Abstract
Background
Accumulating evidence suggests that the balance between pathogenic effector T cells (Teffs) and regulatory T cells (Tregs) may be important for controlling atherosclerotic disease. We hypothesized that a combination therapy with anti‐CD3 antibody (CD3‐Ab) and IL‐2/anti‐IL‐2 monoclonal antibody complex (IL‐2 complex) aimed at increasing the ratio of Tregs to Teffs would effectively inhibit atherosclerosis in mice.
Methods and Results
We treated apolipoprotein E‐deficient mice fed a high‐cholesterol diet with vehicle, CD3‐Ab, IL‐2 complex, or their combination. Mice receiving the combination therapy had markedly reduced atherosclerotic lesions than mice treated with CD3‐Ab or IL‐2 complex alone. In addition, a striking increase in the Treg/Teff ratio of lymphoid organs and atherosclerotic lesions, along with plaque stabilization characterized by decreased macrophage content and increased collagen content was observed. The combination treatment also markedly reduced splenic Ly6Chigh inflammatory monocytes and might induce a favorable macrophage phenotype change in atherosclerotic lesions.
Conclusions
Our results indicate that in addition to suppressing Teff responses, enhancing Treg‐mediated immune responses is more efficacious in preventing atherosclerosis, suggesting a novel therapeutic approach for atherosclerosis.
Keywords: atherosclerosis, immune system, inflammation, T cell
Introduction
It is now widely accepted that inflammatory condition within the vessel wall is one of the most important factors for atherosclerosis development1–2 and contributes toward plaque instability, thrombotic arterial occlusion, and severe clinical events including acute coronary syndrome and stroke. Following accumulation into the subendothelial space or intima through the activated endothelium and differentiation into macrophages, monocytes take up modified low‐density lipoprotein (LDL) particles, and differentiate into foam cells, which secrete pro‐inflammatory cytokines causing activation of immune cells such as T cells. After antigen presentation or activation by macrophages or dendritic cells (DCs), naïve CD4+ T cells differentiate into effector T cells (Teffs) such as T helper type 1 (Th1), T helper type 2 (Th2), and T helper type 17 (Th17) lineages, which all play an important role in atherogenesis in both humans and mice.2 Th1 cells are known to promote atherosclerotic disease by producing inflammatory cytokines such as interferon‐γ.3 However, the roles of Th2 or Th17‐mediated immune responses in atherosclerosis remain controversial. Recently, immunoregulatory CD4+ T‐cell subsets, namely regulatory T cells (Tregs) expressing CD25 (IL‐2 receptor α‐chain) molecule, have been shown to play a protective role in atherogenesis by dampening Teff responses.4–8 The transcription factor Foxp3 (forkhead box P3) is a master regulator and the most reliable molecular marker for natural Tregs.9 Recent study demonstrated that genetic depletion of Foxp3+ Tregs increased atherosclerotic lesions in atherosclerosis‐prone mice by aggravating hypercholesterolemia.10 In consideration of these previous studies, we believe that increasing the Treg/Teff ratio, by suppressing Teff responses and promoting Treg responses, could be a promising therapeutic approach for atherosclerotic disease.
Intravenous administration of anti‐CD3‐specific antibody (CD3‐Ab) was shown to suppress Teff immune responses and to be effective for suppressing atherosclerotic process in mice,11 autoimmune diabetes in mice and humans, and acute transplant rejection in humans.12–13 Previous studies demonstrated induction of CD4+CD25+ Tregs, along with reduced number of Teffs, following CD3‐Ab treatment, explaining the long‐term protective effects observed in mouse models of autoimmune diseases,12 although an increase in CD4+CD25+ Treg number was absent in atherosclerosis‐prone mice.11 Despite these beneficial effects, high doses of CD3‐Ab cannot be used because of severe side effects such as low levels of cytokine release from activated T‐cells.13 Thus, in addition to this antibody treatment, other therapeutic strategies to attain long‐term therapeutic efficacy are needed.
Recent studies have demonstrated that injection of a recombinant mouse IL‐2/anti‐IL‐2 monoclonal antibody complex (IL‐2 complex) could be one promising avenue for the expansion of CD4+CD25+Foxp3+ Tregs14 and studies have shown that this IL‐2 complex therapy suppressed the development and progression of atherosclerosis15–16 and experimental autoimmune encephalitis14 in mice without adverse effects. However, it is reported that Tregs cannot effectively suppress autoimmune reactions in mouse autoimmune disease model, if inflammation in the target organ is not regulated,17 suggesting that under inflammatory conditions such as hypercholesterolemia, suppression of Teff immune responses before expanding Tregs may result in an efficient reduction in atherosclerosis development via augmenting regulatory immune responses.
In the present study, we determined if the combination therapy of CD3‐Ab and IL‐2 complex would effectively inhibit atherosclerosis in ApoE−/− mice by enhancing regulatory immune responses. We propose the novel concept involving modulation of both effector and regulatory arms of T‐cell immune responses could be an attractive therapeutic approach against atherosclerosis.
Methods
Animals and Experimental Design
Six‐week‐old ApoE−/− mice were fed a high‐cholesterol diet containing 0.2% cholesterol and 21% fat (CLEA) and water ad libitum. For blockade of CD3, 50 μg of anti‐CD3 antibody F(ab')2 (145‐2C11; Bio X cell) or 50 μg of isotype‐matched hamster immunoglobulin G F(ab')2 (control IgG) (Bio X cell) was intravenously injected into the mice for 5 consecutive days at 8 weeks of age. For IL‐2 complex therapy, a recombinant mouse IL‐2/anti‐IL‐2 mAb (JES6‐1) complex (1 μg IL‐2 plus 5 μg anti‐IL‐2 mAb) was given i.p. to the 9‐week‐old mice for 3 consecutive days, after which they received once weekly from 10 to 16 weeks of age. Mice were anaesthetized with an isoflurane and an intraperitoneal injection of pentobarbital (30 mg/kg body weight). Mice were housed in a specific pathogen‐free animal facility at Kobe University, and all animal experiments were conducted in accordance to the Guidelines for Animal Experiments at Kobe University School of Medicine.
