Abstract
Background
The onset of plastic bronchitis (PB) can be debilitating in survivors of Fontan surgery. The rarity of this complication makes designing studies to understand risk factors for PB challenging. This 2‐center case‐control study aimed to describe patient outcomes and to assess the association of antecedent patient factors with PB development.
Methods and Results
Using center registries, PB patients (n=25) were matched 1:2 to non‐PB Fontans (n=43) by date of Fontan surgery and center. The groups were compared for baseline characteristics. Association of patient characteristics with PB was assessed using logistic regression and of potential risk factors with onset of PB using time‐to‐event analyses. The median time from Fontan to PB diagnosis was 2.5 years. Overall, 12/25 PB patients died or underwent heart transplant; the median transplant‐free survival was 8.3 years after diagnosis. Factors associated with developing PB included post‐surgical chylothorax (44% PB versus 10% control; odds ratio [OR] 7.3; P=0.003), chest tube (CT) duration at stage 2 (P=0.04) and Fontan (P=0.004), and postoperative ascites (36% PB versus 12% control; OR 4.2; P=0.003). CT drainage >13 days at Fontan was associated with earlier PB onset (P=0.04). Early‐onset PB was associated with an increased risk of death (OR 5.0; P=0.002).
Conclusions
PB is a life‐threatening disorder. A longer duration of CT drainage after surgery, chylothorax, and development of ascites are all associated with developing PB. Understanding the pathophysiology of peri‐operative complications in individual patients and using targeted interventions may delay the onset of the PB phenotype.
Keywords: Fontan procedure, pediatrics, risk factors
Introduction
As survival following congenital heart surgery has improved for even the most complex lesions, attention has turned to important morbidities and their prevention and treatment. In children who have undergone staged Fontan surgery for single ventricle variants, plastic bronchitis (PB) is a particularly debilitating late complication. It is characterized by the formation of large, rubbery plugs or “casts” in the tracheobronchial tree. Previous reports suggest up to a 4% lifetime cumulative incidence in survivors of Fontan surgery.1 The diagnosis is often followed by repeated hospitalizations and invasive procedures with a reported 5‐year mortality as high as 50%.2 Antecedent patient factors that predispose to developing PB have not been defined. Similarly, knowledge of factors associated with eventual disease outcome is lacking. Clearly, a better understanding of factors associated with the development and progression of PB is important to identify Fontan patients at risk of developing this complication. More importantly, improved understanding of PB could lead to identifying new therapeutic targets for prevention and treatment of this complication.
The rarity of PB in survivors of Fontan surgery makes designing studies to understand risk factors for this complication challenging. In this 2‐center case‐control study, we aimed to: (1) assess the association of patient factors at the time of earlier cardiac surgeries with the development of PB; (2) characterize the outcomes of patients who developed PB; and (3) assess the association of antecedent patient factors with their outcome. Our working hypothesis was that patient factors that suggest a more complicated postoperative course during staged single‐ventricle palliation would be associated with the later development and outcome of PB.
Methods
This was a 2‐center, case‐control study designed to evaluate risk factors for development of Fontan‐associated PB. The study was approved by institutional review boards at both the University of Michigan and Boston Children's Hospital.
Subjects
We identified all patients who were status post‐Fontan surgery and were diagnosed with PB at either institution between 1989 and 2012. Patients were matched 1:2 (or 1:1 when 2 matches were not available) with controls (defined as Fontan patients who did not develop PB) by date of Fontan surgery (within 1 year) and by surgical center. A diagnosis of PB required a documented history of airway casts produced either via bronchoscopic removal or expectoration. A Fontan was defined as a patient with single‐ventricle congenital heart disease who had undergone a total cavopulmonary anastomosis.
