Abstract
Background
Atrial fibrillation (AF) remains the most common complication after cardiac surgery. The present study aim was to derive an effective bedside tool to predict postoperative AF and its related complications.
Methods and Results
Data of 17 262 patients undergoing adult cardiac surgery were retrieved at 3 European university hospitals. A risk score for postoperative AF (POAF score) was derived and validated. In the overall series, 4561 patients (26.4%) developed postoperative AF. In the derivation cohort age, chronic obstructive pulmonary disease, emergency operation, preoperative intra‐aortic balloon pump, left ventricular ejection fraction <30%, estimated glomerular filtration rate <15 mL/min per m2 or dialysis, and any heart valve surgery were independent AF predictors. POAF score was calculated by summing weighting points for each independent AF predictor. According to the prediction model, the incidences of postoperative AF in the derivation cohort were 0, 11.1%; 1, 20.1%; 2, 28.7%; and ≥3, 40.9% (P<0.001), and in the validation cohort they were 0, 13.2%; 1, 19.5%; 2, 29.9%; and ≥3, 42.5% (P<0.001). Patients with a POAF score ≥3, compared with those without arrhythmia, revealed an increased risk of hospital mortality (5.5% versus 3.2%, P=0.001), death after the first postoperative day (5.1% versus 2.6%, P<0.001), cerebrovascular accident (7.8% versus 4.2%, P<0.001), acute kidney injury (15.1% versus 7.1%, P<0.001), renal replacement therapy (3.8% versus 1.4%, P<0.001), and length of hospital stay (mean 13.2 versus 10.2 days, P<0.001).
Conclusions
The POAF score is a simple, accurate bedside tool to predict postoperative AF and its related or accompanying complications.
Keywords: antiarrhythmic prevention, atrial fibrillation, cardiac surgery, risk stratification
Introduction
Atrial fibrillation (AF) remarkably remains the most common complication after cardiac surgery.1–2 Postoperative AF (POAF) carries severe clinical and economic implications, increasing early and late morbidity and mortality, and having a great impact on resource utilization.1–5 In addition, the incidence of this complication has been reported to steadily increase, owing mostly to an increasing number of elderly patients with severe comorbidities.1
In light of the importance of POAF for patient outcomes, there has been great interest in preventing this arrhythmia.1–10 Several studies have sought to identify patients at highest risk for the development of AF, but their number is testament to the failure to effectively prevent AF through prophylactic measures in unselected patients.6–10 Routine pharmacological AF prophylaxes could expose as many as 60% to 80% of patients to the side effects of antiarrhythmic drugs for which there is no indication, with unnecessary drug‐related costs.4,11–12
As a result, it is of utmost importance to identify patients whose risk of developing AF is very low and who should not receive routine antiarrhythmic therapy. Accordingly, efforts have been made to develop risk prediction models to identify patients most likely to develop POAF.4–10
However, these models often are statistically underpowered and, because of this, inconclusive.6–7 In addition, they are limited by the exclusion of high‐risk patients with renal and cardiac failure, as well as those undergoing complex procedures, despite the fact that these patients are those most affected by POAF.6–10 These risk models also imply complex calculations without rapid estimation for targeting patients, hampering a prompt prophylactic approach, and confounding the clinical decision making and patient counseling.6–10 The present study aimed to derive an efficient and reliable bedside tool to predict POAF and its related complications.
Methods
Study Population
Between July 1999 and December 2010, all consecutive patients undergoing isolated coronary artery bypass graft surgery (CABG) and valve surgery (with or without concomitant CABG) at 3 European cardiac centers (Bristol Heart Institute, UK; Varese University Hospital, and Centro Cardiologico Monzino IRCCS, Italy) were screened for this observational study. Elective, urgent, or emergency procedures were all included. Patients with a preoperative history of supraventricular arrhythmias requiring treatment were excluded to avoid overestimation of the POAF incidence. The final sample consisted of a total of 17 622 patients. Data used in this analysis were retrieved from institutional databases, which remained consistent over the study period. All data were prospectively collected, and information about demographics, comorbidities, medical and surgical history, operative details, and postoperative events during the hospital stay were registered.
The study protocol was in compliance with the local institutional review boards and received full approval; patient consent was waived.
Patient Management
Preoperative management, anesthetic, and surgical techniques were standardized for all patients and have been previously reported.3,13–16 Preoperative antiarrhythmic therapies were not routinely adopted; in general, β‐blockers were administered on the day of surgery and restarted in the immediate postoperative period unless contraindicated for clinical reasons. At the end of surgery, patients were transferred to a dedicated intensive care unit and managed according to the unit protocol.3,13–16 Patients were monitored daily until discharge with continuous ECG telemetry and standard 12‐lead ECG. Additional recordings were collected at clinical suspicion of AF. Amiodarone, either orally or intravenously administered, constituted the standard pharmacological treatment of POAF. In patients without successful rhythm cardioversion and with persistent AF, warfarin was administered before discharge, with the aim of achieving an international normalized ratio between 2.0 and 3.0, planning an electric cardioversion within 30 days. Symptomatic patients or with ineffective antiarrhythmic therapy to control the ventricular rate underwent cardioversion at any time during the postoperative period.