Atherosclerotic Lesion Assessments
Mice were anesthetized and the aorta was perfused with saline. The samples were cut in the ascending aorta, and the proximal samples containing the aortic sinus were embedded in OCT compounds (Tissue‐Tek; Sakura Finetek). Five consecutive sections (10 μm thickness), spanning 550 μm of the aortic sinus, were collected from each mouse and stained with Oil Red O (Wako Pure Chemical Industries). Total plaque area and Oil Red O stained areas were measured using Image J (National Institutes of Health). The volume of atherosclerosis and lipid accumulation in the aortic sinus was expressed as mean size of the 5 sections for each mouse. Immunohistochemistry was performed on acetone‐fixed or formalin‐fixed cryosections (10 μm) of aortic roots using antibodies to identify macrophages (MOMA‐2, 1:400; BMA Biomedicals), CD4+ T cells (CD4, clone H129.19, 1:100; BD Biosciences) and Foxp3+ cells (Foxp3, clone FJK‐16s, 1:100; eBioscience), followed by detection with biotinylated secondary antibodies and streptavidin‐horseradish peroxidase. Staining with Masson's trichrome was used to delineate the fibrous area. Stained sections were observed under an All‐in‐one Type Fluorescence Microscope (BZ‐8000; Keyence) using the BZ Analyzer Software (Keyence). Stained sections were digitally captured, and the percentage of staining (the stained area per total atherosclerotic lesion area) was calculated. Quantitative analyses of CD4+ T cells and Foxp3+ cells in the atherosclerotic lesion were performed by counting the positive‐stained cells, which was divided by total plaque area.
Flow Cytometric Analysis
Flow cytometry analysis was performed by Attune Acoustic Focusing Cytometer (Life Technologies) using FlowJo software (Tree Star). For Intracellular cytokine staining, cells were stimulated with 20 ng/mL phorbol 12‐myristate 13‐acetate (Sigma) and 1 mmol/L ionomycin (Sigma) for 5 hour in the presence of a GolgiStop (BD Bioscience). The antibodies used were as follows; anti‐CD16/CD32 (clone 2.4G2; BD Bioscience), anti‐CD4 (clone H129.19; BD Bioscience), anti‐CD25 (clone PC61; BD Bioscience), anti‐CD103 (clone M290; BD Bioscience), anti‐GITR (clone DTA1; BD Bioscience), anti‐CTLA‐4 (clone UC10; BD Bioscience), anti‐Foxp3 (clone FJK‐16s; eBioscience), anti‐CD11c (clone HL3; BD Bioscience), anti‐CD80 (clone 16‐10A1; BD Bioscience), anti‐CD86 (clone GL1; BD Bioscience), anti‐CD49b (clone HMa2; BD Bioscience), anti‐LAG3 (clone C9B7W; BD Bioscience), anti‐CD11b (clone M1/70; BD Bioscience), anti‐Ly6C (clone AL‐21; BD Bioscience), anti‐CD115 (clone AFS98; eBioscience), anti‐F4/80 (clone BM8; eBioscience), anti‐CD206 (clone C068C2; BioLegend), anti‐IFNγ (clone XMG1.2; eBioscience), anti‐IL‐4 (clone BVD4‐1D11; eBioscience), anti‐IL‐10 (clone JES5‐16E3; eBioscience) and isotype‐matched control antibodies.
Preparation of Peritoneal Macrophages
Ten‐week‐old ApoE−/− mice of each group were treated with 3% thiogycollate broth i.p. injection and sacrificed with isoflurane for peritoneal macrophage isolation after a 3‐day treatment as described previously.18 Cells were plated onto culture dishes with RPMI medium containing 10% FBS and incubated for 3 to 4 hours at 37°C and 5% CO2. The adhesive cells were used as macrophages for FACS analysis and RT‐PCR analysis.
Real‐Time RT‐PCR Analysis
Total RNA was extracted from aortas or peritoneal macrophages after perfusion with RNA later (Ambion) using the TRIzol reagent (Invitrogen). For RT, a PrimeScript RT reagent Kit (Takara) was used. Quantitative PCR was performed using a SYBER Premix Ex Taq (Takara) and an ABI PRISM 7500 Sequence Detection System (Applied Biosystems) according to comparative threshold cycle method following manufacturer's protocol. The following primers were used to amplify CD4, CD25, Foxp3, CTLA‐4, iNOS, MCP‐1, CXCL10, Arg I, Fizz1, Ym1, and GAPDH: CD4, 5′‐TCA CAC ATG AAG CAT GTC AGG‐3′ and 5′‐GCA CTG GTT AGA ATG TGA GTC TGG‐3′; CD25, 5′‐CTG ATC CCA TGT GCC AGG AA‐3′ and 5′‐AGG GCT TTG AAT GTG GCA TTG ‐3′; Foxp3, 5′‐CTC ATG ATA GTG CCT GTG TCC TCA A‐3′ and 5′‐AGG GCC AGC ATA GGT GCA AG‐3′; CTLA‐4, 5′‐CCT CTG CAA GGT GGA ACT CAT GTA ‐3′ and 5′‐AGC TAA CTG CGA CAA GGA TCC AA‐3′; iNOS, 5′‐GCA GAG ATT GGA GGC CTT GTG ‐3′ and 5′‐GGG TTG TTG CTG AAC TTC CAG TC‐3′; MCP‐1, 5′‐GCA TCC ACG TGT TGG CTC A‐3′ and 5′‐CTC CAG CCT ACT CAT TGG GAT CA‐3′; CXCL10, 5′‐TGA ATC CGG AAT CTA AGA CCA TCA A‐3′ and 5′‐AGG ACT AGC CAT CCA CTG GGT AAA G‐3′; Arg I, 5′‐GGG AAT CTG CAT GGG CAA C‐3′ and 5′‐GCA AGC CAA TGT ACA CGA TGT C‐3′; Fizz1, 5′‐CAG CTG ATG GTC CCA GTG AA‐3′ and 5′‐CAA GCA CAC CCA GTA GCA CTC‐3′; Ym1, 5′‐GTA GGC CTC AAC CTG GAC TG‐3′ and 5′‐CGT CAA TGA TTC CTG CTC CTG‐3′; GAPDH, 5′‐TGT GTC CGT CGT GGA TCT GA‐3′ and 5′‐TTG CTG TTG AAG TCG CAG GAG‐3′. The amplification reactions were performed in duplicate, and the fluorescence curves were analyzed with the software included with the ABI PRISM 7500 system. GAPDH was used as an endogenous control reference.
