Abstract
Background
Obesity is associated with altered atrial electrophysiology and a prominent risk factor for atrial fibrillation. Body mass index, the most widely used adiposity measure, has been related to atrial electrical remodeling. We tested the hypothesis that pericardial fat is independently associated with electrocardiographic measures of atrial conduction.
Methods and Results
We performed a cross‐sectional analysis of 1946 Framingham Heart Study participants (45% women) to determine the relation between pericardial fat and atrial conduction as measured by P wave indices (PWI): PR interval, P wave duration (P‐duration), P wave amplitude (P‐amplitude), P wave area (P‐area), and P wave terminal force (P‐terminal). We performed sex‐stratified linear regression analyses adjusted for relevant clinical variables and ectopic fat depots. Each 1‐SD increase in pericardial fat was significantly associated with PR interval (β=1.7 ms, P=0.049), P‐duration (β=2.3 ms, P<0.001), and P‐terminal (β=297 μV·ms, P<0.001) among women; and P‐duration (β=1.2 ms, P=0.002), P‐amplitude (β=−2.5 μV, P<0. 001), and P‐terminal (β=160 μV·ms, P=0.002) among men. Among both sexes, pericardial fat was significantly associated with P‐duration in analyses additionally adjusting for visceral fat or intrathoracic fat; a similar but non‐significant trend existed with P‐terminal. Among women, pericardial fat was significantly associated with P wave area after adjustment for visceral and intrathoracic fat.
Conclusions
Pericardial fat is associated with atrial conduction as quantified by PWI, even with adjustment for extracardiac fat depots. Further studies are warranted to identify the mechanisms through which pericardial fat may modify atrial electrophysiology and promote subsequent risk for arrhythmogenesis.
Keywords: atrium, conduction, electrocardiography, epidemiology, obesity
Introduction
Obesity is a highly prevalent, well‐established risk factor for atrial fibrillation (AF). Body mass index (BMI) has been associated with AF risk in diverse investigations.1–5 Obesity and AF are increasing in prevalence,6–8 and share co‐morbidities (hypertension, coronary artery disease, congestive heart failure, obstructive sleep apnea). Determining the mechanisms by which obesity increases AF risk is an area of active investigation.
Altered atrial electrical function is considered an important mechanism for the relation of obesity and increased AF risk.9 P wave indices (PWI) constitute ECG endophenotypes that allow for assessment of atrial conduction and may identify subclinical atrial pathology. Altered PWI have been associated with generalized adiposity (as measured by BMI and waist circumference), metabolic syndrome,10 and increased risk of incident AF.11
Pericardial fat is adipose tissue circumscribed by the pericardium, unique for its anatomic proximity to the myocardium and atrial conduction system. Pericardial fat is associated with chemokines (monocyte chemoattractant protein‐1) and inflammatory cytokines (interleukin [IL]‐1, IL‐6, IL‐6sR, and tumor necrosis factor)12 that may alter the atrial myocardium and conduction system through paracrine interactions. Pericardial fat is further related to ventricular diastolic filling, increased filling pressures, and mechanical compression of the heart, all of which may contribute to atrial enlargement.13–14 The extent to which pericardial fat is associated with modification of atrial conduction remains unclear.
As such, we sought to determine the relation between pericardial fat and atrial conduction employing PWI in a community‐based cohort. We hypothesized that increasing pericardial fat would be associated with increased magnitude of PWI given the close proximity of pericardial fat to the myocardium and atrial conduction system. Furthermore, we hypothesized that the relations between pericardial fat and PWI would be independent of adipose tissue depots outside the pericardium, specifically, thoracic and visceral fat.
Methods
Study Participants
The Framingham Heart Study (FHS) is a community‐based, prospective, observational study designed to study cardiovascular disease (CVD) and associated risk factors. FHS began enrollment in 1948; between 2002 and 2005, enrollment was extended to the grandchildren of the original participants, known as the Third Generation cohort.15 Nonpregnant women age ≥40 years and men age ≥35 from the Third Generation participated in a multidetector computed tomography (MDCT) sub‐study. MDCT methods and exclusions are detailed elsewhere.16 The present analysis includes Third Generation participants with an interpretable, concurrent 12‐lead resting ECG and MDCT scans allowing for the measurement of pericardial, visceral, and intrathoracic fat depots. We excluded participants with prevalent AF or flutter, missing baseline covariates, and ECGs precluding reliable PWI measurement: AF or flutter, second degree or higher AV block, nodal rhythm, paced rhythm, or a Wolff‐Parkinson‐White ECG pattern. Consistent with prior FHS analyses16 of pericardial fat, we planned to exclude participants with a history of cardiothoracic surgery although no participants were ultimately excluded on this basis. The Boston University Medical Center Institutional Review Board approved study protocols and all participants provided informed consent at each examination cycle.
