Summary
A 64-year-old man was admitted with massive hemoptysis caused by oropharyngeal carcinoma. Angiography revealed active extravasation from the left carotid bulb. Covered stent-graft placement resolved the bleeding, but the patient presented with recurrent hemorrhage two hours later and was treated with another stent-graft.
Keywords: carotid blow-out, stent-graft
Introduction
Carotid blowout syndrome is the rupture of the extracranial carotid arteries. It is a rare but life-threatening complication of head and neck malignancies and their therapy 1,2. Surgical management is usually technically difficult and limited due to a previously irradiated operation site and hemodynamically unstable patients 3,4. Traditionally, endovascular management of carotid blowout syndrome involves carotid artery sacrifice 2. Current approaches propose stent-directed treatment methods, including the use of covered stents that provide decreased neurological morbidity associated with carotid artery occlusion 2. We present a case of carotid blowout syndrome caused by oropharyngeal carcinoma treated by covered stents.
Case Report
A 64-year-old man was admitted to the emergency unit with massive oral bleeding. The patient had a history of laryngeal carcinoma for which he had previously undergone laryngectomy. He was later diagnosed with primary oropharyngeal carcinoma and received radiation therapy. Oral examination revealed a tumor eroding the oropharynx wall from the left side. Initially oronasal packing was applied with saline and blood transfusion. Then the patient was referred to our interventional radiology unit. Both common carotid arteries were selectively catheterized. There were no abnormal findings in the right common carotid artery injection. Digital subtraction angiography (DSA) of the aortic arch and left common carotid artery injection showed extravasation from the left common carotid artery at the level of the bifurcation (Figure 1A,B). A 6 mm × 50 mm self-expandable stent-graft (Gore Viabahn Endoprosthesis®, W.L. Gore, Flagstaff, AZ, USA) was placed in this segment, with the stent extending from the common carotid artery to the internal carotid artery. Control angiogram following stent deployment revealed partial extravasation (Figure 2). The stent was expanded using a balloon catheter for optimal coverage. Control angiogram showed no extravasation and the patient was transferred to the intensive care unit (Figure 3). However, two hours after the procedure, the patient had a rapid increase in blood pressure and oral bleeding recurred. The patient was transferred to our interventional radiology unit again for re-evaluation and extravasation from the upper end of the covered stent was detected in the left common carotid artery injection (Figure 4). A 6 mm × 30 mm self-expandable stent-graft (Fluency Plus Vascular Stent Graft®, C.R. Bard, Karlsruhe, Germany) was placed overlapping the first stent from the upper end (Figure 5). Five thousand units of heparin were administered during each procedure to prevent thromboembolic complications. Control angiogram showed no extravasation (Figure 6). The patient was discharged from the hospital one week after the procedure. He was prescribed daily 75 mg clopidogrel for three months and daily 100 mg acetylsalicylic acid lifelong to ensure stent patency. At one-month follow-up no re-bleeding had occurred.
Figure 1.
DSA of the aortic arch (A) and left common carotid artery injection (B) showed extravasation from the left common carotid artery at the level of the bifurcation (black arrows).
Figure 2.
Control angiogram after stent deployment revealed partial extravasation (black arrow).
Figure 3.
Control angiogram showed no extravasation following expansion of the stent-graft with a balloon catheter.
Figure 4.
Left common carotid artery injection showed extravasation from the upper end of the covered stent 2 hours after the procedure (black arrow).
