Abstract
The use of nanoparticles in medical treatment has prompted the question of their safety. In this study, the pathophysiology and biodistribution of three different concentrations of intravenously-delivered dextran-coated Fe3O4 iron oxide nanoparticles (IONP) were evaluated in mice. Some groups of mice were exposed to an AC magnetic field (AMF) at levels comparable with those proposed for cancer treatments. Iron biodistribution analysis for both AMF and non-AMF treated mice was performed for all three concentrations used (.6 mg Fe/mouse, 1.8 mg Fe/mouse, and 5.6 mg Fe/mouse). Blood urea nitrogen, alanine transaminase, alkaline phosphatase, total serum protein, and creatinine were also assessed at 4 hours, 7 days, and 14 days post-injection. Histological analysis of lung, spleen, heart, liver, and kidney tissue was conducted at 7 and 14 days post-injection. Prussian blue and H&E stains were used to histomorphometrically assess iron content in the tissues studied. Preliminary results demonstrate small temporary elevation in liver enzymes and hepatocyte vacuolization at all iron concentrations studied. Liver and spleen were the primary sites of IONP deposition. None of the animals demonstrated systemic or local toxicity or illness, with or without AMF activation.
Keywords: Iron oxide, nanoparticle, hyperthermia, alternating magnetic field, mouse, toxicity, biodistribution
2. INTRODUCTION
Interest in hyperthermia-based cancer therapy has been recently revived by iron oxide nanoparticle hyperthermia technology. IONP hyperthermia relies on magnetic nanoparticle “activation” by an alternating magnetic field (AMF) and the subsequent emission of heat via hysteresis loss1, 2 Because of this property, antibody targeted or nontargeted IONPs can be delivered directly or systemically and then activated by AMF when tumor uptake is deemed optimal. IONP size and location with respect to tumor cells provide the potential for a more targeted heating of individual tumor cells. It appears that this method of hyperthermia cancer treatment thus has greater potential tumor specificity and therapeutic ratio than any other hyperthermia technique to date.2
To produce treatment effect in a clinical setting, IONPs delivering hyperthermia doses must be, safe and present at the site of the target tissue in sufficient amounts and possibly select configurations. Differences in particle characteristics, such as size and surface charge, will result in a range of biodistribution profiles.3 Proper modification may even afford the possibility of highly-specific increased particle localization using nanoparticle-bound antibodies and other targeting moieties. Although studies determining optimal particle concentrations and locations among target tissue for nanoparticle hyperthermia have yet to be published, understanding particle biodistribution remains a crucial aspect of successful therapy.
Toxicity and biodistribution studies have been carried out for relatively large imaging based IONPs (<500nm), but not for IONPs with targeting and heating capabilities.2-10 Our studies are designed to provide preliminary biodistribution and relatively short term toxicity data for such moderately-sized (50-100 nm diameter), dextran-coated, high SAR IONPs.
3. MATERIALS AND METHODS
3.1 Animals
Female C3H mice, weighing between 16 and 21 grams, from Jackson Laboratory, Bar Harbor, Maine were used for this study. All study protocols were approved by the Dartmouth College Institutional Animal Care and Use Committee and carried out according to all AAALAC, federal and institutional guidelines.
3.2 Particles and AMF
Iron oxide nanoparticles were manufactured by micromod Partikeltechnologie GmbH in Rostock, Germany and graciously provided by Triton Biosystems of Chelmsford, MA (now Aduro Biotech, Berkley, CA). Particles were dextran-coated with an average diameter of 80-100 nm. Varying concentration doses were achieved by diluting the particles in sterile phosphate-buffered saline.
The alternating magnetic field used to activate the nanoparticles in this experiment was generated by a Huttinger TIG 10/300 generator operating at approximately 160 KHz. The generator was kept at a constant temperature by a Tek-Temo Instruments Inc. chiller set at 22° C.
3.3 Toxicity Treatments
Mice were anesthetized using approximately .1 cc of 90 mg/kg Ketamine 10 mg/kg Xylazine per 10g mouse weight. Particles were then administered by bolus tail vein injection normalized to a total volume of 385 μl. AMF treatment was given 30 minutes post injection at varying field strengths. Rectal and sub dermal flank temperatures (to simulate a solid tumor model site) were measured continuously throughout treatment using a Fiso TMI 4 channel fiber optic signal conditioner with two temperature probes. Sub dermal flank probes were held in place with a 25 gauge catheter. Once temperature probes were placed, mice were situated in the barrel of a 50 ml conical tube and positioned in the AMF coil. The mouse was placed so that the center of the flank probe was positioned at the maximum field strengths area. All parts of the mouse except the head and tail were contained within the coil. Treatment groups were as follows:
Table 1.
