Abstract
Background
Current guidelines recommend that patients with peripheral arterial disease (PAD) cease smoking and be treated with aspirin, statin medications, and angiotensin‐converting enzyme (ACE) inhibitors. The combined effects of multiple guideline‐recommended therapies in patients with symptomatic PAD have not been well characterized.
Methods and Results
We analyzed a comprehensive database of all patients with claudication or critical limb ischemia (CLI) who underwent diagnostic or interventional lower‐extremity angiography between June 1, 2006 and May 1, 2013 at a multidisciplinary vascular center. Baseline demographics, clinical data, and long‐term outcomes were obtained. Inverse probability of treatment propensity weighting was used to determine the 3‐year risk of major adverse cardiovascular or cerebrovascular events (MACE; myocardial infarction, stroke, or death) and major adverse limb events (MALE; major amputation, thrombolysis, or surgical bypass). Among 739 patients with PAD, 325 (44%) had claudication and 414 (56%) had CLI. Guideline‐recommended therapies at baseline included use of aspirin in 651 (88%), statin medications in 496 (67%), ACE inhibitors in 445 (60%), and smoking abstention in 528 (71%) patients. A total of 237 (32%) patients met all four guideline‐recommended therapies. After adjustment for baseline covariates, patients adhering to all four guideline‐recommended therapies had decreased MACE (hazard ratio [HR], 0.64; 95% CI, 0.45 to 0.89; P=0.009), MALE (HR, 0.55; 95% CI, 0.37 to 0.83; P=0.005), and mortality (HR, 0.56; 95% CI, 0.38 to 0.82; P=0.003), compared to patients receiving less than four of the recommended therapies.
Conclusions
In patients with claudication or CLI, combination treatment with four guideline‐recommended therapies is associated with significant reductions in MACE, MALE, and mortality.
Keywords: atherosclerosis, claudication, peripheral vascular disease, prevention, statins
Introduction
Peripheral arterial disease (PAD) affects between 8 and 12 million people in the United States and up to 20% of patients over the age of 75.1–4 Prevalence of PAD has increased by more than 20% in the last decade with an estimated global population burden of 202 million people.5 Patients with even asymptomatic PAD have significantly increased rates of cardiovascular ischemic events, including myocardial infarction (MI), stroke, and death.6–10 Those with critical limb ischemia (CLI), an advanced stage of PAD characterized by ischemic rest pain, ulcers, and gangrene, have both an increased risk of cardiovascular events and adverse limb events, including surgical bypass or major amputation.11–12
Current guidelines established by the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that patients with PAD cease smoking and receive antiplatelet therapy, statin medications, and angiotensin‐converting enzyme (ACE) inhibitors for secondary prevention and cardiovascular risk reduction.13–15 Despite the proven cardiovascular risks of PAD and established guidelines for treatment of this widespread disease, studies have consistently shown that millions of PAD patients are undertreated.4,16–18 For example, patients with PAD have the same or higher risk of long‐term mortality as patients with coronary artery disease (CAD), yet they are less likely to receive guideline‐recommended therapies.19–20 Undertreatment of individuals with PAD may contribute to high rates of preventable cardiovascular morbidity and mortality.
Previous studies have reported on the benefits of individual guideline‐recommended therapies on outcomes in PAD patients, but few studies have examined the effects attributable to adherence to all four of these major guideline‐recommended therapies.21–25 We hypothesized that adherence to guideline‐recommended therapies among patients with symptomatic PAD is associated with reduced subsequent major adverse cardiovascular and cerebrovascular events (MACE; myocardial infarction, stroke, or death) and major adverse limb events (MALE; major amputation, thrombolysis, or surgical bypass).
Methods
Study Design and Data Sources
We conducted a retrospective study utilizing data from the PAD University of California (UC), Davis Registry, which comprises all patients with a clinical diagnosis of PAD who underwent diagnostic angiography and/or therapeutic endovascular intervention at the UC Davis Medical Center between June 1, 2006 and May 1, 2013.26 At the time of data extraction, the registry included 1091 patients and 1719 procedures. Median length of patient follow‐up was 3.1 years. The study protocol was approved by the Institutional Review Board at the UC Davis Medical Center.
Study Population and Data Collection
All patients in the registry with PAD defined by claudication (325; 30% of the total registry) or CLI (414; 38% of the total registry) were analyzed. This patient population consisted of individuals living primarily in northern California or Nevada. All patients underwent diagnostic or interventional lower‐extremity angiography at the UC Davis Medical Center.
Data collection for the registry was based on detailed electronic medical record and angiographic review. Baseline demographic, clinical, laboratory, and procedural data were obtained through preprocedure clinical notes, admission history, and in‐patient documentation. Comorbidities that may affect physician prescribing, including patient history of MI, stroke, CAD, and major bleeding were also recorded. Medical prescribing patterns were verified by pharmacy prescriptions, both preprocedure and during follow‐up. All records were reviewed by trained chart abstractors and verified by a board‐certified cardiologist.
Data Definitions
The ACC/AHA guidelines have indicated aspirin, statins, and smoking abstention as class I recommendations; ACE inhibitors are a class IIa recommendation for treatment of patients with PAD.13,15,27 Each patient's utilization of these four guideline‐recommended therapies (aspirin, statin medications, ACE inhibitors, and smoking abstention) within 3 months preprocedure was assessed. Patients were categorized as adherent to all four guideline‐recommended therapies if their medication list and preprocedural clinic visit note included (1) aspirin, (2) statin medications, (3) ACE inhibitors or angiotensin receptor blockers, and (4) smoking abstinence. This definition of adherence therefore reflects a combination of both physician decision to prescribe the therapy (eg, prescription of an ACE inhibitor) and patient‐reported adherence to that therapy (eg, self‐report of ACE inhibitor use). Patients who were not adherent to all of these guideline‐recommended therapies were categorized into the less than four guideline‐recommended therapy group.