End Point and Definitions
The primary end point of this study was to identify independent predictors of POAF, deriving an effective bedside tool to predict the arrhythmia and its related complications. Briefly, POAF was documented on the basis of a rhythm strip or 12‐lead ECG as previously described.3,13–16 Postoperative acute kidney injury (AKI) was defined according to the RIFLE (Risk, Injury, Failure, Loss of function, and End‐stage renal disease) criteria; estimated glomerular filtration rate (eGFR) was calculated with use of the Chronic Kidney Disease Epidemiology Collaboration equation.17–18 Cerebrovascular accidents were diagnosed on the basis of a new neurological deficit with morphological substrate confirmed by using computed tomography or nuclear magnetic resonance imaging. The decision to perform off‐pump or on‐pump CABG was based on individual surgeon preference.
Statistical Analysis
Clinical data were prospectively recorded and tabulated with Microsoft Excel (Microsoft Corp). Statistical analysis was computed using SPSS version 20 statistical software (IBM SPSS Inc). Fisher exact, χ2, Mann–Whitney, Kruskal–Wallis, and Cochran–Armitage tests were used for univariable analysis. Correlation between continuous variables was estimated by using the Spearman test. No was made attempt to replace missing values. The dataset was randomly divided into a derivation dataset (75% of patients) and a validation dataset (25% of patients).19 Multivariable analysis of data from the derivation dataset was performed using stepwise logistic regression with backward selection. The significance within the models was evaluated with the Wald test, whereas the strength of the association of variables with POAF was estimated by calculating the OR and 95% CIs. The model was calibrated using the Hosmer–Lemeshow goodness‐of‐fit test, as well as residual diagnostics (deviance and dfBetas). Model discrimination was evaluated by using the area under the receiver operating characteristic (ROC) curve. Only variables with a value of P<0.05 in univariable analysis were included in the regression models, to avoid overfitting. Furthermore, dichotomous variables with an OR <1.2 were excluded from the final regression analysis. Additive risk score for the prediction of POAF (POAF score) was calculated by adding weighting points for each independent risk factor. Once the predictive ability of the POAF score was tested in the validation dataset, further analyses were performed only in the overall dataset. In particular, patients in the last stratum of this additive score (POAF score ≥3) were considered for further adjusted analyses. Linear regression was used for adjusted analysis of the length of in‐hospital stay (LOS). Because the LOS variable was not normally distributed, the analysis was performed after it was logarithmically transformed. All tests were 2‐sided with the α level set at 0.05 for statistical significance.
Results
Overall Series
Baseline and operative characteristics of enrolled patients are summarized in Table 1. The final population presented a mean age of 66.3±10.6 years (range, 18 to 92 years), and 4301 (25%) were women. Patients undergoing isolated CABG accounted for 11 685 (68%) patients; isolated valve surgery, 3672 (21%); and valve surgery with concomitant CABG, 1905 (11%).
Table 1.
Presenting Characteristics According to the Occurrence of Postoperative AF
| Variables*,* | Overall Population (N=17 262) | Derivation Cohort (n=12 938) | Validation Cohort (n=4324) | |||||
|---|---|---|---|---|---|---|---|---|
| No AF (n=12 701) | AF (n=4561) | P Value | No AF (n=9515) | AF (n=3423) | No AF (n=3186) | AF (n=1138) | P Value* | |
| Age, y, % | 65.1±10.8 | 69.7±8.9 | <0.001 | 65.1±10.7 | 69.7±9.1 | 65.1±11.1 | 69.8±8.4 | 0.48 |
| <60 | 28.3 | 13.1 | <0.001 | 28.4 | 13.3 | 27.9 | 12.5 | 0.25 |
