Summary
We report our experience in treatment of traumatic direct carotid cavernous fistula (CCF) via endovascular intervention. We hereof recommend an additional classification system for type A CCF and suggest respective treatment strategies.
Only type A CCF patients (Barrow's classification) would be recruited for the study. Based on the angiographic characteristics of the CCF, we classified type A CCF into three subtypes including small size, medium size and large size fistula depending on whether there was presence of the anterior carotid artery (ACA) and/or middle carotid artery (MCA). Angiograms with opacification of both ACA and MCA were categorized as small size fistula. Angiograms with opacification of either ACA or MCA were categorized as medium size fistula and those without opacification of neither ACA nor MCA were classified as large size fiatula. After the confirm angiogram, endovascular embolization would be performed impromptu using detachable balloon, coils or both. All cases were followed up for complication and effect after the embolization.
A total of 172 direct traumatic CCF patients were enrolled. The small size fistula was accountant for 12.8% (22 cases), medium size 35.5% (61 cases) and large size fistula accountant for 51.7% (89 cases). The successful rate of fistula occlusion under endovascular embolization was 94% with preservation of the carotid artery in 70%. For the treatment of each subtype, a total of 21/22 cases of the small size fistulas were successfully treated using coils alone. The other single case of small fistula was defaulted. Most of the medium and large size fistulas were cured using detachable balloons. When the fistula sealing could not be obtained using detachable balloon, coils were added to affirm the embolization of the cavernous sinus via venous access. There were about 2.9% of patient experienced direct carotid artery puncture and 0.6% puncture after carotid artery cut-down exposure. About 30% of cases experienced sacrifice of the parent vessels and it was associated with sizes of the fistula. Total severe complication was about 2.4% which included 1 death (0.6%) due to vagal shock; 1 transient hemiparesis post-sacrifice occlusion of the carotid artery but the patient had recovered after 3 months; 1 acute thrombus embolism and the patient was completely saved with recombinant tissue plaminogen activator (rTPA); 1 balloon dislodgement then got stuck at the anterior communicating artery but the patient was asymptomatic.
Endovascular intervention as the treatment of direct traumatic CCF had high cure rate and low complication with its ability to preserve the carotid artery. It also can supply flexible accesses to the fistulous site with various alternative embolic materials.
The new classification of type A CCF based on angiographic features was helpful for planning for the embolization. Coil should be considered as the first embolic material for small size fistula meanwhile detachable balloons was suggested as the first-choice embolic agent for the medium and large size fistula.
Keywords: carotid cavernous fistula, internal carotid artery ligation, percutaneous cavernous sinus embolization, contralateral internal carotid artery approach
Introduction
Carotid cavernous fistula is abnormal shunting between carotid arteries and the cavernous sinus. It is classified into 4 types including A, B, C and D according to Barrow D in 1985 1. Type A is direct high-flow shunts between the internal carotid artery (ICA) and the cavernous sinus. Type B is dural shunts between meningeal branches of the internal carotid artery and the cavernous sinus. Type C is dural shunts between meningeal branches of the external carotid artery (ECA) and cavernous sinus. Type D is dural shunts between meningeal branches from both internal and external carotid arteries and the cavernous sinus.
The cause of type A is usually head trauma which is called traumatic direct CCF 1. Other causes would be due to rupture of the ICA aneurysm within the cavernous sinus (aka spontaneous direct carotid cavernous fistula) or idiopathic 2.
Before endovascular intervention, type A carotico-cavernous fistula was mainly treated with muscle occlusion, carotid artery ligation or combination of these methods. Beside the procedural complications, surgical repair still carried a risk of recurrence due to collaterals.
Literature Reviews
Traver et al. first described direct traumatic CCF in literature in 1809 3 and the treatment for this entity was introduced as ligation of the ICA. However, most of the CCF cases recurred due to collaterals from the contralateral ICA or from the basilar artery via Acom and Pcom.
In 1930, Brook B 4 introduce another treatment method to occlude the fistula using a piece of muscle and let it flow to the fistula through an opening at the cervical ICA. This method achieved higher successful rate compared to the ICA ligation since it could occlude right to the fistula regardless the collateral flow from any direction from below or from above provided the piece of muscle was big enough. Therefore, this method was likely not to be able to preserve the ICA because the piece of muscle almost always stays within the lumen of the ICA. This always posed a risk of stroke especially if there is not enough collateral. Moreover, the recurrence still could occur due to its in-appropriate position which was above or below the fistula or incomplete sealing of the fistula orifice.
In 1973, Parkinson D 5 suggested a direct operation into the cavernous ICA and suture the torn artery and repair the fistula. However, this method carried lots of risks because of either intraoperative blood loss and/or temporary circulation pauses.
Until 1974, Serbinenko 6 reported a successful method of closing the fistula using detachable balloon. The deflated balloon is small enough to flow through the fistula into the cavernous sinus and it can be inflated as big as being expected. This help settling the disadvantage of fixed dimensions of the piece of the muscle, enable possibility of ICA preservation and further more we also can retrieve back the devices if it is not applicable for the balloon to be detached. Since that evolution, series of studies in treating CCF were reported using this method i.e. Debrun et al. 7 in 1981 with successful rate of 94%, complication rate of 5.5%, Higashida et al. 8 in 1989, Lewis et al. 2 in 1995 with successful rate of 86%, complication rate of 4%. These authors shared common features that endovascular intervention have high successful rates, less complication and less recurrence incidence compared to previous methods.
In Vietnam, before the emergence of endovascular intervention, direct traumatic CCF was also treated with muscle embolization, ICA ligation or combination. Since 1972, Le Xuan Trung and Ton That Tung 9 had initiated Brook's method as the treatment for CCF. In 1999, Truong Van Viet et al. 10,11 reported treatment of direct traumatic CCF by surgical ICA ligation with recurrence rate of 11.36%, hemiparesis rate of 2.27%, death rate of 1.17%.
In 2003, Nguyen Dinh Tung et al. 12 reported the results of treatment of CCF using muscle occlusion with successful rates of about 86,2%, complication rate of about 4.9%, recurrence rate of about 7%, failure of occlusion in 1.62%. In general, surgical treatment for CCF using ICA ligation or muscle occlusion was reported effective. However, the recurrence rate and complication were still high and it was difficult to assess the hemodynamics of the fistula and cerebral vasculatures pre- and post-occlusion. Since the first case of CCF occlusion using detachable balloon performed by Pham Minh Thong in Vietnam in 1999, this technique has been developed and popularized. In 2003, Pham Minh Thong et al. 13 reported 59 cases of direct traumatic CCF which were treated by endovascular intervention. The complete sealing rate of fistula is 98% without any significant complication. The rate of ICA preservation was about 81%. The complication rates of endovascular embolization for CCF were different on various centers. Other authors showed that permenent complication of cerebral infarction is less than 2%. Permanent cranial nerve palsy after the procedure occured in 0-5%. Death also was reported at 1.8 (Kendall et al, 1983) to 3% (Scialfa et al, 1983). Other complications including premature balloon detachment, balloon dislodgement and migration were rare. The advantage of endovascular intervention was the possible preservation of the internal carotid artery which was approximately 70% of cases 14.