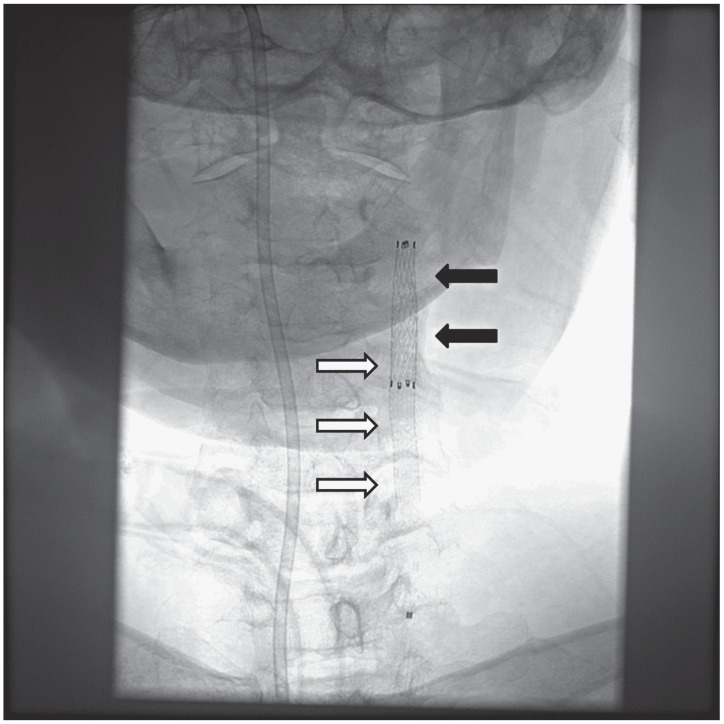
Figure 5.
Radiogram of the neck demonstrated the second stent-graft (black arrows) overlapping the first stent-graft (white arrows) from the upper end.
Figure 6.
Control angiogram after second stent-graft (black arrows) deployment showed no extravasation.
Discussion
Carotid blowout syndrome occurs more frequently in patients with head and neck malignancy and those with radiation-induced necrosis, recurrent tumors, wound complications from neck dissection or vessel erosion from pharyngocutaneous fistulas 5. While the incidence of carotid blowout syndrome was reported to be 4.3% in patients with head and neck malignancy who underwent radical neck dissection, it is related to high rates of morbidity (60%) and mortality (40%) 6. Patients usually present with oronasal bleeding (hemoptysis or epistaxis) and sometimes otorrhagia depending on the site of the tumor 7. Initial management consists of oronasal packing, intranasal Foley balloons, blood transfusion and intubation or tracheostomy for maintaining the airway 7. Emergent surgical carotid artery ligation is the definitive treatment but the application is limited due to previous neck dissection and radiation therapy 4,5.
In 1984, Osguthorpe et al. treated a case of carotid blowout syndrome by endovascular occlusion of the carotid artery using detachable balloons 8. Since then, endovascular management of carotid blowout syndrome has evolved, mainly developing various methods of permanent carotid artery occlusion such as coil embolization. Even though endovascular methods have surpassed surgical treatment in both efficiency and safety, stroke risk secondary to carotid occlusion remained a disadvantage. This problem was overcome in 1995 when Nicholson et al. used covered stent grafts to seal carotid wall defects while maintaining patency of the lumen 9. However, covered stent graft application has various peri-procedural and post-procedural complications. According to the of the literature review by Alaraj et al. in 2011, peri-procedural complications include embolic complications secondary to dissection or atheromatous plaque rupture, acute stent thrombosis and rupture of the target vessel during stent deployment 10. Post-procedural complications include re-bleeding, septic emboli and endoleak during the early stage. Intimal hyperplasia leading to stent stenosis or occlusion and stent thrombosis due to inadequate or inappropriate antiplatelet therapy (with clopidogrel and acetylsalicylic acid) were reported as the late complications 2. A long-term patency rate of 93.2% was reported in endovascular stenting of extracranial carotid artery aneurysms in a literature review 11. But it is difficult to assess patency in this group of patients as in our case due to advanced malignancy. Although stent occlusion or stenosis was reported in some series, use of covered stents is a reasonable choice of treatment in carotid blowout syndrome for immediate hemostatic control 2,10. Another concern with using stent-grafts is the need to use antiplatelet drugs after stent grafting since these drugs may cause bleeding in the tumor. Therefore, for terminal stage patients like our case, treatment options should be evaluated using a multidisciplinary approach as we have done. In our patient, partial extravasation remained after the deployment of the first stent-graft. The stent was expanded with a balloon catheter for optimal coverage which successfully resolved extravasation. However, re-bleeding occurred two hours after the procedure. We attributed this to possible inadequate covered stent placement aggravated by an acute rise in blood pressure. Second stent graft placement overlapping the first stent ensured complete coverage. We did not have any peri-procedural complications.