| 60 to 69 | 35.4 | 33.1 | 35.5 | 33.1 | 35.3 | 33.3 | ||
| 70 to 79 | 32.5 | 44.1 | 31.3 | 43.6 | 32.1 | 45.8 | ||
| ≥80 | 4.8 | 9.7 | 4.9 | 10.1 | 4.7 | 8.4 | ||
| Male, % | 76.2 | 72.1 | <0.001 | 76.3 | 71.7 | 75.6 | 73.1 | 0.85 |
| BMI, kg/m2 | 27.3±4.3 | 27.1±4.5 | 0.17 | 27.2±4.3 | 27.1±4.5 | 27.2±4.3 | 27.1±4.4 | 0.47 |
| BMI >40 kg/m2, % | 0.7 | 1.0 | 0.15 | 0.8 | 1.0 | 0.6 | 1.0 | 0.62 |
| Cardiac presentation, % | ||||||||
| Emergency | 2.8 | 4.8 | <0.001 | 2.8 | 4.7 | 2.5 | 5.3 | 0.94 |
| IABP | 0.7 | 2.0 | <0.001 | 0.7 | 1.9 | 0.8 | 2.0 | 0.49 |
| Previous cardiac surgery | 3.7 | 3.7 | 0.91 | 3.5 | 3.9 | 4.1 | 3.1 | 0.62 |
| Prior AMI | 36.2 | 36.4 | 0.81 | 36.4 | 36.8 | 35.4 | 35.2 | 0.16 |
| LVEF | 55.5±11.3 | 53.4±12.2 | <0.001 | 55.6±11.3 | 53.7±12.2 | 55.3±11.4 | 52.7±12.2 | 0.53 |
| LVEF <30, % | 3.3 | 5.2 | <0.001 | 3.3 | 5.3 | 3.2 | 4.8 | 0.57 |
| Comorbidities, % | ||||||||
| Hypertension | 64.9 | 68.7 | <0.001 | 64.8 | 69.1 | 65.2 | 67.4 | 0.94 |
| Diabetes | 20.0 | 21.7 | 0.012 | 19.9 | 21.5 | 20.1 | 22.2 | 0.69 |
| COPD | 5.2 | 8.4 | <0.001 | 5.4 | 8.3 | 4.5 | 8.8 | 0.26 |
| PVD | 9.7 | 11.5 | <0.001 | 9.8 | 11.8 | 9.4 | 10.7 | 0.25 |
| CVD | 7.3 | 9.1 | <0.001 | 7.3 | 9.6 | 7.1 | 7.5 | 0.13 |
| eGFR, mL/min per 1.73 m2 | 66.8±22.0 | 63.9±23.8 | <0.001 | 66.8±21.9 | 63.9±25.2 | 66.7±22.3 | 63.6±18.9 | 0.53 |
| eGFR <15 mL/min per 1.73 m2 or dialysis, % | 0.4 | 0.7 | 0.003 | 0.5 | 0.9 | 0.6 | 1.1 | 0.22 |
| Additive EuroSCORE, n | 4.0±2.8 | 5.4±3.1 | <0.001 | 4.0±2.8 | 5.5±3.0 | 4.0±2.8 | 5.4±3.1 | 0.89 |
| Preoperative drug regimen, % | ||||||||
| β‐Blockers | 54.8 | 52.9 | 0.17 | 53.7 | 53.7 | 53.0 | 50.3 | 0.16 |
| Calcium antagonists | 21.0 | 22.8 | 0.11 | 21.1 | 23.1 | 20.7 | 22.1 | 0.61 |
| ACE inhibitors | 38.8 | 40.1 | 0.32 | 38.9 | 38.5 | 38.5 | 40.6 | 0.21 |
| Statins | 57.8 | 58.6 | 0.44 | 57.8 | 58.1 | 57.9 | 59.9 | 0.55 |
| Operative data | ||||||||
| CPB time, min | 76.9±53.1 | 88.8±56.1 | <0.001 | 76.8±52.4 | 89.4±55.8 | 76.9±55.1 | 87.0±56.9 | 0.55 |
| ACC time, min | 51.6±39.5 | 59.9±42.5 | <0.001 | 51.6±39.6 | 60.3±41.8 | 51.4±39.3 | 59.0±44.5 | 0.44 |
| Use of CPB, % | 66.4 | 74.0 | <0.001 | 66.2 | 74.0 | 67.0 | 74.2 | 0.33 |
| Valve surgery, % | 29.0 | 41.5 | <0.001 | 28.9 | 41.7 | 29.1 | 41.1 | 0.88 |
| IABP, % | 1.1 | 2.9 | <0.001 | 1.1 | 3.1 | 1.2 | 2.1 | 0.42 |
| Postoperative data | ||||||||
| Ventilation, h | 6 (4 to 11) | 8.5 (5 to 17) | <0.001 | 6 (4 to 11) | 8 (5 to 17) | 6 (4 to 11) | 9 (5 to 17) | 0.39 |
| ICU time, h | 24 (23 to 48) | 43 (23 to 72) | <0.001 | 24 (23 to 48) | 44 (23 to 72) | 25 (23 to 48) | 36 (23 to 72) | 0.73 |
| CVA, % | 1.7 | 4.5 | <0.001 | 1.8 | 4.5 | 1.7 | 4.4 | 0.79 |
| AKI, % | 3.4 | 10.5 | <0.001 | 3.4 | 10.5 | 3.5 | 10.4 | 0.94 |
| RRT, % | 0.8 | 2.6 | <0.001 | 0.8 | 2.5 | 0.8 | 3.0 | 0.85 |
| Blood transfusion, % | 37.7 | 51.0 | <0.001 | 37.5 | 51.4 | 38.5 | 49.9 | 0.88 |
| Hospital outcome | ||||||||
| Length of stay, d | 7 (6 to 8) | 8 (7 to 12) | <0.001 | 7 (6 to 8) | 8 (7 to 12) | 7 (6 to 8) | 8 (7 to 11) | 0.86 |
| Mortality, % | 1.2 | 3.0 | <0.001 | 1.1 | 3.2 | 1.3 | 2.4 | 0.77 |
AF indicates atrial fibrillation; BMI, body mass index; IABP, intra‐aortic balloon pump; AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ACE, angiotensin‐converting enzyme; CPB, cardiopulmonary bypass; ACC, aortic cross‐clamp; ICU, intensive care unit; CVA, cerebrovascular accident; AKI, acute kidney injury; RRT, renal replacement therapy.
For continuous variables, mean±SD or median (25th to 75th percentile); for categorical variables, %.
Other variables considered for the final model and not reported: body surface area, prior percutaneous coronary intervention, left mainstem stenosis, right coronary artery disease, left atrial diameter, dyslipidemia, basal hemoglobin, basal creatinine, creatinine values >2.0 mg/dL, angiotensin receptor blockers.
