Abstract
Background
Reduction of infarct size by ischemic postconditioning (IPost) has been reported in smaller proof‐of‐concept clinical studies, but has not been confirmed in other smaller studies. The principle needs to be evaluated in larger groups of ST‐elevation myocardial infarction (STEMI) patients before being implemented in clinical practice. This study assessed the effect of ischemic postcoditioning (IPost) on infarct size in patients with STEMI treated by primary percutaneous coronary intervention (PCI).
Methods and Results
Patients with first‐time STEMI, <6 hours from symptom onset, referred to primary PCI were randomized to IPost or control groups. IPost was administered by 4 cycles of 1‐minute reocclusion and 1‐minute reperfusion, starting 1 minute after opening, followed by stenting. In the control group, stenting was performed immediately after reperfusion. The primary endpoint was infarct size measured by cardiac magnetic resonance after 4 months. A total of 272 patients were randomized. Infarct size (percent of left ventricular mass) after 4 months (median values and interquartile range) was 14.4% (7.7, 24.6) and 13.5% (8.1, 19.3) in the control group and IPost group, respectively (P=0.18). No significant impact of IPost was found when controlling for baseline risk factors of infarct size in a multivariate linear regression model (P=0.16). The effects of IPost on secondary endpoints, including markers of necrosis, myocardial salvage, and ejection fraction, as well as adverse cardiac events during follow‐up, were consistently neutral.
Conclusions
In contrast to several smaller trials reported previously, we found no significant effects of IPost on infarct size or secondary study outcomes.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov Unique identifier: NCT.No.PO1506.
Keywords: acute myocardial infarction, infarct size, percutaneous coronary intervention, postconditioning, reperfusion injury
Introduction
In spite of improved acute treatment and follow‐up strategy of patients with acute ST‐elevation myocardial infarction (STEMI), the incidence of death or heart failure after STEMI is still high. Reperfusion of the infarct‐related artery (IRA), preferably by primary percutaneous coronary intervention (PCI) if it can be performed within guideline‐recommended time frames, is the most effective treatment to limit infarct size, preserve left ventricular (LV) function, and prevent development of heart failure.1 Paradoxically, however, reperfusion itself may result in further injury to the ischemic myocardium by complex mechanisms collectively termed ischemia‐reperfusion (I/R) injury.2 Numerous different pharmacological principles aimed to reduce I/R injury have been tested in clinical trials and, with a few promising exceptions, have failed to reduce infarct size or occurrence of adverse clinical events.2–4
Cardioprotection by ischemic postconditioning (IPost) was reported on in an animal model in 2003, demonstrating markedly reduced infarct size, compared to controls.5 The first clinical study showing reduction of myocardial necrosis by IPost in STEMI patients was published in 2005.6 This study was followed by several reports on beneficial effects of IPost on release of markers of myocardial necrosis and reduction of infarct size in STEMI patients.7–10 These trials, however, were small, and the efficacy of IPost has not been clarified or implemented in routine treatment of STEMI. Larger clinical studies on the efficacy and safety of IPost in STEMI are needed. The present study was initiated to further evaluate the effects of IPost in a larger group of STEMI patients undergoing primary PCI. The primary endpoint was infarct size, assessed by cardiac magnetic resonance (CMR) after 4 months. Secondary endpoints included the effect of IPost on myocardial necrosis, salvage, LV function, as well as the frequency of adverse cardiac events during follow‐up.
Methods
The Postconditioning in ST‐Elevation Myocardial Infarction (POSTEMI) study was a prospective, randomized, single‐center, open‐label clinical trial with blinded endpoint evaluation in STEMI patients undergoing primary PCI. Two reperfusion strategies, PCI with IPost or conventional PCI, were compared. The study protocol was approved by the Regional Committee for Medical Research Ethics, South‐East Norway, and the trial was registered at www.ClinicalTrials.gov (NCT.No.PO1506). The study was conducted in accord with the ethical principles of the Declaration of Helsinki. All patients gave written informed consent to participate in the study.
