Summary
Flow diverter devices became available in our department in 2009. We considered treatment with flow diverters only in patients with aneurysms not suitable for surgery or conventional endovascular techniques. This paper presents our preliminary experience with flow diverters in a consecutive series of 550 endovascular aneurysm treatments.
Between January 2009 and July 2013, 550 endovascular treatments for intracranial aneurysms were performed. Of these, 490 were first-time aneurysm treatments in 464 patients and 61 were additional treatments of previously coiled aneurysms in 51 patients. Endovascular treatments consisted of selective coiling in 445 (80.8%), stent-assisted coiling in 68 (12.4%), balloon-assisted coiling in 13 (2.4%), parent vessel occlusion in 12 (2.2%) and flow diverter treatment in 12 (2.2%).
Eleven patients with 12 aneurysms were treated with flow diverters. Two patients had ruptured dissecting aneurysms. One patient with a basilar trunk aneurysm died of acute in stent thrombosis and another patient died of brain stem ischaemia at 32 months follow-up. One patient had ischaemia with permanent neurological deficit. Two aneurysms are still open at up to 30 months follow-up.
Flow diversion was used in 2% of all endovascular treatments. Both our own poor results and the high complication rates reported in the literature have converted our initial enthusiasm to apprehension and hesitancy. The safety and efficacy profile of flow diversion should discourage the use of these devices in aneurysms that can be treated with other techniques.
Keywords: flow diverter, endovascular treatment, intracranial aneurysms, complications, outcome
Introduction
Flow diverter devices are new tools for the endovascular treatment of intracranial aneurysms. Flow diverters are intended to reconstruct the parent artery carrying the aneurysm. Reconstruction is supposed to occur in such a way that flow is diverted from the aneurysm resulting in disruption of flow and stasis of blood followed by thrombosis. At the same time, important side branches of the artery covered with the flow diverter should remain open 1,2. The flow diverter is conceptually appealing to many operators and these devices are being deployed in greater numbers of patients with both simple and complex aneurysm morphologies 3. However, the safety and efficacy profiles of flow diverters are still poorly understood 4,5.
In our department flow diverters became available in 2009. We considered treatment with flow diverters only in patients with aneurysms not suitable for surgery or conventional endovascular techniques such as selective coiling with or without balloon or stent assistance or parent vessel occlusion. This paper presents our preliminary experience with flow diverters available as therapeutic devices.
Materials and Methods
Between January 2009 and July 2013, 550 endovascular treatments for intracranial aneurysms were performed. Of these, 490 were first-time aneurysm treatments in 464 patients and 61 were additional treatments of previously coiled aneurysms in 51 patients. Endovascular treatments consisted of selective coiling in 445 (80.8%), stent-assisted coiling in 68 (12.4%), balloon-assisted coiling in 13 (2.4%), parent vessel occlusion in 12 (2.2%) and flow diverter treatment in 11 (2.2%).
The 12 aneurysms in 11 patients treated with flow diverters form the subject of this study. All nine unruptured aneurysms were proctored by peers with ample experience with flow diverters. Patients with unruptured aneurysms were preloaded with dual antiplatelet medication. Patients with ruptured aneurysms received 500 mg Aspirin intravenously just prior to flow diverter placement. Dual antiplatelet medication was continued for six months. One patient had bilateral vertebral dissection aneurysms treated with flow diverters. Patient, aneurysm and treatment characteristics are summarized in Table 1. There were seven women and four men aged between 48 and 68 years. There were five large or giant aneurysms of the carotid artery, three presented with symptoms of mass effect and two had been previously coiled. None of the five patients with large or giant carotid artery aneurysms tolerated angiographic test occlusion. There were four dissection aneurysms, one ruptured supraclinoid carotid artery aneurysm and three vertebral dissection aneurysms of which one had ruptured. Two aneurysms were located on the basilar trunk and presented with mass effect on the brain stem and one incidentally found aneurysm was located on the A1.
Table 1.