Subjects and Methodology
Patients who were clinically diagnosed CCF were proceed for cerebral angiography to confirm the presence and to classify the fistula following Barrow classification system. Only type A CCF patients would be recruited for the study. Based on the angiographic characteristics of the CCF, we classify the type A CCF into three subtypes depending on whether there was presence of the anterior carotid artery and/or middle carotid artery. We postulated that the opacification of the ACA and MCA were corresponding to the size of the fistulas. The angiograms with presence of the anterior cerebral artery and middle cerebral artery in the internal carotid artery run were categorized as small size fistula. The angiograms of the fistula with visualization of either middle or anterior cerebral artery were categorized as medium size fistula. The angiograms of the fistulous internal carotid artery without opacification of both MCA and ACA were categorized as large size fistula. After the confirm angiogram, therapeutic embolization would be performed immediately. The embolic material could be detachable balloons, coils or both depending on the hemodynamic progress. All cases after the embolization were followed up for complication and effect.
Results
A total of 172 cases of traumatic direct carotid cavernous fistula were obtained from Jan 2005 to April 2011. There were 3 cases of bilateral direct CCF, 85 cases of right CCF and 84 cases of left CCF. The causes included occupational injury, motor vehicle accidents, assaults, sport. Most of the patients presented with bruits (98.8%) which were typical for high flow direct CCF. Proptosis happened in 80.8% of cases meanwhile there were only about 55% of cases of eye redness. Other presentations could be cranial nerve palsy (55.2%), vision impairment (38.9%), limb weakness (4.6%), massive bleeding (2.3%).
On ophthalmological examination, there were papilloedema (3%), congested retinal veins (71.6%) and increased intraocular pressure (20%). Radiologically, CCF could present as intracranial hemorrhage including subdural (2.7%), intraparenchyma (2.7%), subarachnoid (1.7%), intracerebellar hemorrhage (2.7%). Other findings could be cerebral infraction, dilatation of ophthalmic veins, cortical veins and cavernous sinuses.
Totally 24 cases had pre-existing treatment including 6 cases of previous CCA ligation, 5 cases of previous ICA ligation, 4 cases of muscle occlusion through ICA, 9 cases of failed previous detachable balloon. The 15 cases of carotid ligation and muscle occlusion had recurrence CCF.
The interval of time from the injury until the admission was ranging from 6 weeks to 58.8 weeks (mean of 12 weeks).
Correlation of time interval to size of fistula was listed in Table 1.
Table 1.
| Fistula |
Average time period (ws) |
Confidence interval |
| Small size | 8.8 +/– 1.3 | 6.1-11.4 |
| Medium size | 20.8 +/– 6.1 | 8.7-32.9 |
| Large size | 38.8 +/– 10.1 | 18.8-58.8 |
The group of patient with small fistula has shortest preadmission time period (injury-to-admission time), the group of medium size fistula has longer preadmission time interval and the group with large size fistula has longest preadmission time interval. It is interesting that the longer the incubation time interval, the larger the fistula size (p<0.05).
Angiographic vasculature features
Among 172 cases of CCF, the angiographic images of the runs with the diagnostic catheter tip at the proximal portion of the ipsilateral ICA showed 22 cases with presence of the ipsilateral ACA and MCA corresponding to small size fistula. A total of 61 patients showed only MCA in angiography corresponding to medium size fistula and 89 patients showed no blood flow to the ipsilateral ACA or MCA corresponding to large size fistula (Table 2).
Table 2.
| Fistula |
Number of cases |
Percentage |
| Small size (visible both ACA and MCA) |
22 | 12.8 |
| Medium size (visible only MCA) |
61 | 35.5 |
| Large size (no ACA or MCA demonstrated in angiography) |
89 | 51.7 |
The collateral supply from the anterior communicating artery (Acom), posterior communicating artery (Pcom) or both Acom and Pcom to the ipsilateral cerebral hemispheres happened in 89% of cases in which both Acom and Pcom suppliers accounted for 65% of all cases. The rest 11% of patients have no collateral and the ipsilateral hemisphere was supplied by the ipsilateral ICA. There was about 11% of pseudoaneurysm in the cervical, cavernous and other intracranial portion of ICA accompanying to the CCF. The draining veins included ophthalmic vein (98%), inferior petrosal sinus (83%), superior petrosal sinus (18.36%), cortical veins (48.8%) in which there were 2 cases (1.2%) had only cortical venous drainage, cerebellar veins (4.6%) and spinal veins (2.3%).
| Accesses for intervention |
Number of cases |
Percentage |
| Routine femoral artery puncture |
154 | 89.5% |
| Through Pcom | 11 | 6.4% |
| Direct ICA punctures | 5 | 2.9% |
| ICA surgical exposure and puncture |
1 | 0.6% |
| Inferior petrosal sinus | 1 | 0.6% |
The interventional routes
Our most common accesses were from the femoral artery punctures and introducing the catheter to the ICA and into the fistula which occurred in 89% of patients. When there were previous treatment of the CCF with CCA, ICA ligation or muscle occlusion via sugery of the ICA, the endovascular treatment could not be proceeded via the conventional femoral puncture, our resolution for this situation was to do direct puncture at the cervical ICA (2.9% of cases) or to do surgical exposure before ICA puncture (0.6%) then to occlude the fistulous ICA or to approach the fistula via the Pcom then occluded the fistulous portion of the ICA (6.4%). Other access route was through femoral vein to petrosal vein and occluded the cavernous sinus (0.6%).
Embolic materials
The embolic materials included detachable balloon (80.2%), coils (10.5%) and both coils and balloons (7.6%). There were 3 cases the embolization was not done due to unsuccessful access to the fistula (1.7%). In cases that the fistula could not be closed totally after balloon detachment, coils can be packed into the cavernous sinus via venous access to affirm the fistula occlusion.