Study Population
The study design has been published in detail previously.11 Briefly, patients with first‐time acute STEMI with symptom duration <6 hours, referred to primary PCI, were considered for inclusion. ST‐segment elevation in ECG ≥1 mm in at least 2 contiguous extremity leads or ≥2 mm in at least 2 precordial leads or new‐onset left‐bundle branch block had to be present. Clinically unstable patients with cardiac arrest, cardiogenic shock, hypotension, or pulmonary congestion were not eligible for inclusion. Patients with previous myocardial infarction, renal failure (serum creatinine >200 μmol/L), inability to provide informed consent, as well as patients with contraindications for CMR were not included. Occlusion of the proximal or middle part of 1 of the 3 main coronary vessels (thrombolysis in myocardial infarction [TIMI] flow 0 to 1) with no or minimal collateral flow to the ischemic myocardium and successful reperfusion after the first balloon inflation (TIMI flow 2 to 3) had to be demonstrated angiographically before 1:1 randomization to IPost or control groups. A permuted block randomization list was computer generated and transferred by people not involved in patient care to a sequence of sealed, opaque, consecutively numbered envelopes before start of the study.
Treatment Protocol
All patients received aspirin 300 mg, clopidogrel 600 mg, and heparin 70 IU/kg before the procedure, and a weight‐adjusted bolus of eptifibatide was given before the first balloon inflation, followed by minimum 12‐hour infusion. Postconditioning was performed by 4 cycles of 1‐minute balloon occlusion of IRA, starting 1 minute after opening and separated by 1‐minute reperfusion intervals, followed by stent implantation. In the control group, stenting was performed immediately after reperfusion. During the inclusion period, it was decided that patients fulfilling the inclusion criteria and with an indication for thrombectomy could be eligible for randomization. The decision to use thrombectomy devices and choice of stent type and dimension were left to the operator. Adjunctive treatment was given according to European guidelines for management of STEMI.1
Study Endpoints
The primary endpoint was infarct size, measured by CMR as percentage of LV mass, after 4 months. Infarct size measured after 4 months was considered a biologically relevant and robust outcome variable. Secondary endpoints were myocardial perfusion grade, ST‐resolution in ECG, peak levels of troponin T, LV ejection fraction (LVEF) by CMR after 4 months, and myocardial salvage index. When feasible, infarct size was also measured by an early CMR in the acute stage. Clinical cardiovascular events until 4 months were recorded.
Angiographic Analysis
Collateral flow to the ischemic myocardium was graded according to Rentrop criteria.12 After PCI, 200 μg of glycerylnitrate was given intracoronary and myocardial perfusion grade was assessed using TIMI definitions.13
ECG Analysis
In a 12‐lead ECG taken before admission, ST elevation was measured manually at the J‐point to the nearest 0.5 mm. For quantification of ST elevation, the single lead with maximal elevation in baseline ECG was chosen. ST elevation was measured in the same lead in ECG taken 60 minutes after reperfusion, and ST resolution (%) was defined as ([ST elevation at baseline−ST elevation after reperfusion]/ST elevation at baseline)×100.
Cardiac Biomarkers
Blood samples were drawn before and at the end of the angiographic procedure for biobanking and for analysis of troponin T, repeated every 8 hours until peak value. Troponin T was determined by electrochemiluminescence technology for quantitative measurement (Elecsys 2010; Roche, Mannheim, Germany). The interassay coefficient of variation was 7%.
CMR Protocol and Analysis
CMR was performed after 4 months, and, if logistics permitted, an early CMR was also undertaken in the acute stage within 1 to 5 days. CMR was performed on a 1.5‐T scanner (Philips Intera, release 11 or Philips Achieva, release 3.2.1.1; Philips Healthcare, Best, the Netherlands), using 5‐element Synergy‐cardiac or Sense‐cardiac coil, respectively, and vector‐based ECG. Patients with 2 CMR studies were examined in the same scanner at both occasions according to a standard clinical CMR protocol. For functional and LV volume analysis, short axis images were acquired. The late gadolinium enhancement study was performed 15 minutes after contrast injection in 2‐ and 4‐chamber long axis view and in short axis view. A dose of 0.15 mmol/kg, gadolinium‐DTPA 469 mg/mL (Magnevist; Schering AG, Berlin, Germany) was used.
