Abstract
Background
Women have less risk of atherosclerotic cardiovascular disease compared with men up until midlife (ages 50 to 60), after which the gap begins to narrow post menopause. We hypothesized that the average lipid profile of women undergoes unfavorable changes compared with men after midlife.
Methods and Results
We examined lipids by sex and age in the Very Large Database of Lipids 10B (VLDL 10B) study. The analysis included 1 350 908 unique consecutive patients clinically referred for lipoprotein testing by density gradient ultracentrifugation from 2009 to 2011. Ratio variables were created for density subclasses of LDL‐C, HDL‐C, and VLDL‐C (LLDR, LHDR, LVDR, respectively). Men showed higher median LDL‐C values than women for ages 20 to 59, with the greatest difference in their 30s: 146 mg/dL in men versus 130 mg/dL in women. In contrast, women consistently had higher values after midlife (age 60), for example ages 70 to 79: 129 mg/dL in women versus 112 mg/dL in men. After age 50, women had higher LDL‐C each decade, for example 14% higher from their 30s to 50s, while HDL‐C concentrations did not differ. Women had more buoyant LDL‐C and HDL‐C (lower LLDR and LHDR) than men at all ages but the gap closed in higher age groups. In contrast, women had a generally denser VLDL‐C (higher LVDR) leading into midlife, with the gap progressively closing in higher age groups, approximating that of men in their 60s and 70s.
Conclusion
The narrowing sex differential in cardiovascular disease risk after midlife is mirrored by a higher total atherogenic lipoprotein cholesterol burden in women and a closer approximation of the less favorable density phenotype characteristic of men.
Keywords: aging, lipids, menopause, prevention, statins
Introduction
As women age, the serum concentrations of triglycerides (TG), low‐density lipoprotein cholesterol (LDL‐C), and total cholesterol (TC) surpass those in men.1 Menopause itself, ie, independent of chronologic age, is associated with an increased prevalence of dyslipidemia,2–3 but not hypertension or insulin resistance,2,4 independent of the effect of chronological aging. The Study of Women's Health Across the Nation (SWAN) showed an increase in TC and LDL‐C within 1 year of the final menstrual period and suggested that increases in these markers leads to an increase in cardiovascular disease (CVD).2 Women do experience an escalation in the incidence of CVD after menopause but continue to have significantly lower cardiovascular morbidity and mortality relative to age‐matched men despite higher median values of TG, LDL‐C, and TC.5 Indeed, after adjusting for multiple risk factors, at any given TC,6 LDL‐C,7–8 HDL‐C level,7,9 CV morbidity and mortality risk is higher in men.
Alternative features such as the length of exposure to hyperlipidemia, biological susceptibility, or the composition of lipoproteins may be similarly important determinants of sex‐related differences in CV risk. In a 26‐year follow‐up of the Framingham cohort of subjects with optimal lipoprotein levels, the incidence of coronary heart disease in men was 4‐fold higher than that in women, suggesting additional estimates of risk are needed.10
The composition of lipoproteins, such as LDL, may shift during menopause. Smaller dense LDL particles (pattern B, predominance of subclasses LDL3+4) may be more atherogenic than larger buoyant LDL particles (pattern A, predominance of subclasses LDL1+2) and are more prevalent in men,11 though the prevalence increases in women after menopause.12 In a large sample of the Framingham Offspring Study, Freedman et al, using nuclear magnetic resonance (NMR) spectroscopy, reported that the increased LDL‐C concentration in older women is largely driven by high concentrations of large, rather than small, LDL particles.13 The study also found that men had smaller HDL particles and larger very low‐density lipoprotein (VLDL) particles. A cross‐sectional analysis of a subset of the Study of Women's Health Across the Nation (SWAN) also found a trend towards smaller HDL particle size after menopause in women.14
The literature for age‐ and sex‐stratified lipoprotein subclass concentrations through menopause is limited. We used the Vertical Auto Profile (VAP) data to characterize differences by sex and age in major lipoproteins and their subclasses from the very large database of lipids. We hypothesized that the composition of major lipoprotein classes in women characterized by distribution of density subclass cholesterol becomes less favorable compared with men after midlife (ages 50 to 60).
Methods
VAP Analysis of Lipoprotein Subclasses
Comparisons in the literature on subclass lipoprotein subclass concentrations are made difficult due to varying assay methods used.15 The gold standard for density subclassification is analytical ultracentrifugation, but it is laborious. Alternatively, LDL size is often measured by gradient gel electrophoresis, which provides the predominant distribution of size but does not directly quantify the number of large and small particles. This is problematic as an overall small LDL phenotype may consist of varying numbers and combinations of large and small particles.16 NMR determines LDL size and subclass particle concentration from proton chemical shift and signal intensity.
We examined a cross‐sectional sample of VAP lipoprotein cholesterol data from 1 350 908 unique consecutive adults in the very large database of lipids, which were collected from 2009 onward and compiled in 2011.17 Vertical Auto Profile density gradient ultracentrifugation is a rapid and scalable method that directly measures major lipoprotein class and subclass cholesterol concentrations.18 Concentrations of lipoprotein classes and subclasses are quantified by deconvoluting the absorbance curve (obtained via centrifugation) using computerized quantitative analysis. Eight density subclass cholesterol concentrations were measured including 4 LDL (LDL1‐C—LDL4‐C), 2 HDL (HDL2‐C, HDL3‐C), and 2 VLDL (VLDL1+2‐C, VLDL3‐C) subclasses. Higher subclass numbers designate higher density. The Johns Hopkins Institutional Review Board declared an exemption from formal ethics approval for this study. Statistical analyses and graphical processing were performed using Stata Version 11.0 (StataCorp).