Post embolization results
The fistulas were totally occluded right after the procedure in 94% of cases. Partial occlusion occurred in 4.7% of cases. Approximately 1.7% (3 cases) was unable to be occluded due to the fistula being too small to be cannulated. Except 11 cases that had preexisting ICA ligation, the treatment was aimed to save the ICA for the rest of 161 cases. As a result, 70% of 161 CCF cases were cured with successful preservation of the ICA. There were about 30% of cases we had to occlude the ICA to ensure the occlusion of the fistulae. After the embolization, 17 patients had recurrence within 1 week and were successfully treated at the second embolization.
The possible causes of these recurrence could be balloon dislodgement within cavernous sinus, early collapse (<6 weeks) of the balloon due to leaking valves. The total successful rate of treatment with total occlusion of the fistula is 97%.
Symptom improvement
Most of cases had improvement of symptoms after 1 month (approximately 99.4% had disappearance of bruit, 98.5% had no more proptosis and eye redness, 95.7% had resolved cranial nerve palsy, 87.5% (7/8) of cases had improvement of neurological deficit and 68.6% of cases had improvement of eyesight). At the end-point of the research, there were no case of eye movement impairment, no recurrence of symptoms.
Complication
Most of the complication were mild and resolved spontaneously. There was 1 case with transient hemiparesis after balloon occlusion of the ICA. In this case, the fistula was large and there was stenosis at M1 of the ipsilateral MCA. Due to a large fistula, we decided to occlude the ICA because we anticipated that the treatment would be unsuccessful if we preserve the ICA. After the embolization, the patient had mild hemiparesis and the patient fully recover after 3 months of follow up. All cases of 3rd nerve palsy were recovered completely after 1 month. There was 1 case encountered acute embolism with thrombus. We injected rTPA directly into the artery and re-canalized the lumen and the patient did not have neurological deficit. There was 1 case of balloon dislodgement. The balloon flew up and got stuck inside the Acom artery and the patient was asymptomatic. There was one case of vagal shock. During resuscitation, the liver had been lacerated and being unable to stop bleeding. The patient experienced volume loss and death.
| Complication |
Number of cases |
Percentage |
| Hematoma at puncture sites | 3 | 1.8% |
| Mild allergy to contrast media | 2 | 1.2% |
| Temporary 3rd nerve palsy | 3 | 1.8% |
| Transient hemiparesis | 1 | 0.6% |
| Vagal shock, death | 1 | 0.6% |
| Acute blood clot embolism | 1 | 0.6% |
| Balloon dislodgement | 1 | 0.6% |
| Total | 12 | 6.9% |
In the data analysis, there was a correlation between size of the fistula and the possibility of ICA sacrifice occlusion (p 0.001). The bigger the fistula, the higher the possibility of ICA sacrifice. The risk of ICA occlusion increased 5 times in the medium size fistula and increased 25 times in the large size fistula. There was direct correlation between the period of time from the injury to the procedure and the size of the fistula. The longer the time, the larger the fistula (Spearman 0.2, p 0.007). This analysis results suggested that the earlier the patient present to treatment, the higher the possibility of ICA preservation.
Discussion
Clinical presentations
The patient with traumatic direct CCF could have no eye proptosis or redness. Those without proptosis and/or redness could be due to multi-compartment of the cavernous sinus with no connection to the ophthalmic vein and blood flows were drained to the inferior petrosal sinuses, superior petrosal sinuses, Sylvian veneous plexus to the cortical veins. Less commonly, the blood flow was to drained into the cerebellar fossa and pre-pontine veins into the spinal veins causing spinal cord compression. According to Gaskill, this draining ways may increase mortality rate 16. Radiographically, when the draining veins were from other veins rather than superior ophthalmic veins, there could be dilatation of cavernous sinus and ectasia of cortical, cerebellar vein or spinal veins (Figure 1). Another presentation of direct CCF was massive bleeding. This was a rare presentation described in the literature with sudden severe epistaxis and rapid death 17. After the accident, the injury can cause fracture of the sphenoid sinus roof and ICA pseudo-aneurysm which can rupture later leading to fatal massive hemorrhage. In our study, we encountered 4 cases (2.3%) of CCF presented with massive nose bleeding (Figure 2). All of these cases were successfully saved after sacrifice of the internal carotid artery. Cranial nerve palsies could be one of the symptoms of the CCF or could be the only presentation. The most common affected cranial nerves were 3rd and 6th nerves. Therefore, if the patient had ptosis or 6th nerve palsy after a trauma, it was necessary to perform a careful clinical examination to detect eye bruit or to do Doppler US, CT scan to check the superior ophthalmic vein to rule out direct CCF.
Figure 1.
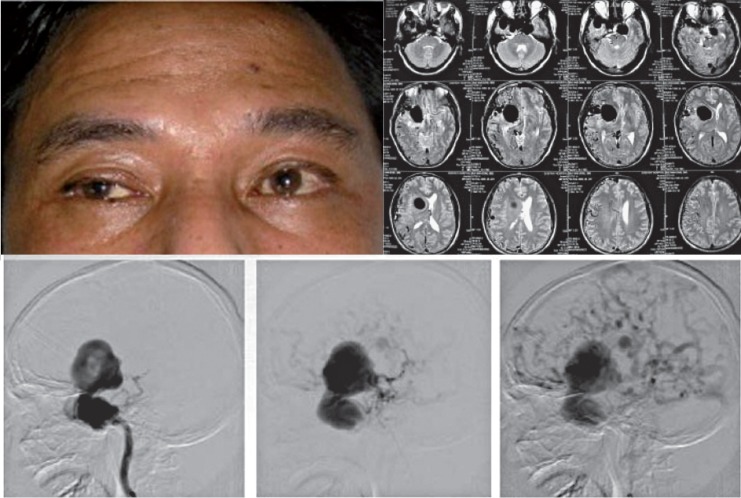
CCF with absence of eye symptom. MRI showed dilated cavernous sinus and huge flowvoids of the ecstatic cortical veins which were confirmed by angiography images.
Figure 2.
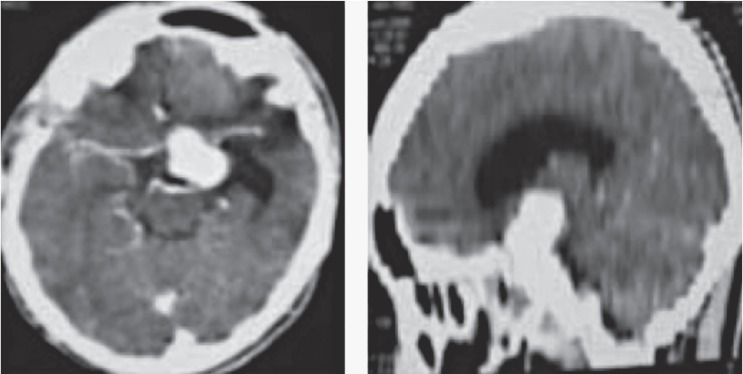
CTA of the brain showed pseudoaneurysm of the left ICA within the cavernous sinus. This patient had massive nose bleeding which need blood transfusion more than 40 pints. The patient was saved with sacrifice of the parent ICA.