Image analyses were performed on an Extended MR Work Space (Philips Medical Systems, Eindhoven, the Netherlands) by an observer blinded to randomization. LVEF and myocardial volume were calculated by assessment of the volumes of the epi‐ and endocardial contours in diastole and systole of the short axis images. Myocardium at risk was measured in the acute stage and myocardial salvage index defined as (%): ([myocardium at risk−infarct size at 4 months]/myocardium at risk)×100. The intraobserver reliability estimated by the intraclass correlation coefficients for myocardium at risk and infarct size were 0.876, 95% CI (0.628 to 0.963) and 0.985, 95% CI (0.963 to 0.994), respectively.
Sample Size and Statistical Analysis
In a previous series of unselected STEMI patients treated by primary PCI in our institution, infarct size measured by single‐photon emission computed tomography was 14±8% of LV mass.14 A 20% relative infarct size reduction (2.8% points) by IPost was considered clinically relevant. To demonstrate this effect with a type I error of 5% and a statistical power of 80%, 127 patients in each treatment group were needed. Categorical variables are presented as proportions, and the chi‐square test was used to analyze differences between groups. Because of skewness, continuous variables are presented as median values with interquartile range and group differences were analyzed by nonparametric tests (Mann‐Whitney). Baseline risk factors of the primary endpoint were controlled for in a multivariate linear model, followed by ANCOVA, also controlling for early infarct size, using a manual backward elimination procedure.15–16 A sensitivity analysis for dropout patients with worst/best case scenarios was done.17 A P<0.05 was considered statistically significant. The EpiInfo (version 3.5.1; Centers for Disease Control and Prevention, Atlanta, GA) software was used.
Results
Study Population and Treatment Assignment
Between May 2009 and August 2012, 2866 STEMI patients were accepted for primary PCI at Oslo University Hospital Ullevål and assessed for eligibility. After screening, 272 patients were randomized in the trial, 136 in each group (Figure 1). Clinical baseline characteristics and angiographic findings were similar between the groups (Table 1). The IPost procedure was well tolerated.
Figure 1.
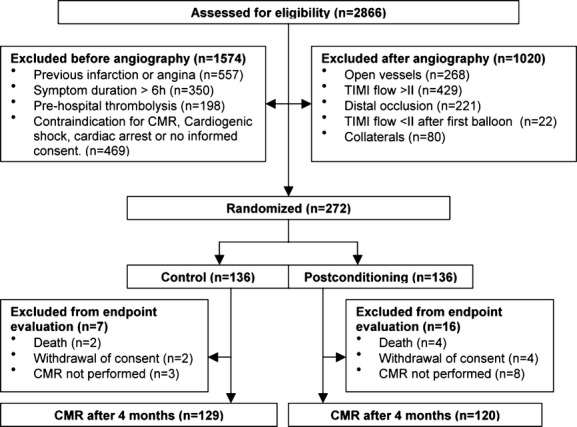
Study flow diagram. CMR indicates cardiac magnetic resonance; TIMI, thrombolysis in myocardial infarction.
Table 1.