Electrocardiography
Electrocardiography was performed according to a standardized protocol. ECGs were recorded digitally on MAC 5000 cardiographs (General Electric) and uploaded to a MUSE 8 ECG Management System (General Electric).17 PWI were measured electronically by Marquette 12SL software algorithms. This study utilized the following PWI: PR interval, P wave duration, P wave amplitude, P wave area, and P wave terminal force. PWI are illustrated in Figure 1. PR interval (ms) was defined as the median duration from the onset of the P wave to the initiation of the QRS complex across the 12‐lead tracing. P wave duration (ms), P wave amplitude (μV), and P wave area (μV·ms) were defined as the maximum value in any lead. P wave terminal force (μV·ms) was defined as the product of the duration of the terminal aspect (eg, negative deflection) of the P wave (measured in ms) and the depth of this same component (measured in μV) in lead V1. MUSE software measurements of PWI are acquired by automated software algorithms and hence have excellent reproducibility.18
Figure 1.
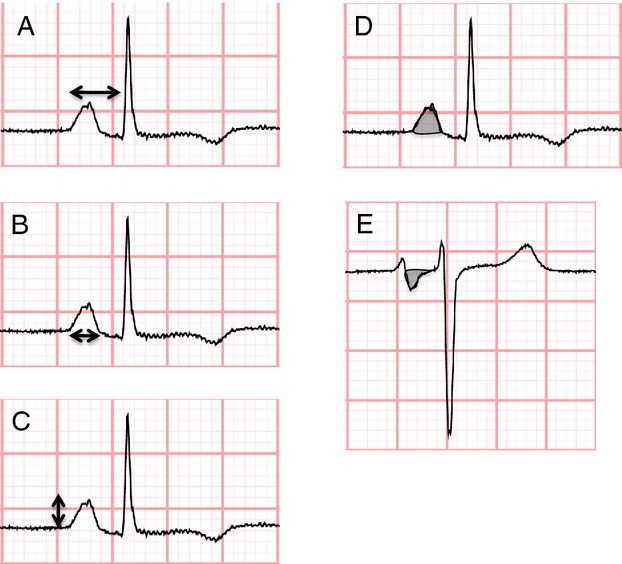
Measurement of (A) PR interval, (B) P wave duration, (C) P wave amplitude, (D) P wave area, and (E) P wave terminal force.
MDCT Scan Protocol and Analysis
The details of the MDCT scan protocol and subsequent analyses have been described.16,19 Briefly, participants were placed in the supine position and assessed with 8‐slice MDCT (LightSpeed Ultra; General Electric). Adipose tissue was defined based on Hounsfield units (HU): window width, −195 to −45 HU; window center, −120 HU. Thoracic, pericardial, and visceral adipose depot volumes were measured with an offline workstation using a semiautomatic segmentation technique (Aquarius 3D Workstation; TeraRecon Inc). Pericardial fat was defined as adipose tissue located within the pericardium. Intrathoracic fat was defined as the total volume of fat within the thorax from the level of the right pulmonary artery to the diaphragm and the chest wall to the descending aorta, minus the volume of fat located within the pericardium. Visceral fat was defined as the total volume of fat within the abdominal wall as defined by the abdominal wall musculature, which was manually traced for all images. Inter‐reader reliability for pericardial fat (interclass correlation 0.95), total thoracic fat (interclass correlation 0.98), and visceral fat (interclass correlation 0.99) have been assessed as excellent.16,19
Risk Factor and Covariate Assessment
Risk factors and covariates were measured during the first examination of the Third Generation cohort (2002–2005).15 Current smoking was defined as smoking at least one cigarette per day within the previous year. Heavy alcohol consumption was defined as ≥15 drinks per week in men and ≥8 drinks per week in women. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or hypertensive treatment. Waist circumference was measured at the level of the umbilicus. BMI was defined as weight (in kilograms) divided by the square of height (in meters). Fasting plasma glucose, total and high‐density cholesterol, and triglycerides were measured on fasting morning samples. Diabetes was defined as fasting plasma glucose level of ≥126 mg/dL or treatment with insulin or a hypoglycemic agent. Menopause was determined by cessation of menses for ≥1 year. CVD includes coronary heart disease, stroke, claudication, and congestive heart failure.5,16 AV nodal medications were defined as beta‐blockers, dihydropyridine and non‐dihydropyridine calcium channel blockers, cardiac glycosides, Class I and III antiarrhythmic medications.
Statistical Analysis
We assessed the distribution and normality of baseline characteristics and presented data as means or medians as appropriate. Correlations between adiposity measures (pericardial fat, intrathoracic fat, visceral fat, waist circumference, and BMI) and PWI were assessed using Pearson partial correlations adjusted for age and sex. We identified an interaction by sex for the relation of PWI to adiposity measures, consistent with prior FHS analysis of adiposity.19 For the primary analysis, we assessed the relation between pericardial fat and PWI using multivariable linear regression analyses adjusting for all relevant covariates: age, heart rate, systolic and diastolic blood pressures, AV nodal medications, hypertension or treatment for hypertension, diabetes, and hormone replacement therapy and menopausal status in women. We performed secondary multivariable linear regression analyses relating pericardial fat and PWI, with further adjustment for visceral fat, intrathoracic fat, and BMI, in separate analyses. Results are presented as per 1‐SD increase in fat. We created restricted cubic splines using three knots to graphically depict the relation between pericardial fat and separate PWI across the continuous ranges. A P value of <0.05 was considered significant for all analyses.