Number of mice per Treatment Group
| No AMF | 350 Oe | 450 Oe | |
|---|---|---|---|
| .62 mg Fe | 6 | 6 | 6 |
| 1.86 mg Fe | 6 | 6 | 6 |
| 5.57 mg Fe | 6 | 6 | 6 |
| 0 mg Fe | 6 | 6 | 6 |
Blood samples were obtained from the retro-orbital sinus at 4 hours, 7 days, and 14 days post injection. Blood samples were immediately centrifuged at 14,000 rpm for seven minutes and serum reserved for enzyme panel analysis. Mice were sacrificed at 7 or 14 days post treatment. At sacrifice lungs, heart, kidney, liver, spleen, and brain were harvested. Subsections of the kidneys, liver, spleen, and brain were frozen and kept for iron content analysis. Remaining liver, spleen, brain, kidneys, lungs, and heart were preserved in 10% formalin for histologic analysis.
3.4 Serum analysis panels
Serum was batched in groups of one to three subjects per panel All samples were analyzed on a VetTest Chemistry Analyzer with slides acquired from IDEXX Laboratories, Inc. to measure alanine transaminase, creatanine, blood urea nitrogen, glucose, alkaline phosphatase, and total protein content.
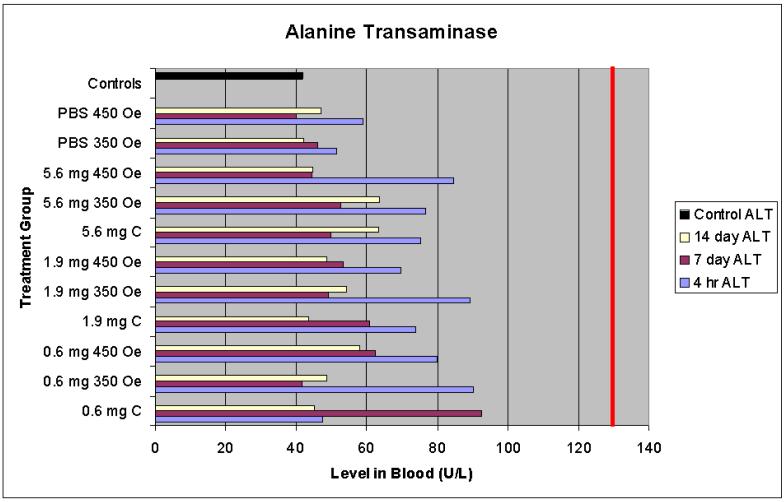
3.4.1 Alanine Transaminase (ALT)
Alanine transaminase was measured because it can be used to indicate liver damage. ALT is an enzyme present primarily in the liver. If the liver is damaged, ALT is released into the bloodstream, and thus elevated levels are used to indicate possible liver damage [11].
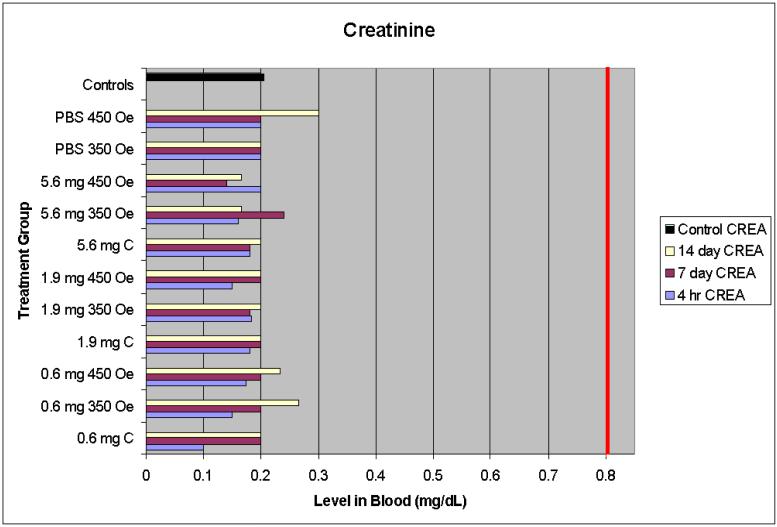
3.4.2 Creatinine (CREA)
Creatinine was measured because it is one of the most sensitive indicators of kidney damage. CREA is a product of the breakdown of creatine phosphate, a component of muscle protein. When the kidney has experienced damage, less CREA will be excreted in the urine, and thus more will be present in the blood [12].