Clinical and imaging characteristics of 11 patients with 12 aneurysms treated with flow diverter devices.
|
Case no. |
Aneurysm location and size |
Clinical presentation |
Device type |
Procedural and late complications |
Clinical result |
Angiographic result |
Duration follow- up |
| 1 | Supraclinoid ICA dissection 5 mm |
Poor grade SAH |
Silk | None | mRs 2 | Grown at 2 mo, aneurysm occluded at 5 mo |
12 mo |
| 2 | Supraclinoid ICA fusiform 15 mm |
Incidental | Pipeline 2nd pipeline at 24 mo |
None | Unchanged | Open | 30 mo |
| 3 | Carotid ophthalmic 12 mm |
Visual loss | Pipeline ×2 |
None | Unchanged | Aneurysm occluded at 12 mo |
12 mo |
| 4 | Carotid ophthalmic 30 mm partially thrombosed |
Incidental 1 previous coiling |
Pipeline ×2 |
None | Unchanged | Aneurysm occluded at 6 mo |
6 mo |
| 5 | Carotid cavernous sinus 30 mm partially thrombosed |
Ophthalmoplegia 3 previous coilings |
Pipeline ×2 |
None | Improved | Aneurysm occluded at 6 mo |
12 mo |
| 6 | Carotid cavernous sinus 30 mm |
Ophthalmoparesis | Pipeline ×2 |
None | Unchanged | Aneurysm occluded at 8 mo |
8 mo |
| 7 | Left vertebral dissection aneurysm 7 mm right vertebral dissection aneurysm 8 mm |
Vertebrobasilar TIAs |
Pipeline | Infarction medulla oblongata at 6 mo, brain stem infarction at 32 mo |
Death | Left aneurysm occluded at 6 mo right V4 occlusion at 6 mo |
32 mo |
| 8 | Dissection (single) left vertebral artery aneurysm 6 mm |
SAH ×3 | Silk | None | mRs 2 | Aneurysm grown at 6 mo |
6 mo |
| 9 | A1 dumbbell aneurysm 10 mm |
Incidental | Pipeline ×2 |
Perforator infarction basal ganglia |
mRs 2 | Aneurysm occluded at 6 mo |
12 mo |
| 10 | Basilar trunk aneurysm 15 mm |
Mass effect brain stem |
Leo stent ×3, Silk, coils |
Acute in-stent thrombosis |
Death | ||
| 11 | Basilar trunk aneurysm 20 mm |
Mass effect brain stem |
Leo stent ×2, Silk, coils |
None | Improved | Aneurysm occluded at 3 mo |
9 mo |
|
Abbreviations: M=male; F= female; ICA=internal carotid artery; SAH=subarachnoid haemorrhage; mRs= modified Rankin Scale; mo=month; TIA=transient ischaemic attack | |||||||
Results
General results
Four aneurysms were treated with SILK (Balt, Montmorency, France) and eight with the Pipeline device (ev3, Irvine, CA, USA). Two devices were placed in five patients and one device was placed in the other six. In one patient with a basilar trunk aneurysm the procedure was complicated by acute in-stent thrombosis resulting in death 6. In another patient, with an aneurysm on the A1, immediate basal ganglia ischaemia occurred resulting in cognition problems and dysphasia 7. Follow-up in the surviving ten patients with 11 aneurysms was a mean 12.3 months (range 6-32 months). Eight aneurysms were occluded at three to 12 months with a patent parent artery. Two aneurysms are still open at 12 and 30 months despite placement of an additional Pipeline in one. In the patient with bilateral vertebral dissection aneurysms, the V4 segment of the right vertebral artery was occluded at six months with small infarctions in the medulla oblongata resulting in partial Wallenberg syndrome. At 32 months, the patient presented with haemorrhagic brain stem infarction and died before angiography could be performed.
Representative cases
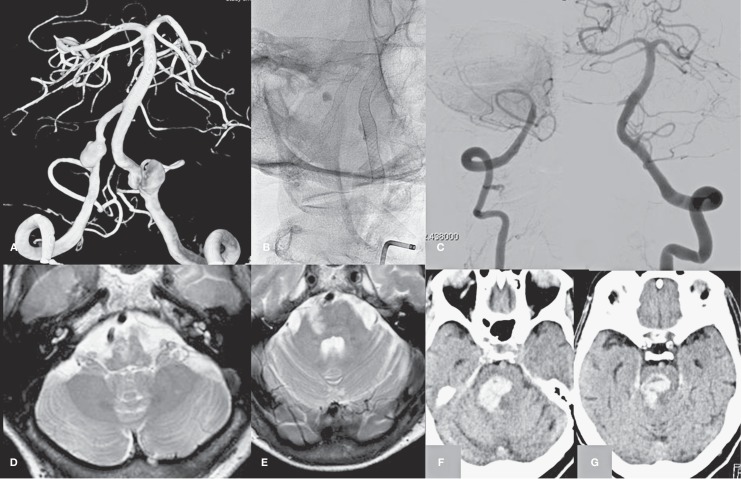
Case 1, Figure 1, patient no. 7
An elderly woman was referred for MRI for repeated vertebrobasilar TIAs. Bilateral vertebral dissection aneurysms were evident and angiography was performed showing dissecting aneurysms in close relation with the PICA on both sides. Deconstructive or reconstructive endovascular therapy with coils and stents was judged not possible and in one session Pipelines were placed in the dissecting segments of both vertebral arteries.