Density Ratios
To compare density ratios (Table 1), the densities of the lipoprotein classes were indexed by calculating the log transformed ratio of higher to lower subclasses of each lipoprotein class. In doing so, we created continuous variables to estimate density. A higher ratio indicates higher relative density within the major lipoprotein class. Log transformation converted density ratios to normally distributed variables. We have previously published this methodology, showing that the LDL density ratio (LLDR) strongly correlates with ultracentrifugation‐determined density (R=0.73, P<106) and LDL Max Time (R2=0.80).19
Table 1.
Determination of Density Ratios
| Abbreviation | Definition | Subclass Ratio* |
|---|---|---|
| LLDR | Logarithmic LDL density ratio | |
| LHDR | Logarithmic HDL density ratio | |
| LVDR | Logarithmic VLDL density ratio |
Lipoprotein subclasses are separated by vertical density gradient ultracentrifugation and their relative cholesterol concentrations are measured by deconvoluting the spectrophotometric absorbance curve.
Timing of Menopause
Information on each woman's final menstrual period was not available, thus age was used as a proxy for menopause. The average ages of perimenopause and menopause are 47.5 years and 51.3 years, respectively, with 97% of women reaching perimenopause by 55 years of age.20
Statistical Methods
In stratified analyses, we examined sex differences in lipoprotein classes and subclasses using linear regression adjusting for age. Non‐Gaussian variables were log‐transformed. With over 1.3 million samples, all hypothesis tests were highly statistically significant (P<0.001), thus relative differences in lipoprotein were presented to allow for clinically relevant interpretation. Relative differences were calculated as median values of men minus women divided by the population median. The interaction term, sex×age, was then used to examine whether sex modified the association between age and each marker.
After stratifying by sex, we calculated Pearson coefficients for the correlations between lipids and lipoprotein subclass cholesterol. The correlations were adjusted for age. Particularly robust associations were represented graphically using the lpoly function of Stata 11.0, which creates a kernel‐weighted local polynomial regression with smoothed values.
By sex, we determined the median, 25th and 75th percentiles for various potential risk markers from ages 30 to 80 to capture sex differences throughout mid‐ and late‐life. Male/female differences in select markers were examined, and linear regression was used to determine whether these sex differences were independent of age. Median values by age group for lipoproteins and ratio variables of interest were expressed graphically.
Results
Differences in Lipids and Lipoproteins
Overall, women had significantly higher median TC, LDL‐C, and HDL‐C concentrations while men had higher concentrations of TG and VLDL‐C (Table 2). All associations were significant at P<0.001 (99.9% confidence level); however, the absolute difference in non‐HDL‐C (6 mg/dL, 5% relative difference) by sex was modest. With adjustment for age, some sex differences were attenuated (Table 2, last column). However, differences remained particularly robust for TC (10% relative difference, age‐adjusted 19 mg/dL), HDL‐C (23%, 12.5 mg/dL), and density ratios (70%, 0.4; 17%, 0.2; and 16%, 0.1) for LLDR, LHDR, and LVDR, respectively. Table 3 presents the results of effect modification by sex. As shown, all measures were significant except HDL‐C (−0.003, P=0.08).
Table 2.
Selected Characteristics, by Sex
| Median (Interquartile Range) | Difference (Men–Women) | ||||
|---|---|---|---|---|---|
| Men (n=645 268) | Women (n=697 815) | Absolute* | % Relative* | Age‐Adjusted* | |
| Age, y | 58.2 | 59.1 | |||
| TC | 179 (150 to 209) | 197 (79 to 158) | −18 | −9.57 | −19 |
| TGs | 120 (85 to 176) | 111 (102.7) | 9 | 7.83 | 9 |
| LDL‐C | 104 (80 to 131) | 112 (89 to 139) | −8 | −7.41 | −8.7 |
| HDL‐C | 46 (39 to 55) | 58 (48 to 70) | −12 | −23.08 | −12.5 |
| VLDL‐C | 23 (18 to 30) | 21 (17 to 28) | 2 | 9.09 | 2.2 |
| Lp(a) | 6 (4 to 9) | 7 (5 to 10) | −1 | −16.67 | −1.4 |
| Non‐HDL‐C | 129 (102 to 160) | 135 (110 to 165) | −6 | −4.51 | −6.5 |
| LDL‐C/HDL‐C | 2.24 (1.66 to 2.96) | 1.90 (1.44 to 2.51) | 0.34 | 16.59 | 0.3 |
| LLDR | 0.88 (0.40 to 1.32) | 0.43 (0.31 to 0.89) | 0.45 | 70.31 | 0.4 |
| LDL4‐C | 13.40 (8.30 to 21.10) | 10.50 (6.40 to 16.20) | 2.9 | 24.58 | 3.4 |
| LDL3‐C | 41.60 (29.40 to 56.40) | 40.90 (29.20 to 55) | 0.7 | 1.69 | 0.4 |
| LDL2‐C | 11.90 (5.40 to 21.70) | 20.90 (11.60 to 32.50) | −9 | −55.21 | −8.4 |
| LDL1‐C | 11.80 (7.70 to 16.90) | 13.70 (9.10 to 19.30) | −1.9 | −14.96 | −2.1 |
| LHDR | 1.24 (1.05 to 1.44) | 1.05 (0.87 to 1.24) | 0.19 | 16.67 | 0.2 |
| HDL3‐C | 36 (30 to 42) | 43 (36 to 51) | −7 | −17.95 | −7.5 |
| HDL2‐C | 10 (8 to 13) | 15 (11 to 20) | −5 | −41.67 | −5.1 |
| LVDR | 0.29 (0.11 to 0.43) | 0.34 (0.19 to 0.45) | −0.05 | −15.63 | −0.1 |
| VLDL3‐C | 13 (11 to 16) | 12 (10 to 15) | 1 | 7.69 | 0.6 |
| VLDL(1+2)‐C | 9.60 (7.10 to 13.90) | 8.70 (6.70 to 12.20) | 0.9 | 9.89 | 1.5 |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio; TC, total cholesterol; TG, triglycerides; VLDL, very large database of lipids.