Patient Variables
A chart review was conducted to collect patient demographic, medical and surgical history, and follow‐up history. Specific patient data reviewed included age and cardiac center at each surgery, cardiac diagnosis, dominance of single ventricle, requirement of aortic arch reconstruction at first surgery, type of superior cavopulmonary anastomosis (Stage 2) and Fontan surgery, duration (in days) of chest tubes (CT) after stage 2 and after Fontan, pre‐ and post‐Fontan ventricular end diastolic pressure (in mm Hg), pre‐ and post‐Fontan pulmonary artery pressure (PAP in mm Hg), pre‐ and post‐stage 2 and Fontan venovenous collateral (VVC) vessel coil placement, pre‐ and post‐stage 2 and Fontan aortopulmonary collateral (APC) vessel coil placement, presence of ascites post‐Fontan, a diagnosis of asthma post‐Fontan, chylothorax after any surgery, a diagnosis of systemic vein occlusion, and a diagnosis of protein losing enteropathy (PLE).
Patient factors from birth through the immediate postoperative period following Fontan were analyzed to determine associations with developing PB. The 2 groups – cases and controls – were also examined for differences in outcomes including heart transplant (HTx), death, and a composite outcome of HTx and death. Factors that were significantly associated with developing PB were analyzed to determine associations with the time of PB onset and with time to outcomes, as described above.
Statistical Analysis
Summary data are presented as median (interquartile range [IQR], 25th, 75th percentile) or number (%). Variables with >50% missing values in either group were not analyzed. Univariate associations of clinical variables with the development of PB were assessed using unconditional logistic regression and odds ratios (OR) with 95% confidence intervals (CI) were calculated. The ability to discriminate patients who developed PB from those who did not was quantified using the area under the receiver‐operating characteristic (ROC) curve (C‐statistic). Pearson's correlation was used to assess correlation (r) between continuous variables. Kaplan‐Meier time‐to‐event analyses and log‐rank test comparisons evaluated temporal influence of factors on outcome.
Statistical analysis was performed using SAS statistical software, version 9.3 (SAS Institute Inc) and GraphPad Prism, version 6.01 (GraphPad Software Inc). All statistical tests were 2‐sided. A P value of <0.05 was deemed significant.
Results
Clinical Outcomes
A total of 25 patients with PB were identified. The median age at diagnosis was 6.0 years (IQR 4.3, 10.7) with a median duration between Fontan and the diagnosis of PB of 2.8 years (IQR 1.6, 6.5). The median follow‐up post‐PB diagnosis was 2.5 years (IQR 0.7, 5.6). Overall, 19 patients (76%) were treated with systemic steroids, 17 (68%) were treated with heparin, 20 (80%) received at least one course of dornase‐alpha, and 15 (56%) received at least one course of inhaled tissue plasminogen activator (56%). Additionally, 17 patients (68%) had casts removed via bronchoscopy. There was no difference in the incidence of death or HTx between patients who required therapeutic bronchoscopy (9/17) and those not requiring bronchoscopy (3/8) (P=0.67). In 7 patients (28%), catheter‐based fenestration creation or enlargement was performed. In the fenestration creation/enlargement group, 1 patient had symptom resolution, 1 patient continues to have PB symptoms, 3 patients later died, and 2 patients were later transplanted. No significant difference in symptom resolution was found between patients with fenestration creation/enlargement and those who did not have an intervention (P=1.0). A higher proportion of patients who underwent fenestration creation/enlargement subsequently died or underwent HTx (5/7) compared with patients without intervention (7/18), although the difference was not significant (P=0.20). Elective fenestration closure also had no significant association with PB diagnosis.
The median transplant‐free survival was 8.3 years after diagnosis of PB (Figure 1). Eight patients died (32%). Among those who died, the median time to death from diagnosis was 0.4 years (IQR 0.2, 0.6). Onset of PB within 1 year of Fontan surgery was significantly associated with death, P=0.041, OR 5.0 (CI 1.1, 26.8), (Figure 2). HTx was performed in 8/25 (32%) patients. Among the PB patients undergoing HTx, the indication for HTx in all cases was severe PB symptoms refractory to treatment. Early post‐HTx mortality was high with 3/8 patients dying prior to hospital discharge in the immediate postoperative period. One additional patient died 3.2 years after HTx. There was resolution of PB in all patients who survived to hospital discharge following HTx. In PB patients who died (with or without HTx), the cause of death was due to complications of PB, including respiratory failure +/− associated heart failure (5/8) severe heart failure (1/8), hemoptysis with severe anoxic brain injury (1/8), and the single exception of the patient dying due to late HTx‐related complications.
