Summary
The Pipeline embolization device (PED) is one of the flow-diverting stents approved for the treatment of unruptured large or wide-necked cerebral aneurysms in 2011 1. Its use has now been extended to the treatment of recently ruptured dissecting cerebral aneurysm, carotid pseudoaneurysm from radiation injury, and blister aneurysms 2,3. We aimed to evaluate the effectiveness of utilizing the PED as a primary treatment for ruptured dissecting intracranial aneurysms.
A single center retrospective review was conducted for all patients primarily treated with PED for acute subarachnoid hemorrhage (SAH) from ruptured dissecting cerebral aneurysms between December 2010 and February 2013. Patients were followed up with CT angiogram (CTA) or digital subtraction angiogram (DSA).
Eight patients with a total of eight dissecting aneurysms were identified. The mean duration from SAH to treatment was 2.5 days. Six of the aneurysms arose from vertebral arteries and two from the basilar artery. Immediate check-DSA confirmed satisfactory contrast stasis in all eight cases, and complete aneurysmal obliteration was achieved at six months. There were two (25%) procedure-related complications, but no major procedure-related complications, such as thromboembolic events or rebleeding from aneurysm were encountered.
The PED is a feasible treatment option for ruptured dissecting cerebral aneurysms in acute phase. According to our experience, using PED as flow-diverters in acute SAH does not significantly increase the complication risks or mortality rate if the antiplatelet regime is carefully monitored. Future studies shall evaluate the optimal antiplatelet regimen for using the PED in the acute phase.
Keywords: pipeline embolization device, dissecting aneurysms, subarachnoid hemorrhage, VerifyNow assay
Introduction
The Pipeline embolization device (PED) is a flow-diverting stent (FDS) approved by the FDA in the USA in 2011 for the treatment of unruptured large or wide-necked cerebral aneurysms. There were a total of 108 PEDs deployed in our center between December 2010 and February 2013. The PED is gaining popularity due to its property of flow modification, eliminating shear stress and reconstruction of the parent artery with subsequent intra-aneurysmal thrombosis, while blood flow to side branch arteries and perforators can be preserved 4,5. Its effectiveness with high complete occlusion rates and low procedure-related morbidity and mortality has been well-demonstrated 6.
As heparin and antiplatelet therapy would be required during and after deployment of a FDS, great caution is advised for its use in the presence of recent hemorrhage 7,8. However, some centers have extended its use for the treatment of recently ruptured dissecting cerebral aneurysm, carotid pseudoaneurysm from radiation injury, and blister aneurysms 9. In patients with recent rupture of aneurysms, complete obliteration upon angiographic follow-up can be achieved in 82.8%, with an overall procedure-related complication rate of 10.3% 10.
The classical treatment for intracranial arterial dissection with hemorrhage is trapping of the involved segment, and thus sacrificing blood supply to the down-stream normal brain tissue. This may result in significant infarction especially if the sacrificed segment is dominant. With the use of FDS, there is a hope that normal blood flow in the arterial lumen can be preserved on the one hand, while the dissected arterial wall can be repaired on the other.
Therefore, we reviewed our experience and aimed to evaluate the safety and effectiveness of utilizing PED as a primary treatment for ruptured dissecting aneurysms in our center.