Difference between men and women: positive values indicate higher in men; P<0.001 for all comparisons.
([Men−women]/(population median))×100.
Adjusted absolute values using linear regression.
Table 3.
Results of Effect Modification by Sex for the Relationship Between Select Lipoproteins and Age
| Coefficient* | P Value | |
|---|---|---|
| TC | −0.61 | <0.001 |
| TG | −1.143 | <0.001 |
| LDL‐C | −0.45 | <0.001 |
| HDL‐C | −0.003 | 0.084 |
| VLDL‐C | −0.151 | <0.001 |
| Lp(a) | −0.002 | 0.004 |
| Non‐HDL‐C | −0.607 | <0.001 |
| LLDR | 0.002 | <0.001 |
| LHDR | −0.001 | <0.001 |
| LVDR | 0.002 | <0.001 |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio; TC, total cholesterol; TG, triglycerides; VLDL, very large database of lipids.
Results determined by linear regression using the interaction term sex×age.
LDL Density Ratio
Men had a higher LLDR (denser phenotype) than women (Table 2). While the concentrations of the denser subclasses, LDL3‐C and LDL4‐C, were similar in women and men, men had lower concentrations of the more buoyant subclasses, LDL1‐C and LDL2‐C.
Stratified by sex, Pearson correlation coefficients for the dependence of select characteristics and subclasses are shown in Table 4. Higher HDL‐C concentrations were associated with a lower, or more buoyant LLDR (−0.34 for men, −0.37 for women). TG was modestly correlated with LLDR (0.21 for both sexes). To explore these correlations further, Figure 1 shows the relationship between LLDR (top panel) across concentrations of TG and HDL‐C. TG and HDL‐C concentrations were restricted from the 5th to 95th percentile to represent the general population, avoiding extreme dyslipidemia phenotypes. LLDR was higher for both sexes with higher TG serum concentrations (top panel, left column). Differences in LLDR by sex persisted when comparisons were made across similar concentrations of HDL‐C (top panel, right column).
Table 4.
Relationship of Select Lipid and Lipoprotein Concentrations With Subclass Ratios and Concentrations
| Subclass | Sex | TG | LDL‐C | HDL‐C | VLDL‐C |
|---|---|---|---|---|---|
| LLDR | Men | 0.21 | −0.12 | −0.34 | 0.1 |
| Women | 0.21 | −0.12 | −0.37 | 0.1 | |
| LDL4‐C | Men | 0.31 | 0.33 | −0.23 | 0.27 |
| Women | 0.18 | 0.17 | −0.18 | 0.12 | |
| LDL3‐C | Men | 0.12 | 0.87 | 0.05 | 0.21 |
| Women | 0.17 | 0.8 | −0.09 | 0.23 | |
| LDL2‐C | Men | −0.28 | 0.43 | 0.43 | −0.17 |
| Women | −0.29 | 0.57 | 0.45 | −0.17 | |
| LDL1‐C | Men | 0.33 | 0.83 | 0 | 0.49 |
| Women | 0.36 | 0.75 | −0.06 | 0.5 | |
| LHDR | Men | 0.2 | 0.1 | −0.47 | 0.17 |
| Women | 0.24 | 0.06 | −0.57 | 0.23 | |
| HDL3‐C | Men | −0.49 | 0.13 | 0.97 | −0.46 |
| Women | −0.5 | 0.1 | 0.97 | −0.49 | |
| HDL2‐C | Men | −0.41 | 0 | 0.87 | −0.38 |
| Women | −0.44 | 0.02 | 0.92 | −0.42 | |
| LVDR | Men | −0.76 | −0.22 | 0.38 | −0.73 |
| Women | −0.61 | −0.21 | 0.29 | −0.62 | |
| VLDL3‐C | Men | 0.87 | −0.43 | −0.43 | 0.97 |
| Women | 0.89 | 0.31 | −0.48 | 0.97 | |
| VLDL(1+2)‐C | Men | 0.93 | −0.46 | −0.46 | 0.98 |
| Women | 0.91 | 0.31 | −0.47 | 0.97 |
Values are Pearson correlation coefficients; negative value represents inverse relationship. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio; TG, triglycerides; VLDL, very large database of lipids.
Figure 1.
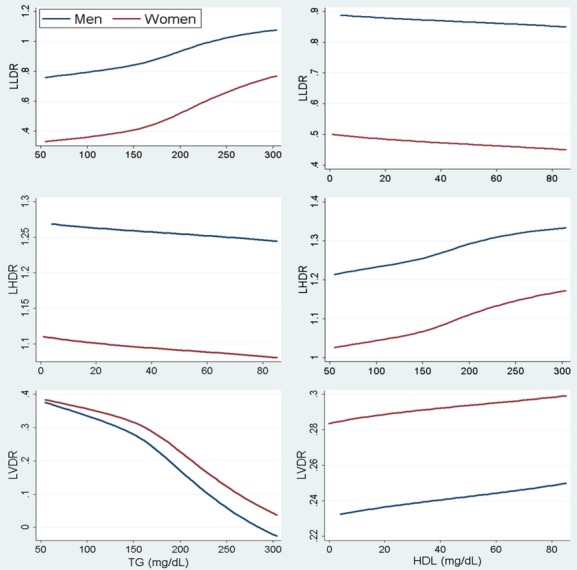
Relationship of LLDR (top), LHDR (middle), and LVDR (bottom) with concentrations of TG (left) and HDL‐C (right). HDL‐C indicates high‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio; TG, triglycerides.