Conclusions
Covered stent application is a safe and efficient method for the treatment of carotid blowout syndrome secondary to head and neck malignancies. This technique preserves carotid artery flow while repairing the arterial wall defect. Although the long-term patency rates of stent grafts are favorable, large prospective randomized studies are warranted.
References
- 1.Lesley WS, Chaloupka JC, Weigele JB, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. Am J Neuroradiol. 2003;24(5):975–981. [PMC free article] [PubMed] [Google Scholar]
- 2.Gaba RC, West DL, Bui JT, et al. Covered stent treatment of carotid blowout syndrome. Semin Intervent Radiol. 2007;24(1):47–52. doi: 10.1055/s-2007-971189. doi: 10.1055/s-2007-971189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fokkema M, den Hartog AG, Bots ML, et al. Stenting versus surgery in patients with carotid stenosis after previous cervical radiation therapy: systematic review and meta-analysis. Stroke. 2012;43(3):793–801. doi: 10.1161/STROKEAHA.111.633743. doi: 10.1161/STROKEAHA.111.633743. [DOI] [PubMed] [Google Scholar]
- 4.Hanakita S, Iijima A, Ishikawa O, et al. Treatment of a cervical carotid pseudoaneurysm that occurred years after laryngectomy and irradiation of a neck tumor. Neurol Med Chir (Tokyo) 2011;51(8):588–591. doi: 10.2176/nmc.51.588. doi: 10.2176/nmc.51.588. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Lee DH, Kim HJ, et al. Life-threatening common carotid artery blowout: rescue treatment with a newly designed self-expanding covered nitinol stent. Br J Radiol. 2006;79(939):226–231. doi: 10.1259/bjr/66917189. doi: 10.1259/bjr/66917189. [DOI] [PubMed] [Google Scholar]
- 6.Chang FC, Lirng JF, Luo CB, et al. Patients with head and neck cancers and associated postirradiated carotid blowout syndrome: endovascular therapeutic methods and outcomes. J Vasc Surg. 2008;47(5):936–945. doi: 10.1016/j.jvs.2007.12.030. doi: 10.1016/j.jvs.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Mak CH, Cheng KM, Cheung YL, et al. Endovascular treatment of ruptured internal carotid artery pseudoaneurysms after irradiation for nasopharyngeal carcinoma patients. Hong Kong Med J. 2013;19(3):229–236. doi: 10.12809/hkmj133833. doi: 10.12809/hkmj133833. [DOI] [PubMed] [Google Scholar]
- 8.Osguthorpe JD, Hungerford GD. Transarterial carotid occlusion: case report and review of the literature. Arch Otolaryngol. 1984;110(10):694–696. doi: 10.1001/archotol.1984.00800360066017. doi: 10.1001/archotol.1984.00800360066017. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson A, Cook AM, Dyet JF, et al. Case report: treatment of a carotid artery pseudoaneursym with a polyester covered nitinol stent. Clin Radiol. 1995;50(12):872–873. doi: 10.1016/s0009-9260(05)83113-7. doi: 10.1016/S0009-9260(05)83113-7. [DOI] [PubMed] [Google Scholar]
- 10.Alaraj A, Wallace A, Amin-Hanjani S, et al. Endovascular implantation of covered stents in the extracranial carotid and vertebral arteries: Case series and review of the literature. Surg Neurol Int. 2011;2:67. doi: 10.4103/2152-7806.81725. doi: 10.4103/2152-7806.81725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Chang G, Yao C, et al. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42(4):419–426. doi: 10.1016/j.ejvs.2011.05.008. doi: 10.1016/j.ejvs.2011.05.008. [DOI] [PubMed] [Google Scholar]