Patient Baseline Characteristics
| Variable | Control (n=136) | Postconditioning (n=136) |
|---|---|---|
| Age, y | 60 (52, 64) | 61 (53, 69) |
| Male gender, % | 80.1 | 83.8 |
| Treated hypertension, % | 25.0 | 28.7 |
| Treated diabetes, % | 2.2 | 3.7 |
| Current smoking, % | 53.7 | 48.5 |
| Symptom‐to‐balloon time, minutes | 176 (124, 255) | 198 (134, 275) |
| Door‐to‐balloon time, minutes | 31 (25, 39) | 32 (29, 38) |
| Systolic blood pressure*, mm Hg | 140 (126, 166) | 140 (125, 160) |
| Heart rate*, bpm | 77 (64, 87) | 75 (63, 89) |
| Infarct‐related artery, % | ||
| LAD | 50.7 | 45.6 |
| LCX | 8.1 | 13.2 |
| RCA | 41.2 | 41.2 |
| 1‐vessel disease, % | 66.9 | 66.9 |
| 2‐vessel disease, % | 24.3 | 19.1 |
| 3‐vessel disease, % | 8.8 | 14.0 |
| TIMI flow before balloon, % | ||
| 0 | 95.6 | 93.4 |
| 1 | 4.4 | 6.6 |
| TIMI flow after balloon, % | ||
| 0 | 0 | 0.7 |
| 2 | 1.5 | 1.5 |
| 3 | 98.5 | 97.8 |
| Type of stent, % | ||
| Bare‐metal stent | 71.3 | 70.9* |
| Drug‐eluting stent | 28.7 | 29.1 |
| Thrombectomy, % | 26.5 | 19.1 |
Data are presented as median values (interquartile range) or percentages. LAD indicates left anterior descending; LCX, left circumflex; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
At admission.
No stent implantation (balloon dilation only) was performed in 2 patients in the postconditioning group.
Infarct Size
CMR examination was performed at a median of 4 (4.4) months after PCI in 249 patients (91.5%) (Table 2). CMR was not accomplished in 23 randomized patients, 6 patients were dead, there were 6 withdrawals of consent, and 11 examinations were rejected because of poor image quality or interrupted because of claustrophobia (Figure 1). Infarct size as percentage of LV mass was 14.4% (7.7, 24.6) in the control group and 13.5% (8.1, 19.3) in the IPost group (P=0.18; Figure 2). Anterior wall infarction and symptom‐to‐balloon time were independent risk factors of infarct size. When controlling for these variables in a multivariate linear model, no significant impact of IPost on infarct size was found (P=0.16). This was followed by another analysis, using ANCOVA to control for early infarct size, infarct location, and symptom‐to‐balloon time, and there was no difference in infarct size after 4 months (P=0.51). A worst/best case simulation of dropouts did not change the primary endpoint evaluation.
Table 2.
Outcome Evaluations in the Acute Phase and After 4 Months
| Variable | Control | Postconditioning | P Value |
|---|---|---|---|
| In the acute phase | (n=136) | (n=136) | |
| Myocardial blush grade* (TMPG) ≥2 | 118 (86.8%) | 112 (82.4%) | 0.20 |
| ≥50% ST‐segment resolution after 1 hour | 122 (89.7%) | 120 (88.2%) | 0.42 |
| Peak troponin T, ng/L | 6251 (3284 to 11 570) | 5693 (3350 to 9488) | 0.63 |
| CMR in the acute phase | (n=119) | (n=113) | |
| Time to CMR, days | 2 | 2 | 0.51 |
| Infarct size (% of LV mass) | 17.4 (11.3 to 28.9) | 16.2 (11.3 to 28.2) | 0.75 |
| Ejection fraction, % | 50 (41 to 59) | 51 (44 to 58) | 0.60 |
| CMR after 4 months | (n=129) | (n=120) | |
| Time to CMR, months | 4 | 4 | 0.37 |
| Primary endpoint: infarct size (% of LV mass) | 14.4% (7.7 to 24.6) | 13.5% (8.1 to 19.3) | 0.18 |
| Ejection fraction, % | 55.0 (48 to 62) | 56.5 (49 to 63) | 0.19 |
| Myocardial salvage index | 49.0 (37 to 61) | 52.4 (41 to 68) | 0.30 |
| Clinical events between randomization and 4‐month follow‐up | |||
| All cause mortality | 2 | 4 | |
| Urgent rehospitalization | |||
| Heart failure | 5 | 2 | |
| Acute coronary syndrome | 9 | 2 |
Data are presented as median values (interquartile range) or n (%). CMR indicates cardiac magnetic resonance; LV, left ventricle; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; TMPG, TIMI‐myocardial perfusion grade.
After completion of PCI.
Figure 2.