Test comparing the prevalence of risk factors between the entire derivation and entire validation cohorts.
The incidence of POAF was 26.4% (4561 of 17262 patients) in the overall series, subdivided into 23% (n=2667), 31% (n=1133), and 40% (n=761) for isolated CABG, isolated valve surgery, and combined procedures, respectively. POAF mainly occurred within 2 days postoperatively (median 2 days). The profile for patients affected by POAF was considerably different with regard to demographics, comorbid conditions, and operative data. Patients affected by POAF in the overall series were older than those who were not ffected (69.7±8.9 versus 65.1±10.8 years, P<0.0001) and were predominantly men (72% versus 76%, P<0.0001) with a lower left ventricular ejection fraction (LVEF, 53.4±12.2% versus 55.5±11.3%, P<0.0001) and eGFR (63.8±23.9 versus 66.7±22.1 mL/min per 1.73 m2, P<0.001), having a more severe profile of comorbidities (additive EuroSCORE: 5.4±3.1 versus 4.0±2.8, P<0.001). Remarkably, preoperative drug regimen did not influence the development of AF. The use of β‐blockers (P=0.174), calcium antagonists (P=0.114), angiotensin‐converting enzyme inhibitors (P=0.321), and statins (P=0.453) was not associated with a decreased AF risk.
Derivation and Validation Cohorts
Results of multivariable analysis for prediction of POAF are reported in Table 2. Ninety‐seven patients (0.6%) in the overall series were not included in the final regression models because of missing values of any of the covariates of interest.
Table 2.
Results of Multivariable Analysis for Prediction of Postoperative AF in the Derivation Cohort
| Variables | OR (95% CI) | Coefficients | Additive Score Points |
|---|---|---|---|
| Age, y | |||
| <60* | |||
| 60 to 69 | 2.04 (1.81 to 2.31) | 0.715 | 1 |
| 70 to 79 | 2.93 (2.60 to 3.30) | 1.076 | 2 |
| ≥80 | 3.94 (3.31 to 4.69) | 1.372 | 3 |
| COPD | 1.33 (1.14 to 1.56) | 0.286 | 1 |
| eGFR <15 mL/min per 1.73 m2 or dialysis | 1.90 (1.17 to 3.10) | 0.643 | 1 |
| Emergency | 1.50 (1.19 to 1.88) | 0.404 | 1 |
| Preoperative IABP | 1.90 (1.28 to 2.83) | 0.644 | 1 |
| LVEF <30% | 1.45 (1.18 to 1.77) | 0.369 | 1 |
| Valve surgery | 1.68 (1.55 to 1.83) | 0.519 | 1 |
| Constant | −2.032 | ||
AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction.
Age <60 y was the reference group.
Among patients in the derivation cohort, logistic regression revealed that age, emergency operation, preoperative intra‐aortic balloon pump (IABP), LVEF <30%, eGFR <15 mL/min per m2 or dialysis, heart valve surgery, and chronic obstructive pulmonary disease were independent predictors of POAF (Hosmer–Lemeshow goodness‐of‐fit test: χ2 [7 df]=4.73, P=0.662; area under the ROC curve: 0.65, 95% CI 0.64 to 0.66). POAF score for POAF was calculated (weighting factors are summarized in Table 2), and its area under the ROC curve was similar to that of the regression model (0.64, 95% CI 0.63 to 0.65). According to this risk score, the incidence of POAF in the derivation cohort was 0, 11.1% (215/1933 patients); 1, 20.1% (799/3970 patients); 2, 28.7% (1141/3982 patients); and ≥3, 40.9% (1223/2992 patients) (P<0.001, Figures 1 and 2).
Figure 1.
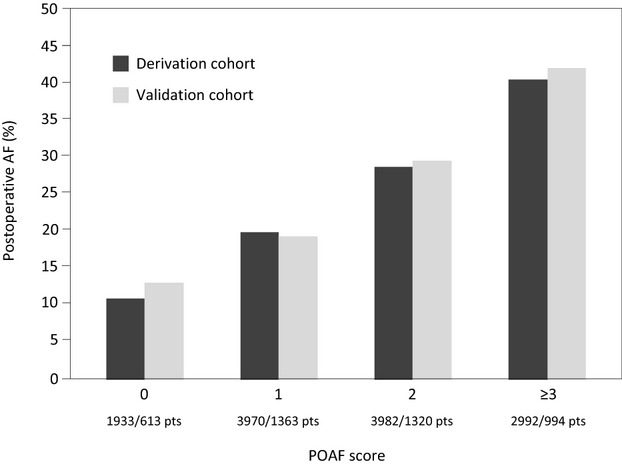
Incidence of postoperative atrial fibrillation (POAF) according to POAF score in the derivation and validation cohorts (both cohorts, Cochran–Armitage test: P<0.001).
Figure 2.