MACE was defined as MI, stroke, or death. MI was defined as symptoms of chest pressure and elevation of troponin with evidence of infarct by stress imaging or cardiac catheterization. Stroke was defined as focal neurologic deficit with computed tomographic or magnetic resonance imaging evidence of cerebral ischemic or hemorrhagic infarct. All mortalities were confirmed by direct chart documentation or the Social Security Death Index. MALE was defined as major lower‐extremity limb amputation above the level of the ankle joint, thrombolysis, or surgical bypass. Claudication was classified as Rutherford category 1 to 3 disease (mild, moderate, or severe claudication, respectively), and CLI was classified as Rutherford category 4 to 6 disease (ischemic rest pain, minor tissue loss, or major tissue loss, respectively).
Outcomes
The primary outcome of the study was the occurrence of MACE during a 3‐year follow‐up period. Prespecified secondary outcomes included the occurrence of MALE, the individual components of MACE and MALE, and the combined incidence of death or amputation during the 3‐year follow‐up period.
Statistical Analysis
Means with standard deviations were used to describe continuous variables and frequencies, and percentages were used for categorical variables. Continuous variables were compared using the Wilcoxon rank‐sum test and categorical values using chi‐squared or Fisher's exact tests.
Propensity scores were developed to adjust for covariates that may influence adherence to four guideline‐recommended therapies.28–30 Baseline covariates in the propensity model included age, gender, and patient‐reported race/ethnicity, as defined by the investigators (Caucasian, Hispanic, African American, and Asian); body mass index, history of diabetes, congestive heart failure, CAD, MI, hypertension, stroke, carotid artery disease, chronic obstructive pulmonary disease, malignancy, abdominal aortic aneurysm, gastrointestinal bleed, and previous above‐ankle amputation; low‐density lipoprotein concentration, hemoglobin A1C level, glomerular filtration rate, left ventricular ejection fraction (in 5% increments from ≤10% to ≥65%), Rutherford score (1 to 6), and ankle brachial index; prescription of concomitant medications, including beta blockers and clopidogrel; and year of procedure. Diagnostic tests to demonstrate balance of covariates after inverse probability of treatment weighting (IPTW) included calculation of the standardized difference before and after weighting and visual inspection of a kernel density plot to verify propensity score overlap between groups. Visual inspection of propensity scores by treatment group preceding weighting also suggested adequate overlap in the two cohorts (Figure 1). Proportional hazards marginal structural models were then developed using weighted regression with IPTW to estimate the causal effect of treatment with four guideline‐recommended therapies. Unadjusted survival curves were estimated using the Kaplan‐Meier method, and adjusted survival curves were estimated using IPTW weighting.31
Figure 1.
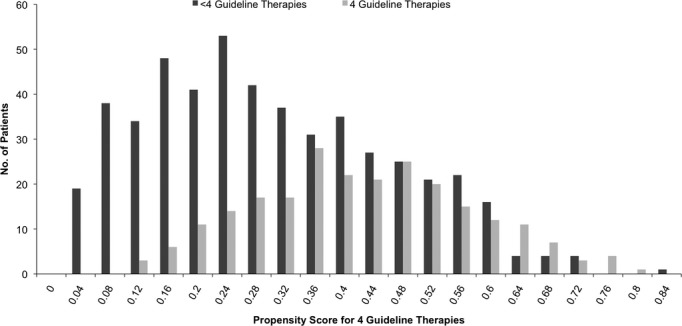
Propensity scores for guideline‐recommended therapies. The propensity score for four guideline‐recommended therapies is the probability given baseline covariates that any patient in either group would be adherent to all four guideline‐recommended therapies.
Several sensitivity analyses were also performed to assess the relationship between guideline‐recommended therapies and cardiovascular outcomes. Hazard ratios (HRs) were recalculated using Cox's proportional hazard modeling without the use of propensity scores, adjusting for the same covariates included as those in the propensity model.32 Propensity modeling was also performed using nearest‐neighbor matching and after trimming the propensity scores to minimize possible effects from outliers. All analyses were performed using STATA software (Version 11.2; STATA Corporation, College Station, TX). HRs are given with 95% confidence intervals (CIs). For all tests, a P value <0.05 was considered significant.
Results
Among the cohort of 739 patients with PAD, 325 (44%) presented with claudication and 414 (56%) with CLI. Baseline characteristics of patients at the time of lower‐extremity angiography are summarized in Table 1. Full adherence to four guideline‐recommended therapies did not differ significantly by gender or race. There were also no significant differences in the Rutherford scores (1 to 6) or baseline ankle brachial indices between the four and less than four guideline‐recommended therapy groups. There was a similar prevalence of treatment with four guideline‐recommended therapies each year throughout the study period (Figure 2).
Table 1.