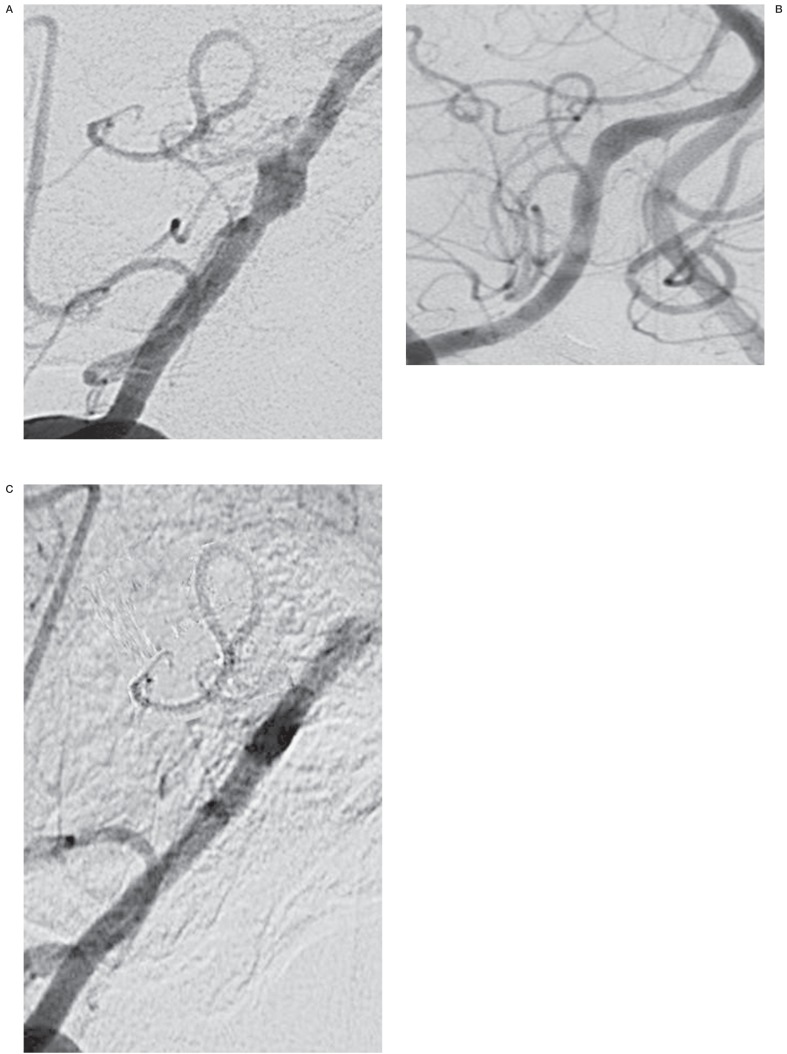
Figure 1.
A) Pre-stenting of a right vertebral artery dissecting aneurysm, with posterior inferior cerebellar artery involvement. B) Immediate angiogram after stenting of the right vertebral artery. C) 4 months post-stenting of the right vertebral artery, showing satisfactory aneurysmal obliteration with patency of the posterior inferior cerebellar artery.
Materials and Methods
A retrospective review from our departmental database was conducted for all patients primarily treated with the PED for acute SAH from ruptured dissecting cerebral aneurysms in Kwong Wah Hospital between December 2010 and February 2013. Relevant data, including aneurysmal characteristics, antiplatelet regimen, technical issues, degree of contrast stasis in immediate post-stented DSA, complications and clinical outcome in Glasgow Outcome Scale (GOS), were collected and analyzed. Patients were followed up with either CTA or DSA at six-month intervals. The images were assessed by independent radiologists.
Results
Eight patients (five female and three male; mean age, 52 years) presented with acute SAH and underwent primary treatment with PED (Table 1). The World Federation of Neurological Surgeons (WFNS) grade on admission was 1 in six (75%) patients and Grade 4 in the remaining two (25%) patients.
Table 1.
Characteristics and outcome of the eight dissecting aneurysms treated with PED.
| Sex |
Day of rupture |
WFNS Grade |
Location |
Size of dissection (mm × mm) |
Post-procedure |
Aneurysm contrast stasis |
Angiographic follow-up |
GOS | Remarks | ||
| Aspirin | Clopidogrel | ||||||||||
| 1 | 47 | 5 | 4 | Rt VA | 6 × 14 | 80 mg × 3 days |
75 mg × 3 months |
Contrast stasis | Complete obliteration at 5 days by CTA |
5 | Lt vitreous hemorrhage at D7, resolved spontaneously |
| 2 | 44 | 2 | 1 | Lt VA | 3.8 × 3.3 | 75 mg × 1 months |
Contrast stasis | Complete obliteration at 1 month by DSA, 4 months by CTA |
3 | Venous infarction at D25 with ICH, requiring craniectomy |
|
| 3* | 63 | 4 | 1 | BA | 4 × 15 | 75 mg × 3 months |
Contrast stasis | Complete obliteration at 2 months by DSA |
2 | Broken 2nd pipeline stent |
|
| 4 | 39 | 1 | 1 | Lt VA | 3 × 3 | 75 mg × 6 months |
Mild contrast stasis |
Complete obliteration in 6 and 18 months by CTA |
5 | ||
| 5 | 56 | 3 | 1 | Lt VA | 4 × 4 | 80 mg × 6 months |
75 mg × 6 months |
Contrast stasis | Complete obliteration at 2 months by CTA, 6 months by DSA |
4 | |
| 6 | 42 | 3 | 1 | Rt VA | 3.9 × 9.4 | 160 mg × 6 months |
Contrast stasis | Complete obliteration at 4 months by DSA |
5 | ||
| 7* | 67 | 1 | 4 | BA | 3 × 6 | 160 mg × 6 months |
Minimal contrast filling |
Complete obliteration at 2 and 10 months by CTA |
2 | ||
| 8 | 56 | 1 | 1 | Rt VA | 2.8 × 3.3 | 160 mg × 6 months |
Contrast stasis | Complete obliteration at 3 and 7 months by DSA |
5 | ||
| WFNS Grade = World Federation of Neurosurgeons classification of SAH; GOS = Glasgow Outcome Scale; * = stent-assisted coiling; VA = vertebral artery; BA = basilar artery.. | |||||||||||
A total of eight dissecting aneurysms were treated, all were from the posterior circulation with major side branch involvement such as the posterior inferior cerebellar artery. Six (75%) were from the vertebral artery, two (25%) from the basilar artery.