Table 5 presents the median (interquartile range) of various lipoprotein variables by sex. Ages 30 through 79 were chosen to highlight the pre‐, peri‐, and post‐menopausal periods in women. Median (25th, 75th percentile) values throughout life are shown in Figure 2. LDL‐C was more strongly correlated with age in women than in men, though both associations were modest (correlation coefficients; 0.29 vs 0.09, respectively). LDL‐C in women trailed men through middle age but this trend reversed beginning in their 50s. Women's higher LDL‐C in ages 50 through 79 was primarily driven by the more buoyant LDL1‐C and LDL2‐C. This, along with lower LLDR for men in late life, accounts for a convergence of LLDR with age. While densities tend to converge, women still maintain a more buoyant LLDR relative to age‐matched men into late life (Figure 3).
Table 5.
Median and Interquartile Range of Select Lipoproteins by Sex and Density Ratios, Stratified by Age
| Age, y | 30 to 39 (n=76 127) | 40 to 49 (n=207 640) | 50 to 59 (n=329 173) | 60 to 69 (n=325 420) | 70 to 79 (n=210 077) | R 2 * | Correlation Coefficient* | |
|---|---|---|---|---|---|---|---|---|
| LDL‐C, mg/dL | Men | 118 (96 to 142) | 118 (95 to 143) | 121 (97 to 146) | 99 (77 to 125) | 89 (70‐115) | 0.01 | 0.09 |
| Women | 108 (89 to 130) | 114 (94 to 138) | 111 (86 to 137) | 116 (91 to 143) | 105 (82 to 133) | 0.08 | 0.29 | |
| Non‐HDL‐C, mg/dL | Men | 146 (121 to 174) | 147 (121 to 175) | 138 (110 to 167) | 123 (98 to 153) | 112 (91 to 141) | 0.08 | −0.28 |
| Women | 130 (108 to 155) | 137 (114 to 163) | 144 (118 to 173) | 139 (112 to 169) | 129 (104 to 160) | 0.00 | −0.04 | |
| LLDR | Men | 0.84 (0.36 to 1.28) | 0.88 (0.41 to 1.31) | 0.90 (0.43 to 1.33) | 0.91 (0.44 to 1.36) | 0.89 (0.42 to 1.33) | 0.01 | 0.09 |
| Women | 0.48 (0.02 to 0.95) | 0.48 (0.01 to 0.96) | 0.40 (−0.07 to 0.87) | 0.42 (−0.03 to 0.89) | 0.45 (−0.01 to 0.90) | 0.00 | −0.06 | |
| LHDR | Men | 1.30 (1.12 to 1.48) | 1.29 (1.10 to 1.48) | 1.27 (1.10 to 1.46) | 1.24 (1.05 to 1.43) | 1.19 (1.00 to 1.39) | 0.01 | −0.12 |
| Women | 1.08 (0.91 to 1.27) | 1.08 (0.90 to 1.26) | 1.07 (0.89 to 1.25) | 1.06 (0.88 to 1.24) | 1.03 (0.85 to 1.22) | 0.00 | −0.06 | |
| LVDR | Men | 0.22 (0.01 to 0.37) | 0.23 (0.03 to 0.38) | 0.27 (0.09 to 0.41) | 0.31 (0.14 to 0.44) | 0.34 (0.18 to 0.46) | 0.03 | 0.17 |
| Women | 0.34 (0.19 to 0.45) | 0.33 (0.18 to 0.45) | 0.33 (0.18 to 0.44) | 0.33 (0.19 to 0.44) | 0.34 (0.20 to 0.45) | 0.01 | 0.10 | |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio.
Statistical tests examined whether sex modified the association between age and each lipoprotein.
Correlation coefficient taken from square root of R2.
Figure 2.
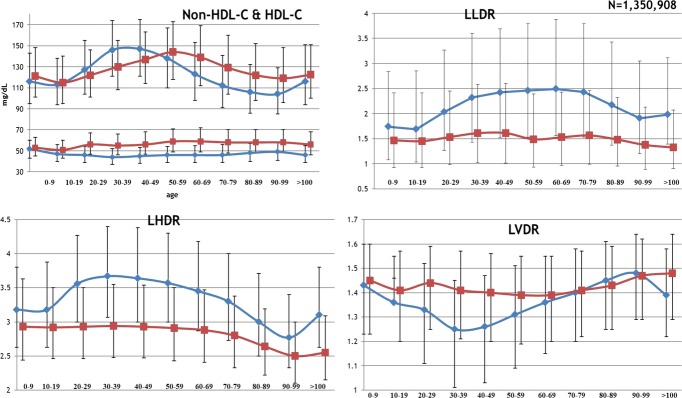
Median and interquartile ranges for selected characteristics. HDL‐C indicates high‐density lipoprotein cholesterol; LHDR, logarithmic HDL density ratio; LLDR, logarithmic LDL density ratio; LVDR, logarithmic VLDL density ratio.
Figure 3.