Baseline Characteristics of Patients With Symptomatic PAD
| Variable | Four Guideline Therapies (N=237) | Less Than Four Guideline Therapies (N=502) | P Value |
|---|---|---|---|
| Age, y | 68.9±10.7 | 66.5±13.2 | 0.02 |
| Male, % | 144 (61) | 279 (55) | 0.2 |
| Race/ethnicity, % | |||
| Caucasian | 178 (75) | 419 (83) | 0.03 |
| Hispanic | 24 (10) | 40 (8) | |
| African American | 24 (10) | 33 (7) | |
| Asian | 11 (5) | 10 (2) | |
| BMI, kg/m2 | 27.8±5.7 | 27.2±6.1 | 0.1 |
| Tobacco, former or current (%) | 166 (72) | 389 (79) | 0.04 |
| CHF, % | 70 (30) | 98 (20) | 0.002 |
| DM, % | 138 (58) | 226 (46) | 0.001 |
| GFR, mL/min | 66.6±33.2 | 71.0±41.8 | 0.4 |
| HTN, % | 224 (95) | 399 (80) | <0.001 |
| CAD, % | 156 (66) | 218 (44) | <0.001 |
| History of MI, % | 58 (24) | 81 (16) | 0.007 |
| Ejection fraction | 53.9±16.4 | 54.8±16.9 | 0.4 |
| History of stroke/TIA, % | 42 (18) | 85 (17) | 0.8 |
| History of malignancy, % | 20 (9) | 68 (14) | 0.05 |
| COPD, % | 30 (13) | 93 (19) | 0.05 |
| History of AAA, % | 13 (6) | 27 (5) | 0.96 |
| History of carotid stenosis, % | 44 (20) | 64 (14) | 0.04 |
| History of GI bleed, % | 10 (4) | 36 (7) | 0.1 |
| History of contralateral amputation, % | 18 (8) | 39 (8) | 0.3 |
| LDL, mg/dL | 78.4±28.7 | 92.0±40.0 | 0.004 |
| HBA1c, % | 7.4±2.2 | 7.9±2.1 | 0.04 |
| Beta blocker, % | 151 (64) | 241 (48) | <0.001 |
| Clopidogrel, % | 167 (70) | 291 (58) | 0.001 |
| Rutherford score | |||
| 1 | 11 (5) | 15 (3) | 0.3 |
| 2 | 49 (21) | 94 (19) | |
| 3 | 49 (21) | 83 (17) | |
| 4 | 17 (7) | 58 (12) | |
| 5 | 91 (39) | 197 (40) | |
| 6 | 16 (7) | 43 (9) | |
| ABI* | 0.54±0.23 | 0.53±0.22 | 0.8 |
AAA indicates abdominal aortic aneurysm; ABI, ankle brachial index; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GFR, glomerular filtration rate; GI, gastrointestinal; HBA1c, hemoglobin A1c; HTN, hypertension; LDL, low‐density lipoprotein; MI, myocardial infarction; PAD, peripheral arterial disease; TIA, transient ischemic attack.
Excluding subjects with ABI >1.2, for whom toe brachial index was also measured.
Figure 2.
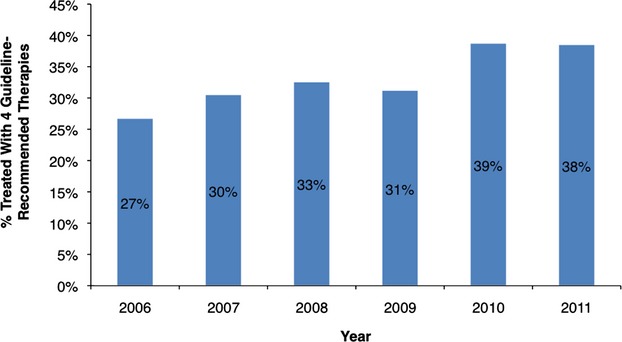
Treatment with four guideline‐recommended therapies by year.
Patients who were being treated with all four guideline‐recommended therapies at the time of angiography had significantly more baseline comorbidities, including congestive heart failure (CHF), diabetes mellitus (DM), hypertension (HTN), previous MI, and history of carotid stenosis than patients who were being treated with less than four guideline‐recommended therapies (all P<0.05). Individuals adhering to all four guideline‐recommended therapies were also, on average, older than individuals adhering to less than four guideline‐recommended therapies (mean ages, 68.9±10.7 versus 66.5±13.2 years).
Medical Therapies at Time of Angiography
A total of 237 (32%) patients were adherent to all four guideline‐recommended therapies. Baseline adherence to guideline‐recommended therapies included aspirin use in 651 (88%), statin use in 496 (67%), ACE inhibitor use in 445 (60%), and smoking abstention in 528 (71%) patients. Patients with CLI were less likely to be prescribed statin medications (62% versus 73%; P=0.002) or ACE inhibitors (57% versus 65%; P=0.02), but had similar rates of aspirin prescription (89% versus 87%; P=0.3) and smoking abstention (73% versus 69%; P=0.2), when compared to patients with claudication.
There was notable variation in the specific guideline therapies adhered to by patients receiving one, two, or three guideline‐recommended therapies (Figure 3). For patients adhering to only one guideline‐recommended therapy, smoking abstention (13%) and aspirin (57%) were the two most prevalent therapies. Among patients adhering to two guideline‐recommended therapies, smoking abstention (52%) and aspirin use (82%) also represented the two most utilized therapies. Among the group receiving three guideline‐recommended therapies, smoking absention (75%), statin medications (75%), and aspirin (90%) were the most utilized therapies. There was no significant difference in prescribing patterns of these individual medications in relation to the year that the procedure was performed.
Figure 3.