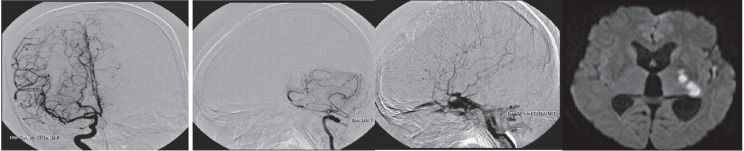
Another rare presentation was subarachnoid hemorrhage and could be resultant to rupture of cortical vein aneurysm secondary to cortical venous reflux. Kanno et al. 18 report a case of direct traumatic CCF causing subarachnoid hemorrhage and was treated with detachable balloon (Figure 3).
Figure 3.
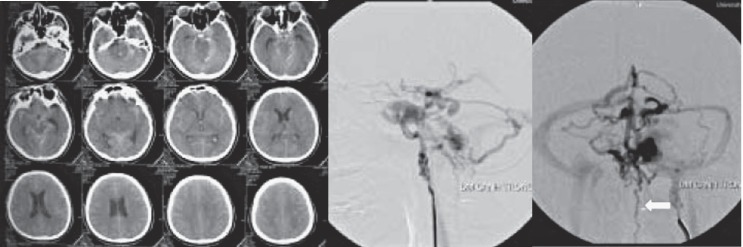
Non-contrast CT images showed diffuse subarachnoid hemorrhage in a patient with a history of direct CCF which was treated previously by ICA muscle occlusion 2002. Angiography showed recurrent CCF with multiple cortical vein aneurysm. There was also reflux into the internal cerebral vein, prepontine and spinal veins (white arrow).
Hemiparesis could also happen due to the blood flow being stolen away from the ICA meanwhile the collaterals were insufficient to supply the ipsilateral hemisphere. It is severe especially when there is no posterior communicating artery or anterior communicating artery
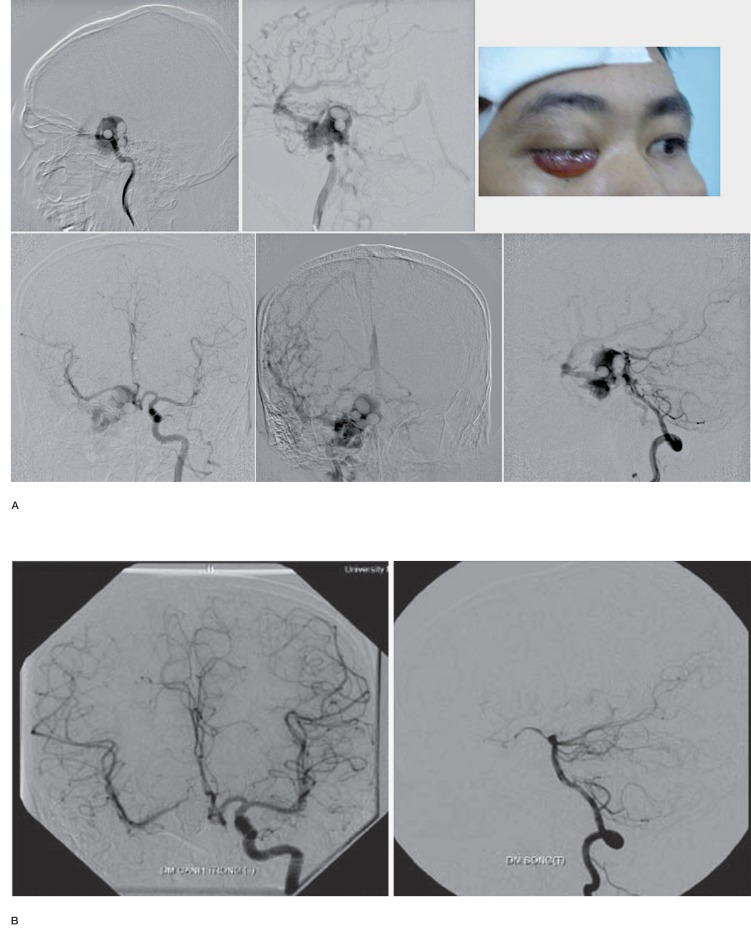
One of the rarest presentation of CCF was a fistula of the trigeminal artery into the cavernous sinus accompanying with hypoplastic ICA. In our review, there were only 2 cases of persistent trigeminal artery in 1500 cases of cerebral angiography (0.13%). CCF in persistent trigeminal artery was extremely rare and was documented as case report. McKenzie JD et al. 19 reported a case of traumatic CCF in the patient with persistent trigeminal artery and was successfully treated with detachable balloon. According to Hattori, there were only 2 cases of persistent trigeminal artery accompanying with hypoplastic ICA, but these two cases had no fistula. Cases of CCF in persistent trigeminal artery and hypoplastic ICA was not reported in literature, we had 1 such case in our study. In this case, the left anterior artery was supplied by the Acom artery. The left MCA was supplied by left Pcom artery and the left common carotid artery supply only for left ECA. There was persistent trigeminal artery which was fistulous to the cavernous sinus (Figure 5).
Figure 4.
Angiography images of a patient with left CCF. There were no correct Pcom artery (which was become fetal Pcom) and there was also hypoplastic left A1 which make less collaterals to the left cerebral hemisphere. DWI image showed acute infarct at the left thalamus and left basal ganglia.
Figure 5.
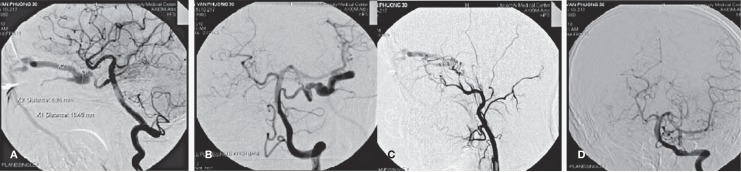
A,B) Agiographic images showed trigeminal artery which was fistulous to the cavernous sinus. (C) The left ICA was hypoplastic. (D) Angiographic image of the left vertebral artery, AP view.
