Abstract
Most nanoparticle-based cancer therapeutic strategies seek to develop an effective individual cancer cell or metastatic tumor treatment. Critical to the success of these therapies is to direct as much of the agent as possible to the targeted tissue while avoiding unacceptable normal tissue complications. In this light, three different cisplatinum/magnetic nanoparticle (mNP) administration regimens were investigated. The most important finding suggests that clinically relevant doses of cisplatinum result in a significant increase in the tumor uptake of systemically delivered mNP. This enhancement of mNP tumor uptake creates the potential for an even greater therapeutic ratio through the addition of mNP based, intracellular hyperthermia.
Keywords: Magnetic nanoparticle, Cisplatinum, CDDP, Hyperthermia
1. INTRODUCTION
Many nanoparticle-based therapeutic strategies have sought to develop an effective individual cancer cell or metastatic tumor treatment. Critical to the success of these therapies is to focus enough of the agent into the targeted tissue while avoiding unacceptable accumulation within normal tissue. It is well established that polyethylene glycol (PEG) slows the removal of nanoparticles by the reticuloendothelial system, resulting in increased circulation time.1 The purpose of increasing the circulation time of nanoparticles is to take advantage of the enhanced permeability and retention (EPR) effect. The EPR effect describes the retention of macromolecules in the tumor tissue due to the irregular nature of tumor blood vessels. Tumor blood vessels are more irregular in geometry and organization, are dilated, and have poor alignment with endothelial cells.1, 2 Unfortunately, despite advanced surface chemistry and protein targeting, the level of systemically injected nanoparticles, including magnetic nanoparticles (mNPs), which reach solid tumors is unacceptably low. In order to improve the concentration of mNPs within the tumor, additional strategies must be developed. Of particular interest are those strategies which enhance the EPR effect by altering the tumor vascular barrier, increasing local blood flow, and reducing interstitial tumor pressure. In addition, agents which have both primary therapeutic effects and EPR enhancement effects are particularly attractive, as they have great potential to ultimately improve the achieved therapeutic ratio. Our lab has demonstrated that radiation (15 Gy) can decrease the pressure within the tumor, increasing the permeability, and increasing the deposition of systemically delivered mNP.3 Tumor modification to increase the uptake of systemically delivered mNP with chemotherapeutics is also an attractive possibility, as it is already standard of care, and may assist in the treatment of metastases, and some have been demonstrated to reduce the interstitial fluid pressure of solid tumors.4 As demonstrated in this study, one such agent is the chemotherapeutic, cisplatinum (CDDP). Cisplatinum has also previously been shown to improve the treatment efficacy of mNP hyperthermia following direct injection into the tumor, in a mouse model (1.3X increase in treatment efficacy over either treatment alone).5
2. METHODS
2.1 Tumor inoculation and measurement
MTGB cells were injected intradermally (100μl) in the right rear flank of C3H mice (Charles River, Wilmington, MA). Cells were suspended at a concentration of ten million cells per ml in 1x Alpha MEM. Tumors were treated when they reached a volume of 150 mm3 +/− 40 mm3. For those animals which were used in efficacy studies, tumors were measured every other day until their volumes were three times their initial treatment volume, which was the study endpoint. Volume was calculated using the measured perpendicular diameters of the ellipsoidal tumor, found with digital calipers. The equation of volume used is listed below.
2.2 Experimental groups
Three different experimental groups were created, with control animals, and are summarized in the chart below. Control animals for groups which received cisplatinum were given an IP injection of cisplatinum and sham injection of PBS for mNP control. Background Fe levels for animals which did not receive cisplatinum were taken from our previously published studies.6 Study 3 included animals with AMF exposure. All efficacy animals, including controls, received cisplatinum. The control group for AMF exposure received a sham IV injection of PBS equivalent to the prescribed volume of mNP. The control group animals for mNP (no AMF) was placed under anesthesia for an hour and was not exposed to an AMF.
2.3 Cisplatinum modification
Cisplatinum (Teva Parenteral Medicines, Inc., Haarlem, Netherlands) was administered IP, diluted in 1 mL of PBS and dosed according to body mass. Groups 1 and 2 received 5 mg of cisplatinum per kg of body mass. Group 3 received 3 mg of cisplatinum per kg of body mass. The first was given dose six days prior to mNP injection, the second 1 hr prior to AMF exposure. Control animals were given an injection of PBS (equivalent volume) at the appropriate times before mNP injection.