Figure 1.
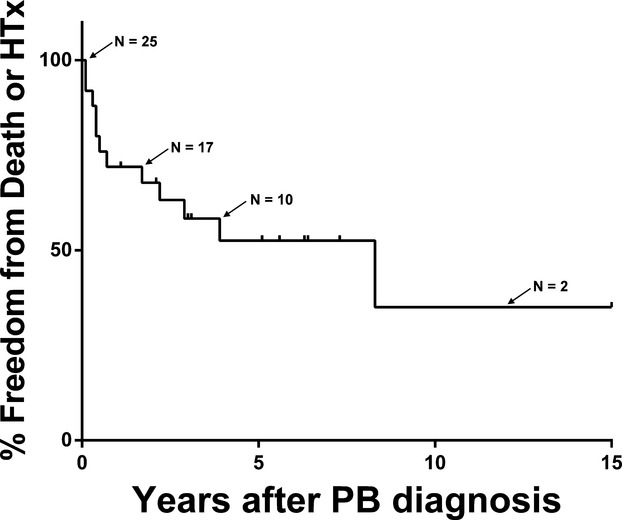
Freedom from death and/or heart transplant (HTx) in plastic bronchitis (PB) patients.
Figure 2.
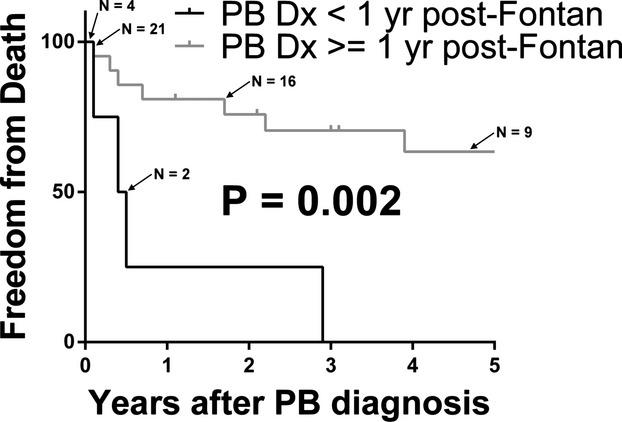
Freedom from death in plastic bronchitis (PB) patients. Subjects are stratified by PB diagnosis <1 year or ≥1 year from Fontan palliation.
Comparison of Groups for Outcomes and Risk Factors
Overall, 12/25 (48%) PB patients died or underwent heart transplant compared with 2/43 (4.6%) controls (P<0.0001, OR 18.9 (CI 3.7, 95.8)). One control patient died after developing PLE whereas the second patient underwent HTx after developing refractory arrhythmias and progressive heart failure. PB patients spent a median of 42 days in the hospital (IQR 19, 98) versus a median of 3 days (IQR 0, 17) for controls following discharge from Fontan surgery (P<0.0001).
A comparison of characteristics with significant differences between PB cases and controls is shown in Table. The following factors were significantly associated with PB in univariate analyses: CT days after stage 2 surgery, CT days after Fontan, ascites following Fontan surgery, post‐Fontan APC coil placement, and chylothorax after any surgery. In addition, aortic arch reconstruction during stage 1 surgery was borderline significant. Several factors that approached but did not reach statistical significance also warrant comment given the relatively small size of the study groups and the factors' potential clinic relevance: PB patients were more likely to be diagnosed with asthma, more likely to have a systemic vein thrombus, and less likely to require pre‐Fontan VVC coiling. Of note, important factors that were not associated include gender, age at any surgery, pre‐Fontan PAP, need for APC coils at pre‐Fontan cath, and Fontan type. Ventricular end‐diastolic pressures were not recorded in the majority of non‐PB Fontans and were not analyzed. In the PB cohort, the median ventricular end‐diastolic pressure was 9 mm Hg (IQR 7.5, 12).