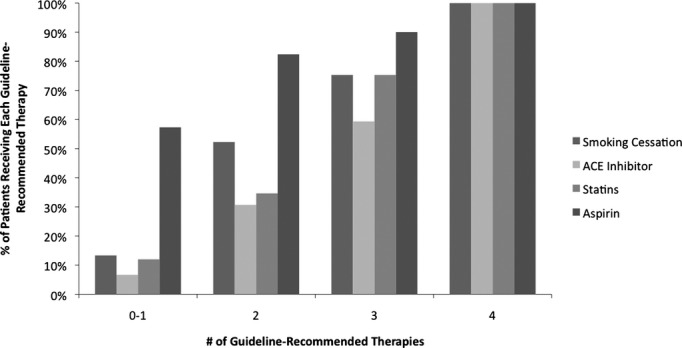
Adherence to guideline‐recommended therapies. ACE indicates angiotensin‐converting enzyme.
Association of Guideline‐Recommended Therapies With Long‐Term Outcomes
The event rates and unadjusted and adjusted HRs for all major outcomes are summarized in Table 2. Despite greater baseline comorbidities, patients receiving all four guideline‐recommended therapies had lower unadjusted rates of all major adverse outcomes, including MACE, MALE, amputation, and death. There was also a dose‐response relationship between the number of guideline‐recommended therapies and outcomes, with 3‐year unadjusted MACE rates of 33%, 30%, 28%, and 26% for patients taking one, two, three, and four guideline‐recommended therapies, respectively.
Table 2.
Three‐Year Outcome Rates and Unadjusted and Adjusted Hazard Ratios
| Variables | Outcome Rates | Unadjusted | IPTW Adjusted | |||
|---|---|---|---|---|---|---|
| Four Guideline Therapies (N=237) | Less Than Four Guideline Therapies (N=502) | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| MACE | 56 (26%) | 137 (30%) | 0.83 (0.60 to 1.13) | 0.2 | 0.64 (0.45 to 0.89) | 0.009 |
| Death | 41 (19%) | 113 (25%) | 0.73 (0.51 to 1.05) | 0.09 | 0.56 (0.38 to 0.82) | 0.003 |
| MI | 13 (9%) | 28 (9%) | 0.95 (0.49 to 1.83) | 0.9 | 0.74 (0.36 to 1.48) | 0.4 |
| Stroke | 5 (4%) | 11 (4%) | 0.95 (0.33 to 2.73) | 0.9 | 0.89 (0.28 to 2.83) | 0.8 |
| MALE | 37 (19%) | 112 (28%) | 0.67 (0.46 to 0.98) | 0.04 | 0.55 (0.37 to 0.83) | 0.005 |
| Lower‐extremity bypass | 20 (10%) | 65 (16%) | 0.63 (0.38 to 1.04) | 0.07 | 0.55 (0.32 to 0.95) | 0.03 |
| Amputation | 19 (10%) | 49 (13%) | 0.82 (0.48 to 1.39) | 0.5 | 0.67 (0.38 to 1.18) | 0.2 |
| Death/amputation | 53 (24%) | 145 (31%) | 0.75 (0.55 to 1.02) | 0.07 | 0.60 (0.42 to 0.84) | 0.003 |
| MACE/MALE | 77 (34%) | 209 (44%) | 0.73 (0.56 to 0.95) | 0.02 | 0.60 (0.45 to 0.80) | 0.001 |
Unadjusted outcome rates are based on Kaplan‐Meier estimates. CI indicates confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; MACE, major adverse cardiovascular and cerebrovascular events (MI, stroke, or death); MALE, major adverse limb events (lower‐extremity bypass or amputation); MI, myocardial infarction.
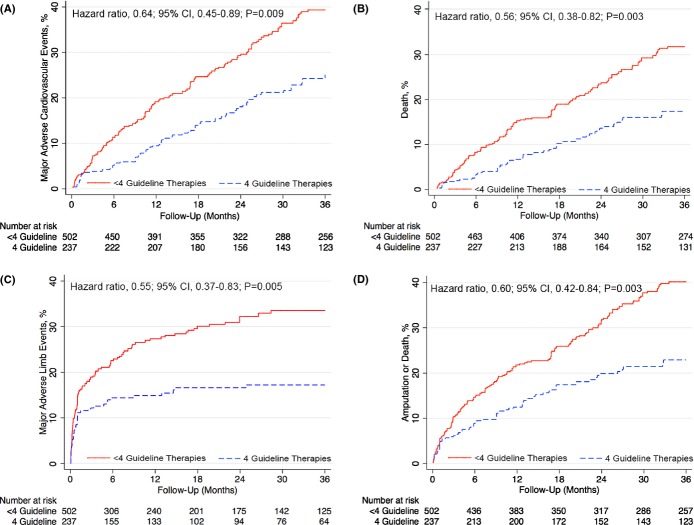
After adjustment using propensity scores, the primary outcome of MACE (HR, 0.64; 95% CI, 0.45 to 0.89; P=0.009) and the secondary outcome of MALE (HR, 0.55; 95% CI, 0.37 to 0.83; P=0.005) were significantly lower during 3‐year follow‐up among patients receiving all four guideline‐recommended therapies (Figure 4). Patients who were adherent to all four guideline therapies also had a significantly decreased risk of death (HR, 0.56; 95% CI, 0.38 to 0.82; P=0.003), compared to patients receiving less than four guideline therapies. Rates of MI (HR, 0.74; P=0.4), stroke (HR, 0.89; P=0.8), and amputation (HR, 0.67; P=0.2) all trended in a direction that favored adherence to four guideline‐recommended therapies, but were not significantly different between the two patient groups. The combined risk of death or major amputation (HR, 0.60; 95% CI, 0.42 to 0.84; P=0.003) and MACE or MALE (HR, 0.60; 95% CI, 0.45 to 0.80; P=0.001) was also significantly decreased in patients receiving all four guideline‐recommended therapies (Table 2).