2.4 MNPs
MNPs (MicroMod GmBH, Rostock, Germany) used for this study produce heat via hysteresis when exposed to an AMF. The mNP have Fe3O4 cores, coated with hydroxyethyl starch (110nm diameter) and PEG 200 at a solid concentration of 22 mg/ml with iron concentration of 12.2 mg/ml. Study 1 received 0.10 mg of Fe per gram of body mass three days after CDDP injection. Studies 2 and 3 received 0.25 mg per gram body mass, three and six days after CDDP injections, respectively.
2.5 Tissue Collection
Mice were given 5 units heparin IP 5 minutes prior to sacrifice. Mice were perfused with 25mL 1X PBS. Tissues were digested in a 1:3 volumetric ratio of trace metal grade hydrochloric acid to nitric acid. Vials were incubated on a hot plate at 65°C for 1hr or more until tissues were digested and were then allowed to sit at room temperature for twenty four hrs. Inductively-coupled plasma mass spectrometry (ICP-MS) was used for Fe analysis. DORM-3 fish protein standards (National Research Council, Canada) were digested to provide iron controls. Blood and tumors were taken for Studies 1, 2 and 3. In addition, livers and spleens were taken for Studies 2 and 3.
2.6 Administration of AMF, temperature recording and thermal dose
The AMF field was generated by a water cooled, whole body circular coil (Fluxtrol Inc., Auburn Hills, MI) powered by a Huttinger TIG 10/300 generator operating at 165 KHz and 450 Oe and a constant temperature of 20° C (chiller /Tek-Temo Instruments Inc.). Mouse rectal and tumor temperatures were recorded throughout the treatment using FISO fiber optic probes (FISO Inc., Quebec, Canada). Three temperature measurements were taken within the tumor with a single fiber optic probe with 3 sensors, centering the middle sensor within the tumor. At the initiation of treatment, the core body temperatures of the mice were 36°C ± 1°C. Using the fiber optic probe, the cumulative equivalent minute relationship (CEM) was calculated real-time in the tumor tissue as a means of measuring thermal dose.7 AMF exposure was 1 hr with core body temperatures maintained at 38°C± 1°C. Mice were anesthetized using 1–3% isoflurane gas and 95% O2.
2.7 Histology
Sections of tumors from three mice (each group) from Study 3 were reserved for histological analysis and stained with hematoxylin and eosin stain (H&E). Multiple, random high magnification images were taken from all regions of each tumor. Each digitized histologic image was assessed for quantification of cellularity using the NIH ImageJ program.
2.8 Statistical Analysis
A two-tailed, two sample, t-test was used to assess statistical significance, using Matlab software (The MathWorks, Inc. Natick, Massachusetts).
3. RESULTS
3.1 Fe concentrations in tumors
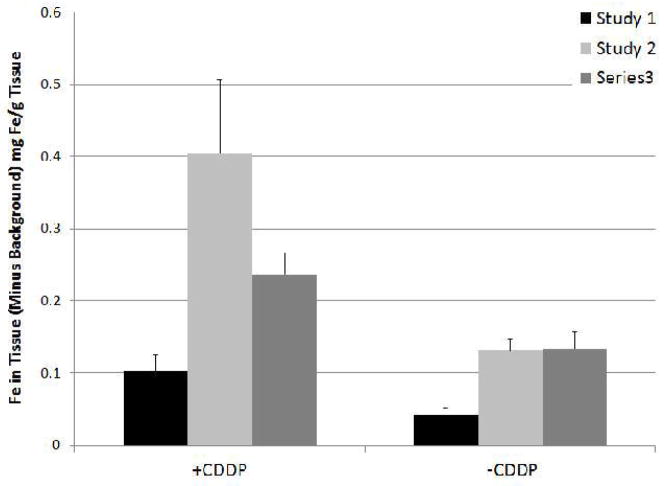
Increased concentrations of Fe were found in all cisplatinum treated tumors, as compared to non-cisplatinum treated tumors. Study 1 resulted in 2.5X more Fe than no pretreatment (p=0.04), Study 2 in 3.1X (p=0.02) and Study 3 in 1.8X (p=0.11) in tumor tissue. Studies 2 and 3 received more Fe than Study 1 which is reflected in the lower Fe concentrations in both the pretreated and control tumors (p<0.03 for all). Though not statistically significant (p=0.14) pretreated tumors from Study 2 resulted in 1.7X more Fe than Study 3. Average background Fe levels from control animals were subtracted from Fe concentrations, which are shown in the following graphs.