Table 1.
Patient Characteristics
| Plastic Bronchitis | Non‐Plastic Bronchitis Fontan | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Total patients | 25 | 43 | ||
| Male gender | 17 (68%) | 27 (63%) | 0.81 | |
| Right dominant ventricle | 18 (75%) | 22 (51.1%) | 0.061 | 2.86 (0.99, 9.18) |
| Age at 1st surgery (days) | 0 (0 to 0) | 0 (0 to 0) | 0.48 | |
| Neonatal arch reconstruction | 19 (76%) | 22 (52.4%) | 0.060 | 2.88 (1.00, 9.22) |
| Age at Stage 2 | 0.4 (0.3 to 0.6) | 0.5 (0.4 to 0.6) | 0.31 | |
| Stage 2 type | 0.28 | |||
| Bidirectional Glenn | 15 (62.5%) | 20 (50%) | ||
| Hemifontan | 9 (37.5%) | 19 (47.5%) | ||
| Other | 0 | 1 (2.5%) | ||
| Stage 2 CT duration, days | 6.5 (3 to 14) | 4 (3 to 5) | 0.04 | |
| Pre‐Fontan PAP, mm Hg | 12.1 (3.3) | 11.6 (2.9) | 0.58 | |
| Pre‐Fontan APC coil | 8 (35%) | 10 (23%) | 0.32 | |
| Pre‐Fontan VVC coil | 2 (9%) | 11 (26%) | 0.12 | 0.28 (0.04, 1.17) |
| Age at Fontan, y | 2.4 (2 to 3) | 2.4 (2 to 3.2) | 0.87 | |
| Fontan type | 0.74 | |||
| Lateral tunnel | 20 (80%) | 38 (88%) | ||
| Extra‐cardiac conduit | 5 (20%) | 3 (7%) | ||
| Other | 0 | 2 (5%) | ||
| Fontan CT days | 15 (8 to 25) | 7.5 (5.5 to 12.5) | 0.004 | |
| Post‐Fontan Ascites | 9 (36%) | 5 (12%) | 0.024 | 4.16 (1.24, 15.46) |
| Post‐Fontan PAP* | 16.5 (12 to 22) | 13 (12 to 15) | 0.03 | |
| Post‐Fontan APC coil | 13 (52%) | 3 (7.3%) | 0.0003 | 13.72 (3.71, 67.67) |
| Post‐Fontan VVC coil | 6 (24%) | 8 (20%) | 0.66 | |
| Chylothorax | 11 (44%) | 4 (10%) | 0.003 | 7.27 (2.11, 28.88) |
| Asthma | 8 (32%) | 5 (12%) | 0.085 | 3.50 (0.86, 15.78) |
| Systemic vein thrombus | 8 (32%) | 7 (17%) | 0.15 | 2.35 (0.73, 7.79) |
| PLE | 4 (16%) | 2 (5%) | 0.25 | 3.8 (0.50, 45.50) |
Data presented as N (%), median (IQR), or mean (SD). APC indicates aortopulmonary collateral; CT, chest tube; OR, odds ratio; PAP, pulmonary artery pressure; PLE, protein losing enteropathy; VVC, venovenous collateral.
Value missing on 39% of non‐plastic bronchitis patients.
By ROC analysis, CT duration >6 days at stage 2 (C‐statistic 0.68) and >13 days at Fontan (C‐statistic 0.73) optimally discriminated between PB and non‐PB patients. Given the potential covariance between prolonged CT duration at stage 2, prolonged CT duration at Fontan, and ascites, these variables were analyzed for correlation. Pairwise correlation was negligible between CT output at either stage and ascites (r=0.14 for stage 2, r=0.15 for Fontan). Correlation between prolonged CT output at Stage 2 and Fontan was moderate (r=0.48).