Follow-up MRI and angiography showed that the left aneurysm was occluded with a patent vertebral artery. However, the right vertebral artery was completely occluded with a patent PICA. The patient had small infarctions in the medulla oblongata and a partial Wallenberg syndrome. In addition, there were new small peripheral cerebellar infarctions. At 32 months follow-up, the patient presented with extensive haemorrhagic brain stem infarction and died before angiography could be performed. We speculate that late occlusion of the flow diverter in the left vertebral artery was the cause for this event.
Figure 1.
An elderly woman with bilateral vertebral dissection aneurysms and vertebrobasilar TIAs (patient no.7). A) 3D angiogram demonstrates bilateral vertebral dissection aneurysms in close relation to the PICA origins. B) Position of flow diverters in the vertebral arteries. C,D) Angiogram at 6 months shows occlusion of the right V4 segment (C), the left aneurysm is occluded (D). E-F) T2-weighted MRI image with infarctions in the medulla oblongata. G-H) CT scan at 32 months shows hemorrhagic brain stem infarction.
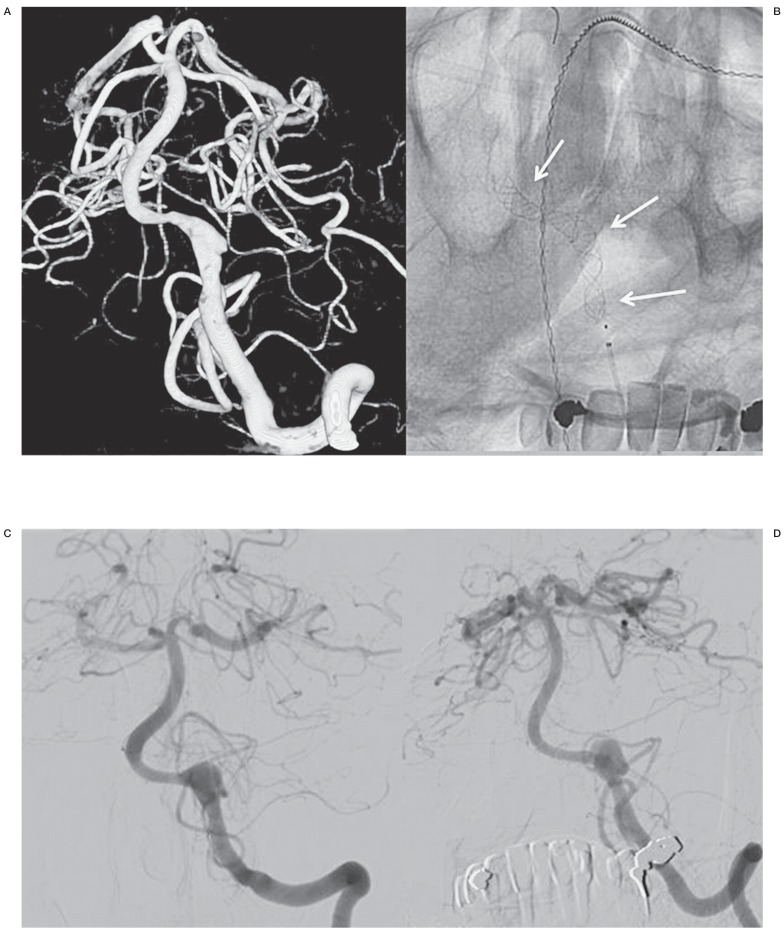
Case 2, Figure 2, patient no.8
A middle-aged man was admitted in good grade SAH from a left vertebral dissection aneurysm. The right vertebral artery had a PICA ending. Conventional reconstructive therapy was not possible. In the following days the patient had two recurrent SAHs and on day 6 a Pipeline device was placed across the dissecting vertebral aneurysm. The patient gradually completely recovered and follow-up angiography at six months showed that the aneurysm had grown and was still open. Further follow-up is scheduled.
Figure 2.