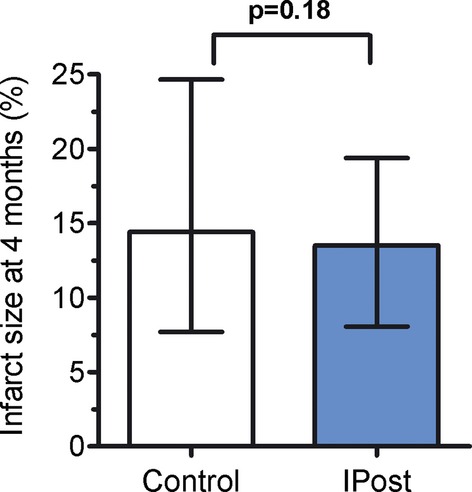
Final infarct size (percent of left ventricular mass) in the control (n=129) and ischemic postconditioning (IPost, n=120) groups, measured by cardiac magnetic resonance after 4 months. Median values and interquartile range are given.
Secondary Endpoints
There were no between‐group differences in myocardial perfusion grade immediately after PCI, ST resolution 60 minutes after reperfusion, peak troponin T values, or LVEF at 4 months (Table 2). Myocardium at risk and myocardial salvage index were determined in a subgroup of patients who had been evaluated by CMR both in the acute stage and after 4 months (n=185). There was no significant effect of IPost on these variables (Figure 3A and 3B; Table 2). A total of 259 included patients had clinical follow‐up visit after 4 months. Six patients had died between inclusion and the 4‐month visit, 2 in the control group and 4 in the IPost group. There was no significant difference between the groups regarding rehospitalization for acute coronary syndromes or heart failure (Table 2).
Figure 3.
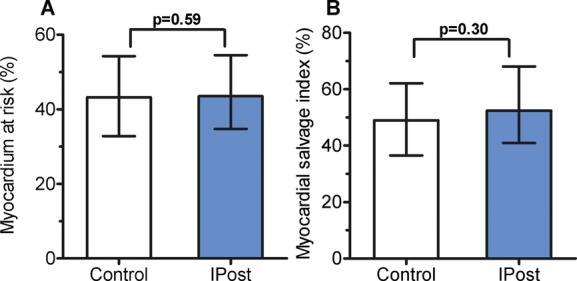
A, Myocardium at risk (percent of left ventricle) in the control (n=91) and ischemic postconditioning (IPost; n=94) groups, measured by cardiac magnetic resonance in the acute stage. Median values and interquartile range are given. B, Myocardial salvage index (%), determined in‐hospital and after 4 months in the control (n=89) and ischemic postconditioning (IPost; n=88) groups. Median values and interquartile range are given.
In a post‐hoc, hypothesis‐generating approach, the impact of IPost on infarct size was evaluated in subgroups, according to gender, age (over/below 60 years), infarct location (anterior/nonanterior), and symptom‐to‐balloon time (over/below 3 hours). The effect of IPost in these subgroups was neutral in multivariate analyses (data not shown).
Discussion
The main result of the present trial is that IPost in STEMI patients undergoing primary PCI did not reduce infarct size after 4 months. Also, the effect of IPost on secondary endpoints in the study, including myocardial perfusion grade, ST resolution, peak levels of troponin T, LVEF, and myocardial salvage, as well as the frequency of clinical adverse events, was consistently neutral. When we adjusted, in multivariate analyses, for baseline variables influencing infarct size, there was still no impact of IPost. Also, there was no effect of IPost on early infarct size, measured by CMR in the acute stage in approximately 85% of the patients.
This is, to date, the largest randomized trial on IPost in STEMI with infarct size as the primary endpoint. Since the launch of our study, the results of several other, smaller trials have been published.18–24 In some of these studies, cardioprotective effects of IPost have been reported.18–19,21,23 Other investigators, however, have not been able to confirm any protection by IPost,20,22,24 and a trend toward adverse effects has even been suggested.22,24 Recently, Hahn and coworkers reported no effect on ST‐segment resolution by IPost in 700 STEMI patients. However, data on final infarct size were lacking.25 With the results of our trial, the evidence supporting the use of IPost in the routine treatment of STEMI has been further weakened.