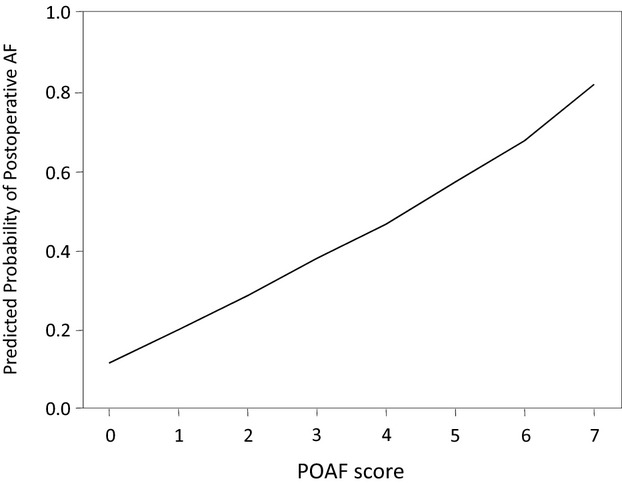
Correlation between postoperative atrial fibrillation (POAF) score and predicted probability of POAFoccurrence.
In the validation cohort, AF incidence was 0, 13.2% (81/613 patients); 1, 19.5% (266/1363 patients); 2, 29.9% (395/1320 patients); and ≥3, 42.5% (422/994 patients) (P<0.001, Figure 1). The area under the ROC curve of this risk score for prediction of POAF in the validation cohort was 0.64 (95% CI 0.63 to 0.66).
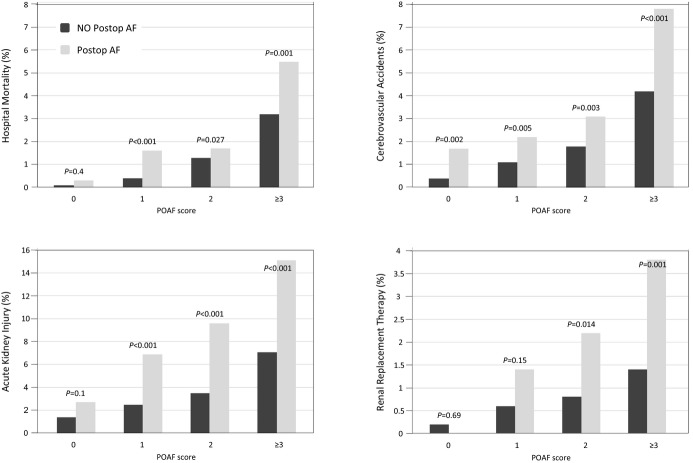
In the overall series, the risk of hospital mortality (P<0.001), stroke (P<0.001), AKI (P<0.001), renal replacement therapy (RRT, P<0.001), and LOS (P<0.001) increased along with the risk of AF and its occurrence. The risk of adverse events was increased in patients with POAF in all strata. However, the absolute difference in the rate of adverse events was most evident in the stratum with ≥3 risk factors for POAF. The POAF score was predictive also of hospital mortality (area under the ROC curve 0.73, 95% CI 0.70 to 0.76), CVA (0.71, 95% CI 0.68 to 0.73), AKI (0.67, 95% CI 0.65 to 0.68), and RRT (0.66, 95% CI 0.61 to 0.72) (Figure 3). A significant correlation was observed between POAF score and LOS (ρ 0.32, P<0.001).
Figure 3.
Postoperative complication (hospital mortality, cerebrovascular accident, acute kidney injury, and renal replacement therapy) rates according to the postoperative atrial fibrillation (POAF) score and the occurrence of POAF. P values are within each risk class.
Adjusted Analysis in High‐Risk Patients of the Overall Series
Analyzing the prognostic impact of POAF in the last stratum of POAF score (score ≥3), which included 3986 patients from the overall series, the arrhythmia was associated in univariable analysis with increased risk of hospital mortality (5.5% versus 3.2%, P=0.001, for mortality after the first postoperative day: 5.1% versus 2.6%, P<0.001), CVA (7.8% versus 4.2%, P<0.001), AKI (15.1% versus 7.1%, P<0.001), RRT (3.8% versus 1.4%, P<0.001), and LOS (mean 13.2 versus 10.2 days, P<0.001).
Additive EuroSCORE adjusted analysis showed that the last stratum of POAF score (score for AF ≥3) had the highest risk of mortality (OR 3.61, 95% CI 1.27 to 10.26). The area under the ROC curve of additive EuroSCORE for predicting hospital mortality in this stratum was 0.80 (95% CI 0.77 to 0.82). When adjusted for additive EuroSCORE, POAF was associated with an increased risk of hospital mortality (OR 1.49, 95% CI 1.08 to 2.05). Similarly, AF was an independent predictor of hospital mortality and/or CVA (OR 1.59, 95% CI 1.27 to 1.99). Because POAF usually occurs a mean of 2 to 3 days after the procedure, its impact on the hospital mortality occurring >1 day after surgery was also evaluated. The impact of this arrhythmia was even larger in predicting hospital mortality occurring >1 day after surgery (OR 1.74, 95% CI 1.24 to 2.45). When adjusted for additive EuroSCORE, POAF was also associated with an increased risk of CVA (OR 1.76, 95% CI 1.34 to 2.31), AKI (OR 2.21, 95% CI 1.72 to 2.62), RRT (OR 2.68, 95% CI 1.42 to 5.08), and LOS (coefficient 0.09, 95% CI 0.07 to 0.10) in the last stratum of the POAF score.