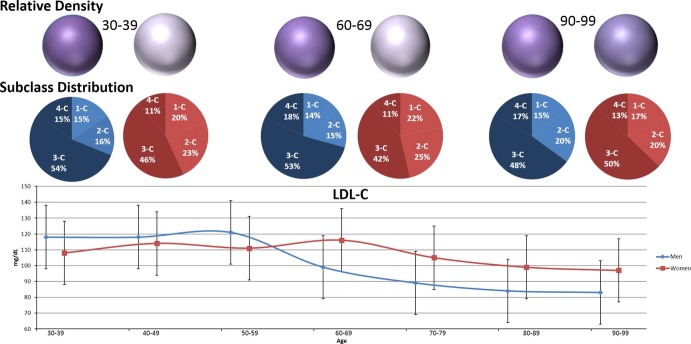
LDL‐C and density through late life, by sex. Lower panel shows median LDL‐C (directly measured) with interquartile range, by sex through life. Middle panel shows the ratio of dense subclasses (darker) to buoyant subclasses (lighter) at select ages to highlight the pre‐, peri‐, and post‐menopausal time periods. Upper panel shows density relative to men, age 30 to 39. Upper panel shows LLDR or density expressed as color saturation. Darker, or more saturated purple reflects higher LLDR. As shown, while LDL‐C is higher for women than for men from age 60 and beyond, this is driven by a greater proportion of the buoyant subclasses leading to a preservation of the advantageous density phenotype. With advanced age, sex differences in density narrow. LDL‐C indicates low‐density lipoprotein cholesterol; LLDR, logarithmic LDL density ratio.
HDL Density Ratio
Men had a higher LHDR and thus denser HDL‐C phenotype than women (Table 2).
Higher HDL‐C concentrations (Table 4) were associated with a lower (more buoyant) LHDR (Pearson coefficient −0.47 for men and −0.57 for women). Similar to LLDR, the relationship between TG concentrations and LHDR was modestly correlated (0.20, 0.24 for men and women, respectively). The relationship across TG concentrations (Figure 1, middle panel, left column) was linear. Men had higher LHDR throughout but the sex difference was reduced by taking into account TG concentrations. As with LLDR, sex differences in LHDR were not reduced across HDL‐C concentrations (middle panel, right column).
Although HDL‐C concentrations in both men and women were relatively unchanged from age 30 through 79 (Figure 2) both sexes had a more buoyant phenotype (lower LHDR) from age 30 forward. The differences in LHDR by sex converged with late age but, like LLDR, women maintained a lower LHDR, and thus a more buoyant phenotype, into late life.
VLDL Density Ratio
Table 2 shows that men had a lower LVDR (more buoyant VLDL‐C phenotype) than women by a relative difference of 16%.
TG and VLDL‐C concentrations correlated inversely with LVDR and positively with VLDL‐C subclasses (Table 4). However, a higher HDL‐C was associated with higher LVDR in men (0.38) and women (0.29). Pearson coefficients showed a stronger inverse relationship of LVDR to TG concentrations in men (−0.76) than in women (−0.61). LVDR significantly decreased as TG concentrations increased (Figure 1; bottom panel, left column). The differences in LVDR by sex were also greatly reduced when comparisons were made at similar TG concentrations. Comparisons at similar HDL‐C concentrations did not appreciably affect the differences in LVDR between men and women.
Table 5 shows that across ages 30 through 79, women held a higher LVDR (denser phenotype), but this difference narrowed with late age. Unlike both LLDR and LHDR, the curves converge to the point where sex differences were minimal at ages 80 and older (Figure 2).
Discussion
In an analysis of ultracentrifugation data from over 1.3 million subjects, we found that men tend to have denser LDL and HDL, but more buoyant VLDL relative to age‐matched women. By age 60, at which point nearly all women have reached menopause, and throughout the remainder of life, women have higher non‐HDL‐C and LDL‐C. The higher LDL‐C in women is primarily driven by the more buoyant subclasses. Total HDL‐C does not appreciably change with age for both sexes but in late life HDL2‐C predominates. Thus, at the time of menopause and beyond, women's lipid and lipoprotein advantage narrows.
Despite higher conventional risk markers (non‐HDL‐C, LDL‐C), women retain advantageous density ratios, which may partly explain the residual CVD risk differential. Alternatively, the higher CVD burden into late age for men may relate to earlier peaking LDL‐C and non‐HDL‐C levels and, thus, a longer period of exposure to conventional lipid and lipoprotein related atherogenic risk (Figure 2).
The strengths of this study include its large size and the physical separation of lipoprotein subclasses by ultracentrifugation. The findings are concordant with other cross‐sectional,21–29 and prospective cohort studies29 on major lipoproteins and closely parallel subclass analyses by NMR13 and GGE.12
Of the potential lipoprotein density distribution associations examined in our study, the small and dense LDL phenotype has the most robust support for associations with CVD.30–32 Our results show an age‐related decrease in density for men. The SWAN study showed an increase in LDL‐C in women within one year of the final menstrual period that was independent of chronological aging.2 Our findings are compatible with the SWAN study. Additionally, using measures beyond the standard lipid profile, we show that the driving force behind women's higher LDL‐C at peri‐menopause is the more buoyant (“larger”) subclass. We postulate that this could partly explain why, at equivalent concentrations of LDL‐C, women continue to retain a survival advantage in coronary artery disease outcomes beyond middle age.7,33 Indeed, in a large sub‐analysis of the Women's Health Study, Mora et al recently showed that women who had a discordantly low LDL particle concentration relative to LDL‐C had a better coronary prognosis than their LDL‐C alone would predict.34 Such discordance likely reflects larger, more buoyant LDL particles.
The independence of LDL density phenotype or size as a predictor of CV risk has been questioned due to residual confounding from other lipoproteins and triglycerides and heterogeneity in methods of measurement.16 Not all studies report CVD risk associated with small, dense LDL particles independent of TG, HDL‐C, and apoB concentrations.32,35–37 In the present analysis, sex differences in LDL density phenotype were minimally reduced when associations at equivalent HDL‐C concentrations are made; indeed, we found that only 14% of the variance in LLDR could be attributed to HDL‐C. For TG, the associations were also weak (4% variance) and the correlations between LLDR and TG concentrations were modest in both sexes (0.21, both sexes).