Figure 4.
Major adverse cardiovascular events and limb outcomes among patients adhering to 4 guideline‐recommended therapies. Cumulative hazard curves to 3 years postprocedure showing the proportion free of (A) MACE (MI, stroke, or death; P=0.009), (B) death (P=0.003), (C) MALE (bypass graft surgery, thrombolysis, or major amputation; P=0.005), and (D) amputation or death (P=0.003). All curves are after propensity weighting. CI indicates confidence interval; MACE, major adverse cardiovascular or cerebrovascular events; MALE, major adverse limb events; MI, myocardial infarction.
Several sensitivity analyses revealed similar point estimates for the primary and secondary endpoints; standardized difference calculation also confirmed covariate balance after propensity weighting (Tables 3 through 6). Similar point estimates of the primary endpoint were also obtained when the results were stratified by clinical presentation. For patients with claudication, the unadjusted HR for MACE was 0.85 (95% CI, 0.40 to 1.80), and the adjusted HR was 0.60 (95% CI, 0.27 to 1.35). For patients with CLI, the unadjusted HR for MACE was 0.91 (95% CI, 0.65 to 1.29), and the adjusted HR was 0.77 (95% CI, 0.53 to 1.11).
Table 5.
Propensity Score Matching for Guideline‐Recommended Therapies
| Outcome | Unadjusted HR | Adjusted HR |
|---|---|---|
| MACE | 0.83 (0.60 to 1.13) | 0.61 (0.45 to 0.83) |
| MALE | 0.67 (0.46 to 0.98) | 0.68 (0.46 to 1.00) |
| Death | 0.73 (0.51 to 1.05) | 0.56 (0.39 to 0.80) |
| Amputation/death | 0.75 (0.55 to 1.02) | 0.62 (0.45 to 0.85) |
Nearest‐neighbor matching was performed using a caliper width equal to 0.25× standard deviation of the propensity score. Unadjusted hazard ratios represent rates of MACE, MALE, death, and amputation/death before adjustment of baseline covariates. Adjusted hazard ratios were calculated using the same covariates included in the main propensity model. HR indicates hazard ratio; MACE, major adverse cardiovascular or cerebrovascular events; MALE, major adverse limb events.
Table 6.
Inverse Probability of Treatment Weighting After Trimming Propensity Scores <0.1
| Outcome | Unadjusted HR | Adjusted HR |
|---|---|---|
| MACE | 0.83 (0.60 to 1.13) | 0.62 (0.44 to 0.87) |
| MALE | 0.67 (0.46 to 0.98) | 0.56 (0.37 to 0.86) |
| Death | 0.73 (0.51 to 1.05) | 0.55 (0.37 to 0.81) |
| Amputation/death | 0.75 (0.55 to 1.02) | 0.60 (0.42 to 0.84) |
After trimming propensity weights with values <0.1, there were a total of 234 patients with four guideline‐recommended therapies and 435 with less than four guideline‐recommended therapies. Unadjusted hazard ratios represent rates of MACE, MALE, death, and amputation/death before adjustment of baseline covariates. Adjusted hazard ratios were calculated using the same covariates included in the main propensity model. HR indicates hazard ratio; MACE, major adverse cardiovascular or cerebrovascular events; MALE, major adverse limb events.
Table 3.
Standardized Differences in Observed Characteristics for Guideline‐Recommended Therapies Before and After Inverse Probability of Treatment Weighting Adjustment
| Variable | Standardized Difference Before Adjustment (%) | Standardized Difference After Adjustment (%) |
|---|---|---|
| Hypertension | 40.4 | 9.8 |
| Coronary artery disease | 37.8 | 6.7 |
| Beta blocker | 26.3 | 5.1 |
| Heart failure | 22.4 | 6.3 |
| Clopidogrel | 21.8 | 2.6 |
| Diabetes | 21.1 | 5.2 |
| Age | 20.7 | 5.8 |
| Caucasian | 16.7 | 2.6 |
| Myocardial infarction | 16.1 | 3.2 |
| Previous cancer | 13.8 | 3.7 |
| COPD | 13.4 | 8.9 |
| Carotid artery disease | 12.9 | 4.9 |
| GFR | 12.2 | 4.9 |
| Ejection fraction | 12.2 | 4.8 |
| BMI | 10.2 | 7.1 |
| Gender | 8.6 | 1.8 |
| Stroke/TIA | 2.1 | 3.0 |
| AAA | 1.1 | 1.0 |
| Previous amputation | 1.0 | 1.0 |
AAA indicates abdominal aortic aneurysm; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; TIA, transient ischemic attack.
Table 4.