Discussion
AF after cardiac surgery remains a vexing complication, resulting in prolonged hospital stay, and causes additional morbidity and mortality in a substantial number of patients.1–10 Consequently, considerable efforts have been directed toward reduction of the risk and POAF management, mainly focusing on pharmacological agents.1–10 However, a caveat with the use of the antiarrhythmic approaches is that most patients undergoing cardiac surgery do not develop POAF, and 60% to 80% of them are exposed to the costs and potential side effects of unnecessary prophylaxes.1–10 On the one hand, it is critical that before such pharmacological managements are implemented in all cardiac surgery patients, the safety and effectiveness of these strategies in improving patient outcomes must be proved. On the other hand, a rapid, accurate estimation of individual patients' risk for POAF may facilitate correct identification of patients who are at the lowest risk of the development of AF and should not be treated with preventive strategies. In this setting, our study demonstrated that the POAF score is a simple, accurate bedside risk tool, allowing for the identification of high‐risk AF patients in whom preventive antiarrhythmic therapies could be justified. The POAF score was also found to be of value in developing an easy‐to‐use risk scoring method for AF‐related or accompanying complications, suggesting possible simultaneous preventive approaches.
Other risk prediction models have been previously proposed to identify patients most likely to develop POAF.4–10 Zaman et al6 first enrolled 326 elective isolated CABG patients and created a model for preoperative risk stratification in patients affected by AF, demonstrating that P‐wave duration >155 ms, age, and male sex were able to predict POAF in 59% of their patient population. Amar et al7 reported in 1851 patients undergoing isolated CABG that 4 preoperative and postoperative variables were independently associated with AF development. Using their prediction model, 3 risk categories for AF were identified, suggesting increased AF occurrence (from 14% to 60%) with category worsening.7 Mathew et al8 performed a prospective multicenter observational study of 4657 CABG patients, obtaining a final model with 3 AF risk classes (low, medium, and high risk) based on 17 preoperative, intraoperative, and postoperative variables. More recently, Magee et al9 extracted 19 083 patients undergoing isolated CABG from the Society of Thoracic Surgeons Database, using perioperative risk factors to develop a predictive risk algorithm of AF development. The final model included 14 preoperative, intraoperative, and postoperative variables, which provided statistically significant coefficients and ORs.9
However, all of these prediction models provided controversial results, revealing important limitations, and limiting their widespread adoption in the clinical practice of AF preventive strategies.6–10 Some of these studies were computationally complex due to the relevant number of variables considered and underpowered due to their small sample size.4,6–8,10 In addition, considered risk factors of POAF such as P‐wave duration, male sex, or angiotensin‐converting enzyme inhibitor treatment have been demonstrated to be poorly associated with the arrhythmia, while other well‐known AF predictors were surprisingly not included.6–10 Patients with deteriorated left ventricular function, compromised renal function, or complex operations including valve surgery were often excluded from these AF prediction models. Nevertheless, these subjects are the ones mostly affected by AF, as demonstrated in several studies, and would benefit the most from antiarrhythmic measures.4,6–7,6–10 On the other hand, the same risk prediction models included postoperative variables such as low cardiac output, postoperative drug treatment, and prolonged ventilation, hampering prompt prophylactic treatment, especially in the case of urgent or emergent operations.7–9 In fact, Magee et al9 included prolonged ventilation use as one of the most relevant AF predictors, limiting the possibility to adopt preventive measures before such a condition develops and postponing the usefulness of their model to the postoperative period only. However, it is well known that POAF most commonly occurs within the first 48 hours after surgery, and it remains distinctly possible that AF occurred as a consequence of the observed prolonged ventilation rather than being a cause of it, confounding the predictive value of this variable and consequently invalidating the final model. Furthermore, patients with preexistent AF were inopportunely included in the earlier mentioned prediction models, ignoring the fact that these subjects represent a specific surgical subgroup, being at higher risk for mortality and morbidity.7–8,10,20–23 Preexisting AF is often encountered in older patients with significant mitral valve disease and left atrial enlargement, and such patients are certainly more prone to develop AF after surgery than are those without this preexisting condition.19–20 In fact, both Amar et al7 and Mathew et al8 included the history of AF as a relevant risk factor in their prediction models, although patients with preexisting arrhythmia were largely affected by POAF (68% and 53%, respectively). Consonant data are obtained in the prediction model of Magee et al,9 with inclusion of the preoperative arrhythmia variable in the final prediction model, although patients affected by it experienced POAF in 70% of cases. In our study, patients with a preoperative history of supraventricular arrhythmias were excluded to avoid confusing the incidence of POAF with recurrence of a preexisting arrhythmic condition and because these patients have been demonstrated to benefit from extensive antiarrhythmic prophylaxis or a contemporary surgical ablation.22–24
The present study has important indirect clinical implications with reference to possible AF preventive strategies, although it was not designed to specifically address the complex AF preoperative prophylaxis issue. Our aim was to identify a patient population more likely to develop AF and its related complications who may most benefit from of any preventive strategy.