Statistical Analysis
Data were expressed as the mean±SEM. The Mann‐Whitney U test was used to detect significant differences between 2 groups. The Kruskal‐Wallis test was used to detect significant differences when comparing more than 3 groups, followed by post hoc Dunn's multi‐comparison test. A value of P<0.05 was considered statistically significant. For statistical analysis, GraphPad Prism version 6.0 (GraphPad Software) was used.
Results
Effects of CD3 Ab, IL‐2 Complex, or Combination Therapy on CD4+ T Cell Immune Responses
To compare the efficacy of CD3‐Ab, IL‐2 complex, and the combination therapy with CD3‐Ab and IL‐2 complex, 4 experimental groups were made as follows: IgG plus PBS‐treated (control‐treated), CD3‐Ab plus PBS‐treated (CD3‐Ab‐treated), IgG plus IL‐2 complex‐treated (IL‐2 complex‐treated), CD3‐Ab plus IL‐2 complex‐treated (combination‐treated) groups. As described in Figure 1A, ApoE−/− mice were intravenously treated with CD3‐Ab or control IgG daily for 5 consecutive days at 8 weeks of age, and then were intraperitoneally treated with IL‐2 complex or control PBS for 3 consecutive days at 9 weeks of age. At 10 weeks of age, lymphoid cells from spleen and LNs were analyzed by flow cytometry. We first investigated the efficacy of CD3‐Ab treatment on CD4+ T cells, and observed a marked reduction in CD4+ T cells in the spleen and LNs of CD3‐Ab‐treated mice, whereas the percentage of Foxp3+ Tregs and CD25+Foxp3+ Tregs within the CD4+ T cell population was not different between control‐treated and CD3‐Ab‐treated mice (Figures 1B through 1E). These results indicate that the atheroprotective effect of CD3‐Ab treatment could be mainly due to the depletion of Teffs in our experiments, although short‐term treatment with CD3‐Ab has been shown to increase the proportion of Foxp3+ Tregs at later time points.13 As treatment with IL‐2 complex has been reported to systemically increase Tregs but not Teffs,14–15 we next investigated the impact of IL‐2 complex on immune responses including Tregs and Teffs according to the protocol described in Figure 1A. The percentages of CD4+ T cells in the spleen and LNs were not different between control‐treated and IL‐2 complex‐treated mice (Figures 1B and 1C). We found a trend towards increase in CD4+Foxp3+ Tregs and CD4+CD25+Foxp3+ Tregs in the spleen and LNs of IL‐2 complex‐treated mice compared to control‐treated mice (Figures 1B, 1D, and 1E). To examine the combined effects of Teff elimination and Treg induction on immune responses, we treated ApoE−/− mice fed a high‐cholesterol diet with both CD3‐Ab and IL‐2 complex. We found a dramatic increase in CD4+Foxp3+ Tregs and CD4+CD25+Foxp3+ Tregs in the spleen and LNs of CD3‐Ab/IL‐2 complex‐treated mice, along with a trend towards decrease in CD4+ T cells in the spleen and LNs (Figures 1B through 1E). Notably, the Treg/Teff ratio was much higher in the spleen and LNs of the mice with the combination therapy compared with control‐treated or CD3‐Ab‐treated mice (Figure 1F). T regulatory type 1 (Tr1) cell is another Treg subset that does not express Foxp3 and can clearly be detected by surface markers CD49b and lymphocyte activation gene 3 (LAG‐3).19 We observed that the combination therapy markedly increased the number of splenic Tr1 cells (Figure 1G). Accordingly, these results indicate that the combination therapy can efficiently shift the Treg/Teff balance to Tregs.
Figure 1.

Effect of a novel combination therapy on Teffs and Tregs. A, Experimental design is shown. Arrows with circle represent intravenous injection with 50 μg anti‐CD3 antibody and arrows with square represent intraperitoneal injection with IL‐2/anti‐IL‐2 mAb complex (1 μg IL‐2 plus 5 μg anti‐IL‐2 mAb). For analysis of systemic immune responses, lymphocytes from spleen and LNs were prepared at 10 weeks of age. B, Representative results of CD4 and Foxp3 expressions in the spleen assessed by FACS. The graphs represent the percentage of CD4+ cells within the total splenocytes and the percentage of Foxp3+ cells within the CD4+ population. C through F, The percentage of (C) CD4+ T cells within total cells, (D) Foxp3+ Tregs and (E) CD25+ Foxp3+ Tregs within the CD4+ T cell population were determined by FACS. F, The ratio of CD4+Foxp3+ Tregs to CD4+Foxp3− Teffs was also determined as Treg/Teff ratio. G, The percentage of +CD49b+LAG‐3+ cells within CD4+ T cells were determined by FACS. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001. FACS indicates fluorescence activated cell sorting; IgG, immunoglobulin G; IL, interleukin; LAG‐3+, lymphocyte activation gene 3; LNs, lymph nodes; PBS, phosphate buffered saline; Teffs, effector T cells; Tregs, regulatory T cells.