Results
A total of 2047 participants in the MDCT sub‐study had adequate quality CT scans allowing for the measurement of pericardial, visceral, and intrathoracic fat depots. Participants were excluded for prevalent AF or flutter (n=85) or missing baseline covariates or PWI (n=16). The remaining sample consisted of 1946 participants (age 44.0±6.4, 45% women). The mean BMI was 28.2±4.5 kg/m2. Overall and sex‐stratified characteristics are displayed in Table 1. PWI were non‐normally distributed and are presented as medians (Q1 to Q3).
Table 1.
Baseline Characteristics
| Characteristics | Overall (N=1946) | Women (N=879) | Men (N=1067) |
|---|---|---|---|
| Age, y | 45.0±6.2 | 46.2±5.7 | 44.0±6.4 |
| Current smoking | 288 (14.8) | 126 (14.3) | 162 (15.2) |
| Heavy drinking | 251 (13) | 102 (11.7) | 149 (14.2) |
| Systolic blood pressure, mm Hg | 119±14 | 117±16 | 121±13 |
| Diastolic blood pressure, mm Hg | 77±9 | 74±9 | 79±9 |
| Waist circumference, cm | 95.4±14.4 | 90.4±15.4 | 99.5±11.9 |
| Body mass index, kg/m2 | 27.4±5.3 | 26.5±5.9 | 28.2±4.5 |
| Heart rate, beats per minute | 62±10 | 63±10 | 61±10 |
| Weight, kg | 81.1±18.2 | 71.3±16.6 | 89.2±15.2 |
| AV nodal medications | 132 (6.8) | 62 (7.1) | 70 (6.6) |
| Prevalent hypertension | 411 (21.1) | 148 (16.8) | 263 (24.6) |
| Diabetes | 71 (3.6) | 24 (2.7) | 47 (4.4) |
| Hormone replacement treatment | 85 (9.7) | — | |
| Post‐menopausal | 221 (25.1) | — | |
| Pericardial fat, cm3 | 105±38 | 92±34 | 116±38 |
| Visceral fat, cm3 | 1603±920 | 1127±718 | 1995±882 |
| Intrathoracic fat, cm3 | 88±60 | 52±32 | 117±61 |
| PR interval, ms | 160 (140 to 170) | 150 (140 to 160) | 160 (150 to 180) |
| P wave duration, ms | 104 (98 to 112) | 102 (94 to 110) | 108 (100 to 114) |
| P wave amplitude, μV | 112 (92 to 131) | 112 (92 to 136) | 110 (92 to 126) |
| P wave area, μV·ms | 307 (248 to 365) | 308 (238 to 367) | 307 (252 to 363) |
| P wave terminal force, μV·ms | 1488 (0 to 2457) | 1368 (0 to 2340) | 1584 (0 to 2584) |
Continuous measures are mean±SD and categorical measures No (%). Heavy alcohol consumption is defined as ≥15 drinks per week in men and ≥8 drinks per week in women. Atrioventricular (AV) nodal medications include beta‐blockers, calcium channel blockers (dihydropyridine and non‐dihydropyridine), cardiac glycosides, and Class I and III antiarrhythmic medications. P wave indices: expressed as median (Q1 to Q3).
Correlations
P wave duration and P wave terminal force were significantly (P<0.001) correlated with pericardial fat. P wave duration and P wave terminal force were also associated with WC, BMI, intrathoracic fat, and visceral fat (P<0.001). PR interval was significantly correlated with both WC and BMI and P wave amplitude was significantly associated with BMI, intrathoracic, and visceral fat. All significant correlations were mild or moderate in magnitude (0.06 to 0.21). The complete list of correlations is displayed in Table 2.
Table 2.
Age and Sex Adjusted Pearson Partial Correlation Coefficients Relating P Wave Indices and Adipose Measures
| PR Interval (N=1944) | P Wave Duration (N=1911) | P Wave Amplitude (N=1911) | P Wave Area (N=1911) | P Wave Terminal Force (N=1909) | |
|---|---|---|---|---|---|
| WC | 0.06‡ | 0.21* | −0.04 | 0.03 | 0.19* |
| BMI | 0.07† | 0.20* | −0.06‡ | 0.01 | 0.20* |
| Pericardial fat | −0.01 | 0.15* | −0.04 | 0.02 | 0.17* |
| Visceral fat | 0.02 | 0.15* | −0.07† | −0.01 | 0.21* |
| Intrathoracic fat | −0.002 | 0.13* | −0.07† | −0.01 | 0.17* |
P wave indices as defined by text. BMI indicates body mass index; WC, waist circumference.
*P<0.0001, †P<0.01, †P<0.05.
Multivariable Analyses
We performed multivariable linear regression analyses to assess the relation between pericardial fat and PWI. Sex‐stratified analyses were employed because a significant sex interaction was demonstrated between PWI and pericardial fat (Table 3). The results of the primary analysis are displayed in Table 4. (Table 4 contains results from primary and secondary analyses to facilitate comparison.) Among women, pericardial fat was associated with PR interval (β=1.7 ms, P=0.049), P wave duration (β=2.3 ms, P<0.001), and P wave terminal force (β=297 μV·ms, P<0.001). Among men, pericardial fat was associated with P wave duration (β=1.2 ms, P=0.002), P wave amplitude (β=−2.5 μV, P<0.001), and P wave terminal force (β=160 μV·ms, P=0.002). The relation between pericardial fat and other PWI were nonsignificant.