Each variable associated with PB diagnosis was evaluated for its influence on the timing of PB development. Only chest tube duration at Fontan was found to be significantly associated with time‐to‐onset of PB; among PB patients, those patients who had prolonged chest tube duration at Fontan had significantly shorter freedom from PB (Figure 3).
Figure 3.
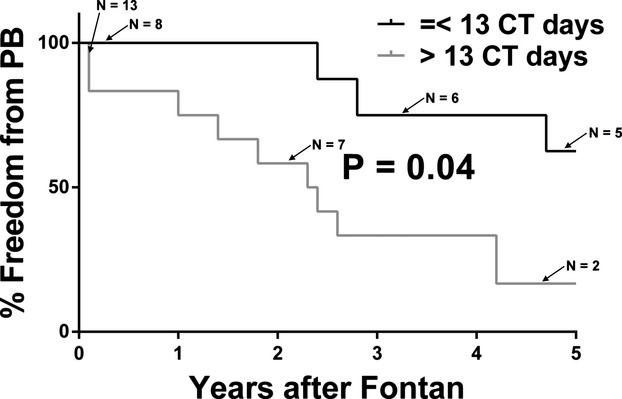
Freedom from plastic bronchitis stratified by post‐Fontan CT duration. Subjects are stratified by ≤13 CT days or >13 CT days. CT indicates chest tube; PB, plastic bronchitis.
Discussion
In this study, we analyzed patient factors associated with the development of PB following Fontan surgery. We found that prolonged chest tube drainage after Stage 2 and after Fontan surgery, history of chylothorax associated with staged palliation, ascites, and history of significant aorto‐pulmonary collaterals (important enough to require catheter coil occlusion) are all associated with higher odds of developing PB on follow‐up. Furthermore, prolonged CT drainage was associated with earlier development of PB. In essence, these findings suggest that Fontan patients who have experienced higher morbidity in the perioperative periods of staged single ventricular palliation are at higher long‐term risk of developing PB. Thus, the development of PB after Fontan surgery is not a random phenomenon but is another manifestation of suboptimal adaptation to cavopulmonary circulation. Unfortunately, the onset of PB may not only be debilitating but – as the results show – often leads to poor outcomes, particularly if the onset is early after Fontan surgery. A close follow‐up of high‐risk patients with a goal of optimum hemodynamics may prevent or delay the onset of PB.
This is the first study to analyze factors associated with PB following the Fontan palliation. Previous studies were either descriptive, single‐center studies1,3–5 or were case‐series of patients from a transplant registry so that all subjects were already listed for heart transplant after developing PB.6 An ideal study design to better understand rare, late‐onset complications such as PB after Fontan surgery would be to follow a large, multicenter cohort of Fontan patients prospectively. While cohort studies are considered essential in determining the absolute risk of developing a disease for subjects in the population (or the risk of a complication when following a diseased cohort), they are impractical for studying rare diseases. The resources required for studying a large cohort for a long duration can be prohibitive. In contrast, case control studies are efficient in detecting associations between patient factors and rare diseases. Although such studies cannot detect absolute measures of risk, the associations found are insightful, particularly if they provide a plausible framework for the disease, as the current study does.