Cox's Proportional Hazards Model for Guideline‐Recommended Therapies
| Outcome | Unadjusted HR | Adjusted HR |
|---|---|---|
| MACE | 0.83 (0.60 to 1.13) | 0.64 (0.46 to 0.89) |
| MALE | 0.67 (0.46 to 0.98) | 0.61 (0.41 to 0.91) |
| Death | 0.73 (0.51 to 1.05) | 0.58 (0.40 to 0.86) |
| Amputation/death | 0.75 (0.55 to 1.02) | 0.62 (0.44 to 0.86) |
Unadjusted hazard ratios represent rates of MACE, MALE, death, and amputation/death before adjustment of baseline covariates. Adjusted hazard ratios were calculated using the same covariates included in the main propensity model. HR indicates hazard ratio; MACE, major adverse cardiovascular or cerebrovascular events; MALE, major adverse limb events.
Discussion
The major finding of this study is that patients with PAD who were receiving all four guideline‐recommended therapies at the time of lower‐extremity angiography had significantly reduced risk of subsequent major adverse cardiovascular, cerebrovascular, and limb events up to 3 years postprocedure. In this cohort, adherence to all four guideline‐recommended therapies was associated with a 36% reduction in MACE, a 45% reduction in MALE, and a 44% reduction in death within 3 years of original angiography. We also found that patients who were treated with all four guideline‐recommended therapies had significantly more baseline comorbidies (CHF, DM, HTN, previous MI, or history of carotid stenosis) than patients who were treated with less than four guideline‐recommended therapies, yet these patients had lower 3‐year MACE and MALE rates than the presumably healthier patients who were not fully adherent to the guidelines. These findings suggest that optimal adherence by physicians and patients to guideline‐recommended therapies could significantly improve long‐term health outcomes for patients with symptomatic PAD.
Underuse of Guideline‐Recommended Therapies
PAD has been well established as a prevalent disease associated with significant impairment in quality of life and increased mortality.6–12 A 10‐year prospective study by Criqui et al.8 showed that patients with symptomatic PAD have a 10 to 15 times greater risk for cardiovascular‐related death, compared to subjects without PAD. Despite this evidence, studies have reported that patients with PAD continue to receive suboptimal treatment, compared to patients with CAD.4,17–18,20,33–34 Multiple factors likely contribute to this disparity, including lack of physician awareness, presence of atypical symptoms, and focus on limb‐related, rather than cardiovascular, outcomes. The results of this study suggest the importance of adhering to multiple guideline‐therapies when treating patients with PAD severe enough to necessitate lower‐extremity angiography. Even among this cohort of patients with multiple comorbidities and symptomatic PAD, full adherence to these secondary prevention therapies was associated with a reduction in risk for death or major amputation by 40% and MACE or MALE by 40%.
Suboptimal adherence to guideline‐recommended therapies has also been explored with national physician surveys, which have revealed deficiencies in physician awareness of, and attitudes toward, the importance of atherosclerotic risk factor reduction in patients with PAD.33,35–36 In the PAD Awareness, Risk, and Treatment: New Resources for Survival program, PAD was detected in 1865 individuals during screening, but only 49% of their physicians were aware of the diagnosis.4 Physicians also often do not recognize the adverse cardiovascular risks of PAD and therefore prescribe risk‐reduction therapies less intensively than they do for CAD patients.14,35,37 Furthermore, through a national public PAD awareness survey, Hirsch et al.38 showed that individuals with PAD were not aware of the associated risks of heart attack, stroke, amputation, or death. Though a national PAD public awareness campaign, Stay in Circulation, has been implemented by the National Heart, Lung and Blood Institute to reduce knowledge gaps among both patients and physicians,38 there continues to be a shortage of research that addresses the lack of clearly defined, guideline‐based treatment plans for the optimal management of PAD.
Secondary Prevention in PAD
To assess the effect of full adherence to major recommended secondary prevention guidelines, we chose to analyze whether patients were fully or less than fully adherent to these recommendations. Our results extend the findings of multiple earlier observational studies and randomized, clinical trials that have shown the beneficial effects of adherence to single guideline‐recommended therapies. Aspirin and statin medications, both class I recommended guideline therapies, have each been associated with significantly reduced risks for MACE.13,21–23,21–43 Smoking has been identified as a major risk factor for developing PAD44–45 as well as a significant risk factor for subsequent MI and cardiac mortality after PAD diagnosis,25,46 thereby making smoking abstention a class I recommendation.13,43 ACE inhibitors, as a class IIa recommendation, have also been proven to reduce rates of MI, stroke, and death among patients with PAD.13,21,24,43 Additionally, ramipril was recently reported to increase walking times in patients with intermittent claudication, indicating a potential additional benefit of ACE inhibitors.47 Our study built upon the well‐established efficacies of individual guideline‐recommended therapies for primarily asymptomatic patients and demonstrated that the combination of these four therapies is associated with a substantial outcome advantage for patients with symptomatic PAD.
Our results are also in accord with, and extend the findings of, previous studies that have analyzed the additive effects of multiple guideline‐recommended therapies. Utilizing data from the National Health and Nutrition Examination Survey, Pande et al.17 reported that treatment with more than two preventive therapies (aspirin, statin, and/or ACE inhibitors/angiotensin receptor blockers) was associated with a 65% reduced risk of all‐cause mortality in individuals with an ankle brachial index ≤0.90. Another study by Hoeks et al.33 found that patients taking aspirin, statin medications, and beta blockers at baseline had significantly decreased 3‐year mortality rates, compared to patients who did not adhere to these three medical therapies. A more recent study by Ardati et al.16 concluded that patients taking aspirin and statin medications on admission had significantly decreased rates of repeat peripheral intervention, amputation, or limb salvage surgery within 6 months of peripheral vascular intervention. To our knowledge, our study is the first to examine the long‐term, additive effects of both medical‐ and lifestyle‐associated, guideline‐recommended therapies on outcomes of MACE, MALE, and mortality in symptomatic PAD.