Effects of CD3 Ab, IL‐2 Complex, or Combination Therapy on Treg‐Associated Molecules, DC Maturation and Th1/Th2 Balance
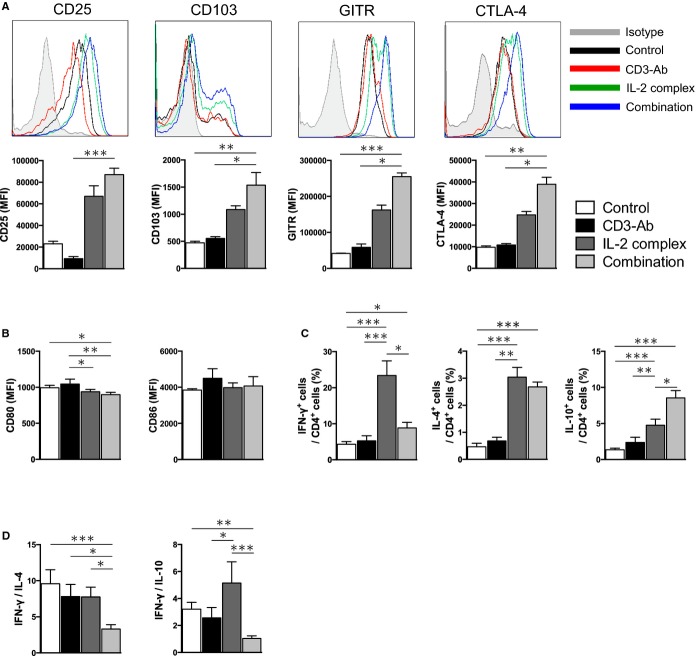
Next, the effects of each therapy on the expression of Treg‐associated molecules in Foxp3+ Tregs were determined by flow cytometry. Notably, Foxp3+ Tregs from CD3‐Ab/IL‐2 complex‐treated mice expressed higher levels of CD25, CD103, glucocorticoid‐induced TNF receptor family‐related gene/protein (GITR), cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4) compared to those from control‐treated or CD3‐Ab‐treated mice (Figure 2A), implying an activated phenotype of Tregs after the combination therapy. In addition, a trend towards increased expression of Treg‐associated molecules was seen in mice with IL‐2 complex alone. We also investigated the effect of each therapy on surface maturation markers CD80 and CD86 in splenic CD11c+ DCs. Neither CD3‐Ab nor IL‐2 complex monotherapy altered the expression of these maturation markers in splenic DCs, whereas we observed a modest but significant decrease in the expression of CD80, but not CD86, in splenic DCs of CD3‐Ab/IL‐2 complex‐treated mice (Figure 2B). To determine whether the combination treatment changed T‐cell responses and polarization, we examined cytokine secretion from CD4+ T cells by intracellular cytokine staining. We found that IL‐2 complex mono‐therapy dramatically increased the percentage of splenic IFN‐γ producing‐Th1 cells, IL‐4‐producing Th2 cells, and IL‐10‐producing CD4+ T cells compared to control‐treated mice (Figure 2C). Notably, co‐treatment with CD3‐Ab canceled the increase in IFN‐γ producing‐CD4+ T cells by IL‐2 complex treatment, while the increase in IL‐4 or IL‐10‐producing‐CD4+ T cells was still observed (Figure 2C). The Th1/Th2 ratio was much lower in the spleen of the mice with the combination therapy compared with mice in other groups (Figure 2D). Taken together, these results indicate that the combination therapy can induce activated phenotype of Tregs and the Th1/Th2 balance to Th2, which may contribute to a reduction in atherosclerosis development via suppressing inflammatory responses.
Figure 2.
Effects of the combination therapy on Treg‐associated molecules, DC maturation and Th1/Th2 responses. A, The expression levels of Treg‐associated markers were analyzed by FACS gating on CD4+Foxp3+ Tregs in the spleen. Histograms show mean fluorescence intensity (MFI). B, The expression levels of CD80 and CD86 were analyzed gating on CD11c+ DCs in spleens. C, Splenocytes from 10‐week‐old mice in each group were prepared and intracellular cytokine staining was performed. The graphs represent the frequencies of IFN‐γ, IL‐4, and IL‐10‐producing CD4+ T cells. D, The ratio of IFN‐γ‐producing CD4+ T cells to IL‐4 or IL‐10‐producing CD4+ T cells in the spleen was determined as Th1/Th2 ratio. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001. CTLA‐4 indicates cytotoxic T lymphocyte‐associated protein 4; DC, dendritic cell; GITR, glucocorticoid‐induced TNF receptor; IFN, interferon; IL, interleukin; Th, T helper type.
The Combination Therapy With CD3 Ab and IL‐2 Complex Inhibits Atherosclerotic Lesion Formation and Induces Stable Plaque Phenotype
To determine the effects of CD3‐Ab, IL‐2 complex, or combination therapy on atherosclerosis development, we assessed the atherosclerotic lesion formation of the 4 experimental groups. No adverse effects were observed in all groups throughout the experiments. The mice receiving combined therapy showed a decrease in plasma total cholesterol and LDL cholesterol levels and an increase in triglyceride levels compared with control‐treated mice, whereas no statistical differences in plasma lipid profiles were detected among the other 3 groups (Table). CD3‐Ab or IL‐2 complex monotherapy caused a modest but significant reduction in atherosclerotic lesion formation in the aortic root compared with control‐treated mice (61.6±2.8×104 μm2 in control‐treated mice, 50.6±1.7×104 μm2 in CD3‐Ab‐treated mice, 50.7±1.9×104 μm2 in IL‐2 complex‐treated mice; Figures 3A and 3B). Notably, CD3‐Ab/IL‐2 complex‐treated mice showed a further reduction in atherosclerotic lesion formation (37.2±1.9×104 μm2, Figures 3A and 3B) compared with control‐treated and each monotherapy mice. Consistent with this, the lipid content of the plaques in the aortic sinus was also significantly decreased in the CD3‐Ab/IL‐2 complex‐treated mice compared to control‐treated and each monotherapy mice (Figure 3C). In parallel with the cross‐sectional studies, we performed en face analysis of thoracic aortas, revealing a significant reduction in aortic plaque burden in CD3‐Ab/IL‐2 complex‐treated mice (3.74±0.12%) compared with control mice (5.53±0.23%) (Figure 3D).
Table 1.
Body Weight and Plasma Lipid Profiles
| Control | CD3‐Ab | IL‐2 Complex | Combination | |
|---|---|---|---|---|
| Body weight, g | 21.0±0.4 | 21.6±0.5 | 21.4±0.3 | 22.2±0.5 |
| Total‐cholesterol, mg/dL | 854.7±52.1 | 716.3±45.7 | 696.2±34.1 | 568.1±37.5* |
| HDL‐cholesterol, mg/dL | 5.1±0.7 | 7.0±0.6 | 5.5±0.3 | 7.1±0.6 |
| LDL‐cholesterol, mg/dL | 196.9±12.6 | 168.8±12.7 | 165.2±9.0 | 137.5±12.8* |
| Triglycerides, mg/dL | 26.0±3.8 | 37.6±4.0 | 35.7±4.8 | 42.5±4.7 |
Results are expressed as means±SEM. n=10 to 12 mice per group. HDL indicates high‐density lipoprotein; IL, interleukin; LDL, low‐density lipoprotein.
P<0.05 vs Control.
Figure 3.