It is suggested that the HDL‐C gap between sexes should narrow with age secondary to progressive estrogen withdrawal, resulting in lower serum HDL particle concentration.38 Further, “andropause” is thought to lead to increased HDL‐C concentration in older men39 as was shown in the SWAN and Rancho Bernardo studies, both of which used longitudinal samples.2,29 The SWAN study showed a decrease in HDL‐C within one year from the final menstrual period that was independent of age. Our study did not confirm this finding. Rather, higher HDL levels persisted for women across all ages with minimal change for both sexes beyond middle age raising the possibility of survival bias in our cross‐sectional analysis. We found HDLR lowered with age in both sexes.
The relationship of HDL subclasses to cardiovascular disease is less clear and the inverse association between density and size is less correlated than it is for the LDL subclasses.40 A commonly cited belief is that the more buoyant HDL2‐C is characteristic of lower CV risk, as it is over‐represented in centenarians41–43 and low HDL2‐C is linked to coronary heart disease in some studies.44–45 However, as HDL2‐C is the more buoyant, cholesterol‐rich particle, an over‐representation of that subclass may represent inefficiency or impaired rates of reverse cholesterol transport. Low HDL3‐C is also a key driver of risk in primary and secondary prevention populations, and mounting evidence supports it as being associated with atheroprotection and reduced risk for CV events.46
The role that cholesteryl ester transfer protein (CETP) plays in HDL subclass metabolism in older age is controversial.47 Whether CETP deficiency is anti‐atherogenic through over‐representation of HDL2‐C or the opposite due to a deficiency in reverse transport is unresolved. With advanced aging, CETP activity decreases,48 which may account for our findings of higher HDL2‐C and lower HDL3‐C with age in both sexes. The finding of lower density and larger size was also reported by Freedman et al using NMR.13
The relationship of VLDL density to cardiovascular disease risk is complex. Increased VLDL size (TG loaded, measured by NMR, composite of apoB100 and B48 particles), independent of aggregate TG concentration, is associated with coronary artery disease and coronary calcium.44,49 However, small, dense VLDL is cholesterol rich (remnant particle of VLDL) and is also atherogenic as it is considered a remnant lipoprotein.50 In this study, at equivalent TG concentrations above 40 mg/dL, men maintained lower LVDR or density than did women. With age, LVDR in men increased, matching that of women by age 70. Accordingly, the age‐adjusted correlation was stronger in men (R=0.17 vs 0.08) for this trend.
High TG and LDL‐C concentrations correlated with a lower LVDR. Physiologically, this makes sense. In the setting of hypertriglyceridemia, VLDL particles are highly enriched with triglycerides. If the hypertriglyceridemia results from a functional lipoprotein lipase deficiency state, there will be activation of cholesterol ester transfer protein, which engages in a 1:1 stoichiometric exchange of cholesterol ester for triglyceride. This will lead to the enrichment of LDL particles with triglycerides, making them better substrates for lipolysis by hepatic lipase, resulting in the production of large numbers of small, dense LDL particles that are cholesterol depleted. LDL density is high since there is greater protein content relative to cholesterol ester and TG content. Increased hepatic VLDL secretion and VLDL size (decreased density) have been associated with conditions such as insulin resistance (also associated with reduced lipoprotein lipase activity) that promote the formation of small, dense LDL particles.51 In this study, we found a Pearson correlation of −0.31 between LVDR and LLDR supporting this previously described association.
Limitations
This analysis is cross‐sectional and thus subject to all biases associated with observational studies, especially survivor bias (ie, people with higher HDL‐C may survive longer; thus we are unable to detect age‐related HDL‐C decline within individuals). Cohort bias, referring to secular changes in lipids such that persons born in different periods have differences in lipoproteins at any given age, is also unaccounted for in our cross‐sectional analysis. Prospective studies are necessary to truly detect age‐related changes, though our results are concordant with most prospective studies. While our data allow cross‐sectional lipid and lipoprotein comparisons across the various stages of life, we do not have cardiovascular morbidity or mortality data with which to correlate our findings. Further, we do not have access to data on use and duration of lipid‐modifying therapies or specific prevalent medical diagnoses, extended demographics, or biometric data such as weights and body mass indices. Similarly, we do not have data on indications for diagnostic lipid testing. Thus, we cannot exclude treatment effects or participation bias.
The Rancho Bernardo study, whose findings match those of the present analysis, reported that behavioral factors, such as weight change and smoking status, are the most important factors associated with lipid change in the elderly; however, these analyses did not completely account for age‐related decrements.29 As we do not have information on menopausal status or estrogen levels, we are unable to precisely adjust for the timing of menopause, so it is unclear exactly what sex differences are driven by age vs. estrogen/progesterone withdrawal. Finally, our results were obtained through ultracentrifugation and while they closely match those of Freedman et al using NMR, we recognize, especially when considering size and density relationships, that these methods are not transposable.
Conclusion
In conclusion, after middle age we show a higher total atherogenic lipoprotein cholesterol burden in women and a simultaneous shift towards the lipoprotein phenotype characteristic of men. Although subclass phenotypes converge, they do not cross and this may account, in part, for the residual sex differential in CVD outcomes in late life.
Sources of Funding
Atherotech provided the investigators with de‐identified data generated from commercial lipid analyses. This was an investigator‐initiated study and Atherotech did not provide payments for the research or writing of the manuscript. Atherotech did not participate in the analysis of the data or influence the conclusions. Drs Swiger, Martin, and Jones had unrestricted access to study data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. Dr Martin is supported by the Pollin Cardiovascular Prevention Fellowship, as well as the Marie‐Josée and Henry R. Kravis endowed fellowship. Dr Blumenthal is supported by the Kenneth Jay Pollin Professorship in Cardiology.