Table 3.
P Values for Sex‐Specific Interactions in the Multivariable‐Adjusted* Relations Between P Wave Indices and Adipose Measures
| Adipose Variable | PR Interval | P Wave Duration | P Wave Amplitude | P Wave Area | P Wave Terminal Force |
|---|---|---|---|---|---|
| Waist circumference | 0.54 | 0.48 | 0.41 | 0.14 | 0.050 |
| Body mass index | 0.66 | 0.20 | 0.45 | 0.041 | 0.042 |
| Pericardial fat | 0.12 | 0.042 | 0.029 | 0.010 | 0.051 |
| Visceral fat | 0.29 | 0.002 | 0.89 | 0.17 | 0.007 |
| Intrathoracic fat | 0.41 | 0.001 | 0.48 | 0.39 | <0.001 |
Multivariable adjustment includes, age, heart rate, systolic and diastolic blood pressures, AV nodal medications (beta‐blocker, dihydropyridine and non‐dihydropyridine calcium channel blockers, cardiac glycosides, Class I and III antiarrhythmic medications), hormone replacement therapy, prevalent hypertension or hypertension treatment, diabetes, and menopausal status in women.
Table 4.
Sex‐Stratified Multivariable* Models of Pericardial Fat and P Wave Indices With Additional Adjustment (+) for Visceral Fat, Intrathoracic Fat, and BMI
| Model | Adipose Variable Measures | Women | Male | ||||
|---|---|---|---|---|---|---|---|
| Beta | SE | P Value | Beta | SE | P Value | ||
| PR interval | |||||||
| Pericardial fat | Pericardial | 1.7 | 0.9 | 0.049 | −0.2 | 0.7 | 0.82 |
| +Visceral fat | Pericardial | 0.6 | 1.0 | 0.59 | −1.1 | 0.8 | 0.18 |
| Visceral | 2.4 | 1.3 | 0.06 | 2.1 | 0.9 | 0.023 | |
| +Intrathoracic fat | Pericardial | 1.5 | 1.1 | 0.18 | −0.8 | 0.9 | 0.35 |
| Intrathoracic | 0.5 | 1.9 | 0.78 | 1.2 | 0.8 | 0.17 | |
| +BMI | Pericardial | 0.5 | 0.9 | 0.58 | −1.3 | 0.8 | 0.09 |
| BMI | 2.2 | 0.8 | 0.005 | 3.5 | 0.9 | <0.001 | |
| P wave duration | |||||||
| Pericardial fat | Pericardial | 2.3 | 0.4 | <0.001 | 1.2 | 0.4 | 0.002 |
| +Visceral fat | Pericardial | 1.4 | 0.5 | 0.009 | 1.0 | 0.4 | 0.033 |
| Visceral | 2.1 | 0.7 | 0.002 | 0.5 | 0.5 | 0.28 | |
| +Intrathoracic fat | Pericardial | 1.5 | 0.6 | 0.009 | 1.0 | 0.5 | 0.030 |
| Intrathoracic | 2.3 | 1.0 | 0.018 | 0.4 | 0.5 | 0.43 | |
| +BMI | Pericardial | 1.3 | 0.5 | 0.005 | 0.7 | 0.4 | 0.08 |
| BMI | 1.8 | 0.4 | <0.001 | 1.5 | 0.5 | 0.002 | |
| P wave amplitude | |||||||
| Pericardial fat | Pericardial | −1.1 | 1.3 | 0.38 | −3.2 | 0.9 | <0.001 |
| +Visceral fat | Pericardial | 3.0 | 1.6 | 0.06 | −2.1 | 1.0 | 0.043 |
| Visceral | −8.9 | 1.9 | <0.001 | −2.5 | 1.1 | 0.029 | |
| +Intrathoracic fat | Pericardial | 2.6 | 1.7 | 0.12 | −2.0 | 1.1 | 0.06 |
| Intrathoracic | −10.1 | 2.9 | <0.001 | −2.2 | 1.0 | 0.037 | |
| +BMI | Pericardial | 1.1 | 1.4 | 0.45 | −2.7 | 1.0 | 0.005 |
| BMI | −4.2 | 1.2 | <0.001 | −1.6 | 1.1 | 0.16 | |
| P wave area | |||||||
| Pericardial fat | Pericardial | 4.7 | 3.7 | 0.21 | −5.6 | 2.9 | 0.06 |
| +Visceral fat | Pericardial | 11.3 | 4.5 | 0.012 | −1.6 | 3.4 | 0.64 |
| Visceral | −14.4 | 5.6 | 0.010 | −8.7 | 3.7 | 0.020 | |
| +Intrathoracic fat | Pericardial | 10.5 | 4.8 | 0.029 | −2.8 | 3.5 | 0.43 |
| Intrathoracic | −15.6 | 8.3 | 0.06 | −5.1 | 3.4 | 0.14 | |
| +BMI | Pericardial | 7.2 | 4.1 | 0.08 | −3.7 | 3.2 | 0.25 |
| BMI | −4.6 | 3.4 | 0.17 | −5.9 | 3.7 | 0.10 | |
| P wave terminal force | |||||||
| Pericardial fat | Pericardial | 297.3 | 51.5 | <0.001 | 160 | 51.1 | 0.002 |
| +Visceral fat | Pericardial | 140.9 | 61.9 | 0.023 | 84.2 | 59.1 | 0.15 |
| Visceral | 339.8 | 76.6 | <0.001 | 164.4 | 64.9 | 0.011 | |
| +Intrathoracic fat | Pericardial | 117.2 | 66.2 | 0.08 | 104.1 | 60.8 | 0.09 |
| Intrathoracic | 483.3 | 113.3 | <0.001 | 100.2 | 59.3 | 0.09 | |
| +BMI | Pericardial | 169.6 | 56.4 | 0.003 | 127.9 | 55.1 | 0.020 |
| BMI | 239.3 | 46 | <0.001 | 98.1 | 63.6 | 0.12 | |
BMI indicates body mass index; SE, standard error.