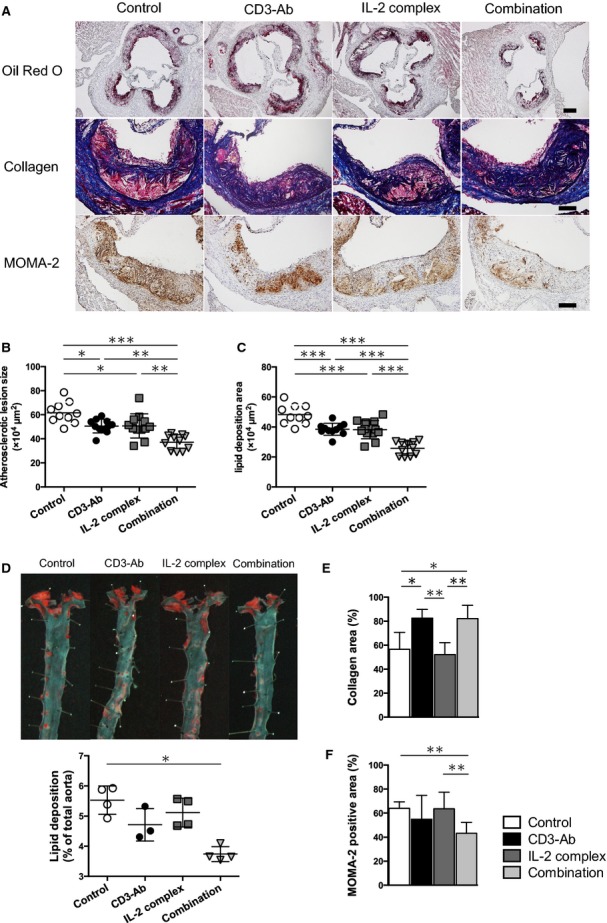
Effects of the combination therapy on the development of atherosclerosis and plaque compositions. A, Representative photomicrographs of Oil Red O staining, Masson's trichrome staining, MOMA‐2 staining in the aortic sinus of mice in each group. The black bar on Oil Red O staining represents 200 μm and black bars on the others represent 100 μm. B, Quantitative analysis of atherosclerotic lesion size in the aortic sinus was performed using Oil red O‐stained sections. Horizontal bars represent mean. n=10 to 12 mice per group. C, Quantitative analysis of Oil red O‐stained positive area (lipid content) in the aortic sinus. Horizontal bars represent mean. n=10 to 12 mice per group. D, Representative photomicrographs of Oil red O staining and quantitative analysis of atherosclerotic lesion size in the thoracic aortas in each group. Horizontal bars represent mean. n=3 to 4 mice per group. E and F, Quantitative analyses of Masson's trichrome staining (E, n=8 mice per group) and MOMA‐2 staining (F, n=10 to 12 mice per group) in the aortic sinus of mice in each group. *P<0.05, **P<0.01, ***P<0.001. IL indicates interleukin; MOMA‐2, monocyte/macrophage marker.
To determine the effects of each therapy on plaque composition, immunohistochemical studies of atherosclerotic lesions in the aortic sinus were performed. Collagen contents in atherosclerotic lesions were significantly increased in CD3‐Ab‐treated and CD3‐Ab/IL‐2 complex‐treated mice compared to control‐treated mice (Figures 3A and 3E). Although CD3‐Ab or IL‐2 complex monotherapy did not affect the recruitment of macrophages in atherosclerotic plaques, the lesions of CD3‐Ab/IL‐2 complex‐treated mice showed a significant reduction in the macrophage accumulation compared to control mice (Figures 3A and 3F).
The Combination Therapy With CD3 Ab and IL‐2 Complex Dramatically Enhances Regulatory Immune Responses in the Lesions
It has been reported that Tregs and Teffs migrate into atherosclerotic plaques and the lesional Treg/Teff balance may be important in controlling atherosclerotic process via diminishing inflammation within atherosclerotic plaques.20 Immunohistochemical analysis revealed that IL‐2 complex monotherapy did not affect the number of CD4+ T cells in atherosclerotic plaques, whereas CD3‐Ab monotherapy or combination therapy showed a trend toward reduction in CD4+ T cell infiltration compared with control mice (Figures 4A and 4B). Immunohistochemical studies of atherosclerotic lesions using anti‐Foxp3 antibody demonstrated a marked increase in the number of Foxp3+ Tregs within the plaque of IL‐2 complex‐treated or CD3‐Ab/IL‐2 complex‐treated mice (Figures 4A and 4C). In addition, expression analyses of T cell and Treg‐associated markers in the lesions by quantitative RT‐PCR revealed that the combination therapy significantly decreased the relative mRNA expression of CD4 and markedly increased the expressions of Treg‐associated markers such Foxp3, CD25, and CTLA‐4 (Figure 4D), which is consistent with the results of immunohistochemical analyses. Collectively, these results indicate that this novel combination therapy with CD3‐Ab and IL‐2 complex inhibits the infiltration of effector CD4+ T cells and promotes the migration of systemically expanded Tregs into the plaques in ApoE−/− mice, and a subsequent increase in the Treg/Teff ratio in the atherosclerotic lesions, as well as in the lymphoid organs, may contribute to the reduction of macrophage accumulation, the increment of collagen content and the substantial inhibition of the atherosclerotic lesion formation by regulating systemic and local inflammatory responses.
Figure 4.
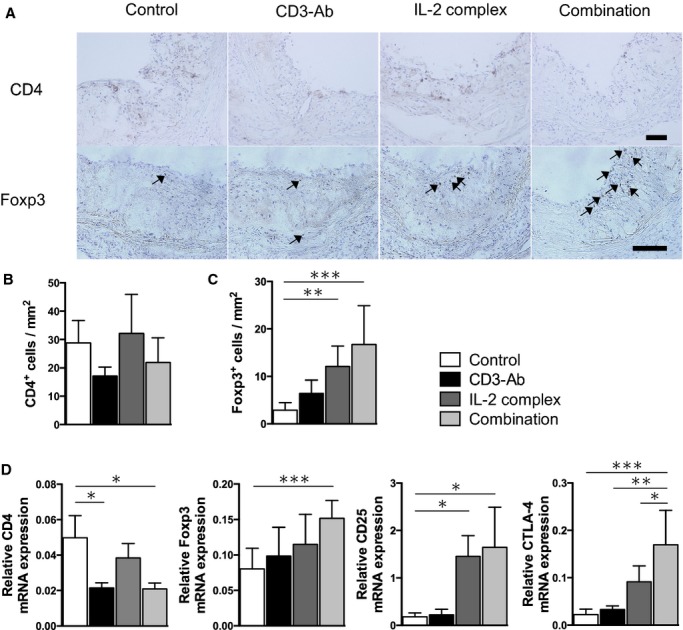
Effects of the combination therapy on aortic Teffs and Tregs. A, Representative photomicrographs of CD4 staining and Foxp3 staining in the aortic sinus of mice in each group. The black bar on each staining represents 100 μm. B and C, Quantitative analyses of CD4+ T cells (B) and Foxp3+ Tregs (C) in the aortic sinus of mice in each group. n=8 mice per group. D, Messenger RNA expressions of CD4, Foxp3, CD25 and CTLA‐4 in atherosclerotic aortas were quantified by quantitative RT‐PCR and normalized to GAPDH. n=5 to 6 mice per group. *P<0.05, **P<0.01, ***P<0.001. CTLA‐4 indicates cytotoxic T lymphocyte‐associated protein 4; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; IL, interleukin; RT‐PCR, real‐time polymerase chain reaction; Teffs, effector T cells; Tregs, regulatory T cells.