Effect of CCF on eye included proptosis, mucous edema, eye redness, mucosal hemorrhage, vision impairment, glaucoma. These symptoms were due to congestion of the draining pathway of the ophthalmic veins due to high flow from the ICA. In the retinal layer, the venous congestion can cause exudative optic disc edema, retinal hemorrhage and optic disc atrophy. In our study, the later the patient presented to the hospital, the worse the eye symptoms. There was 80% of the patients with severe proptosis, 39% of cases with eyesight reduction <5/10. In fundoscopy, there was 22% of cases having optic disc atrophy. Naesens R et al. 20 stated that without treatment, the eye vision would be reduced in 89% of cases. His study also discussed that vision improvement after complete occlusion of the fistula can be up to 94% of cases. In our study, the vision improvement within 1 month happened in about 69% in the group of vision <5/10 provided the patients were not completely blind.
Treatment
In treatment of CCF, endovascular intervention can provide good assessment of the hemodynamics of the fistula with accurate information in collateral and anatomical arterial appearance. This enabled us to decide whether we should preserve or sacrifice the ICA. When there were insufficient collateral supplies from contra-lateral vessels, the ICA preservation must be prioritized and this could probably be obtained only with endovascular intervention. Under angiography, we could always be able to assess the fistula during inflating the balloon and before balloon detachment. It could provide information and orientation for the interventionist to select embolic materials and decide when we should stop the procedure. At the end point of the study, we achieved the complete fistula occlusion rate of about 94%. The severe neurological complication rate was about 2.4%, in which 1 patient was dead. The rate of ICA preservation was 70%. In general, the result of our study was favourable compared to the surgical results and relatively identical to the other radiological results.
Features of the fistula size
Most of the author choosed detachable balloon as initial occlusion devices to treat traumatic direct CCF because of it potency to completely seal the fistula in only one balloon detachment which is much cheaper than using other embolic materials. However, each study reported a rate of failure because of being unable to push the balloon through the fistula. Naesens R et al. 20 reported an unsuccessful rate of 5-10% and the author attributed them to small fistula. However, he did not show or interpret the angiography images of the fistula. In these cases, the author suggested to use coils. This may increase procedure cost due to extra pay from the discarded balloon. On the other hand, pulling the catheter attaching into the balloon also carries risk of unexpected balloon detachment which might cause embolism and stroke. Therefore, anticipation of the necessary embolic material before the procedure is really critical to avoid balloon dislodge or unexpected consumables. To get a good anticipation of choosing embolic material for the fistula, it necessitated insightful understanding of the fistula sizes. Infact, we were unable to measure the fistula sizes directly, however, in our study, we did divide the fistula into 3 groups which are small, medium and large depending on the angiographic features. In the group of large fistula, we only could see the cut off appearance of the ICA at the cavernous sinus and all the blood flow was stolen by the cavernous sinus (Figure 6).
Figure 6.
A) (left): Angiographic appearance of the large size CCF. There was cut off of the ICA flow. Reflux of blood to the cavernous sinus and the draining veins. MCA and ACA were absent. (B) (right): cerebral vasculature after balloon detachment.
In this case, the embolic agent should be balloon. The important issue was the risk of being unable to preserve the ICA during the treatment in this group. Therefore, we needed to carefully assess the collateral from Acom, Pcom and the tolerance of the ICA occlusion test before the real ICA occlusion. Similarly, if occlusion of the ICA was needed, it was also imperative to perform an assessment of collateral from the Pcom and Acom before balloon detachment to avoid residual flow from the Acom or Pcom which still supplied the fistula meanwhile the ICA was occluded at the lower portion. This situation would make difficult to totally occlude the fistula that necessitated accesses from Pcom which was not always possible to obtain. In our study, there were 89 large size fistula, accordingly, the rate of ICA occlusion was 53.9% and the risk of ICA occlusion in the large size fistula was 25 times more than that of the small ones. In the cases with large fistula size, the risk of cerebral infarct was also high especially in patient with poor supplies from collaterals. In these cases, preservation of the ICA was prioritized and stent can be considered the first as embolic agent.
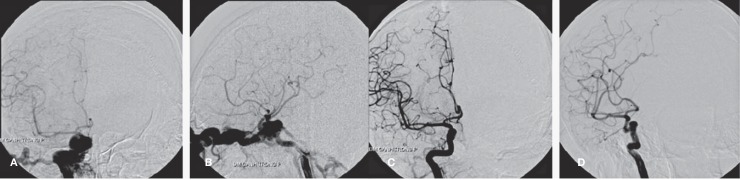
In the group of those we classified as medium fistula, the ICA still had some blood flow supplying to the ipsilateral hemisphere. The blood flow went partially to the cavernous sinus and partially up to the either ACA or MCA. Some cases we can still see both MCA and ACA but one was clearly seen and the other branch was attenuated. More over, the flow to the cavernous sinus was quite abundant that we could not identify exactly where the fistula was. (Figure 7).
Figure 7.
Angiographical images of the patient who was classified as medium fistula. A and B (upper row): AP and lateral views showing MCA quite clearly and the flow of the ACA is reduced. The flow of ICA to the cavernous sinus is abundant with obviously dilated draining ophthalmic vein and inferior petrosal sinus. C and D (lower row): angiographical images of the patient after balloon embolization. The fistula was totally closed with increase vascularization of the MCA and ACA territories.
In this group, the suggested embolic agent was detachable balloon. In case it was unable to close the fistula and it was necessary to occlude the ICA, it was crucial to assess the collaterals from the ipsilateral Acom and Pcom as well as to perform the occlusion test before occluding the ICA. In our study, there were 61 cases with medium fistula. The incident of necessary ICA occlusion to close the fistula was 18% which was less than the group with large fistula. The risk for Ica occlusion was 5 times more than the group of small fistula.