The present prediction model includes variables such as COPD, valve surgery, emergency status, preoperative IABP need, advanced renal failure, and reduce LVEF, which have normally constituted exclusion criteria in several observational and randomized studies testing different AF prophylactic regimens, because the adverse drug effects registered in such patient classes.13,21,25–26 Low‐risk patients (POAF score <3) may benefit from an extensive preoperative AF prophylaxis based on β‐blockers and statins.13,21,25 However, high‐risk patients (POAF score ≥3) are likely to be the most appropriate candidates for amiodarone prophylaxis, also for subjects already receiving β‐blockers.21,25–26 Amiodarone has demonstrated a large compliance in the routine antiarrhythmic care of cardiac surgery patients, because of its low incidence of acute complications and a large effectiveness independently from dose (high or low) or administration time (preoperative or intraoperative).4,21,25–26
In addition, high‐risk patients (POAF score ≥3) may benefit from a prolonged postoperative surveillance because of the possible occurrence of late AF episodes.3,27–28
Finally, in comparison with previous prediction models, the POAF score for the first time was also tested in predicting related or accompanying complications of POAF.4,6–10 Having the highest AF risk as identified by POAF score ≥3 was strongly associated with higher hospital mortality, postoperative CVA, AKI, and RRT, suggesting a wider preventive approach based on both antiarrhythmic prophylaxes and other protective measures for AF‐related morbidities. In particular, patients with a POAF score ≥3 and affected by POAF experienced a 2‐ to 4‐fold increased risk of postoperative CVA compared with those in other POAF score classes. Patients with a POAF score ≥3 may largely benefit from antiarrhythmic prophylaxis accompanied by routine postoperative use of low‐molecular‐weight heparin, starting in the immediate period after surgery, especially in CABG patients as previously anticipated.22 The use of oral anticoagulation only at the onset of AF has been demonstrated to be inadequate to prevent stroke.22 Interestingly, oral anticoagulation in patients affected by POAF has been demonstrated to reduce early and late mortality associated with thromboembolic AF sequelae.3,5,28–30 Therefore, it should be considered in high‐risk group patients (POAF score ≥3) immediately after cardiac surgery. In this patient class, the ligation of the let atrial appendage should also be considered.31
Study Limitations
Although the AF predictors were highly significant and assessed in a validation cohort, the accuracy of the present model is moderate, a result shared with other similar studies. Mahoney et al4 examined more than 10 000 cardiac surgery patients, investigating predictors of POAF in subjects undergoing isolated CABG and valve surgery with or without concomitant coronary surgery, and obtained 3 different models with an area under the curve of 0.67, 0.65, and 0.64, respectively. Similar results were also obtained by Thorén et al10 in a cohort of 7115 isolated CABG patients in an effort to identify patients at high risk of developing AF, and the predictive ability of their final prediction model was moderate (area under the ROC curve, 0.62). This is likely due to the complex and multifactorial nature of this arrhythmia. Furthermore, the present data were unable to clearly identify the temporal relationship between the onset of AF after surgery and the occurrence of adverse clinical events. It cannot be excluded that POAF occurred as a consequence of an observed complication rather than it being a cause of the complication.
Conclusions
The POAF score based on routinely available preoperative data is a simple, accurate bedside tool, allowing for identification of patients at high risk for POAF and in whom preventive antiarrhythmic therapies seem justified. Permitting identification of patients at high risk for AF and related or accompanying complications, the POAF score may also help to develop and update clinical and therapeutic strategies that not only would minimize the occurrence of POAF but also would result in an improvement in the otherwise dismal morbidity and mortality rates observed among patients affected by this arrhythmia.
Sources of Funding
The study was supported by The British Heart Foundation and the NIHR Bristol Cardiovascular Biomedical Research Unit. The authors also thank the Fondazione Cesare Bartorelli (Milan, Italy) for his support.
Disclosures
None.
References
- 1.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Schuessler RB. The persistent problem of new‐onset postoperative atrial fibrillation: a single‐institution experience over two decades. J Thorac Cardiovasc Surg. 2011; 141:559-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004; 43:742-748 [DOI] [PubMed] [Google Scholar]
- 3.Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, Cantore C, Biglioli P, Sala A. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008; 118:1612-1618 [DOI] [PubMed] [Google Scholar]
- 4.Mahoney EM, Thompson TD, Veledar E, Williams J, Weintraub WS. Cost‐effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002; 40:737-745 [DOI] [PubMed] [Google Scholar]
- 5.Mariscalco G, Engström KG. Atrial fibrillation after cardiac surgery: risk factors and their temporal relationship in prophylactic drug strategy decision. Int J Cardiol. 2008; 129:354-362 [DOI] [PubMed] [Google Scholar]
- 6.Zaman AG, Archbold A, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative stratification. Circulation. 2000; 101:1403-1408 [DOI] [PubMed] [Google Scholar]