It is not clear why the results of clinical trials on IPost in STEMI diverge. Patient baseline characteristics, selection and determination of relevant endpoints, and the IPost procedure may be issues of importance.26–27
Patient Population
Clinical studies on IPost in STEMI have, thus far, been designed to include individuals supposed to benefit most from the procedure, as will be the case in explanatory trials aimed to test whether an intervention can work.28 Accordingly, all patients included in IPost trials are highly selected from the acute STEMI population, albeit according to somewhat different criteria. In the POSTEMI trial, we included patients with relatively short symptom duration, with the potentially largest areas at risk, without collaterals, and patients without preinfarction symptoms. Because of the endpoint evaluation with CMR, we excluded patients with a history or sign of previous infarction. Thus, our results are not representative for the general STEMI population, but in accord with experimental data, our included patients would be among those expected to gain most from IPost.2,26–27 Our inclusion and exclusion criteria compare well with those of studies reporting positive effects of IPost, although some of these have included patients up to 12 hours from symptom onset. The median total ischemia time in our study is relatively short, but there is no evident relation between this time period and study outcome.6,18–19,21,23 Patient comorbidities and medication may represent confounding factors in trials on IPost,26–27 but our patients seem to be comparable with other study populations in these respects.6,9,18,20–24
Study Endpoints
Ideally, novel treatment strategies in STEMI should be evaluated by randomized trials with new clinical events, including mortality, as primary endpoints. Such studies would necessarily require a large number of patients. Consequently, the use of surrogate endpoints to test the potential of a novel treatment is reasonable. Release of markers of myocardial necrosis, myocardial perfusion grade, and ST resolution may be considered less‐precise measures of myocardial injury than infarct size. Infarct size has been shown to correlate with prognosis after STEMI.29 CMR permits a high spatial resolution, and infarct size determined by this modality has been advocated as a suitable surrogate endpoint in medium‐size clinical trials in STEMI.30 A reduction, as well as no impact, of IPost on infarct size measured by CMR has been reported previously.18,20,22,24 Myocardial salvage may be a relevant variable in the assessment of a new reperfusion therapy.27,31 Salvage takes myocardium at risk, the most important determinant of final infarct size, into account. Because the size of myocardium at risk may be influenced by the reperfusion strategy itself, it should be determined by an imaging technique that can be applied before reperfusion.23,27,31 Our study, however, included so many patients that the size of myocardium at risk would be expected to be similarly distributed in the 2 groups at baseline; we therefore included salvage as a secondary outcome in patients undergoing both early and late CMR, and no effect of IPost was found. Our results are consistent in that the use of IPost did not affect any of the outcome variables.
Postconditioning Procedure
Differences in IPost protocols could possibly influence the results of studies. Similar to other groups, who have reported positive as well as neutral effects of IPost,9,19–20,19–25 we used the same reperfusion‐reocclusion intervals as Staat and coworkers.6 However, we did not perform direct stenting of occluded vessels, which, in our experience, is often not suitable in these patients. Up‐front clopidogrel loading and administration of eptifibatide to all patients in our study could be thought to reduce the risk of microembolization during PCI. Direct stenting or stenting after predilation had no impact on the results in a previous study.24 The use of thrombectomy device or type of stent did not affect the primary endpoint in our trial.
Limitations
The results of our study apply to a highly selected group of STEMI patients, but this is inherent in the explanatory trial design.27 According to the inclusion criteria, the patients should represent a group expected to profit from IPost.2,26–27 Nevertheless, there were more patients with small infarcts than we anticipated. Also, there were few adverse clinical events during follow‐up, altogether reflecting a low‐risk STEMI population. Of ethical and practical reasons, we did not include patients with cardiac arrest or cardiogenic shock nor unstable patients with pulmonary congestion. We do not know whether IPost could have an effect in these patients. Drop‐out patients are not at random, but it seems that the causes of lost subjects for primary endpoint determination are similar in the 2 groups, making selection bias probability low.17
Conclusions
IPost had no effect on infarct size, assessed by CMR after 4 months, in STEMI patients treated by primary PCI. The results on secondary endpoints, including myocardial perfusion grade, 60‐minutes ST resolution in ECG, peak levels of troponin T in the acute stage, and LVEF and myocardial salvage index after 4 months, were consistently neutral. Cardiac adverse events were few and similarly distributed between the groups. At this stage, IPost adjunctive to primary PCI in the treatment of STEMI is not substantiated.
