on the subcellular location and amount of NO release (15, 25). Low NO concentrations increase cell shortening, whereas high NO concentrations (induced by exogenous NO donors or activation of inducible NOS) decrease cell shortening (25). NO can modulate contractility via thiol nitrosylation of proteins involved in excitation-contraction coupling or via cGMP-PKG signaling, which in turn may affect proteins involved in excitation-contraction coupling (15, 27). cGMP can modulate cAMP-PKA signaling via regulation of cyclic nucleotide phosphodiesterases (PDE). cGMP stimulates PDE2 and inhibits PDE3 (11). PDE3 is a major cardiac isoform, which is highly expressed in rabbit heart (30). Low levels of NO and cGMP increase cardiac L-type Ca2+ currents in a cAMP-PKA-dependent manner via inhibition of PDE3 (11, 22, 38). Here we observed that Ucn2 caused rather small increases in NO and cGMP levels (compared with the exogenous NO donor), suggesting that cGMP-dependent inhibition of PDE3 might have contributed to the positive inotropic effect of Ucn2 in rabbit ventricular myocytes. Further experiments are required, however, to confirm this notion.
Conclusions.
Ucn2 stimulates eNOS-mediated NO and cGMP signaling and contractility in ventricular myocytes. This may contribute to the beneficial effects of urocortins in normal hearts. The redundancy in eNOS phosphorylation at Ser1177, its phosphorylation by two separate signaling pathways (PI3K-PDK-Akt and cAMP-PKA), may ensure robust phosphorylation of eNOS even under pathological conditions.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (MA 1982/4–2, TPA03 SFB 1002 to L. S. Maier); Early Career Fellowship from the National Health and Medical Research Council of Australia (to J. N. Edwards); the Ernst und Berta Grimmke-Stiftung (to J. Kockskämper and B. Pieske), Fondation Leducq (to L. S. Maier); the Max-Planck-Society, SNRP2 (5U54NS039406–9 to J. Spiess), National Heart, Lung, and Blood Institute Grants R01-HL-062231, P01-HL-080101, and R01-HL-101235 and the Leducq Foundation (to L. A. Blatter); the Shanghai Pujiang Program (No. 10PJ1406900 to L.-Z. Yang); and a State of Styria grant (to S. Walther).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W. and J.K. conception and design of research; S.W., F.P., S.R., Y.N., J.T.M., and K.S. performed experiments; S.W., F.P., S.R., Y.N., J.T.M., K.S., and J.K. analyzed data; S.W., F.P., S.R., Y.N., J.T.M., L.-Z.Y., K.S., J.N.E., P.W., K.G., L.S.M., J.S., L.A.B., B.P., and J.K. interpreted results of experiments; S.W., F.P., and J.K. prepared figures; S.W., J.N.E., and J.K. drafted manuscript; S.W., F.P., K.S., J.S., L.A.B., and J.K. edited and revised manuscript; S.W., F.P., S.R., Y.N., J.T.M., L.-Z.Y., K.S., J.N.E., P.W., K.G., L.S.M., J.S., L.A.B., B.P., and J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The expert technical assistance of Brigitte Korff, Esther Messerschmidt, Juliane Stuth, and Eva-Maria Thon-Gutschi is gratefully acknowledged.
REFERENCES
- 1.Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101: 3697–3702, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjos OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 145: 24–35; discussion 21–23, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem 275: 8508–8514, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol 34: 483–492, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Calderon-Sanchez E, Delgado C, Ruiz-Hurtado G, Dominguez-Rodriguez A, Cachofeiro V, Rodriguez-Moyano M, Gomez AM, Ordonez A, Smani T. Urocortin induces positive inotropic effect in rat heart. Cardiovasc Res 83: 717–725, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335: 1–13, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards M. Urocortin 2 infusion in human heart failure. Eur Heart J 28: 2589–2597, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol 28: 1–27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol 142: 136–143, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Grossini E, Molinari C, Mary DA, Marino P, Vacca G. The effect of urocortin II administration on the coronary circulation and cardiac function in the anaesthetized pig is nitric-oxide-dependent. Eur J Pharmacol 578: 242–248, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Grossini E, Molinari C, Mary DA, Uberti F, Ribichini F, Caimmi PP, Vacca G. Urocortin II induces nitric oxide production through cAMP and Ca2+ related pathways in endothelial cells. Cell Physiol Biochem 23: 87–96, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol 35: 719–729, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, Theoharides TC. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J Mol Endocrinol 42: 397–405, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Chan FL, Lau CW, Tsang SY, He GW, Chen ZY, Yao X. Urocortin-induced endothelium-dependent relaxation of rat coronary artery: role of nitric oxide and K+ channels. Br J Pharmacol 135: 1467–1476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda K, Tojo K, Otsubo C, Udagawa T, Hosoya T, Tajima N, Nakao K, Kawamura M. Effects of urocortin II on neonatal rat cardiac myocytes and non-myocytes. Peptides 26: 2473–2481, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Tojo K, Sato S, Ebisawa T, Tokudome G, Hosoya T, Harada M, Nakagawa O, Nakao K. Urocortin, a newly identified corticotropin-releasing factor-related mammalian peptide, stimulates atrial natriuretic peptide and brain natriuretic peptide secretions from neonatal rat cardiomyocytes. Biochem Biophys Res Commun 250: 298–304, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Jo SH, Leblais V, Wang PH, Crow MT, Xiao RP. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent G(s) signaling during beta2-adrenergic stimulation. Circ Res 91: 46–53, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab 87: 340–346, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kirstein M, Rivet-Bastide M, Hatem S, Benardeau A, Mercadier JJ, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J Clin Invest 95: 794–802, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kockskamper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci 121: 186–195, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Kockskamper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. Activation and propagation of Ca(2+) release during excitation-contraction coupling in atrial myocytes. Biophys J 81: 2590–2605, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res 78: 91–101, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Leblais V, Jo SH, Chakir K, Maltsev V, Zheng M, Crow MT, Wang W, Lakatta EG, Xiao RP. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ Res 95: 1183–1190, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Sadoshima J, Trimarco B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res 96: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Movsesian MA, Komas N, Krall J, Manganiello VC. Expression and activity of low Km, cGMP-inhibited cAMP phosphodiesterase in cardiac and skeletal muscle. Biochem Biophys Res Commun 225: 1058–1062, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation 112: 3624–3632, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Satoh H, Delbridge LM, Blatter LA, Bers DM. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J 70: 1494–1504, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schillinger W, Teucher N, Sossalla S, Kettlewell S, Werner C, Raddatz D, Elgner A, Tenderich G, Pieske B, Ramadori G, Schondube FA, Kogler H, Kockskamper J, Maier LS, Schworer H, Smith GL, Hasenfuss G. Negative inotropy of the gastric proton pump inhibitor pantoprazole in myocardium from humans and rabbits: evaluation of mechanisms. Circulation 116: 57–66, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt K, Baer HP. Purification of radioiodinated succinyl cyclic nucleotide tyrosine methyl esters by anion-exchange thin-layer chromatography. Anal Biochem 141: 499–502, 1984 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt K, Mayer B, Kukovetz WR. Effect of calcium on endothelium-derived relaxing factor formation and cGMP levels in endothelial cells. Eur J Pharmacol 170: 157–166, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Smani T, Calderon-Sanchez E, Gomez-Hurtado N, Fernandez-Velasco M, Cachofeiro V, Lahera V, Ordonez A, Delgado C. Mechanisms underlying the activation of L-type calcium channels by urocortin in rat ventricular myocytes. Cardiovasc Res 87: 459–466, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Terui K, Higashiyama A, Horiba N, Furukawa KI, Motomura S, Suda T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J Endocrinol 169: 177–183, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Vandecasteele G, Verde I, Rucker-Martin C, Donzeau-Gouge P, Fischmeister R. Cyclic GMP regulation of the L-type Ca(2+) channel current in human atrial myocytes. J Physiol 533: 329–340, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346: 561–576, 2000 [PMC free article] [PubMed] [Google Scholar]
- 40.Walther S, Awad S, Lonchyna VA, Blatter LA. NFAT transcription factor regulation by urocortin II in cardiac myocytes and heart failure. Am J Physiol Heart Circ Physiol 306: H856–H866, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol 143: 508–514, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang LZ, Kockskamper J, Heinzel FR, Hauber M, Walther S, Spiess J, Pieske B. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A. Cardiovasc Res 69: 402–411, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Yang LZ, Kockskamper J, Khan S, Suarez J, Walther S, Doleschal B, Unterer G, Khafaga M, Machler H, Heinzel FR, Dillmann WH, Pieske B, Spiess J. cAMP- and Ca(2+)/calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes. Br J Pharmacol 162: 544–556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang LZ, Tovote P, Rayner M, Kockskamper J, Pieske B, Spiess J. Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol 632: 1–6, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Yi FX, Magness RR, Bird IM. Simultaneous imaging of [Ca2+]i and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol 288: R140–R148, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Zhang XP, Hintze TH. cAMP signal transduction induces eNOS activation by promoting PKB phosphorylation. Am J Physiol Heart Circ Physiol 290: H2376–H2384, 2006 [DOI] [PubMed] [Google Scholar]