The pathophysiology of PB is largely unknown. Several mechanisms have been postulated including hyper secretion of mucus due to chronically elevated systemic venous pressures,7 thoracic lymphatic malformation or lymph leak into the airways,3,8 low cardiac output,9–10 and an abnormal inflammatory response.11 However, none of these has been adequately supported with evidence. Given the association of PB with Fontan physiology and the resolution in PB in patients who survive heart transplant, some relationship with Fontan physiology must exist. However, because many Fontan patients with chronically elevated PAP do not develop PB suggests that additional mechanisms are important. The findings of the current study suggest an abnormality in the production or drainage of pulmonary fluid for most patients. Several potential sources exist and may differ among patients. Lymphatic abnormalities have attracted the most attention. There are reports of lymphatic derangements in some patients with PB3,8,12–13 while others have described relief of symptoms with interventions expected to stop abnormal lymphatic flow.14–15 Perhaps significant lymphatic derangement in the presence of chronically elevated pulmonary artery pressure and/or non‐pulsatile flow leads to lymph stasis which results in bronchial lymph leakage and cast formation. Alternatively, a role for inflammation has been proposed both in patients with prolonged chest tube drainage (or ascites) early after Fontan11,16–17 and in patients with PB on follow‐up.11 Perhaps prolonged postoperative chest tube drainage identifies a patient with a particularly pronounced or pathologic inflammatory response that may prompt airway cast formation at a later date. Fontan physiology has been associated with chronically elevated systemic inflammation,18 which in some individuals with Fontan physiology maybe sufficient to result in cast formation and PB. Characterizing the mechanism associated with prolonged chest tube drainage after stage 2 palliation or after Fontan in an individual patient (abnormality of hemodynamics versus lymphatic system versus inflammatory response) may not only provide the basis for individualized treatment of peri‐operative complications but also for the longer‐term management to prevent or delay the onset of PB in these patients.
Thus, a patient with prolonged chest tube drainage after Fontan completion requires more vigilant monitoring of their symptoms, signs, and functional status as they are at increased risk of developing PB. Diagnostic evaluation at the onset of PB could include assessment of hemodynamics and other potential mechanisms such as evidence of systemic inflammation and lymphatic abnormalities3; any significant findings may direct subsequent treatment. Our results demonstrate that some patients are at risk of early death while many go on to require heart transplant. Early involvement of heart failure teams to help optimize their medical therapy and discuss heart transplant candidacy may improve outcomes in these patients.
Limitations
This study has several limitations. First, despite the use of registries from 2 large congenital heart centers, the sample size is still relatively small, which may result in the study being underpowered to detect associations. This is to be expected considering the rare nature of this complication after Fontan surgery. Second, matching criteria were limited to year and center and were chosen to control for era effect, allow a reasonable chance of finding suitable controls for most patients while also permitting evaluation of other associations. Further, to maintain study power, the logistic regression analyses used were unconditional, meaning the matching parameters were not specifically accounted for during each risk factor's analysis. Third, post‐Fontan hemodynamic data were not available for many controls as they had not undergone heart catheterization since their Fontan surgery. Thus, full characterization of some significant associations may be limited. Finally, the small sample size precluded multivariable analysis meaning that independent risk factors have not been demonstrated and confounding may be present.
Conclusion
In the current era, post‐Fontan PB remains a life‐threatening disorder. This study identified several factors associated with the development of PB in post‐Fontan patients. A longer duration of CT drainage after surgery, chylothorax, and development of significant aortopulmonary collaterals are all associated with subsequent development of PB. In particular, prolonged duration of chest tube drainage at Fontan was associated not only with developing PB but also with earlier onset of the disease. Understanding the pathophysiology of peri‐operative complications in individual patients may allow targeted interventions and may delay the onset of the PB phenotype.
Sources of Funding
Heart Transplant Research and Education Fund, Department of Cardiology, Boston Children's Hospital, Boston, MA.
Disclosures
None.