Sources of Funding
This work was supported by The Norwegian Health and Rehabilitation Foundation and Center for Heart Failure Research, University of Oslo, Oslo, Norway.
Disclosures
None.
Acknowledgments
We cordially thank the interventionists as well as the rest of the staff at the Section of Interventional Cardiology. We appreciate the help and support of study nurses Anne‐Kari Brun and Charlotte Holst Hansen, CMR technicians Vigdis Rosseland and Marianne Nesheim, and the staff at the Intensive Coronary Care Unit and Center for Clinical Heart Research, Oslo University Hospital. We express our gratitude to Dan Atar, MD, PhD, and Bjørn Bendz, MD, PhD, of the Data and Safety Monitoring Board.
References
- 1.Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van`t Hof A, Widimsky P, Zahger D. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation. Eur Heart J. 2012; 33:2569-2619 [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007; 357:1121-1135 [DOI] [PubMed] [Google Scholar]
- 3.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André‐Fouët X, Revel D, Kirkorian G, Monassier J‐P, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008; 359:473-481 [DOI] [PubMed] [Google Scholar]
- 4.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Køber L, Treiman M, Holst JJ, Engstrøm T. Exenatide reduces reperfusion injury in patients with ST‐segment elevation myocardial infarction. Eur Heart J. 2012; 33:1491-1499 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z‐Q, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten‐Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003; 285:H579-H588 [DOI] [PubMed] [Google Scholar]
- 6.Staat P, Rifoul G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, André‐Fouët X, Ovize M. Postconditioning the human heart. Circulation. 2005; 112:2143-2148 [DOI] [PubMed] [Google Scholar]
- 7.Ma XJ, Zhang XH, Li CM, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function in patients with acute myocardial infarction. Scand Cardiovasc J. 2006; 40:327-333 [DOI] [PubMed] [Google Scholar]
- 8.Yang XC, Liu Y, Wang LF, Cui L, Wang T, Ge YG, Wang HS, Li WM, Xu L, Ni ZH, Liu SH, Zhang L, Jia HM, Vinten‐Johansen J, Zhao ZQ. Reduction in myocardial infarct size by postconditioning in patients after percutaneous coronary intervention. J Invasive Cardiol. 2007; 19:424-430 [PubMed] [Google Scholar]
- 9.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, André‐Fouët X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long‐term benefit of postconditioning. Circulation. 2008; 117:1037-1044 [DOI] [PubMed] [Google Scholar]
- 10.Laskey WK, Yoon S, Calzada N, Ricciardi MJ. Concordant improvements in coronary flow reserve and ST‐segment resolution during percutaneous coronary intervention for acute myocardial infarction: a benefit of postconditioning. Catheter Cardiovasc Interv. 2008; 72:212-220 [DOI] [PubMed] [Google Scholar]
- 11.Limalanathan S, Andersen GØ, Hoffmann P, Kløw NE, Abdelnoor M, Eritsland J. Rationale and design of the POSTEMI (POstconditioning in ST‐Elevation Myocardial Infarction) Study. Cardiology. 2010; 116:103-109 [DOI] [PubMed] [Google Scholar]
- 12.Rentrop KP, Cohen M, Blanke H, Philips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985; 5:587-592 [DOI] [PubMed] [Google Scholar]
- 13.TIMI Study Group. Available at: http//www.timi.org/education/definitions Accessed March 7, 2014.