3.4.3 Blood Urea Nitrogen (BUN)
Blood urea nitrogen is used as an indicator of kidney and liver damage. When protein breaks down it forms urea nitrogen, which can be found in the blood. BUN tests are usually used to test for kidney function as elevated levels indicate kidney malfunction. Levels lower than the normal range can indicate liver damage, low protein, or malnutrition [13].
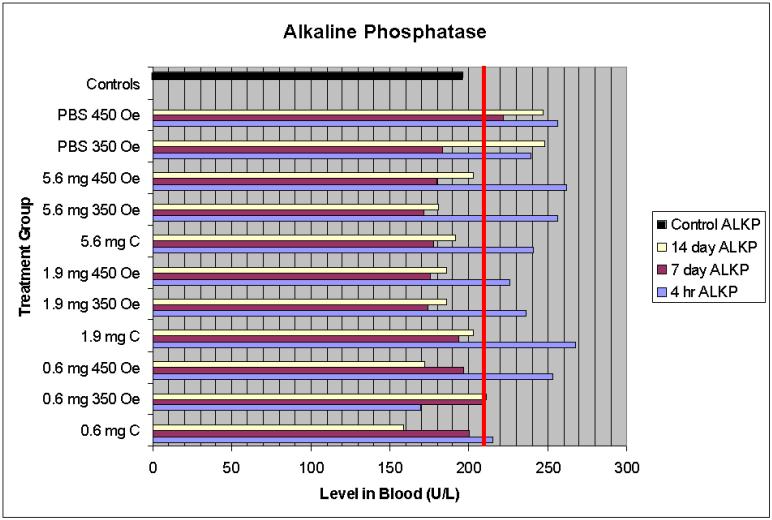
3.4.4 Alkaline Phosphatase (ALP)
Alkaline phosphatase is a sensitive indicator of liver and bone damage. ALP is present in all tissue; however, particularly high amounts are present in the liver. When the liver is damaged, ALP is released into the blood, thus elevated ALP levels indicate possible liver damage [14].
3.4.5 Total Serum Protein (TP)
Total protein was measured because it can be used as an additional indicator of liver or kidney damage. This panel measures the total albumin and globulin protein in the blood. Lower than normal levels of TP can indicate possible liver damage while higher than normal values may indicate renal damage [15].
3.5 Histology
Mice were sacrificed seven or fourteen days post treatment. At sacrifice, lungs, heart, kidney, liver, and spleen were harvested and fixed in 10% neutral buffered formaldehyde. Histologic slides using hematoxylin and eosin stain (H&E), were prepared for all organs. Each slide was evaluated via light microscopy at a number of magnifications. The same blocks were also cut and stained with an iron specific stain, Prussian Blue (PB). To quantify iron content via histomorphometry, photomicrographs of a preset number of microscopic fields from each PB stained section were analyzed using ImageJ image processing and analysis software to determine the average 2-D area iron fraction.
4. RESULTS
4.1 Serum chemistry analysis
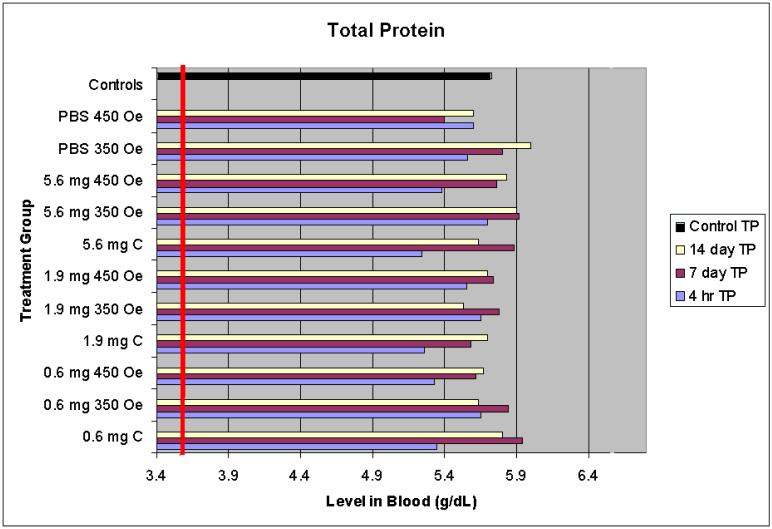
Overall, the serum chemistry data indicated that no significant renal or hepatic damage resulted from the nanoparticle presence or hyperthermia treatment. Normal serum levels were provided by the VetTest analyzer and are shown as a vertical line on all graphs except in Figure 5.