The Combination Therapy Induces Macrophage Polarization Toward a Less Inflammatory M2 Phenotype
To further reveal the mechanisms for reduced macrophage accumulation in the plaques, we examined the effects of the combination therapy on the monocyte number or macrophage phenotype change. We found that the number of CD11b+Ly6Chigh monocytes were significantly decreased in the spleen of CD3‐Ab/IL‐2 complex‐treated mice compared with control‐treated mice (Figure 5A) and those monocytes expressed lower levels of CD115 (CSF‐1 receptor), a growth factor receptor for CSF‐1 involved in promoting atherosclerosis.21 To examine whether the combination therapy affected the phenotype of macrophages in vivo, we induced inflammatory macrophage infiltration in the peritoneal cavity by thioglycollate injection as described previously.18 We collected thioglycollate‐induced peritoneal macrophages and performed flow cytometry and quantitative RT‐PCR analysis. Flow cytometry analysis of peritoneal macrophages revealed that the combination therapy significantly reduced CD11c+ M1 macrophages and increased CD206+ M2 macrophages compared with control mice (Figure 5B). In contrast, there were no significant differences in the percentage of CD11c+ M1 and CD206+ M2 macrophages among the other 3 groups. Consistent with these results, quantitative RT‐PCR analyses of peritoneal macrophages revealed that the combination therapy significantly decreased the expressions of M1 macrophage markers including iNOS, MCP‐1, and CXCL‐10, and increased the expressions of M2 macrophage markers including Arg I, Fizz1, and Ym‐1 (Figure 5C). Furthermore, we examined the mRNA expressions of iNOS and Arg I in atherosclerotic aortas by quantitative RT‐PCR and found decreased expression of iNOS and increased expression of Arg I in CD3‐Ab/IL‐2 complex‐treated mice compared with control‐treated mice (Figure 5D). These results suggest that athero‐protective effects of the combination therapy may be partly due to the reduced number of inflammatory monocytes or polarization of macrophages toward a less inflammatory phenotype, as a result of dramatic up‐regulation of regulatory immune responses.
Figure 5.
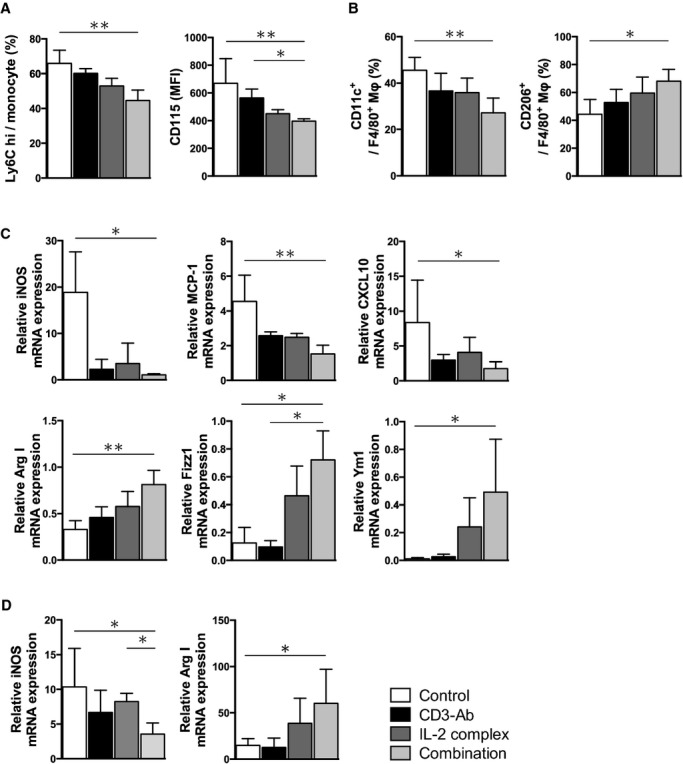
The combination therapy polarizes macrophages from the M1 to M2 state. A, The percentage of Ly6Chigh monocyte subset (left) and the expression levels of CD115 (CSF‐1 receptor) (right) in the spleen of 10‐week‐old mice in each group were examined by FACS. n=5 mice per group. B, The percentage of CD11c or CD206 in thioglycollate‐induced peritoneal macrophages (F4/80+) was analyzed by FACS. n=5 mice per group. C, Messenger RNA expressions of M1 macrophage markers (iNOS, MCP‐1, CXCL10) and M2 macrophage markers (Arg I, Fizz1, Ym‐1) in thioglycollate‐induced peritoneal macrophages were quantified by quantitative RT‐PCR and normalized to GAPDH. n=4 to 5 mice per group. D, Messenger RNA expressions of iNOS and Arg I in atherosclerotic aortas were quantified by quantitative RT‐PCR and normalized to GAPDH. n=5 to 6 mice per group. *P<0.05, **P<0.01, ***P<0.001. Arg indicates arginase; CSF, colony stimulating factor; CXCL, C‐X‐C motif chemokine; IL, interleukin; iNOS, inducible nitric oxide synthase; MCP, monocyte chemotactic protein; MFI, mean fluorescence intensity; RT‐PCR, real‐time polymerase chain reaction.