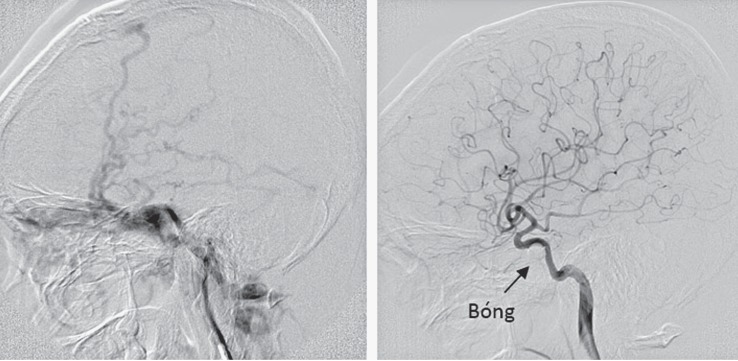
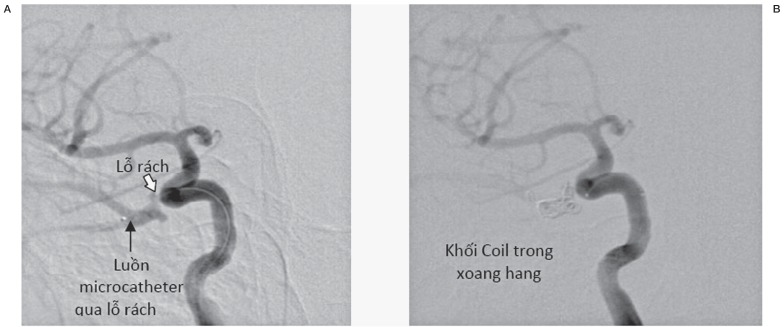
In the group of patient with fistula classified as small size, the hemodynomics of the vessel was almost unchanged or minimally affected. Angiographically, the ICA has almost normal vasculature to the hemisphere. The fistulous point with extravasation of contrast from the ICA to the cavernous sinus could be demonstrated. The draining veins could be mildly dilated (Figure 8).
Figure 8.
Angiography of the small size fistula. (A) (left): before embolization. The fistula (white arrow). The tip of the microcatheter within the cavernous sinus (black arrow). (B) after embolization. Presence of coils within the cavernous sinus and the fistulous site.
In this patient group, if detachable balloon was initially selected as embolic agent, the risk of being unsuccessful was high because we may not be able to introduce the balloon through the fistula. In our study, we have totally 22 cases of small size fistula among which, 9 cases were attempted to be treated with balloon and all those 9 cases were failed to be treated. 8/9 patients in this group were then successfully treated using coils. The other patient had then defaulted from the treatment. After the first 9 cases of treatment failure with balloon, the rest of the 13 cases with small size CCF were intentionally indicated for coil usage as embolic agent. By this way, 11/13 cases of CCF (86.3%) were successfully cannulated with microcetheter and the fistula were closed with coils. The other 2 cases were unsuccessful because of failure to insert the catheter to the cavernous sinus.
When the fistula was small, it was difficult to cannulate the microcatheter and similarly, the balloon may encounter the same situation. The diameter of the collapse balloon was usually larger than a microcatheter with luminal diameter about 0.5 mm. With using of detachable balloon, there was a risk of inadvertent balloon detachment and dislodgement. Another risk of using balloon was that the balloons dwelled partially inside and partially outside the fistula leading to the risk of dislodgement into the lumen after balloon inflation and detachment. Therefore, the suggested embolic agent for this group was coils.
According to the data analysis, the size of the fistula was directly associated with the injury-to-admission time (spearman 0.2, p 0.007). On the other hand, the risk of being sacrifying to perform total occlusion of the internal carotid artery has increased with the size of the fistula. Therefore, the longer the waiting time before the admission, the chance of preservation of the ICA during embolization would be deminished. Therefore, it was advisable that the CCF patient should be referred for treatment as early as possible to reduce the risk of total ICA occlusion which was dangerous for patients with less collaterals.
Beside coils and detachable balloon which has been usually selected as embolic agents for direct traumatic CCF, histoacryl glue (N-butyl-2 cyanoacrylate n-BCA) and Onyx (new liquid polymeric embolic agent) can also be used for treatment of the CCF 21. These materials were used for embolization of AVM or slow flow DF. However, there was the risk of embolic agent reflux into the ICA during injection. Chao Bao Luo et al. reported 18 cases of using glue in treatment of CCF among 176 CCF cases in his study. The indication for using glue was to assure the occlusion of the CCF after coils and balloon were unable to seal the fistula orifice. Injection of the glue was to fill up all spaces and slit among the coils and between the coils and balloon to enforce the consolidation of the embolic mass. To avoid refluxing of glue into the ICA, a balloon should be inflated within the lumen at the fistulous portion of the ICA to close the fistula tract before injection. However, to avoid the need to inject extra glue after using 1 balloon, we can use 2 balloons which should be inflated only about 50-70%.
The best treatment was to occlude the fistula and preserve the ICA. However, when the fistula was too large, getting complicated or when the ICA is contused, it was difficult to completely close the fistula. In this case, it was necessary to occlude the ICA for eradication of the fistula. In this study, the rate of ICA preservation is 70%.
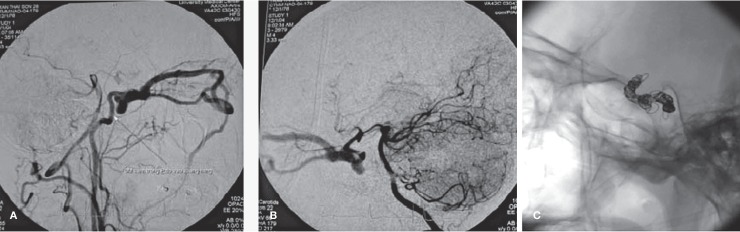
In cases of previous ICA ligation, recurrence of CCF can happen due to reflux of flow from the Acom and Pcom. If the CCA was ligated, the flow can feed the fistula via the contralateral ECA or ipsilateral vertebral artery anastomotic to the ipsilateral ECA down to the ICA (Figure 9A,B).
Figure 9.
Angiography of the recurrence CCF patient 6 months after ligation of the common carotid artery. (A) run from the right subclavian artery showed anastomosis of the right vertebral artery to the occipital branch of the right ECA, going to right ICA and supplying back to the fistula. (B) run from the left vertebral artery showed recurrent right CCF with supply from the right PCA via right Pcom artery. The right ICA was punctured and the fistulous portion of the right ICA was embolized. (C) image of the coils after total occlusion of the fistulous portion of the right ICA. No fistula was seen in the contrasted images.
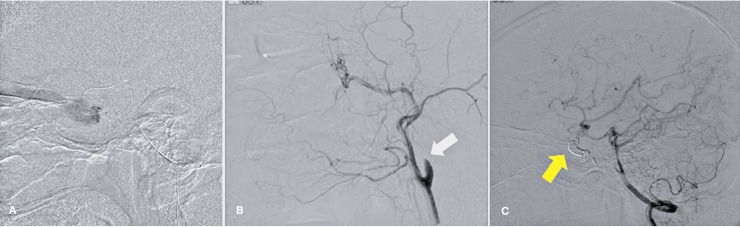
The possible treatment could be a repeat of another ligation at the intracranial ICA 22, but mechanically, endovascular intervention was more superior with multiple options for fistulous repair. The fistula can be accessed via the ipsilateral ICA if the recanalized lumen of the previously ligated ICA if it was still spacious enough. If the common carotid artery was totally occluded, the direct ICA puncture can be an alternative and we could occlude the ICA using coils (Figure 6C) or using balloon (Figure 10A,B,C).
