Abstract
Background
Decreased arterial elasticity is a risk factor for several cardiovascular outcomes. Longitudinal data on the effect of physical activity in youth on adult arterial elasticity are limited. The aim of this study was to determine the long‐term effects of physical activity in children and young adults on carotid artery elasticity after 21 years of follow‐up.
Methods and Results
Participants were 1417 children (aged 9 to 15 years) and 999 young adults (aged 18 to 24 years) from the prospective Cardiovascular Risk in Young Finns Study. Participants had questionnaire measures of leisure‐time physical activity available from 1986 and ultrasound‐derived indices of carotid artery elasticity measured in 2007. Carotid artery elasticity indices were distensibility (%/10 mm Hg), Young's elastic modulus (kPa), and stiffness index (unitless). Physical activity at age 18 to 24 years was directly associated with distensibility (β=0.068, P=0.014) and inversely with Young's elastic modulus (β=−0.057, P=0.0037) and indirectly with stiffness index (β=−0.050, P=0.0028) 21 years later in males and females. The associations remained after adjustment for age, sex, body mass index, smoking, systolic blood pressure, serum lipids and insulin, and 21‐year change in physical activity. At age 9 to 15 years, the favorable association, remaining after adjustment, was found in males (distensibility [β=0.097, P=0.010], Young's elastic modulus [β=−0.060, P=0.028], and stiffness index [β=−0.062, P=0.007]) but not in females (P=0.70, P=0.85, and P=0.91, respectively).
Conclusions
Leisure‐time physical activity in boys and young adults is associated with carotid artery elasticity later in life, suggesting that higher levels of physical activity in youth may benefit future cardiovascular health.
Keywords: atherosclerosis, exercise, prevention
Introduction
Central arteries, such as the aorta and carotid, lose their elasticity with age.1–3 Decreased arterial elasticity is a risk factor for several cardiovascular outcomes: hypertension, atherosclerosis, and coronary heart disease.4 It has been shown in cross‐sectional studies that high levels of habitual physical activity is associated with increased arterial elasticity, such as improved carotid artery compliance5–6 and reduced arterial stiffening in older adults.6–7 However, longitudinal data on the effect of physical activity in children and young adults on later vascular health are limited. Van de Laar et al have shown that lifetime vigorous, but not light‐to‐moderate, habitual physical activity is favorably associated with brachial and femoral artery compliance among 373 participants, aged 13 years at baseline, during 24 years of follow‐up.8 Chen et al have shown that a decrease in physical activity in males aged 14 years at baseline is associated with increased arterial stiffness after 3 years of follow‐up.9
We10–12 and others13–15 have previously shown in adults that habitual physical activity is related to increased insulin sensitivity, a more favorable lipid profile, decreased prevalence of the metabolic syndrome, and lower blood pressure and reduced risk for the development of hypertension. All these characteristics may have a positive effect on arterial wall structure and function.16–18
The purpose of this study was to examine the effect of physical activity in >2000 children and young adults on carotid artery elasticity measured 21 years later.
Material and Methods
Subjects
The Cardiovascular Risk in Young Finns Study is a prospective follow‐up of atherosclerosis risk factors from youth. The first cross‐sectional survey was conducted in 1980. The original sample size was 4320 children and adolescents aged 3, 6, 9, 12, 15, and 18 years. Of these, 3596 participated (83.2% of those invited) in 1980. Full details of the study design have been published.19 The entire cohort was followed in 1983, 1986, 2001, and 2007. Vascular ultrasound measurements were performed in 2001 and 2007. The sample for this analysis included those subjects who took part in the study in 1986 and in 2007 (N=2416). Due to missing data for some of the analysis covariates, such as serum lipids, the number of subjects varied between 1603 and 2416 in the analyses. Participants were aged 9 to 24 years at baseline and 30 to 45 years at follow‐up. The 1986 study was chosen as baseline because this was the first year that physical activity was inquired using the same questionnaire for all participants. Clinical measurements and physical activity data collections were performed simultaneously for each follow‐up, during winter months, to minimize seasonal variation. Participants gave written informed consent, and the study was approved by local ethics committees.