Figure 5.
Total protein serum levels
4.1.1 Alanine transaminase
Observed levels of ALT for all treatment groups at all time points were well within the normal range. The four hour time points showed levels consistently higher than the seven and fourteen day time points (as can be seen in Figure 1); however, the greatest difference between a four hour sample level and the control was 15%. These data suggest the treatments resulted in no acute or chronic (defined as 14 days) liver damage.
Figure 1.
Alanine transaminase serum levels
4.1.2 Creatinine
CREA levels remained within the normal range for all treatments and doses at all time points (Figure 2). The highest observed serum level was .3 mg/dL while the normal range includes levels as high as .8 mg/dL, and the control mice averaged .2 mg/dL. The normal CREA levels observed indicates no kidney damage resulting from any of the nanoparticle presence or hyperthermia treatments.
Figure 2.
Creatinine serum levels
4.1.3 Blood urea nitrogen
BUN levels remained within the normal range for all treatment groups at all times (Figure 3). The highest observed level occurred in the group that received 1.9 mg of iron and no AMF treatment. Although this group did not receive the greatest amount of iron oxide, it did have the highest BUN average (15% above control level), suggesting BUN levels, when within the normal range, do not indicate adverse effects of IONP treatment.
Figure 3.
Blood urea nitrogen serum levels
4.1.4 Alkaline phosphatase
At four hours post injection, ALP levels were slightly above the normal maximum for the range reported by the VetTest Chemistry Analyzer (Figure 4, shown by the bar line at 210 U/L). In general, these levels return to the normal range by seven days and were not accompanied by any additional symptoms of organ damage or corresponding elevated levels of other enzymes.
Figure 4.
Alkaline phosphatase serum levels
4.1.5 Total serum protein
Total serum protein levels were all above the minimum normal value (shown as a bar at 3.6 g/dL) for all treatment groups at all time points including the controls (Figure 5).
4.2 Histopathology and IONP biodistribution assessment
Lung and heart tissues were histologically normal (no iron observed) in all IONP treated mice (Images not shown). Occasionally iron was observed in the kidney of nanoparticle-injected mice; however morphologic renal damage was not observed. As expected, large quantities of iron were observed in the spleen and liver of all nanoparticle-injected mice. Modest cytoplasmic vacuolization was observed in a number of hepatocytes at all assessment times.
4. Discussion
Overall, the data presented in these studies suggest that the IONPs studied are biocompatible and nontoxic, at least in the short term. Although ALP levels (Figure 6) were slightly above the normal maximum at the 4 hour post treatment time point, the magnitude of change was so small and temporary that the change was considered insignificant. It is possible that the use of anesthetics resulted in these minor ALP elevations, as some drugs are known to have this effect. Since elevated ALP levels are seen in control mice, mice that received particles and no AMF treatment (and thus no heat) and also AMF treatment but no particles, there does not appear to be a correlation between treatment and elevated levels. All other serum panels show normal levels.
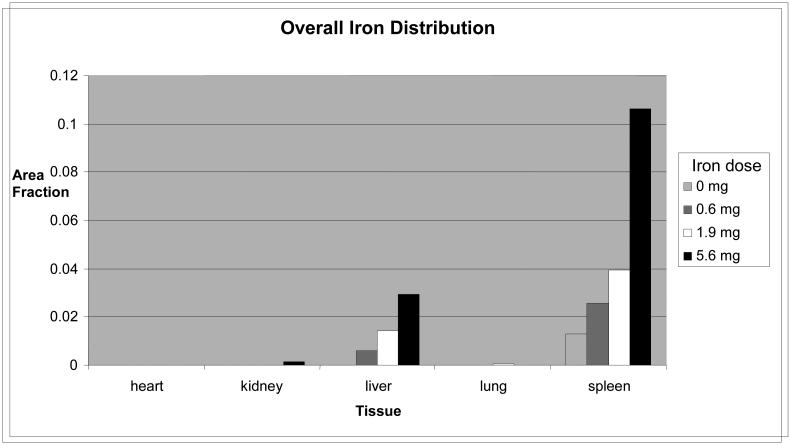
Figure 6.