A middle-aged man with SAH from a left vertebral dissection aneurysm (patient no.8). A) 3D angiogram shows a dissecting aneurysm in the V4 segment. Occlusion of this segment was not possible since the right vertebral artery had a PICA ending. B) Position of flow diverters. C,D) Follow-up angiograms after 2 (C) and 6 (D) months showing a slight enlargement of the dissecting aneurysm.
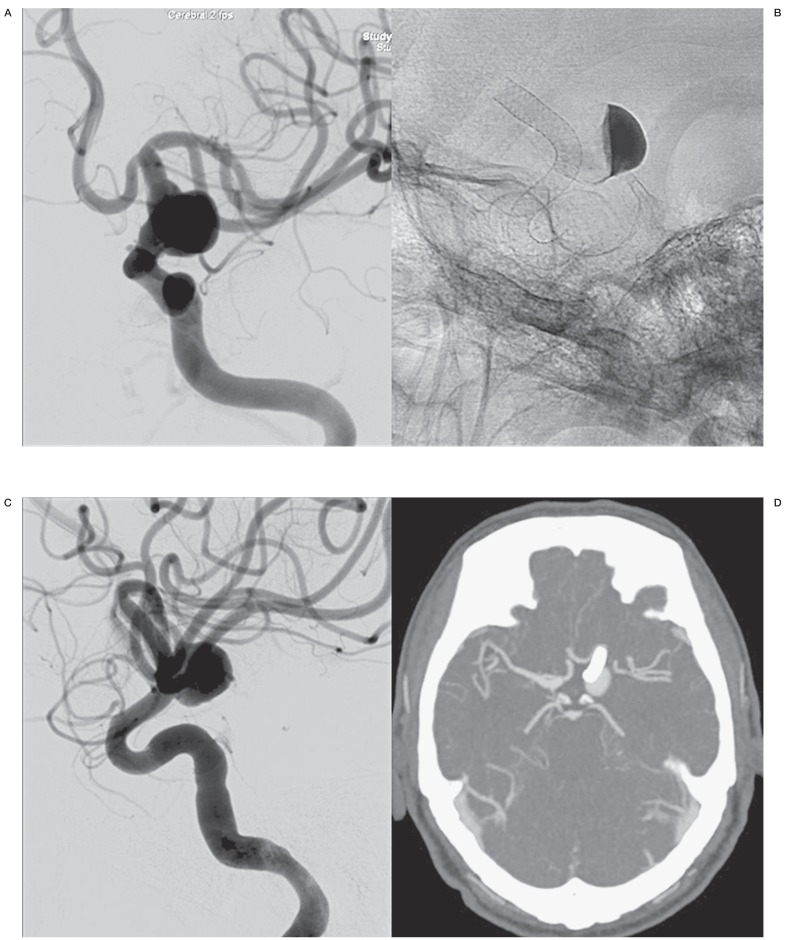
Case 3, Figure 3, patient no. 2
An elderly man was referred for CT scan of the head after a minor trauma and an aneurysm was suspected. Angiography demonstrated a large fusiform aneurysm of the left supraclinoid carotid artery.
A test occlusion of the left internal carotid artery was not tolerated. After several consultations at the outpatient clinic, it was finally decided to treat the aneurysm by placing a Pipeline flow diverter. Follow-up CT angiography at six, 12, 18 and 24 months revealed that the aneurysm was still open and at 24 months a second Pipeline was telescopically placed across the aneurysm. CT angiography six months later showed that the aneurysm is still open.
Figure 3.
A man in his sixties with an incidentally discovered fusiform aneurysm of the left supraclinoid carotid artery (patient no.2). A) Left carotid artery angiogram demonstrates the large fusiform aneurysm. B) Position of flow diverter and stasis of contrast inside the aneurysm. C) Angiogram at 24 months shows that the aneurysm is still open. A second flow diverter was telescopically placed inside the first one. D) CT angiogram 6 months later reveals that the aneurysm is still not occluded.
Discussion
When the flow diverter became available for the endovascular treatment of intracranial aneurysms in our hospital the device was welcomed as a promising additional tool for aneurysms with difficult morphology. Since at that time no or little clinical data on efficacy and safety were available, we decided to use the flow diverter only in cases were conventional endovascular techniques or surgery were impossible or undesirable. This restraint indication resulted in only 11 treatments with flow diverters among 550 endovascular treatments in a 4.5 year period. Our preliminary results are disappointing: of ten patients, two died and one had permanent morbidity, and two aneurysms are still open at up to 30 months follow-up.