Considering the established clinical benefits of guideline‐recommended therapies and the consistent underuse of these therapies in patients with PAD, widespread implementation of performance measures has the potential to significantly improve health outcomes and quality of life. Recently published performance measures for patients with PAD recommend systematic assessment of risk‐reduction interventions, including the use of antiplatelet therapy, statin therapy, and smoking‐cessation programs.14 Increased adoption of such performance measures at important points of patient care (eg, at time of angiography or discharge from the hospital) has the potential to improve overall secondary prevention measures among patients with symptomatic PAD. The data from these performance measures may then be utilized as a framework to build quality improvement initiatives that aim to bridge the current gap between guideline‐recommended therapies and clinical practice.
Limitations
This study should be interpreted in the context of its design. First, inherent to all observational studies without randomization is the limitation that reported associations may not represent causality. However, propensity‐score–based methods account for both confounding and bias and are established methods for estimating causal effects using observational data. A number of sensitivity analyses, including Cox's proportional hazard modeling and nearest‐neighbor matching, also provided similar results and point estimates of effect size. Given the severe comorbidities associated with CLI, a randomized trial of guideline‐recommended therapies in this population of patients is also very unlikely. Second, though we adjusted for comprehensive baseline covariates through propensity score weighting, the possibility for unmeasured confounding factors remains. The similar point estimates obtained through both Cox's regression and nearest‐neighbor matching strengthens the likelihood of our results. Third, this study was conducted at a multidisciplinary vascular center where patients had already been referred for symptomatic PAD. Future studies will be necessary to assess reasons for nonadherence at both the provider and patient level in the overall PAD patient population. Fourth, though physician and patient‐reported adherence to guideline‐recommended therapies was carefully assessed by prescription records and clinical chart notes, adherence can never be fully assessed. Fifth, it is also important to note that the lack of statistically significant relationships between adherence to guideline‐recommended therapies and MI, stroke, and amputation may be the result of the low overall rates for these events and the relatively small size of the cohort. Therefore, this study may have been underpowered to detect clinically meaningful differences for these secondary outcomes.
Conclusions
This study demonstrated that in PAD patients with claudication or CLI, adherence to four guideline therapies (aspirin, statin medications, ACE inhibitors, and smoking abstention) was associated with significantly reduced 3‐year rates of MACE, MALE, and all‐cause mortality. These findings highlight the importance of risk‐factor management in patients with symptomatic PAD and indicate a potential opportunity to reduce atherothrombotic events and decrease mortality in this patient population. Further studies are needed to determine the optimal doses for combinatory use of guideline medical therapies and investigate barriers to implementation of combination guideline‐recommended therapies.
Sources of Funding
This study did not receive any specific financial support. Dr. Armstrong is supported by the American Heart Association (11CRP7260031). Chen was supported by the UC Davis Medical Student Research Fellowship.
Disclosures
Dr Laird reports being a consultant for Boston Scientific, Covidien, Abbott, Bard, and Medtronic. Dr Yeo reports being a speaker for Abbott Vascular and receiving funding from Medtronic. None: Armstrong, Chen, Westin, Singh, McCoach, Bang, Anderson, and Amsterdam.
References
- 1.Criqui MH, Fronek A, Barrett‐Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985; 71:510-515 [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991; 20:384-392 [DOI] [PubMed] [Google Scholar]
- 3.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998; 18:185-192 [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001; 286:1317-1324 [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013http://linkinghub.elsevier.com/retrieve/pii/S0140673613612490 [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH. Peripheral arterial disease—epidemiological aspects. Vasc Med. 2001; 6:3-7 [DOI] [PubMed] [Google Scholar]
- 7.Saw J, Bhatt DL, Moliterno DJ, Brener SJ, Steinhubl SR, Lincoff AM, Tcheng JE, Harrington RA, Simoons M, Hu T, Sheikh MA, Kereiakes DJ, Topol EJ. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006; 48:1567-1572 [DOI] [PubMed] [Google Scholar]
- 8.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992; 326:381-386 [DOI] [PubMed] [Google Scholar]
- 9.Fowkes FGR, Price JF, Stewart MCW, Butcher I, Leng GC, Pell ACH, Sandercock PAG, Fox KAA, Lowe GDO, Murray GDAspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010; 303:841-848 [DOI] [PubMed] [Google Scholar]
- 10.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008; 52:1736-1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGRTASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007; 45suppl S:S5-S67 [DOI] [PubMed] [Google Scholar]
- 12.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010; 51:230-241 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel BAmerican Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter‐Society Consensus, Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463-e654 [DOI] [PubMed] [Google Scholar]
- 14.Olin JW, Allie DE, Belkin M, Bonow RO, Casey DE, Jr, Creager MA, Gerber TC, Hirsch AT, Jaff MR, Kaufman JA, Lewis CA, Martin ET, Martin LG, Sheehan P, Stewart KJ, Treat‐Jacobson D, White CJ, Zheng Z‐J, Masoudi FA. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). Circulation. 2010; 122:2583-2618 [DOI] [PubMed] [Google Scholar]