Figure 10.
Angiography of the patient with recurrence CCF after ICA ligation. (A) run from the right ICA showed left CCF supplied from the right ICA via Acom artery. (B) direct left ICA puncture. (C) run from the right ICA after left ICA occlusion using balloons.
Other approach can be from the Pcom artery via the vertebral artery 23 then occlude the cavernous sinus and the fistulous portion of the ipsilateral ICA (Figure 11A,B,C).
Figure 11.
Angiogram of the patient with recurrence left CCF after left ICA ligation. The diagnostic angio showed recurrence CCF supplied from the left Pcom and sphenopalatine branch of left ECA (images were not showed). (A) selective cannulation of the left Pcom artery to the cavernous sinus and putting coils. (B) run from the left CCA post embolization of the cavernous sinus showed no more fistula from the ECA branches. Presence of the ICA stump after ligation (arrow). (C) run from the left vertebral artery showed complete occlusion of the left cavernous sinus and fistulous portion of the left ICA (arrow).
Otherwise, the embolization could be performed via venous access through the inferior petrosal sinus or the superior ophthalmic veins and pack the cavernous sinus with coils. In 1991, Monsein et al. 24 reported 4 cases of CCF treatment using superior ophthalmic vein approach without complication. In 1989, Halbach et al. 25 reported 3 cases of CCF treatment using direct ICA puncture distal to the ligated portion and to occlude the fistula using coils or detachable balloon with no complication. There were 14 cases of recurrent fistula after balloon detachment in the cavernous sinus. All these cases were large size fistula but we did try to preserve the ICA. The recurrence could be due to low-pressure opposition of the balloon to the arterial wall or due to pulsation of the artery. In the second intervention for all these 14 patients, we can preserve the ICA in 7 patients. The other 7 patient had to have the ICA occluded to cure the CCF. If the preservation of the ICA did not successfully occlude the large size fistula, the presence of the balloon within the cavernous sinus may cause alteration of the normal drainage pathway along the dural sinuses. This would be one of the causes of flow reflux to the cortical veins, cerebellar veins and spinal veins which was associated with intracranial hemorrhage (Pierre Lasjaunias). Our study had 5 cases of intracranial bleed (subarachnoid bleed, intraparenchymal bleed). In this situation, if the collateral to the same side hemisphere was good, a total occlusion of the ICA may be a better choice rather than trying to preserve the ICA. Another solution was occlusion the cavernous sinus using coils to achieve the eradication of the fistula. In our study, we encounter a case with large CCF causing cortical vein reflux. He experience balloon embolization in the other center and achieve partial fistula occlusion (Figure 12A,B).
Figure 12.
A) (left): Angiography from the right ICA after the first balloon embolization which show persistent right CCF with cortical vein reflux and presence of multiple balloons within the right cavernous sinus. The right ICA did not supply the right ACA and right MCA. The right MCA and ACA was supplied from the left ICA. The fistula was also demonstrated from the left ICA run via Acom. (B) (right): Angiography images from the left ICA after the second embolization. The right right ICA was occluded and the fistula was sealed. The right MCA and ACA was also well visualized from the left ICA run.
He went to our center after 1 month with recurrent headache, proptosis and eye redness. Under DSA, his CCF became worsened with more cortical reflux and new formation of cavernous pseudoaneurysm. In this patient, it was possible that the balloon within cavernous sinus may hinder the flow to the draining veins and sinuses which caused worsening of the cortical reflux. The patient was repeated another embolization. The right ICA was occluded and the fistula was completely sealed (Figure 12B).
The question was that when we should occlude the ICA and when we should preserve them. In our experience, if the total occlusion of the fistula could not be achieved at the first embolization, occlusion of the ICA should be considered as long as there were good collaterals from the contralateral ICA, rather than to partially seal the fistula which may lead to dangerous cortical venous reflux. In case there were insufficient collaterals, occlusion of the ICA and the fistula was infeasible and expensive. In that case, stent could be placed within the ICA and followed by coiling of the cavernous sinus. Other authors reported treatment of direct CCF using stent graft 26.
Kocer et al. 27 reported a case of CCF secondary to a pituitary surgery and the fistula was cured using stent graft which was a type of stent used for treatment of coronary perforation due to coronary intervention. However, after placement, the patients had to use antiplatelet forever to prevent intrastent- restenosis. Fernando Gomez reported 7 case of direct CCF in 2007 which were treated with stent graft. The stent placements were successful in all 7 cases. Among which, there was 1 case of stent occlusion after a moth due to incompliant discontinuation of antiplatelet. However, this patient had no symptom as there were good collaterals. Another case had intra-stent stenosis due to intimal hyperplasia. In general, stents could be used when it was unable to treat the CCF with detachable balloon or coils. Likewise it could be considered when it was necessary to preserve the ICA in large fistula but there were poor collaterals from the other ICA. However, using stent as treatments for direct CCF was a new method which still had a risk of intra-stent re-stenosis, costly devices and the patients had to use anticoagulation for life. This needs further investigation.
Conclusion
As being demonstrated in our study, the size of the fistula was directly related to the risk of ICA sacrifice which was 5 times more in the medium fistula and 25 times more in the large fistula. There was direct relation between the accidence-to-admission time periods and the size of fistula. The longer the accidence-to-admission time, the larger the fistula orifice tends to be. Through which, the patients should be treated early to reduce the eye and brain complication. Endovascular intervention has been currently a treatment of choice for direct traumatic CCF because of its high cured rate, ability to preserve the carotid artery and flexibility to gain various accesses to the fistulous sites.
The new grading system of type A CCF was helpful for treatment plan via endovascular intervention. Detachable balloons were suggested for medium and large size fistula meanwhile coils should be used for small size fistula because it was difficult to introduce the balloon through small fistulous holes and could increase the risk of balloon dislodgement when pulling the catheter up and down. In our study, the rate of cured fistula was about 94% in the first embolization. The possibility of ICA preservation was about 70% and complication rate was 2.4%.