References
- 1.Caruthers RL, Kempa M, Loo A, Gulbransen E, Kelly E, Erickson SR, Hirsch JC, Schumacher KR, Stringer KA. Demographic characteristics and estimated prevalence of Fontan‐associated plastic bronchitis. Pediatr Cardiol. 2013; 34:256-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larue M, Gossett JG, Stewart RD, Backer CL, Mavroudis C, Jacobs ML. Plastic bronchitis in patients with Fontan physiology: review of the literature and preliminary experience with Fontan conversion and cardiac transplantation. World J Pediatr Congenit Heart Surg. 2012; 3:364-372 [DOI] [PubMed] [Google Scholar]
- 3.Ezmigna DR, Morgan WJ, Witte MH, Brown MA. Lymphoscintigraphy in plastic bronchitis, a pediatric case report. Pediatr Pulmonol. 2013; 48:515-518 [DOI] [PubMed] [Google Scholar]
- 4.Grutter G, Di Carlo D, Gandolfo F, Adorisio R, Alfieri S, Michielon G, Carotti A, Pongiglione G. Plastic bronchitis after extracardiac Fontan operation. Ann Thorac Surg. 2012; 94:860-864 [DOI] [PubMed] [Google Scholar]
- 5.Verghese S, Jackson M, Vaughns J, Preciado D. Plastic bronchitis in a child with Fontan's physiology presenting for urgent rigid bronchoscopy. Anesth Analg. 2008; 107:1446-1447 [DOI] [PubMed] [Google Scholar]
- 6.Gossett JG, Almond CS, Kirk R, Zangwill S, Richmond ME, Kantor PF, Tresler MA, Lenderman SM, Naftel DC, Matthews KL, Pahl E. Outcomes of cardiac transplantation in single‐ventricle patients with plastic bronchitis: a multicenter study. J Am Coll Cardiol. 2013; 61:985-986 [DOI] [PubMed] [Google Scholar]
- 7.Seear M. Acellular bronchial casts in children after cardiac surgery. Crit Care Med. 2001; 29:465-466 [DOI] [PubMed] [Google Scholar]
- 8.Languepin J, Scheinmann P, Mahut B, Le Bourgeois M, Jaubert F, Brunelle F, Sidi D, de Blic J. Bronchial casts in children with cardiopathies: the role of pulmonary lymphatic abnormalities. Pediatr Pulmonol. 1999; 28:329-336 [DOI] [PubMed] [Google Scholar]
- 9.Wilson J, Russell J, Williams W, Benson L. Fenestration of the Fontan circuit as treatment for plastic bronchitis. Pediatr Cardiol. 2005; 26:717-719 [DOI] [PubMed] [Google Scholar]
- 10.Chaudhari M, Stumper O. Plastic bronchitis after Fontan operation: treatment with stent fenestration of the Fontan circuit. Heart. 2004; 90:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racz J, Mane G, Ford M, Schmidt L, Myers J, Standiford TJ, Schumacher KR, Fifer C, Russell MW, Stringer KA. Immunophenotyping and protein profiling of Fontan‐associated plastic bronchitis airway casts. Ann Am Thorac Soc. 2013; 10:98-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005; 6:292-300 [DOI] [PubMed] [Google Scholar]
- 13.Hug MI, Ersch J, Moenkhoff M, Burger R, Fanconi S, Bauersfeld U. Chylous bronchial casts after Fontan operation. Circulation. 2001; 103:1031-1033 [DOI] [PubMed] [Google Scholar]
- 14.Parikh K, Witte MH, Samson R, Teodori M, Carpenter JB, Lowe MC, Morgan W, Hardin C, Brown M, Naughton Y, Sinha S, Barber BJ. Successful treatment of plastic bronchitis with low fat diet and subsequent thoracic duct ligation in child with Fontan physiology. Lymphology. 2012; 45:47-52 [PubMed] [Google Scholar]
- 15.Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: is thoracic duct ligation a real surgical option? Ann Thorac Surg. 2006; 81:2281-2283 [DOI] [PubMed] [Google Scholar]
- 16.Schroeder VA, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation. 2003; 107:2823-2828 [DOI] [PubMed] [Google Scholar]
- 17.Clarizia NA, Manlhiot C, Schwartz SM, Sivarajan VB, Maratta R, Holtby HM, Gruenwald CE, Caldarone CA, Van Arsdell GS, McCrindle BW. Improved outcomes associated with intraoperative steroid use in high‐risk pediatric cardiac surgery. Ann Thorac Surg. 2011; 91:1222-1227 [DOI] [PubMed] [Google Scholar]
- 18.Rychik J. Protein‐losing enteropathy after Fontan operation. Congenit Heart Dis. 2007; 2:288-300 [DOI] [PubMed] [Google Scholar]