Discussion
In the present study, we examined the impact of Teff depletion, Treg expansion, and both intervention on the Teff and Treg immune responses in both lymphoid organs and atherosclerotic plaque and on the development of atherosclerosis, using CD3‐Ab, IL‐2 complex, or the combination of CD3‐Ab and IL‐2 complex, respectively. Monotherapy with CD3‐Ab or IL‐2 complex alone induced a modest but significant reduction in atherosclerotic plaque formation compared with untreated mice, whereas therapeutic intervention of both Teffs and Tregs using CD3‐Ab/IL‐2 complex combination therapy further prevented atherosclerosis development compared with each monotherapy group. The beneficial effects of the combination therapy were associated with a dramatic increase in the Treg/Teff ratio, up‐regulation of Treg activation markers in lymphoid organs and atherosclerotic plaques, the shift of Th1/Th2 balance to Th2 in lymphoid organs, a reduced number of proinflammatory Ly6Chigh monocytes in the spleen, and a conversion of the M1 to M2 macrophage state. This is the first report demonstrating that efficacy of IL‐2 complex therapy in prevention of atherosclerosis can be greatly enhanced when combined with CD3‐Ab. We believe that our findings are highly relevant for shaping future clinical strategies for preventing atherosclerotic diseases.
It is now clear that Teff immune responses are involved in accelerating atherosclerosis development and progression.2 Furthermore, based on previous reports and our findings, Foxp3+ Tregs play a critical role in suppressing atherogenesis via attenuating inflammatory processes, suggesting that modulation of Treg‐mediated immune responses represent a promising therapeutic approach for atherosclerosis. It has been demonstrated that local inflammatory responses in the target tissue should be controlled to preserve the suppressive function of Tregs.17 Because there may be many inflammatory cells including Teffs infiltrated in advanced atherosclerotic plaques, causing severe tissue inflammation, we should first eliminate such accumulated Teffs and down‐regulate the Teff immune responses to effectively inhibit inflammatory responses before Treg therapy. To address this issue, we examined the impact of Teff depletion, Treg expansion, and both interventions on the Teff and Treg immune responses in both lymphoid organs and atherosclerotic plaque and on the development of atherosclerosis, using CD3‐Ab, IL‐2 complex, or the combination of CD3‐Ab and IL‐2 complex, respectively. The finding that the combination therapy led to a significant reduction in atherosclerosis compared with CD3‐Ab or IL‐2 complex monotherapy was very striking. The beneficial effects of the combination therapy included a dramatic change in the Treg/Teff ratio and up‐regulation of Treg activation markers in lymphoid organs and atherosclerotic plaques. We found that the efficacy of IL‐2 complex therapy to induce Tregs can be greatly enhanced when combined with CD3‐Ab in ApoE−/− mice. Based on the above findings and previous data, we suppose that suppression of CD4+ T cell‐mediated immune responses before expanding Tregs may be essential for the induction of Tregs in plaques, maintenance of their anti‐inflammatory effects, and subsequent reduction of atherosclerosis. Our observations suggest that therapeutic intervention aimed at enhancing Treg‐mediated immune responses and limiting Teff‐mediated immune responses might represent a novel therapeutic approach for atherosclerotic diseases.
Recent studies have demonstrated that treatment with IL‐2 complex selectively increases CD4+CD25+Foxp3+ Tregs without affecting other immune cells including CD4+ T cells, CD8+ T cells, or natural killer cells, although the immune response and activation status of helper CD4+ T cells were not examined after treatment.14–15 However, as shown in Figure 2A and 2B, we unexpectedly observed that IL‐2 complex significantly induced the activation of both Teffs and Tregs. CD25 molecule is a component of the high‐affinity IL‐2 receptor (IL‐2R) and is functionally essential for Treg development by binding IL‐2.9 However, this molecule is expressed not only on Tregs but also on activated immune cells such as Teffs, for proliferation through IL‐2R signaling.22 Taking this into account, it is possible that IL‐2 complex affects the activation and proliferation of both Teffs and Tregs. Importantly, we showed that the additional treatment with CD3‐Ab in IL‐2 complex‐treated mice abolished the up‐regulation of atherogenic Th1 immune responses, while the up‐regulation of the immune responses such as IL‐4 or IL‐10 production from CD4+ T cells remained unchanged, suggesting that addition of CD3‐Ab may enhance the efficacy and reduce the risk of side effects associated with IL‐2 complex treatment. Recent evidence suggests that several subsets of Tregs differentiate from naïve T cells in the periphery under certain conditions and have similar immunological properties with thymus‐derived Foxp3+ Tregs.9 Previous studies showed that peripherally generated Tregs such as Tr1 cells or CD4+LAP+ (latency‐associated peptide) Tregs inhibit atherosclerosis in atherosclerosis‐prone mice by producing IL‐10 or TGF‐β, respectively.7,23 Our data indicate that induction of Tr1 cells may also contribute to the reduction of atherosclerosis following CD3‐Ab/IL‐2 complex combination therapy.
In the present study, we found that combination therapy dramatically increased not only the number of Foxp3+ Tregs but also the expressions of Treg activation markers in both lymphoid organs and atherosclerotic plaques. Our finding of marked up‐regulation of CTLA‐4, one of Treg activation markers, in Foxp3+ Tregs in CD3‐Ab/IL‐2 complex‐treated mice is interesting, because CTLA‐4‐dependent suppression of antigen‐presenting cell (APC) function including DCs by CD80 or CD86 down‐regulation is supposed to be a key mechanism for Treg‐mediated suppression.24 Such DCs with low expression levels of CD80/CD86 are called “tolerogenic DCs” and have been shown to contribute to the inhibition of atherosclerosis development and the regression of established plaques by inducing Tregs and inhibiting Teffs.25–26 Although we observed a modest decrease in the CD80 expression in splenic DCs of CD3‐Ab/IL‐2 complex‐treated mice, whether the lesional suppression mechanisms through this pathway are involved in the reduction of atherosclerosis remains unclear and further studies are needed. To the best of our knowledge, we believe that this combination therapy is the most effective approach for specifically promoting regulatory immune responses.
A recent study has demonstrated that hypercholesterolemia decreases the Treg/Teff ratio in atherosclerotic lesions via inhibiting Treg accumulation within plaques.20 This implies the possibility that increasing proportion of Tregs in atherosclerotic lesions could be a hopeful strategy to dampen plaque inflammation and prevent atherosclerosis, although further extensive experiments are required to identify the exact role of intraplaque Tregs in atherogenesis. In this study, we showed that the marked reduction of atherosclerosis from combination therapy was associated with a dramatic increase in the Treg/Teff ratio and up‐regulation of Treg activation markers not only in lymphoid organs but also in atherosclerotic plaques, implying that the beneficial effects of the combination therapy may partly be due to the induction of Tregs with activated phenotype in atherosclerotic plaques.