Multivariable models adjusted for indicated adiposity measures and age, heart rate, systolic and diastolic blood pressures, AV nodal medications (beta‐blocker, dihydropyridine and non‐dihydropyridine calcium channel blockers, cardiac glycosides, Class I and III antiarrhythmic medications), hormone replacement therapy in women, prevalent hypertension or hypertension treatment, diabetes, and menopausal status in women.
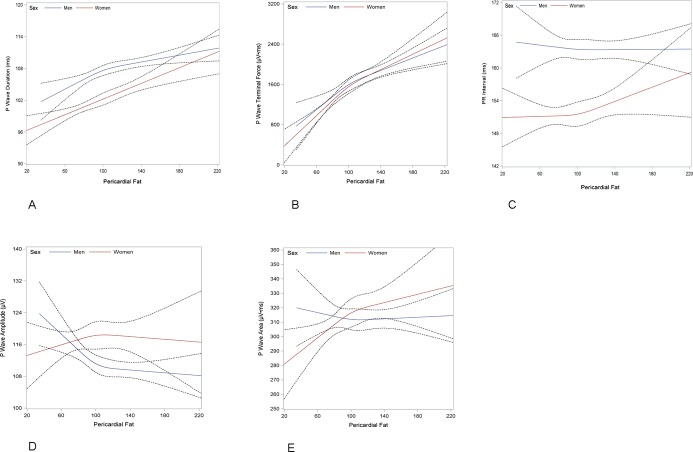
Restricted cubic spline functions relating pericardial fat and PWI are depicted in Figure 2. Functions plotting P wave duration (Figure 2A) and P wave terminal force (Figure 2B) demonstrated linear relations. Increasing pericardial fat was associated with longer P wave duration and increased P wave terminal force. The relations between pericardial fat and PR interval (Figure 2C), P wave amplitude (Figure 2D), and P wave area (Figure 2E), are non‐linear.
Figure 2.
Sex‐stratified cubic restricted splines depicting the association of pericardial fat and (A) P wave duration, (B) P wave terminal force, (C) PR interval, (D) P wave amplitude, and (E) P wave area. Dotted lines represent 95% confidence intervals surrounding the estimate.
We subsequently tested the hypothesis that the association between pericardial fat and PWI was independent of adipose depots located outside of the pericardial space (ie, distal from the myocardium and atrial conduction system). We performed multivariable models adjusting for intrathoracic and visceral fat in separate multivariable analyses that are detailed in Table 4. Our analyses demonstrated that relations between pericardial fat and P wave duration were independent of both intrathoracic and visceral fat depots. Among women, pericardial fat was associated with P wave area after adjustment for intrathoracic fat and visceral fat. Among women, pericardial fat remained significantly associated with P wave terminal force after adjustment for visceral but not intrathoracic fat. In multivariable models adjusting for BMI, pericardial fat remained associated with P wave duration and P wave terminal force in women, and P wave amplitude and P wave terminal force in men.
Additionally, Table 5 contains descriptions of the multivariable adjusted relations between other adipose measures and PWI. When all primary and secondary analyses were repeated excluding participants with prevalent CVD (n=37), the results were similar.
Table 5.