Average overall iron distribution for 7 and 14 mice
The majority of the injected iron was found in the spleen and liver, with very small amounts in the kidney (high iron dose groups only). This might be expected considering the size of the particles and the filtering properties of these organs. Co-registration of histology-derived biodistribution data is currently being done using mass spectrometry. A long term parallel study for biodistribution and clearance is also underway with endpoints up to one year.
Although only focused on the short term, these data carry strong implications for the safety of IONP hyperthermia treatment. It is now clear that AMF delivery to organs with high particle concentrations will produce significant heating effects, therefore targeted IONPs and AMF will likely have the greatest therapeutic ratio. The time between injection and treatment is also a concern as different periods of circulation will result in different biodistributions, even between 7 and 14 days post-treatment (data not shown).
As research into IONP hyperthermia continues, improved particle design and targeting will increase the efficacy and safety of treatment. If targeting can be accomplished successfully, however, smaller AMF doses and fewer particles may be necessary to achieve a successful treatment.
6. Acknowledgements
The authors would like to thank R Ivkov (Johns Hopkins University) and A Foreman of Aduro Biotech Corp., both formerly of Triton Biosystems, for providing the particles used in this experiment.
5. References
- 1.Berry CC, Curtis ASG. Functionalization of magnetic nanoparticles for applications in biomedicine. J. Phys D: Appl Phys. 2003;36:R198–R206. [Google Scholar]
- 2.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FKH, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neuro-Oncol. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 3.Briley-Saebo KC, Johansson LO, Hustvedt SO, Hardorsen AG, Bjørnerud A, Fayad ZA, Ahlstrom HK. Clearance of iron oxide particles in rat liver. Invest Radiol. 2006;41(7):560–571. doi: 10.1097/01.rli.0000221321.90261.09. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon LL, Sándor M, Pinkston KE, Neuwelt EA. Imaging, distribution and toxicity of superparamagnetic iron oxide nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796. doi: 10.1093/neurosurgery/57.4.785. [DOI] [PubMed] [Google Scholar]
- 5.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229(3):838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 6.Sun R, Dittrich J, Le-Huu M, Mueller MM, Bedke J, Kartenbeck J, Lehmann WD, Krueger R, Bock M, Huss R, Seliger C, Gröne H-J, Misselwitz B, Semmler W, Kiessing F. Physical and biological characterization of superparamagnetic iron oxide- and ultrasmall superparamagnetic iron oxide-labeled cells. Invest Radiol. 2005;40(8):504–513. doi: 10.1097/01.rli.0000162925.26703.3a. [DOI] [PubMed] [Google Scholar]
- 7.Corot C, Robert P, Idée JM, Port M. Recent advances in iron odixe nanocrystal technology for medical imaging. Adv Drug Deliver Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Moore A, Marecos E, Bogdanov A, Weissleder R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214(2):568–574. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 9.Briley-Saebo K, Bjørnerud A, Grant D, Ahlstrom H, Berg T, Kindberg GM. Hepatic cellular distribution and degredation of iron oxide nanoparticles following sincle intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res. 2004;316:315–323. doi: 10.1007/s00441-004-0884-8. [DOI] [PubMed] [Google Scholar]
- 10.Van Beers BE, Sempoux C, Materne R, Delos M, Smith AM. Biodistribution of ultrasmall iron oxide particles in the rat liver. JMRI-J Magn Reson Im. 2001;13:594–599. doi: 10.1002/jmri.1083. [DOI] [PubMed] [Google Scholar]
- 11.Medical Encyclopedia. The National Institute of Health; 2007. ALT. [Google Scholar]
- 12.Medical Encyclopedia. The National Institute of Health; 2007. Creatinine - Serum. [Google Scholar]
- 13.Medical Encyclopedia. The National Institute of Health; 2007. BUN. [Google Scholar]
- 14.Medical Encyclopedia. The National Institute of Health; 2007. ALP. [Google Scholar]
- 15.Medical Encyclopedia. The National Institute of Health; 2007. Total Protein. [Google Scholar]