Disclosures
Drs Martin and Jones are listed as co‐inventors on a pending patent filed by Johns Hopkins University for a method of low‐density lipoprotein cholesterol estimation. Dr Toth: medical advisory board for Atherotech, Inc, compensation for consultancy and lecturers from Abbott Laboratories, Aegerion, Amgen, Amylin, AstraZeneca, GlaxoSmithKline, Kowa, and Merck & Co.
References
- 1.Godsland IF, Wynn V, Crook D, Miller NE. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J. 1987; 114:1467-1503 [DOI] [PubMed] [Google Scholar]
- 2.Matthews K a, Crawford SL, Chae CU, Everson‐Rose SA, Sowers MF, Sternfeld B, Sutton‐Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009; 54:2366-2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torng P‐L, Su T‐C, Sung FC, Chien K‐L, Huang S‐C, Chow S‐N, Lee Y‐T. Effects of menopause on intraindividual changes in serum lipids, blood pressure, and body weight–the Chin‐Shan Community Cardiovascular Cohort study. Atherosclerosis. 2002; 161:409-415 [DOI] [PubMed] [Google Scholar]
- 4.Guthrie JR, Ball M, Dudley EC, Garamszegi CV, Wahlqvist ML, Dennerstein L, Burger HG. Impaired fasting glycaemia in middle‐aged women: a prospective study. Int J Obes Relat Metab Disord. 2001; 25:646-651 [DOI] [PubMed] [Google Scholar]
- 5.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age‐related cardiovascular mortality. PLoS One. 2013; 8:e63347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isles CG, Hole DJ, Gillis CR, Hawthorne VM, Lever AF. Plasma cholesterol, coronary heart disease, and cancer in the Renfrew and Paisley survey. BMJ. 1989; 298:920-924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs DR, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow‐up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990; 131:32-47 [DOI] [PubMed] [Google Scholar]
- 8.Gordon DJ. Role of circulating high‐density lipoprotein and triglycerides in coronary artery disease: risk and prevention. Endocrinol Metab Clin North Am. 1990; 19:299-309 [PubMed] [Google Scholar]
- 9.Stensvold I, Urdal P, Thürmer H, Tverdal A, Lund‐Larsen PG, Foss OP. High‐density lipoprotein cholesterol and coronary, cardiovascular and all cause mortality among middle‐aged Norwegian men and women. Eur Heart J. 1992; 13:1155-1163 [DOI] [PubMed] [Google Scholar]
- 10.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26‐year follow‐up of the Framingham population. Am Heart J. 1986; 111:383-390 [DOI] [PubMed] [Google Scholar]
- 11.McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PW, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein subfraction distribution. Results from the Framingham Offspring Study. Arteriosclerosis; 7:483-490 [DOI] [PubMed] [Google Scholar]
- 12.Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, Castelli WP, Schaefer EJ. LDL particle size distribution. Results from the Framingham Offspring Study. Arterioscler Thromb. 1992; 12:1410-1419 [DOI] [PubMed] [Google Scholar]
- 13.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D'Agostino RB, Wilson PWF, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004; 50:1189-1200 [DOI] [PubMed] [Google Scholar]
- 14.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009; 169:1352-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks FM. Low‐density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003; 88:4525-4532 [DOI] [PubMed] [Google Scholar]
- 16.Mora S. Advanced lipoprotein testing and subfractionation are not (yet) ready for routine clinical use. Circulation. 2009; 119:2396-2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin SS, Blaha MJ, Toth PP, Joshi PH, McEvoy JW, Ahmed HM, Elshazly MB, Swiger KJ, Michos ED, Kwiterovich PO, Kulkarni KR, Chimera J, Cannon CP, Blumenthal RS, Jones SR. Very large database of lipids: rationale and design. Clin Cardiol. 2013; 36:641-64810.1002/clc.22214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006; 26:787-802 [DOI] [PubMed] [Google Scholar]
- 19.Ahmed HM, Elshazly MB, Martin SS, Blaha MJ, Kulkarni KR, Jones SR. Ratio of dense to buoyant LDL subclass is associated with LDL density phenotype (VLDLx20105). 2013;:1–5
- 20.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992; 14:103-115 [DOI] [PubMed] [Google Scholar]
- 21.Heiss G, Tamir I, Davis CE, Tyroler HA, Rifkand BM, Schonfeld G, Jacobs D, Frantz ID. Lipoprotein‐cholesterol distributions in selected North American populations: the lipid research clinics program prevalence study. Circulation. 1980; 61:302-315 [DOI] [PubMed] [Google Scholar]
- 22.Moulopoulos SD, Adamopoulos PN, Diamantopoulos EI, Nanas SN, Anthopoulos LN, Iliadi‐Alexandrou M. Coronary heart disease risk factors in a random sample of Athenian adults. The Athens Study. Am J Epidemiol. 1987; 126:882-892 [DOI] [PubMed] [Google Scholar]
- 23.Heitmann BL. The effects of gender and age on associations between blood lipid levels and obesity in Danish men and women aged 35‐65 years. J Clin Epidemiol. 1992; 45:693-702 [DOI] [PubMed] [Google Scholar]
- 24.Schaefer EJ, Lamon‐Fava S, Cohn SD, Schaefer MM, Ordovas JM, Castelli WP, Wilson PW. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res. 1994; 35:779-792 [PubMed] [Google Scholar]
- 25. Plasma lipid distributions in selected North American populations: the Lipid Research Clinics Program Prevalence Study. The Lipid Research Clinics Program Epidemiology Committee. Circulation. 1979; 60:427-439 [DOI] [PubMed] [Google Scholar]