The Association of P Wave Indices and Adiposity Measures in Sex‐Stratified, Multivariable‐Adjusted* Analyses
| Adipose Variable | Women | Men | ||||
|---|---|---|---|---|---|---|
| β | SE | P Value | β | SE | P Value | |
| PR interval | ||||||
| Waist circumference | 2.4 | 0.7 | 0.001 | 3.0 | 0.9 | <0.001 |
| BMI | 2.3 | 0.7 | <0.001 | 3.0 | 0.8 | <0.001 |
| Pericardial fat | 1.7 | 0.9 | 0.049 | −0.2 | 0.7 | 0.82 |
| Visceral fat | 2.8 | 1.1 | 0.007 | 1.5 | 0.8 | 0.07 |
| Intrathoracic fat | 2.1 | 1.5 | 0.14 | 0.7 | 0.7 | 0.31 |
| P wave duration | ||||||
| Waist circumference | 2.5 | 0.4 | <0.001 | 2.2 | 0.5 | <0.001 |
| BMI | 2.3 | 0.4 | <0.001 | 1.8 | 0.4 | <0.001 |
| Pericardial fat | 2.3 | 0.4 | <0.001 | 1.2 | 0.4 | 0.002 |
| Visceral fat | 3.0 | 0.5 | <0.001 | 1.1 | 0.4 | 0.013 |
| Intrathoracic fat | 3.9 | 0.8 | <0.001 | 0.9 | 0.4 | 0.020 |
| P wave amplitude | ||||||
| Waist circumference | −3.6 | 1.1 | 0.001 | −2.5 | 1.1 | 0.020 |
| BMI | −3.8 | 1.1 | <0.001 | −2.8 | 1.0 | 0.008 |
| Pericardial fat | −1.1 | 1.3 | 0.38 | −3.2 | 0.9 | <0.001 |
| Visceral fat | −6.8 | 1.6 | <0.001 | −3.6 | 1.0 | <0.001 |
| Intrathoracic fat | −7.2 | 2.2 | 0.001 | −3.2 | 0.9 | <0.001 |
| P wave area | ||||||
| Waist circumference | −0.9 | 3.2 | 0.78 | −4.1 | 3.6 | 0.24 |
| BMI | −2.1 | 3.0 | 0.49 | −7.5 | 3.4 | 0.027 |
| Pericardial fat | 4.7 | 3.7 | 0.21 | −5.6 | 2.9 | 0.06 |
| Visceral fat | −6.4 | 4.6 | 0.16 | −9.6 | 3.2 | 0.003 |
| Intrathoracic fat | −4.1 | 6.4 | 0.52 | −6.5 | 2.9 | 0.023 |
| P wave terminal force | ||||||
| Waist circumference | 297.7 | 44.6 | <0.001 | 134.3 | 61.7 | 0.030 |
| BMI | 299.7 | 41.6 | <0.001 | 153.9 | 59.1 | 0.009 |
| Pericardial fat | 297.3 | 51.5 | <0.001 | 160.0 | 51.1 | 0.002 |
| Visceral fat | 439.0 | 63.2 | <0.001 | 211.3 | 56.0 | <0.001 |
| Intrathoracic fat | 611.3 | 87.4 | <0.001 | 155.5 | 49.8 | 0.002 |
BMI indicates body mass index; SE, standard error.
Multivariable‐adjustment includes age, heart rate, systolic and diastolic blood pressures, AV nodal medications (beta‐blocker, dihydropyridine and non‐dihydropyridine calcium channel blockers, cardiac glycosides, Class I and III antiarrhythmic medications), hormone replacement therapy in women, prevalent hypertension or hypertension treatment, diabetes, and menopausal status in women.
Discussion
Principle Findings
In this large community‐based cohort, pericardial fat was associated with atrial conduction as measured by PWI. Pericardial fat was associated with P wave duration and P wave terminal force in men and women, PR interval in women, and P wave amplitude in men. Pericardial fat remained associated with P wave duration even after adjustment for intrathoracic fat and visceral fat in subsequent analyses; a similar nonsignificant trend existed with pericardial fat and P wave terminal force. Among women, pericardial fat was associated with P wave area after adjusting for intrathoracic and visceral fat, in separate analyses. Finally, we found that the relation between pericardial fat and many PWI was independent of BMI.
In the Context of the Current Literature
Previous studies have linked increases in generalized adiposity (as measured by BMI and WC) with alterations in atrial conduction.10 Our study extends our understanding of the relation between adiposity and atrial conduction by demonstrating that a specific fat depot – pericardial fat – was independently associated with atrial conduction. The observed independent association between pericardial fat and atrial conduction is consistent with the hypothesis that the proximity of pericardial fat and the atrial myocardium may be associated with atrial conduction via a mechanism that is unique relative to other ectopic fat depots.
The relation between PWI and pericardial fat was assessed in the Multiethnic Study of Atherosclerosis (MESA) study.20 Investigators demonstrated a significant relation between pericardial fat and PR duration, P wave duration, and P wave terminal force, in unadjusted analyses. In multivariable models including adjustment for surrogates of total body adiposity (BMI or waist circumference), pericardial fat was no longer significantly associated with PWI. These results are in contrast to our study, which found pericardial fat to be independently associated with PWI; the association between pericardial fat and selected PWI remained after adjustment for visceral fat, intrathoracic fat, and BMI, in separate analyses.
The current investigation differs from the MESA analysis in a number of key ways that explain our differing results and extend our understanding of the relation between pericardial fat and atrial conduction. The prior study performed in MESA defined pericardial fat as all fat surrounding the heart and included fat outside of the pericardium; the anterior border of the depot was defined by the chest wall and the posterior border by the aorta and the bronchus.20 In contrast, we defined pericardial fat as exclusively located within the pericardium and excluded other thoracic fat depots. Thus, our analyses used a more specific measure of the fat depot that is more closely located to and shares a blood supply with the myocardium.21 We consider that such an approach increases the sensitivity to detect a unique association between pericardial fat and atrial conduction. Our study further compared the relative association of PWI and other ectopic fat depots (visceral and intrathoracic fat). In secondary analyses we demonstrated that the relations between pericardial fat and many PWI were independent of ectopic visceral and intrathoracic fat depots. Our results support the hypothesis that pericardial fat (eg, the fat depot most proximal to the atrial tissue) has an important association with atrial conduction.