All eight patients were treated with PED in the acute phase (<five days). Mean duration from SAH was 2.5 days (Range, 1-5 days). The mean number of PED deployed was 1.4 (range, 1-2). One PED was broken on deployment. Two patients received stent-assisted coiling in view of large daughter sacs identified. Contrast stasis was achieved in all eight patients.
All our patients received intraprocedural systemic heparinization and a loading dose of aspirin 320 mg and clopidogrel 300 mg via nasogastric tube just before deployment of PED, followed by low-molecular weight heparin for one day. After the first few cases, we standardized our maintenance antiplatelet regimen as a single agent, either aspirin or clopidogrel for three to six months more. We also confirmed the effectiveness of aspirin or clopidogrel with the VerifyNow test (Accumetrics, San Diego, CA, USA). If the patient was resistant or a poor responder to one of the antiplatelet agents, we switched to the other alternative. If the initial CT brain showed ventricular dilatation, endovascular interventions were performed only after external ventricular drains were inserted, preferably under the same general anesthesia.
There were two (25%) procedure-related symptomatic complications. One patient was admitted for acute right vertebral artery dissection (WFNS Grade 3). Immediate post-operative recovery was uneventful. CTA done five days later showed complete obliteration of the dissection. She complained of visual blurring at day 7 and ophthalmological examination showed left vitreous hemorrhage. It resolved spontaneously afterwards. She had a Glasgow Outcome Scale of 5 at two months' follow-up. However she subsequently defaulted and follow-up angiogram could not be performed. Another patient presented with left vertebral artery dissection (WFNS Grade 1). One PED was deployed and she was put on clopidogrel 75 mg daily post-operatively. However, she developed cortical vein thrombosis in the right parietal region, which resulted in intracerebral hematoma 25 days later. Despite this, the follow-up DSA showed complete obliteration of the stented aneurysm, with no in-stent thrombosis. Clopidogrel was then switched to warfarin for eight months more. She made a satisfactory recovery and could manage to take care of her daily living.
There was one technical failure in a patient with a 4 mm × 15 mm basilar trunk dissection. A 3.75 mm × 30 mm PED was first successfully deployed across the dissection. When a second PED (3.75 mm × 20 mm) was deployed in a telescoping manner inside the first stent, the tip of the guide-wire broke and became lodged at the basilar tip. The wire tip was successfully retrieved with an Amplatz gooseneck snare, and the patient remained asymptomatic afterwards.
There were no hemorrhagic complications encountered from the aneurysms or when external ventricular drains were removed. Four patients had their ventriculo-peritoneal shunts placed after an average of 24 days from stenting (range, 14-31 days). All were uneventful.
The median clinical follow-up time was 11.5 months (range, 2-32 months). No symptomatic delayed complications or delayed rebleeding occurred. Upon last follow-up, five patients (63%) were independent (GOS >/= 4). There was no procedure-related mortality.
The median angiographic follow-up was five months (range: 2-10 months; CTA in three, DSA in five). All of the dissecting aneurysms showed complete obliteration and no recanalization was detected at intermediate angiogram.
Discussion
Data on the use of PED in acute or subacute SAH from posterior circulation dissecting aneurysms are limited to a few case reports or small series (Table 2) 2,11-14. Great caution is generally advised due to thromboembolic risks and the use of heparin or antiplatelet therapy 13.