Our unsatisfactory results in our limited experience with flow diverters seem to be in agreement with other studies. Although some small single centre studies report good results 8,9, pooled data from registries and meta-analyses indicate complications in a substantial proportion of patients and suboptimal treatment efficacy 4,5,10,11. In the meta analysis of Brinjikji et al. 5, comprising 1654 unruptured aneurysms in 1451 patients, treatment mortality was 4% and permanent morbidity was 5%. Complications consisted of early SAH (<30 days) in 3%, late SAH in 2%, early parenchymal haemorrhage in 3% and late in 2%, early ischaemic stroke in 5% and late ischaemic stroke in 3%. Perforator infarction occurred in 3%. SAH occurred more often in large and giant aneurysms and ischaemic events more often in the posterior circulation. At six months, the aneurysm occlusion rate was 76%. Since the cumulative proportion of complications exceeds the overall morbidity and mortality rate in this analysis, apparently many complications occur that do not lead to permanent morbidity or death. Another meta analysis 4 comprising 1018 aneurysms in 897 patients and largely overlapping the Brinjikji study reported an overall mortality rate of 4.1% and neurological morbidity was 9.9%. At a mean follow-up of nine months, 76% of aneurysms were occluded. Both meta-analyses comprised early studies with limited follow-up periods. It remains unknown what proportion of aneurysms will occlude beyond the six to nine months interval. Furthermore, the frequency of late adverse events like late in-stent stenosis and parent vessel occlusion and haemorrhage is unknown but these events will likely increase the overall complication rate of treatment with flow diverters. This issue of late events was addressed by Velioglu et al. 12, who combined their results with four other studies and found that in more than 11% (22 of 197) of aneurysms treated with a flow diverter the parent artery became occluded during follow-up.
Because of the need for anti aggregation for patients undergoing treatment with a flow diverter, the majority of treatments are performed in patients with unruptured aneurysms. The preliminary results of endovascular treatment of intracranial aneurysms with flow diverters are much worse than for conventional endovascular techniques, in terms of both safety and efficacy 13,14. In view of the high ischaemic event rate in posterior circulation aneurysms, the use of flow diverters in this location is strongly discouraged 4,5. In anterior circulation aneurysms, flow diversion is predominantly used for large and giant aneurysms of the internal carotid artery and M1 segment. Most of these aneurysms can be treated with conventional endovascular techniques or direct surgery at lower risk and with an equal or better chance of success. Especially in cavernous sinus aneurysms, that generally exhibit a benign clinical course, the treatment complication rate should approach 0%, a safety profile that flow diverters cannot offer. For resolution of symptoms of mass effect, flow diversion is not better than conventional endovascular techniques. Most patients with aneurysms presenting with mass effect do well after conventional endovascular treatment such as selective coiling or parent vessel occlusion 15-18.
Since the flow diverter became available in our practice, our initial enthusiasm for the concept of flow diversion has not been sustained. Both our own poor results and the high complication rates reported in the literature have converted our enthusiasm to apprehension and hesitancy 19,20. We cannot offer a patient with an unruptured aneurysm a treatment with a more than one in ten chance of mortality or permanent neurological deficit and at the same time with a chance of one in four that the aneurysm does not occlude, at least in the first year. In our view, the indication for a flow diverter is restricted to those unruptured aneurysms with a poor natural history that cannot be treated otherwise. Our initial experience indicates that flow diversion can thus be restricted to less than 2% of all endovascular treatments for aneurysms.
In patients presenting with unruptured aneurysms, our first goal is to do no harm. A treatment strategy with parent vessel occlusion when tolerated, selective coiling when necessary and conservative treatment when possible (cavernous sinus aneurysms) has a very low complication rate approaching 0% and is effective in preventing first-time SAH or in alleviating symptoms of mass effect and has no negative consequences in the long term. With over 10% combined morbidity and mortality together with the risk of delayed aneurysm rupture and delayed parent vessel occlusion, flow diverters should for now be considered a dangerous therapy for unruptured aneurysms, especially for posterior circulation aneurysms.