- 26.Newschaffer CJ, Bush TL, Hale WE. Aging and total cholesterol levels: cohort, period, and survivorship effects. Am J Epidemiol. 1992; 136:23-34 [DOI] [PubMed] [Google Scholar]
- 27.Curb JD, Reed DM, Yano K, Kautz JA, Albers JJ. Plasma lipids and lipoproteins in elderly Japanese‐American men. J Am Geriatr Soc. 1986; 34:773-780 [DOI] [PubMed] [Google Scholar]
- 28.Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA, Borhani NO, Wong ND, O'Leary DH. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation. 1992; 86:858-869 [DOI] [PubMed] [Google Scholar]
- 29.Ferrara A, Barrett‐Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women : the Rancho Bernardo Study 1984 1994. Circulation. 1997; 96:37-43 [DOI] [PubMed] [Google Scholar]
- 30.Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low‐density lipoprotein size, pravastatin treatment, and coronary events. JAMA. 2001; 286:1468-1474 [DOI] [PubMed] [Google Scholar]
- 31.Hulthe J, Wiklund O, Bondjers G, Wikstrand J. LDL particle size in relation to intima‐media thickness and plaque occurrence in the carotid and femoral arteries in patients with hypercholesterolaemia. J Intern Med. 2000; 248:42-52 [DOI] [PubMed] [Google Scholar]
- 32.Gardner CD, Fortmann SP, Krauss RM. Association of small low‐density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996; 276:875-881 [PubMed] [Google Scholar]
- 33.Criqui MH, Cowan LD, Tyroler HA, Bangdiwala S, Heiss G, Wallace RB, Cohn R. Lipoproteins as mediators for the effects of alcohol consumption and cigarette smoking on cardiovascular mortality: results form the Lipid Research Clinics Follow‐up Study. Am J Epidemiol. 1987; 126:629-637 [DOI] [PubMed] [Google Scholar]
- 34.Mora S, Buring JE, Ridker PM. Discordance of LDL cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2013; 129:553-61.10.1161/CIRCULATIONAHA.113.005873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low‐density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996; 276:882-888 [PubMed] [Google Scholar]
- 36.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP. Small, dense low‐density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation. 1997; 95:69-75 [DOI] [PubMed] [Google Scholar]
- 37.Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, Sharp DS. Low‐density lipoprotein particle size, triglycerides, and high‐density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese‐American men. Am J Cardiol. 2000; 86:412-416 [DOI] [PubMed] [Google Scholar]
- 38.Kolovou GD, Anagnostopoulou KK, Salpea KD, Pilatis ND, Iraklianou S, Grapsa G, Pantelakis A, Tsarpalis K, Kapnia E, Cokkinos DV. Postprandial lipemia in postmenopausal women with high fasting high‐density lipoprotein cholesterol. Am J Med Sci. 2006; 331:10-16 [DOI] [PubMed] [Google Scholar]
- 39.Münzer T, Harman SM, Sorkin JD, Blackman MR. Growth hormone and sex steroid effects on serum glucose, insulin, and lipid concentrations in healthy older women and men. J Clin Endocrinol Metab. 2009; 94:3833-3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Superko HR, Pendyala L, Williams PT, Momary KM, King SB, Garrett BC. High‐density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012; 6:496-523 [DOI] [PubMed] [Google Scholar]
- 41.Barbagallo CM, Averna MR, Fradà G, Noto D, Cavera G, Notarbartolo A. Lipoprotein profile and high‐density lipoproteins: subfractions distribution in centenarians. Gerontology. 1998; 44:106-110 [DOI] [PubMed] [Google Scholar]
- 42.Luc G, Bard JM, Lussier‐Cacan S, Bouthillier D, Parra HJ, Fruchart JC, Davignon J. High‐density lipoprotein particles in octogenarians. Metabolism. 1991; 40:1238-1243 [DOI] [PubMed] [Google Scholar]
- 43.Ettinger WH, Verdery RB, Wahl PW, Fried LP. High density lipoprotein cholesterol subfractions in older people. J Gerontol. 1994; 49:M116-M122 [DOI] [PubMed] [Google Scholar]
- 44.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998; 18:1046-1053 [DOI] [PubMed] [Google Scholar]
- 45.Sich D, Saïdi Y, Giral P, Lagrost L, Egloff M, Auer C, Gautier V, Turpin G, Beucler I. Hyperalphalipoproteinemia: characterization of a cardioprotective profile associating increased high‐density lipoprotein2 levels and decreased hepatic lipase activity. Metabolism. 1998; 47:965-973 [DOI] [PubMed] [Google Scholar]
- 46.Sweetnam PM, Bolton CH, Yarnell JW, Bainton D, Baker IA, Elwood PC, Miller NE. Associations of the HDL2 and HDL3 cholesterol subfractions with the development of ischemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Circulation. 1994; 90:769-774 [DOI] [PubMed] [Google Scholar]
- 47.Curb JD, Abbott RD, Rodriguez BL, Masaki K, Chen R, Sharp DS, Tall AR. A prospective study of HDL‐C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J Lipid Res. 2004; 45:948-953 [DOI] [PubMed] [Google Scholar]
- 48.Ordovas JM, Cupples LA, Corella D, Otvos JD, Osgood D, Martinez A, Lahoz C, Coltell O, Wilson PW, Schaefer EJ. Association of cholesteryl ester transfer protein‐TaqIB polymorphism with variations in lipoprotein subclasses and coronary heart disease risk: the Framingham study. Arterioscler Thromb Vasc Biol. 2000; 20:1323-1329 [DOI] [PubMed] [Google Scholar]
- 49.Mackey RH, Kuller LH, Sutton‐Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002; 90:71i-76i [DOI] [PubMed] [Google Scholar]
- 50.Mahley RW. Atherogenic lipoproteins and coronary artery disease: concepts derived from recent advances in cellular and molecular biology. Circulation. 1985; 72:943-948 [DOI] [PubMed] [Google Scholar]
- 51.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003; 52:453-462 [DOI] [PubMed] [Google Scholar]