Table 2.
Characteristics and outcome of the posterior circulation dissecting aneurysms treated with PED in previous studies.
| Reference |
Number of patients |
Locations |
Complete aneurysmal occlusion at 6 months |
Clinical outcome |
Complications |
| McAuliffe and Wenderoth (2012) 12 |
4 | 1 VA, 3 BA |
4 (100%) | 2 mRS of 0; 1 mRS of 1; 1 mRS of 2 |
One PED migration; One cerebellar bleeding |
| Narata et al. (2012) 13 |
2 | 2 VA | 2 (100%) | No neurological deficit |
None reported |
| Martin AR et al. (2012) 2 |
1 | BA | 1 (100%) | No neurological deficit |
None reported |
| McTaggart et al. (2013) 14 |
1 | VA | Not available | Returned to neurological baseline |
PED migration |
| VA = vertebral artery; BA = basilar artery. | |||||
Ruptured vertebrobasilar dissecting aneurysms have a high rate of early rehemorrhage, up to 70% in some series 15,16. They carry a poor clinical outcome with a high mortality rate of 46.7% in rerupture cases 17. Hence, early treatment is warranted.
The classical treatment of choice for dissecting aneurysms remains trapping or parent artery occlusion with a success rate of 62-73% 18-20. However, in the setting when there is insufficient collateral flow or major branches such as involvement of the posterior inferior cerebellar artery, parent vessel preservation is paramount. PED provides a novel treatment option for such devastating aneurysms by wall reconstruction and aneurysmal sac thrombosis at a later stage.
The optimal antiplatelet regimen in the acute phase of SAH has yet to be determined. The widely accepted standard regimen of dual antiplatelets for stent placement in unruptured cases carries great concern in these acute dissection cases, so we came to the conclusion that a single maintenance antiplatelet agent either aspirin or clopidogrel should be used.
Aspirin resistance has been reported to occur in 5% to 45% of the general population 21 while the prevalence of clopidogrel non-response was 21% 22 . Since the VerifyNow assay became available in our neurosurgical department in November 2011, we have routinely checked the aspirin-reaction unit (ARU) for aspirin and percentage inhibition of P2Y12 reaction units (PRU) for clopidogrel to determine the effect of antiplatelet agents. We use a widely-adopted cut-off value of <550 ARU for aspirin and PRU <230 and >/= 40% inhibition for clopidogrel as indicators of acceptable antiplatelet effect for both drugs 23. Patients with values below these cut-offs were usually switched to another antiplatelet agent to ensure adequate protection against thromboembolic complications.
Our study has several limitations, including its retrospective design, small sample size, short follow-up period and the heterogeneity of the patient group, which included patients with different WFNS grading, size of aneurysms and various antiplatelet regimens, though we tried to standardize it after the first few cases. There was no validation of each patient's compliance with the individual antiplatelet regimen. However, we believe that this case series can help further validate the role of the PED in managing acute dissection of the posterior circulation.
Conclusions
The Pipeline embolization device (PED) has revolutionized the endovascular treatment of intracranial aneurysms by its property of parent artery remodeling to achieve flow diversion and side branch preservation. As many published studies showed, the PED should be used with caution in the acute setting of ruptured aneurysms carrying a high rate of rebleeding and high morbidity afterwards. Our case series demonstrated that PED can be a feasible treatment option in a carefully selected patient group and does not significantly increase the complication risks or mortality rate. Future studies should evaluate the optimal antiplatelet regimen for using the PED in the acute phase.