- 14.Andersen GØ, Knudsen EC, Aukrust P, Yndestad A, Oie E, Müller C, Seljeflot I, Ueland T. Elevated serum osteoprotegerin levels, measured early after acute ST‐elevation myocardial infarction predict final infarct size. Heart. 2011; 97:460-465 [DOI] [PubMed] [Google Scholar]
- 15.Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001; 323:1123-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002; 21:2917-2930 [DOI] [PubMed] [Google Scholar]
- 17.Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ. 2013; 346:e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lønborg J, Kelbæk H, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrøm T. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010; 3:34-41 [DOI] [PubMed] [Google Scholar]
- 19.Xue F, Yang X, Zhang B, Zhao C, Song J, Jiang T, Jiang W. Postconditioning in human heart in percutaneous coronary intervention. Clin Cardiol. 2010; 33:439-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sörensson P, Saleh N, Bouvier F, Böhm F, Settergren M, Caidahl K, Tornvall P, Arheden H, Rydén L, Pernow J. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010; 96:1710-1715 [DOI] [PubMed] [Google Scholar]
- 21.Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, Shroff GR, Moore L, Traverse JH. Long‐term follow‐up of patients undergoing postconditioning during ST‐elevation myocardial infarction. J Cardiovasc Trans Res. 2011; 4:92-98 [DOI] [PubMed] [Google Scholar]
- 22.Tarantini G, Favaretto E, Marra MP, Frigo AC, Napodano M, Cacciavillani L, Giovagnoni A, Renda P, De Biasio V, Plebani M, Mion M, Zaninotto M, Isabella G, Bilato C, Iliceto S. Postconditioning during coronary angioplasty in acute myocardial infarction: the POST‐AMI trial. Int J Cardiol. 2012; 162:33-38 [DOI] [PubMed] [Google Scholar]
- 23.Thuny F, Lairez O, Roubille F, Mewton N, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Thibault H, Cung TT, Finet G, Argaud L, Revel D, Derumeaux G, Bonnefoy E, Elbaz M, Piot C, Ovize M, Croisille P. Post‐conditioning reduces infarct size and edema in patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2012; 59:2175-2181 [DOI] [PubMed] [Google Scholar]
- 24.Freixa X, Bellera N, Ortiz‐Pérez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012; 33:103-112 [DOI] [PubMed] [Google Scholar]
- 25.Hahn J‐Y, Song YB, Kim EK, Yu CW, Bae J‐W, Chung W‐Y, Choi S‐H, Choi J‐H, Bae J‐H, An KJ, Park J‐S, Oh JH, Kim S‐W, Hwang J‐Y, Ryu JK, Park HS, Lim D‐S, Gwon H‐C. Ischemic postconditioning during primary percutaneous coronary intervention. The effects of postconditioning on myocardial reperfusion in patients with ST‐segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013; 128:1889-1896 [DOI] [PubMed] [Google Scholar]
- 26.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia‐Dorado D, Hausenloy DJ, Heusch G, Vinten‐Johansen J, Yellon DM, Schulz R. Postconditioning and protection from reperfusion injury: where do we stand? Cardiovasc Res. 2010; 87:406-423 [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Bøtker HE, Condorelli G, Ferdinandy P, Garcia‐Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz‐Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013; 98:7-27 [DOI] [PubMed] [Google Scholar]
- 28.Swarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008; 337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eitel I, Wöhrle J, Suenkel H, Meissner J, Kerber S, Lauer B, Pauschinger M, Birkemeyer R, Axthelm C, Zimmermann R, Neuhaus P, Brosteanu O, de Waha S, Desch S, Gutberlet M, Schuler G, Thiele H. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2013; 61:1447-1454 [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim T, Bülow HP, Hackl T, Hörnke M, Nekolla SG, Breuer M, Schömig A, Schwaiger M. Diagnostic value of contrast‐enhanced magnetic resonance imaging and single‐photon emission computed tomography for detection of myocardial necrosis early after acute myocardial infarction. J Am Coll Cardiol. 2007; 49:208-216 [DOI] [PubMed] [Google Scholar]
- 31.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013; 381:166-175 [DOI] [PubMed] [Google Scholar]