References
- 1.Fiorella D, Lylyk P, Szikora I, et al. Curative cerebrovascular reconstruction with the Pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. 2009;1(1):56–65. doi: 10.1136/jnis.2009.000083. doi: 10.1136/jnis.2009.000083. [DOI] [PubMed] [Google Scholar]
- 2.Szikora I, Nelson PK, Berentei Z, et al. The potential of flow modification in the treatment of intracranial aneurysms. Interv Neuroradiol. 2008;14(Suppl 1):77–80. doi: 10.1177/15910199080140S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Urso PI, Karadeli HH, Kallmes DF, et al. Coiling for paraclinoid aneurysms: time to make way for flow diverters? Am J Neuroradiol. 2012;33(8):1470–1474. doi: 10.3174/ajnr.A3009. doi: 10.3174/ajnr.A3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73(2):193–200. doi: 10.1227/01.neu.0000430297.17961.f1. doi: 10.1227/01.neu.0000430297.17961.f1. [DOI] [PubMed] [Google Scholar]
- 5.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442–447. doi: 10.1161/STROKEAHA.112.678151. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 6.van Oel LI, van Rooij WJ, Sluzewski M, et al. Reconstructive endovascular treatment of fusiform and dissecting basilar trunk aneurysms with flow diverters, stents, and coils. Am J Neuroradiol. 2013;34(3):589–595. doi: 10.3174/ajnr.A3255. doi: 10.3174/ajnr.A3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij WJ, Sluzewski M. Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured A1 aneurysm. Am J Neuroradiol. 2010;31(4):E43–44. doi: 10.3174/ajnr.A2034. doi: 10.3174/ajnr.A2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. Am J Neuroradiol. 2012;33(8):1436–1446. doi: 10.3174/ajnr.A3246. doi: 10.3174/ajnr.A3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–642. doi: 10.1227/01.NEU.0000339109.98070.65. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 10.Briganti F, Napoli M, Tortora F, et al. Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications-a retrospective data analysis. Neuroradiology. 2012;54(10):1145–1152. doi: 10.1007/s00234-012-1047-3. doi: 10.1007/s00234-012-1047-3. [DOI] [PubMed] [Google Scholar]
- 11.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. Am J Neuroradiol. 2012;33(6):1150–1155. doi: 10.3174/ajnr.A2907. doi: 10.3174/ajnr.A2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velioglu M, Kizilkilic O, Selcuk H, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology. 2012;54(12):1355–1365. doi: 10.1007/s00234-012-1051-7. doi: 10.1007/s00234-012-1051-7. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi M, Cirillo L, Toni F, et al. Treatment of intracranial aneurysms using flow-diverting silk stents (BALT): a single centre experience. Interv Neuroradiol. 2011;17(3):306–315. doi: 10.1177/159101991101700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirillo L, Leonardi M, Dall'Olio M, et al. Complications in the treatment of intracranial aneurysms with silk stents: an analysis of 30 consecutive patients. Interv Neuroradiol. 2012;18(4):413–425. doi: 10.1177/159101991201800407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Rooij WJ, Sluzewski M. Endovascular treatment of large and giant aneurysms. Am J Neuroradiol. 2009;30(1):12–18. doi: 10.3174/ajnr.A1267. doi: 10.3174/ajnr.A1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rooij WJ, Sluzewski M. Unruptured large and giant carotid artery aneurysms presenting with cranial nerve palsy: comparison of clinical recovery after selective aneurysm coiling and therapeutic carotid artery occlusion. Am J Neuroradiol. 2008;29(5):997–1002. doi: 10.3174/ajnr.A1023. doi: 10.3174/ajnr.A1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij WJ. Endovascular treatment of cavernous sinus aneurysms. Am J Neuroradiol. 2012;33(2):323–326. doi: 10.3174/ajnr.A2759. doi: 10.3174/ajnr.A2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooij WJ, Sluzewski M. Unruptured carotid artery aneurysms presenting with symptoms of mass effect: outcome after selective coiling, parent vessel occlusion, and flow diversion. Am J Neuroradiol. 2013;34(5):940–941. doi: 10.3174/ajnr.A3594. doi: 10.3174/ajnr.A3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rooij WJ, Sluzewski M, van der Laak C. Flow diverters for unruptured internal carotid artery aneurysms: dangerous and not yet an alternative for conventional endovascular techniques. Am J Neuroradiol. 2013;34(1):3–4. doi: 10.3174/ajnr.A3317. doi: 10.3174/ajnr.A3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooij WJ. Flow diverters for unruptured aneurysms: are they safe enough? Neuroradiology. 2012;54 (10:1179–1180. doi: 10.1007/s00234-012-1066-0. doi: 10.1007/s00234-012-1066-0. [DOI] [PubMed] [Google Scholar]