3.2 Fe concentrations in blood, liver and spleen
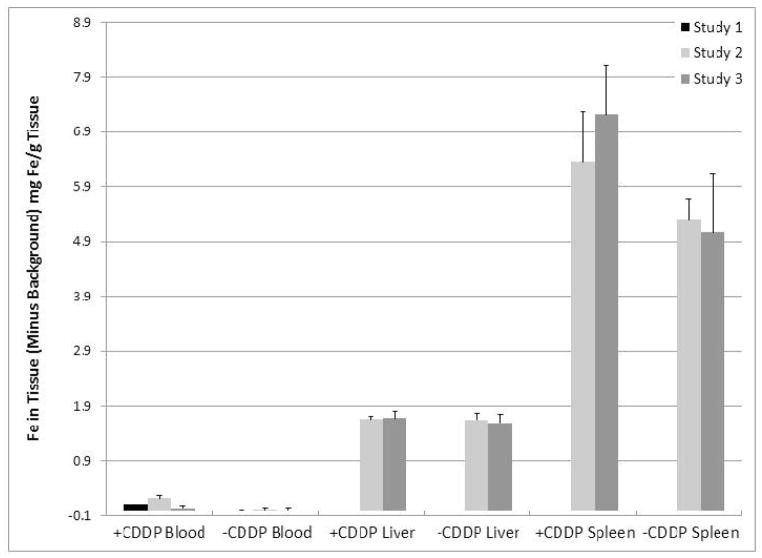
Although inconsistent, blood Fe levels in cisplatinum treated groups were elevated compared to non-treated groups (Studies 1 and 2, p=0.0002 and 0.002 respectively). Fe levels within the liver and the spleen were not statistically significant (p>0.68 and p>0.06 respectively).
3.3 Study 3: AMF exposure and treatment efficacy
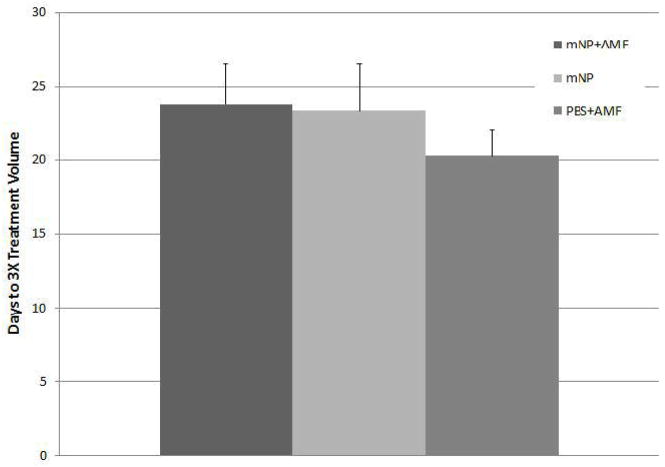
The graph below demonstrates the number of days required to reach 3X initial treatment volume. Cisplatinum was delivered 3mg/kg intraparietally, 6 days before IV delivery of mNP at 250ug Fe/g body weight. Twenty three hrs after IV injection of mNP (or PBS) an additional 3mg/kg of CDDP was given IP. Twenty four hrs following mNP injection (or PBS), mNP and PBS treated mice were exposed to AMF at 450 Oe for 1hr. An identically treated mNP control group did not receive AMF. No elevation in tumor temperature was observed. No statistical differences exist between groups (p > 0.3).
3.4 Histology
The presence of mNP within tumors was visible ex vivo and microscopically. Computer-based analysis of cellularity demonstrates a slightly reduced cellularity for low-dose cisplatinum treated tumors (36% vs. 41%), p=0.17. Although tumors treated at the higher platinum dose demonstrated higher mNP/Fe uptake, cellularity assessments are not available at this time.
4. DISCUSSION
These results demonstrate the increased uptake of systemically delivered mNP following intravenous chemotherapy. The most significant increase (3.1X) occurred following 5mg/kg cisplatinum delivered three days prior to the administration of mNP. Although no significant regrowth delay differences were observed between groups, those receiving cisplatinum and mNP treatment demonstrated reduced tumor cellularity. Statistically significant improvements in tumor concentrations were achieved with all three treatment regimens investigated. The relative percentage of total mNP reaching the tumor for Study 2, which reached the highest concentration of mNP, and greatest improvement over control tumors (3.1X), saw a modest improvement in total dose reaching the tumor (1.24% vs. 0.88%).