Potential Mechanisms
There are several potential mechanisms by which increases in pericardial fat may lead to changes in atrial conduction.22 Altered PWI indicate prolonged atrial depolarization, diminished voltage, and heterogeneous atrial activation22 related to fibrosis,23 hypertrophy, and fatty myocardial infiltration. PWI represent a summation of the electrical vectors of atrial depolarization reflecting the atrial activation sequence and as such are subclinical markers of atrial remodeling.22 The electrical vectors are additionally influenced by sinus node anatomy and exit site, electrical propagation from right to left atrium via the interatrial conduction fibers (eg, Bachmann's bundle, Wenckebach's bundle, and Thorel's bundle), and shape and geometry of the atrial chambers.22
Pericardial fat is likely associated with the representation of these vectors on the 12‐lead ECG in a number of complex ways. The appearance of the voltage‐dependent PWI (P wave amplitude, P wave area, P wave terminal force) on the surface ECG is affected by multiple competing factors. For example, although pericardial fat may have the potential to increase vector magnitude via atrial hypertrophy it may also decrease the vector via fibrosis and decrease the ability to detect the summed vector on the surface ECG due to the insulating effects of adipose tissue. In contrast, the voltage‐independent PWI (P wave duration and PR interval) are not affected by the insulating effects of adiposity but are affected by factors that influence conduction time, for example hypertrophy and fibrosis.
Of note, although P wave terminal force is a voltage‐dependent PWI, it has been hypothesized that it is strongly influenced by interatrial conduction.22 This hypothesis posits that block in the posterior interatrial bundles leads to atrial breakthrough in the anterior aspect of the interatrial septum. The anterior to posterior activation of the left atrium results in a terminal negative deflection in V1. This hypothesis may explain why P wave terminal force is more closely associated with pericardial fat than other voltage‐dependent PWI.
The Relation Between Pericardial Fat and AF
Previous work has associated abnormal atrial conduction with the development of AF.11,18,24 AF is promoted by slowed tissue conduction and longer electrical path lengths, which result in prolonged atrial conduction.25 PWI partially reflect prolonged or altered atrial conduction and thus PWI may be associated with atrial electrical remodeling. Given that pericardial fat has been associated with AF,26–27 our work thus suggests a potential intermediate mechanism for the relation of pericardial fat and AF pathogenesis.
Multiple intermediate mechanisms likely relate obesity and AF.9 These mechanisms may include: inflammation,12,28 vascular adaptation, fibrosis,23 increased left atrial pressures (with accompanying atrial remodeling) secondary to left ventricular hypertrophy and diastolic adaptation19 and altered compliance or diastolic dysfunction from pericardial adiposity.19,29 Studies have demonstrated that different fat depots (pericardial, thoracic, visceral) are associated with variable risks of cardiovascular disease including AF.16,26 Pericardial fat has been proposed to have a unique paracrine effect on the myocardium and conduction system because of its comparatively small volume and close proximity to the myocardial surface,21 and lack of independent association with circulating markers of inflammation.12
The influence of pericardial fat on the development of AF may be amplified relative to general adiposity and other ectopic fat stores due to its unique anatomic characteristics.21 The inhomogeneous contact between pericardial fat and atrial myocardium21 is likely associated with spatial heterogeneity of tissue injury and thus atrial conduction. Heterogeneity of atrial conduction is associated with risk of AF30 and thus pericardial fat may promote the fibrillatory state.
Clinical Implications and Future Directions
This study demonstrates that pericardial fat is associated with atrial conduction. Our results are consistent with the hypothesis that pericardial fat induced changes in atrial conduction may represent an important mechanism by which pericardial fat predisposes towards AF. Longitudinal and mechanistic studies are needed to assess the temporal relation between pericardial fat and changes in atrial conduction, atrial chamber size, and development of AF.
Further studies are needed for more refined assessments of the impact of pericardial fat on atrial electrophysiology, such as how pericardial fat is related to modification of the action potential duration, refractory period, and changes in automaticity. Investigation is warranted to assess the contribution of pericardial fat towards scarring, fibrosis, and ion channel expression. Modification of such components of the myocardial substrate could result in altered PWI and facilitate AF. Finally, an improved understanding of how pericardial fat modifies atrial electrophysiology may yield novel approaches towards preventing AF in obesity.
Strengths and Limitations
This study has several strengths that include its large sample size, precise and rigorous definition of pericardial fat, and PWI quantification by automated software algorithm. The availability of abdominal CT scans allowed us to demonstrate that the relation between pericardial fat and selected PWI was independent of visceral fat.