- 7.Amar D, Shi W, Hogue CW, Zhang H, Passman RS, Thomas B, Bach PB, Damiano R, Thaler HT. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004; 44:1248-1253 [DOI] [PubMed] [Google Scholar]
- 8.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004; 291:1720-1729 [DOI] [PubMed] [Google Scholar]
- 9.Magee MJ, Herbert MA, Dewey TM, Edgerton JR, Ryan WH, Prince S, Mack MJ. Atrial fibrillation after coronary artery bypass grafting surgery: development of a predictive risk algorithm. Ann Thorac Surg. 2007; 83:1707-1712 [DOI] [PubMed] [Google Scholar]
- 10.Thorén E, Hellgren L, Jidéus L, Ståhle E. Prediction of postoperative atrial fibrillation in a large coronary artery bypass grafting cohort. Interact Cardiovasc Thorac Surg. 2012; 14:588-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber UK, Osswald S, Huber M, Buser P, Skarvan K, Stulz P, Schmidhauser C, Pfisterer M. Selective versus non‐selective antiarrhythmic approach for prevention of atrial fibrillation after coronary surgery: is there a need for pre‐operative risk stratification? A prospective placebo‐controlled study using low‐dose sotalol. Eur Heart J. 1998; 19:794-800 [DOI] [PubMed] [Google Scholar]
- 12.Mariscalco G, Cederlund B, Engström KG. The clinical noncompliance of oral sotalol/magnesium for prophylactic treatment of atrial fibrillation after coronary artery bypass grafting. J Card Surg. 2007; 22:281-286 [DOI] [PubMed] [Google Scholar]
- 13.Mariscalco G, Lorusso R, Klersy C, Ferrarese S, Tozzi M, Vanoli D, Domenico BV, Sala A. Observational study on the beneficial effect of preoperative statins in reducing atrial fibrillation after coronary surgery. Ann Thorac Surg. 2007; 84:1158-1164 [DOI] [PubMed] [Google Scholar]
- 14.Miceli A, Fino C, Fiorani B, Yeatman M, Narayan P, Angelini GD, Caputo M. Effects of preoperative statin treatment on the incidence of postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2009; 87:1853-1858 [DOI] [PubMed] [Google Scholar]
- 15.Ascione R, Caputo M, Calori G, Lloyd CT, Underwood MJ, Angelini GD. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: a prospective randomized study. Circulation. 2000; 102:1530-1535 [DOI] [PubMed] [Google Scholar]
- 16.Watters M, Ascione R, Ryder IG, Ciulli F, Pitsis AA, Angelini GD. Hemodynamic changes during beating heart coronary surgery with the “Bristol Technique”. Eur J Cardiothorac Surg. 2001; 19:34-40 [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky PAcute Dialysis Quality Initiative workgroup. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204-R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461-470 [DOI] [PubMed] [Google Scholar]
- 19.Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower‐extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011; 34:1695-1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banach M, Misztal M, Goch A, Rysz J, Goch JH. Predictors of atrial fibrillation in patients following isolated surgical revascularization. A metaanalysis of 9 studies with 28 786 patients. Arch Med Sci. 2007; 3:229-239 [Google Scholar]
- 21.Banach M, Kourliouros A, Reinhart KM, Benussi S, Mikhailidis DP, Jahangiri M, Baker WL, Galanti A, Rysz J, Camm JA, White CM, Alfieri O. Postoperative atrial fibrillation—what do we really know? Curr Vasc Pharmacol. 2010; 8:553-572 [DOI] [PubMed] [Google Scholar]
- 22.Banach M, Mariscalco G, Ugurlucan M, Mikhailidis DP, Barylski M, Rysz J. The significance of preoperative atrial fibrillation in patients undergoing cardiac surgery: preoperative atrial fibrillation—still underestimated opponent. Europace. 2008; 10:1266-1270 [DOI] [PubMed] [Google Scholar]
- 23.Ad N, Barnett SD, Hann CK, O'Brien SM, Milford‐Beland S, Speir AM. Does preoperative atrial fibrillation increase the risk for mortality and morbidity after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2009; 137:901-906 [DOI] [PubMed] [Google Scholar]
- 24.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta‐analysis. J Thorac Cardiovasc Surg. 2007; 133:182-189 [DOI] [PubMed] [Google Scholar]
- 25.Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013; 1:CD003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley MS, Nolan PE, Jr, Slack MK, Tisdale JE, Hilleman DE, Copeland JG. Amiodarone prophylaxis after cardiac surgery: meta‐analysis of dose response and timing of initiation. Pharmacotherapy. 2007; 27:360-368 [DOI] [PubMed] [Google Scholar]
- 27.Mariscalco G, Sarzi‐Braga S, Banach M, Borsani P, Bruno VD, Napoleone M, Vitale C, Piffaretti G, Pedretti RFE, Sala A. Preoperative n‐3 polyunsatured fatty acids are associated with a decrease in the incidence of early atrial fibrillation following cardiac surgery. Angiology. 2010; 61:643-650 [DOI] [PubMed] [Google Scholar]
- 28.Mariscalco G, Engström KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009; 88:1871-1876 [DOI] [PubMed] [Google Scholar]
- 29.Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, Rimpiläinen J, Lepojärvi M, Juvonen T. Postoperative atrial fibrillation is a major cause of stroke after on‐pump coronary artery bypass surgery. Ann Thorac Surg. 2004; 77:1241-1244 [DOI] [PubMed] [Google Scholar]
- 30.Kollar A, Lick SD, Vasquez KN, Conti VR. Relationship of atrial fibrillation and stroke after coronary artery bypass graft surgery: when is anticoagulation indicated? Ann Thorac Surg. 2006; 82:515-523 [DOI] [PubMed] [Google Scholar]
- 31.Kim R, Baumgartner N, Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation‐related cerebrovascular accident. J Thorac Cardiovasc Surg. 2013; 145:582-589 [DOI] [PubMed] [Google Scholar]