Although no additional regrowth delay or thermal dose was measured in the efficacy group of Study 3, potential remains to incorporate mNP hyperthermia into established chemotherapy regimens, utilizing both the ability of chemotherapy to aid tumor uptake of mNP and the adjuvant benefits of chemotherapy with mNP hyperthermia. The thermal data collected shows that the amount of Fe found in the pretreated tumors (0.24mg Fe/g tissue) was below that which could generate measurable thermal doses. However, with optimization of tumor environment modification strategies (Study 2 produced 0.41mg Fe/g tissue) the amount of Fe will continue to approach the concentrations which have been shown to be therapeutically relevant (~3mg Fe/g tissue with direct injection).
Figure 1.
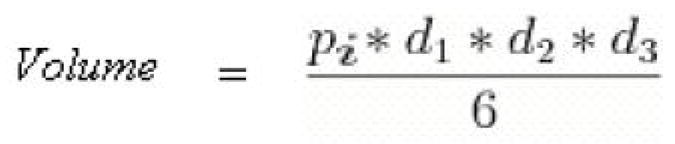
Formula for ellipsoid. Used for calculating tumor volume (d=diameter).
Figure 2.
Summary of Fe concentrations in tumors. Control values of background Fe subtracted. Study 1 resulted in 2.5X more Fe than no pretreatment (p=0.04), Study 2 in 3.1X (p=0.02) and Study 3 in 1.8X (p=0.11) in tumor tissue.
Figure 3.
No statistically significant difference was found between any of the Fe concentrations for liver and spleen (p>0.68 and 0.06 respectively).
Figure 4.
This graph demonstrates the number of days required for the tumors to reach 3X treatment volume following two doses of cisplatinum (3mg/kg), separated by six days, with mNP given between doses. Twenty-four hrs after mNP injection (or PBS), mNP+AMF and PBS+AMF mice were exposed to AMF at 450 Oe for 1hr. No statistical differences exist between tumor regrowth delays for any groups (p > 0.3)
Figure 5.

These tumor images (ex vivo) of MTGB tumors represent the difference between cisplatinum pretreatment and no pretreatment prior to mNP administration. Cisplatinum treatment (left) 5mg/kg and non-cisplatinum treated tumor (right). Tissues were collected twenty four hrs after IV injection. Average tumor Fe concentrations were 3.1X greater in pretreated tumors in comparison to non-treated tumor (Study 2).
Figure 6.
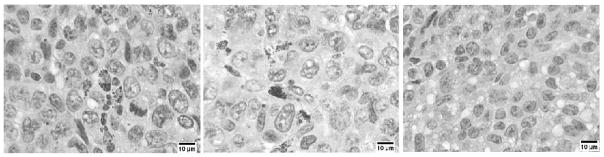
Representative photomicrographs from Study 3 demonstrating decreased cellularity following cisplatinum treatment. Although not quantitatively different in these photographs, both the non-cisplatinum and cisplatinum treated tumors, left, center respectively, demonstrate mNP uptake. The figure at right received cisplatinum, but no mNP.
Table 1.
Experimental Groups
| CDDP | MNP Dose | Tissue Collected/AMF | AMF ? | # of mice per group | |
|---|---|---|---|---|---|
| Study 1 | 5mg/kg (Day −3) | 0.10 mg/g (Day 0) | Day +2 | No | 4 |
| Study 2 | 5mg/kg (Day −3) | 0.25 mg/g (Day 0) | Day +1 | No | 6–7 |
| Study 3 | 3mg/kg (Day −6 and +1) | 0.25 mg/g (Day 0) | Day +1 | Yes | Distribution: 6 Efficacy: 3–4 |
Acknowledgments
Supported by the Dartmouth Center for Cancer Nanotechnology Excellence: NCI-CCNE U54CA151662-03
References
- 1.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends in Biotechnology. 2008;26(10):562. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano LIFE. 2010;1&2:17. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giustini AJ, Petryk AA, Hoopes PJ. Ionizing radiation increases systemic nanoparticle tumor accumulation. Nanomedicine: Nanotechnology, Biology and Medicine. 2012 doi: 10.1016/j.nano.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced Apoptosis Decompresses Blood Vessels and Lowers Interstitial Fluid Pressure in Solid Tumors Clinical Implications. Cancer Res. 1999;59(15):3776. [PubMed] [Google Scholar]
- 5.Petryk AA, Giustini AJ, Gottesman RE, Hoopes PJ. Magnetic nanoparticle hyperthermia enhancement of cisplatinum chemotherapy cancer treatment. 2013 doi: 10.3109/02656736.2013.825014. Unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate JA, Petryk AA, Giustini AJ, Hoopes PJ. In vivo biodistribution of iron oxide nanoparticles: an overview. Proc SPIE. 2011:790117. doi: 10.1117/12.876414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]