In cases occlusion of the fistula can not be obtained using detachable balloon, coil can be used to occlude the cavernous sinus via arterial or venous access. Direct puncture of carotid artery can be indicated when the patient had carotid artery ligation or when there was difficulty in fistula approach from the femoral artery. Ophthalmic vein could also be used as an access route when it was immpossible to get any other vascular access. Finally, if the fistula was failed to be treated with traditional ways using balloons or coils, occlusion of the parent vessels could be another option.
References
- 1.Barrow DL, Spector RH, Braun IF, et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248–256. doi: 10.3171/jns.1985.62.2.0248. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 2.Lewis AI, Tomsick TA, Tew JM., Jr Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36(2):239–244. doi: 10.1227/00006123-199502000-00001. doi: 10.1227/00006123-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Viñuela F, Briganti F, et al. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm: endovascular treatment by electrothrombosis with detachable coils. Neurosurgery. 1992;31(3):591–596. doi: 10.1227/00006123-199209000-00026. doi: 10.1227/00006123-199209000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Brooks B. The Treatment of Traumatic Arteriovenous Fistula. Southern Medical Journal. 1930;23(2):100–106. doi: 10.1097/00007611-193002000-00004. [Google Scholar]
- 5.Parkinson D. Carotid cavernous fistula: direct repair with preservation of the carotid artery. Technical note. J Neurosurg. 1973;38(1):99–106. doi: 10.3171/jns.1973.38.1.0099. doi: 10.3171/jns.1973.38.1.0099. [DOI] [PubMed] [Google Scholar]
- 6.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:50–55. doi: 10.3171/jns.1974.41.2.0125. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 7.Debrun G, Lacour P. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55(5):678–692. doi: 10.3171/jns.1981.55.5.0678. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 8.Higashida RT, Halbach VV. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: result of 87 cases. J Neurosurg. 1990;72(6):857–863. doi: 10.3171/jns.1990.72.6.0857. doi: 10.3171/jns.1990.72.6.0857. [DOI] [PubMed] [Google Scholar]
- 9.Le Xuan Trung. Carotid cavernous fistula orifices. Vietnam Journal of Med. 1966;2:101–107. [Google Scholar]
- 10.Truong Van Viet, Nguyen Dinh Tung. Carotid cavernous fistula. Vietnam Australia Neurosurgical Conference Bulletine. :24–25. [Google Scholar]
- 11.Truong Van Viet, Nguyen Dinh Tung. Cartotid cavernous fistula. Vietnam J Medicine. :409–411. [Google Scholar]
- 12.Nguyen Dinh Tung. Treatment for carotid-cavernous fistula. Thesis for the course of specialization level II in neurosurgery. Medical University of Ho Chi Minh City. 2003:2–80. [Google Scholar]
- 13.Pham Minh Thong, Bui Van Giang. Treatment of direct carotid cavernous fistula under endovascular intervention. Vietnam J Practical Med. 2003;459(9):54–56. [Google Scholar]
- 14.Berenstein A, Lasjaunias P, Ter Brugge KG. Traumatic carotico-cavernous fistula. Surgical neuroangiography. Springer. Second edition. 2004:279–333. doi: 10.1007/978-3-642-18888-6. [Google Scholar]
- 15.Capo H, Kupersmith MJ, Berenstein A, et al. The clinical importance of the inferolateral trunk of the internal carotid artery. Neurosurgery. 1991;28(8):733–737. doi: 10.1097/00006123-199105000-00018. doi: 10.1227/00006123-199105000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Gaskill Shipley MF, Tomsick TA. Angiography in the evaluation of head and neck trauma. Neuroimaging clinics of North America. 1996;6(3):607–624. [PubMed] [Google Scholar]
- 17.Argo A. Post-traumatic lethal carotid cavernous fistula. J Forensic and Legal Medicine. 2008;15(4):266–268. doi: 10.1016/j.jflm.2007.07.004. doi: 10.1016/j.jflm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Kanno H, Inomori S, Chiba Y, et al. Traumatic carotid cavernous fistula presenting subarachnoid hemorrhage 5 year after head injury: case report. No Shinkei Geka. 1991;19(8):767–771. [PubMed] [Google Scholar]
- 19.McKenzie JD, Dean BL, Flom RA. Trigeminal cavernous fistula. Saltzman Anatomy Revisited. Am J Neuroradiol. 1996;17(2):280–282. [PMC free article] [PubMed] [Google Scholar]
- 20.Naesens R, Mestdagh C, Breemersch M, et al. Direct carotid-cavernous fistula: a case report and review of the literature. Bull Soc Belge Ophtalmol. 2006;299:43–54. [PubMed] [Google Scholar]
- 21.Luo CB, Teng MM, Chang FC, et al. Transarterial balloon assisted N-butyl-2-cyanoacrylate embolization of direct carotid cavenous fistula. Am J Neuroradiol. 2006;27(7):1535–1540. [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal A, Forrell A, Nimjee S, et al. Unusual case of recurrent carotid cavernous fistula after 47 year, treated with direct clip ligation of petrous internal carotid artery [Internet] NASBS 2013 annual metting. 2013 Available from http://nasbs2013.org/unusual-case-of-recurrent-carotid-cavernous-fistula-after-47-years-treated-with-direct-clip-ligation-of-the-petrous-internal-carotid-artery/ [Google Scholar]
- 23.Garcia-Cervigon E, Bien S, Laurent A, et al. Treatment of a recurrent carotid-cavernous fistula: vertebro-basilar approach after surgical occlusion of the internal carotid artery. Neuroradiology. 1988;30(4):355–357. doi: 10.1007/BF00328189. doi: 10.1007/BF00328189. [DOI] [PubMed] [Google Scholar]
- 24.Monsein LH, Debrun GM. Treatment of dural carotid cavernous fistula via superior ophthalmic vein. Am J Neuroradiol. 1991;12(3):435–439. [PMC free article] [PubMed] [Google Scholar]
- 25.Halbach VV, Higashida RT, Hieshima GB, et al. Direct puncture of the proximmally occluded internal carotid artery for treatment of carotid cavernous fistula. Am J Neuroradiol. 1989;10(1):151–154. [PMC free article] [PubMed] [Google Scholar]
- 26.Archondakis E, Pero G, Valvassory L. Angiographic follow-up of tramatic carotid cavernous fistula treated with endovascular stent graft placement. Am J Neuroradiol. 2007;28:342–347. [PMC free article] [PubMed] [Google Scholar]
- 27.Kocer N, Kizilkilic O, Albayram S. Treatment of iatrogenic internal carotid artery laceration and carotid three dimensional cavernous fistula with endovascular stengraft placement. Am J Neuroradiol. 2002;23(3):442–446. [PMC free article] [PubMed] [Google Scholar]