References
- 1.Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012;54(4):369–382. doi: 10.1007/s00234-011-0948-x. doi: 10.1007/s00234-011-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin AR, Cruz JP, Matouk CC, et al. The pipeline flow-diverting stent for exclusion of ruptured intracranial aneurysms with difficult morphologies. Neurosurgery. 2012;70(1 Suppl Operative):21–28. doi: 10.1227/NEU.0b013e3182315ee3. discussion 28. [DOI] [PubMed] [Google Scholar]
- 3.Rasskazoff S, Silvaggio J, Brouwer PA, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interv Neuroradiol. 2010;16(3):255–258. doi: 10.1177/159101991001600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augsburger L, Farhat M, Reymond P, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol. 2009;19(3):204–214. doi: 10.1007/s00062-009-9005-0. doi: 10.1007/s00062-009-9005-0. [DOI] [PubMed] [Google Scholar]
- 5.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38(8):2346–2352. doi: 10.1161/STROKEAHA.106.479576. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442–447. doi: 10.1161/STROKEAHA.112.678151. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 7.McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. Am J Neuroradiol. 2012;33(3):487–493. doi: 10.3174/ajnr.A2797. doi: 10.3174/ajnr.A2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz JP, O'Kelly C, Kelly M, et al. Pipeline embolization device in aneurysmal subarachnoid hemorrhage. Am J Neuroradiol. 2013;34(2):271–276. doi: 10.3174/ajnr.A3380. doi: 10.3174/ajnr.A3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amenta PS, Starke RM, Jabbour PM, et al. Successful treatment of a traumatic carotid pseudoaneurysm with the Pipeline stent: Case report and review of the literature. Surg Neurol Int. 2012;3:160. doi: 10.4103/2152-7806.105099. doi: 10.4103/2152-7806.105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung GK, Tsang AC, Lui WM. Pipeline embolization device for intracranial aneurysm: a systematic review. Clin Neuroradiol. 2012;22(4):295–303. doi: 10.1007/s00062-012-0178-6. doi: 10.1007/s00062-012-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Barros Faria M, Castro RN, Lundquist J, et al. The role of the pipeline embolization device for the treatment of dissecting intracranial aneurysms. Am J Neuroradiol. 2011;32(11):2192–2195. doi: 10.3174/ajnr.A2671. doi: 10.3174/ajnr.A2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. Am J Neuroradiol. 2012;33(3):487–493. doi: 10.3174/ajnr.A2797. doi: 10.3174/ajnr.A2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narata AP, Yilmaz H, Schaller K, et al. Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery. 2012;70(4):982–988. doi: 10.1227/NEU.0b013e318236715e. discussion 988-9. doi: 10.1227/NEU.0b013e318236715e. [DOI] [PubMed] [Google Scholar]
- 14.McTaggart RA, Santarelli JG, Marcellus ML, et al. Delayed retraction of the pipeline embolization device and corking failure: pitfalls of pipeline embolization device placement in the setting of a ruptured aneurysm. Neurosurgery. 2013;72(2 Suppl Operative):onsE245–250. doi: 10.1227/NEU.0b013e31827fc9be. discussion onsE250-251. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani T, Aruga T, Kirino T, et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36(5):905–911. doi: 10.1227/00006123-199505000-00003. discussion 912-913. doi: 10.1227/00006123-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Rabinov JD, Hellinger FR, Morris PP, et al. Endovascular management of vertebrobasilar dissecting aneurysms. Am J Neuroradiol. 2003;24(7):1421–1428. [PMC free article] [PubMed] [Google Scholar]
- 17.Ramgren B, Cronqvist M, Romner B, et al. Vertebrobasilar dissection with subarachnoid hemorrhage: a retrospective study of 29 patients. Neuroradiology. 2005;47(2):97–104. doi: 10.1007/s00234-005-1346-z. doi: 10.1007/s00234-005-1346-z. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Yang Y, Miao H, et al. Endovascular treatment for large and giant fusiform aneurysms of the vertebrobasilar arteries. Clin Imaging. 2013;37(2):227–231. doi: 10.1016/j.clinimag.2012.05.002. doi: 10.1016/j.clinimag.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Rabinov JD, Hellinger FR, Morris PP, et al. Endovascular management of vertebrobasilar dissecting aneurysms. Am J Neuroradiol. 2003;24(7):1421–1428. [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79(2):161–173. doi: 10.3171/jns.1993.79.2.0161. doi: 10.3171/jns.1993.79.2.0161. [DOI] [PubMed] [Google Scholar]
- 21.Mason PJ, Freedman JE, Jacobs AK. Aspirin resistance: current concepts. Rev Cardiovasc Med. 2004;5(3):156–163. [PubMed] [Google Scholar]
- 22.Snoep JD, Hovens MMC, Eikenboom JCJ, et al. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154(2):221–231. doi: 10.1016/j.ahj.2007.04.014. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Arat A, Morsi H, et al. Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. Am J Neuroradiol. 2008;29(7):1389–1394. doi: 10.3174/ajnr.A1070. doi: 10.3174/ajnr.A1070. [DOI] [PMC free article] [PubMed] [Google Scholar]