The study has multiple limitations. The cross‐sectional nature of the study precludes determination of the temporal relation or causality regarding the development of ectopic fat depots and changes in PWI. The FHS is comprised primarily of individuals of white, European descent and, as such, the results may not be applicable to those of other racial or ethnic backgrounds. The MDCT substudy excluded those >320 lbs due to scanner limitations and, as such, our results may not be generalizable to individuals above this weight threshold. While PWI were measured using digital software algorithms, the P wave is a low amplitude signal and may be vulnerable to distortion. However, we expect measurement error to be non‐differential with respect to pericardial fat and therefore bias our results towards the null. We did not adjust for multiple comparisons and, as such, the results should be considered exploratory rather than confirmatory. Finally, we used linear regression analyses for primary and secondary analyses relating PWI and pericardial fat, although not all relations were linear as depicted by spline functions. Given the biologic plausibility that there are no clear relations between pericardial fat and PR interval, P wave area, and P wave amplitude, we abstained from testing multiple statistical models and presented linear models as they were part of our a priori statistical plan.
Conclusions
Pericardial fat is associated with atrial conduction as measured by PWI. The relation is independent of associations with adipose depots outside of the pericardium (thoracic and visceral fat). Our findings are consistent with the hypothesis that pericardial fat may modify the atrial substrate and subsequent risk for arrhythmogenesis.
Sources of Funding
Dr Magnani is supported by American Heart Association award 09FTF219028 and a Boston University School of Medicine Department of Medicine Career Investment Award, and Dr Meigs by NIH grant 2K24 DK080140. This work was supported by National Heart, Lung, and Blood Institute grant R21HL1060926 and Framingham Heart Study contract N01‐HC25195.
Disclosures
Dr Massaro receives support from Cardiovascular Clinical Sciences, Abbott Vascular, Cordis, Medtronic, Merck, and Harvard Clinical Research Institute. There are no perceived conflicts between these funding sources and the investigation and manuscript presented here. The remaining authors report no disclosures.
References
- 1.Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM, Smith NL, Heckbert SR. Risk of new‐onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006; 166:2322-2328 [DOI] [PubMed] [Google Scholar]
- 2.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005; 118:489-495 [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009; 30:1113-1120 [DOI] [PubMed] [Google Scholar]
- 4.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol. 2010; 55:2319-2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004; 292:2471-2477 [DOI] [PubMed] [Google Scholar]
- 6. From the Centers for Disease Control and Prevention. Update: prevalence of overweight among children, adolescents, and adults–United States, 1988–1994. JAMA. 1997; 277:1111. [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012; 307:491-497 [DOI] [PubMed] [Google Scholar]
- 8.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119-125 [DOI] [PubMed] [Google Scholar]
- 9.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013; 128:401-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the Atherosclerosis Risk in Communities study. Obesity (Silver Spring). 2012; 20:666-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ. P wave duration and risk of longitudinal atrial fibrillation in persons >/= 60 years old (from the Framingham Heart Study). Am J Cardiol. 2011; 107:917-921.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov‐Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003; 108:2460-2466 [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Leonetti F, Singh N, Sharma AM. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007; 115:272-273 [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008; 16:1693-1697 [DOI] [PubMed] [Google Scholar]
- 15.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007; 165:1328-1335 [DOI] [PubMed] [Google Scholar]
- 16.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009; 30:850-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnani JW, Newton‐Cheh C, O'Donnell CJ, Levy D. Development and application of a longitudinal electrocardiogram repository: the Framingham Heart Study. J Electrocardiol. 2012; 45:673-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009; 40:1204-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D'Agostino RB, Sr, O'Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009; 119:1586-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babcock MJ, Soliman EZ, Ding J, R AK, Goff DC., Jr Pericardial fat and atrial conduction abnormalities in the Multiethnic Study of Atherosclerosis (MESA). Obesity (Silver Spring). 2011; 19:179-184 [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005; 2:536-543 [DOI] [PubMed] [Google Scholar]
- 22.Platonov PG. P‐wave morphology: underlying mechanisms and clinical implications. Ann Noninvasive Electrocardiol. 2012; 17:161-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo‐fibrokines. Eur Heart J. 2013 [DOI] [PubMed] [Google Scholar]
- 24.Magnani JW, Wang N, Nelson KP, Connelly S, Deo R, Rodondi N, Schelbert EB, Garcia ME, Phillips CL, Shlipak MG, Harris TB, Ellinor PT, Benjamin EJHealth A, Body Composition S. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2013; 6:84-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Teh AW, Medi C, Kistler PM, Morton JB, Kalman JM. Atrial remodeling in varying clinical substrates within beating human hearts: relevance to atrial fibrillation. Prog Biophys Mol Biol. 2012; 110:278-294 [DOI] [PubMed] [Google Scholar]
- 26.Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010; 3:345-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010; 56:784-788 [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010; 2010:535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, Leonetti F. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002; 10:767-773 [DOI] [PubMed] [Google Scholar]
- 30.Matsuyama TA, Tanaka H, Adachi T, Jiang Y, Ishibashi‐Ueda H, Takamatsu T. Intrinsic left atrial histoanatomy as the basis for reentrant excitation causing atrial fibrillation/flutter in rats. Heart Rhythm. 2013; 10:1342-1348 [DOI] [PubMed] [Google Scholar]