Monocytes infiltration from the peripheral blood to the subendothelial space or intima followed by macrophage differentiation is believed to be critical in initiation of atherosclerosis in humans and animals. The extent of macrophage recruitment into atherosclerotic plaques may depend on the cholesterol levels or monocyte number in the blood.27 In the present study, we found that the combination therapy significantly reduced proinflammatory Ly6Chigh monocytes compared to untreated or monotherapy groups, suggesting a possible contribution to decreased macrophage accumulation in atherosclerotic plaques. We found that thioglycollate‐induced peritoneal macrophages from CD3‐Ab/IL‐2 complex‐treated mice showed lower mRNA expressions of M1 markers and higher expressions of M2 markers. Regarding macrophage markers, a similar trend was observed in the atherosclerotic aortas of CD3‐Ab/IL‐2 complex‐treated mice. Th1 cells are reported to contribute to generation of M1 macrophages, whereas Th2 cells promote generation of M2 macrophages.28 In addition, IL‐10 derived from Tregs induces STAT3 (Signal Transducer and Activator of Transcription 3) activation and drives M2 polarization in mice with severe combined immunodeficiency.29 Collectively, we suppose that in CD3‐Ab/IL‐2 complex‐treated mice, phenotype change of thioglycollate‐induced peritoneal macrophages toward M2 phenotype may be attributable to dramatic expansion of Tregs as well as a shift from Th1 to Th2 immune responses, which represents one possible explanation for the athero‐protective effects of the combination therapy.
In conclusion, we have demonstrated that combined intervention using CD3‐Ab and IL‐2 complex induced a remarkable inhibition of atherosclerosis via dramatically increasing the Treg/Teff ratio in lymphoid organs and atherosclerotic plaques, which caused macrophage polarization toward a less inflammatory M2 phenotype. Our data imply that therapeutic intervention aimed at enhancing Treg‐mediated immune responses and limiting Teff‐mediated immune responses might represent a novel therapeutic approach for atherosclerotic diseases.
Sources of Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Number 23790849 (N. Sasaki), 25860601 (N. Sasaki), and 24591114 (Yamashita), research grants from the Global Center of Excellence (Kasahara), Suzuken Memorial Foundation (N. Sasaki), ONO Medical Research Foundation (N. Sasaki), Takeda Scientific Foundation (Yamashita and N. Sasaki), Senshin Medical Research Foundation (Yamashita), Mitsui Life Social Welfare Foundation (Yamashita), Yakult Bioscience Research Foundation (Yamashita), Uehara Memorial Foundation (Hirata), and The Japan Circulation Society Translational Research Foundation (Hirata).
Disclosures
None.
Acknowledgments
We would like to thank Tomomi Minami for technical assistance and Naoki Kitano for critical reading of the manuscript.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999; 340:115-126 [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204-212 [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN‐gamma potentiates atherosclerosis in apoE knock‐out mice. J Clin Invest. 1997; 99:2752-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ait‐Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006; 12:178-180 [DOI] [PubMed] [Google Scholar]
- 5.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T‐cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006; 114:2047-2055 [DOI] [PubMed] [Google Scholar]
- 6.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek‐Shaul T, Keren G, George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007; 27:893-900 [DOI] [PubMed] [Google Scholar]
- 7.Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K. Oral anti‐CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009; 120:1996-2005 [DOI] [PubMed] [Google Scholar]
- 8.Sasaki N, Yamashita T, Takeda M, Hirata K. Regulatory T cells in atherogenesis. J Atheroscler Thromb. 2012; 19:503-515 [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133:775-787 [DOI] [PubMed] [Google Scholar]
- 10.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013; 123:1323-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffens S, Burger F, Pelli G, Dean Y, Elson G, Kosco‐Vilbois M, Chatenoud L, Mach F. Short‐term treatment with anti‐CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation. 2006; 114:1977-1984 [DOI] [PubMed] [Google Scholar]
- 12.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF‐beta‐dependent mechanisms mediate restoration of self‐tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003; 9:1202-1208 [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L, Bluestone JA. CD3‐specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007; 7:622-632 [DOI] [PubMed] [Google Scholar]
- 14.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL‐2‐mAb complexes: induction of resistance to EAE and long‐term acceptance of islet allografts without immunosuppression. J Exp Med. 2009; 206:751-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A, Agrotis A. Cytokine therapy with interleukin‐2/anti‐interleukin‐2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012; 126:1256-1266 [DOI] [PubMed] [Google Scholar]
- 16.Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, van Eck M, van Berkel TJ, Kuiper J, van Puijvelde GH. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011; 218:53-60 [DOI] [PubMed] [Google Scholar]
- 17.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin‐specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007; 13:423-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita T, Kawashima S, Hirase T, Shinohara M, Takaya T, Sasaki N, Takeda M, Tawa H, Inoue N, Hirata K, Yokoyama M. Xenogenic macrophage immunization reduces atherosclerosis in apolipoprotein E knockout mice. Am J Physiol Cell Physiol. 2007; 293:C865-C873 [DOI] [PubMed] [Google Scholar]
- 19.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona‐Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG‐3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013; 19:739-746 [DOI] [PubMed] [Google Scholar]
- 20.Maganto‐Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet‐induced hypercholesterolemia. Circulation. 2011; 124:185-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaposhnik Z, Wang X, Lusis AJ. Arterial colony stimulating factor‐1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J Lipid Res. 2010; 51:1962-1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyman O, Sprent J. The role of interleukin‐2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012; 12:180-190 [DOI] [PubMed] [Google Scholar]
- 23.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E‐knockout mice. Circulation. 2003; 108:1232-1237 [DOI] [PubMed] [Google Scholar]
- 24.Takeda M, Yamashita T, Sasaki N, Hirata K. Dendritic cells in atherogenesis: possible novel targets for prevention of atherosclerosis. J Atheroscler Thromb. 2012; 19:953-961 [DOI] [PubMed] [Google Scholar]
- 25.Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Ishida T, Hirata K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010; 30:2495-2503 [DOI] [PubMed] [Google Scholar]
- 26.Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, Hirata K. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor‐deficient mice. Arterioscler Thromb Vasc Biol. 2011; 31:1963-1972 [DOI] [PubMed] [Google Scholar]
- 27.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly‐6Chi monocytes dominate hypercholesterolemia‐associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007; 117:195-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010; 11:889-896 [DOI] [PubMed] [Google Scholar]