- 15.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011; 58:2432-2446 [DOI] [PubMed] [Google Scholar]
- 16.Ardati AK, Kaufman SR, Aronow HD, Nypaver TJ, Bove PG, Gurm HS, Grossman PM. The quality and impact of risk factor control in patients with stable claudication presenting for peripheral vascular interventions. Circ Cardiovasc Interv. 2012; 5:850-855 [DOI] [PubMed] [Google Scholar]
- 17.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011; 124:17-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, Berger J, Torp‐Pedersen C, Fosbol EL. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower‐extremity peripheral artery disease, underuse remains. Circulation. 2012; 126:1345-1354 [DOI] [PubMed] [Google Scholar]
- 19.Welten GMJM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, Bax JJ, van Sambeek MRHM, Poldermans D. Long‐term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008; 51:1588-1596 [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997; 12:209-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feringa HHH, van Waning VH, Bax JJ, Elhendy A, Boersma E, Schouten O, Galal W, Vidakovic RV, Tangelder MJ, Poldermans D. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. J Am Coll Cardiol. 2006; 47:1182-1187 [DOI] [PubMed] [Google Scholar]
- 22.Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002; 324:71-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002; 360:7-2212114036 [Google Scholar]
- 24.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000; 342:145-153 [DOI] [PubMed] [Google Scholar]
- 25.Jonason T, Bergström R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987; 221:253-260 [PubMed] [Google Scholar]
- 26.McCoach CE, Armstrong EJ, Singh S, Javed U, Anderson D, Yeo KK, Westin GG, Hedayati N, Amsterdam EA, Laird JR. Gender‐related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med. 2013; 18:19-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KAAHA, ACC, National Heart, Lung, and Blood Institute. AHA, ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006; 113:2363-2372 [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70:41-55 [Google Scholar]
- 29.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability‐weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007; 45:S103-S107 [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000; 11:550-560 [DOI] [PubMed] [Google Scholar]
- 31.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004; 75:45-49 [DOI] [PubMed] [Google Scholar]
- 32.Therneau TM. Modeling Survival Data: Extending the Cox Model. 2000New York, NY: Springer [Google Scholar]
- 33.Hoeks SE, Scholte op Reimer WJM, van Gestel YRBM, Schouten O, Lenzen MJ, Flu W‐J, van Kuijk J‐P, Latour C, Bax JJ, van Urk H, Poldermans D. Medication underuse during long‐term follow‐up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009; 2:338-343 [DOI] [PubMed] [Google Scholar]
- 34.Aspry KE, Holcroft JW, Amsterdam EA. Physician recognition of hypercholesterolemia in patients undergoing peripheral and carotid artery revascularization. Am J Prev Med. 1995; 11:336-341 [PubMed] [Google Scholar]
- 35.McDermott MM, Hahn EA, Greenland P, Cella D, Ockene JK, Brogan D, Pearce WH, Hirsch AT, Hanley K, Odom L, Khan S, Criqui MH, Lipsky MS, Hudgens S. Atherosclerotic risk factor reduction in peripheral arterial diseasea: results of a national physician survey. J Gen Intern Med. 2002; 17:895-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al‐Omran M. Knowledge and attitude of physicians in a major teaching hospital towards atherosclerotic risk reduction therapy in patients with peripheral arterial disease. Vasc Health Risk Manag. 2007; 3:1019-1027 [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MM, Mandapat AL, Moates A, Albay M, Chiou E, Celic L, Greenland P. Knowledge and attitudes regarding cardiovascular disease risk and prevention in patients with coronary or peripheral arterial disease. Arch Intern Med. 2003; 163:2157-2162 [DOI] [PubMed] [Google Scholar]
- 38.Hirsch AT, Murphy TP, Lovell MB, Twillman G, Treat‐Jacobson D, Harwood EM, Mohler ER, III, Creager MA, Hobson RW, II, Robertson RM, Howard WJ, Schroeder P, Criqui MHPeripheral Arterial Disease Coalition. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007; 116:2086-2094 [DOI] [PubMed] [Google Scholar]
- 39.Heart Protection Study Collaborative Group. Bulbulia R, Bowman L, Wallendszus K, Parish S, Armitage J, Peto R, Collins R. Effects on 11‐year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high‐risk individuals: a randomised controlled trial. Lancet. 2011; 378:2013-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feringa HHH, Karagiannis SE, van Waning VH, Boersma E, Schouten O, Bax JJ, Poldermans D. The effect of intensified lipid‐lowering therapy on long‐term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007; 45:936-943 [DOI] [PubMed] [Google Scholar]
- 41.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, Upchurch GR, Jr, Stanley JC, Eagle KA. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg. 2004; 39:357-365 [DOI] [PubMed] [Google Scholar]
- 42.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008; 47:774-781 [DOI] [PubMed] [Google Scholar]
- 43.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W‐K. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:1425-1443 [DOI] [PubMed] [Google Scholar]
- 44.Agarwal S. The association of active and passive smoking with peripheral arterial disease: results from NHANES 1999–2004. Angiology. 2009; 60:335-345 [DOI] [PubMed] [Google Scholar]
- 45.Conen D, Everett BM, Kurth T, Creager MA, Buring JE, Ridker PM, Pradhan AD. Smoking, smoking cessation, [corrected] and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med. 2011; 154:719-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faulkner KW, House AK, Castleden WM. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. Med J Aust. 1983; 1:217-219 [DOI] [PubMed] [Google Scholar]
- 47.Ahimastos AA, Walker PJ, Askew C, Leicht A, Pappas E, Blombery P, Reid CM, Golledge J, Kingwell BA. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA. 2013; 309:453-460 [DOI] [PubMed] [Google Scholar]