Physical Activity
Physical activity was assessed by using a self‐administered questionnaire in 1986 and in 2007.10,20 The participants were asked to report their habitual leisure‐time physical activity intensity, frequency, and duration. A metabolic equivalent (MET) index for leisure‐time physical activity (later called “MET‐index”) was calculated from the product of intensity×frequency×duration (MET h/wk) for both study years. The coefficients for the intensity of physical activity were estimated from the existing tables.21 One MET is the consumption of 1 kcal of a person per weight kilogram per hour in rest. The MET‐index ranged between 0 and 52 MET h/wk in 1986 and between 0 and 93 MET h/wk in 2007.
The validation of the questionnaire is published previously.10 In brief, we performed an experimental study including 45 adults (age range 23 to 55 years, 48% females). The participants filled in the questionnaire, and their physical activity was measured with accelerometers and pedometers for a period of 1 week. The MET‐index and its components (ie, intensity, frequency, and duration of physical activity) correlated significantly with data assessed using accelerometers (r=0.26 to 0.40) and pedometers (r=0.30 to 0.39). These correlation coefficients were of similar magnitude to those demonstrated in other studies.22 We also collected physical activity data in 2007 using validated pedometers in 1934 individuals from the entire study population. Participants wore an Omron Walking Style One (HJ‐152R‐E; Omron Healthcare Europe) pedometer for a period of 1 week. The correlation between the number of steps measured with pedometers and the amount of movement measured with ActiGraph accelerometers (GT1M) was r=0.97 (P<0.001, n=45).
Carotid Artery Ultrasound
The left common carotid artery was scanned by ultrasound technicians following a similar, standardized protocol in 5 study centers.3 A high‐resolution ultrasound imaging device (Sequoia 512; Acuson) with a 13‐MHz linear‐array transducer was used to assess arterial elasticity and intima‐media thickness (IMT). The image was focused on the posterior (far) wall, and gain settings were used to optimize image quality. Measurements were made offline from stored digital images. All ultrasound scans were analyzed by 1 reader blinded to the subjects' details. To assess carotid artery elasticity indices and IMT, the best‐quality cardiac cycle was selected from a continuous 5‐second image file. From this image, at least 4 measurements of the common carotid far wall were taken ≈10 mm proximal to the bifurcation to derive mean carotid IMT. The common carotid diameter was measured at least twice during end diastole and end systole. The means of the measurements were used as the end‐diastolic and end‐systolic diameters. The methods have been described in detail previously.23–24 Ultrasound and concomitant brachial blood pressure measurements were used to calculate the following indices of arterial elasticity: carotid artery distensibility (Cdist [%/10 mm Hg]=[(Ds−Dd)/Dd]/(Ps−Pd), Young's elastic modulus (YEM [kPa]=[(Ps−Pd)Dd2]/[2(Ds−Dd)IMT], and stiffness index [SI]=ln(Ps/Pd)/[(Ds−Dd)/Dd], where Dd is diastolic diameter, Ds is systolic diameter, Ps is systolic blood pressure, and Pd is diastolic blood pressure.25 Note that higher levels of both the YEM and SI represent decreased arterial elasticity, while a higher Cdist value represents a better arterial elasticity.
Clinical Characteristics and Laboratory Methods
Weight was measured in light clothing without shoes with a digital scale to the nearest 0.1 kg, and height was measured with a wall‐mounted stadiometer to the nearest 0.1 cm. Body mass index (BMI) was calculated as BMI=weight (kg)/[height (m)]2. Blood pressure was measured with a random zero sphygmomanometer. Korotkoff's fifth sound was used as a sign of diastolic blood pressure (DBP), and first sound was used as the sign of systolic blood pressure (SBP). Readings to the nearest even number of millimeters of mercury were performed at least 3 times on each subject. The average of these measurements was used in the analyses. Mean arterial pressure was calculated as mean arterial pressure=[(2×DBP)+SBP]/3. Total energy intake was assessed by a food frequency questionnaire in 2007. Alcohol consumption, estimated as number of standard drinks per day during the previous week, was assessed using a questionnaire in 2007. Smoking habits were assessed by a self‐administered questionnaire beginning at age 12 years. Those smoking daily were considered as smokers. Pack‐years of smoking were calculated in 2007. Socioeconomic status was assessed by the level of education (total school years) in 2007. The use of medications were assessed by using self‐administered questionnaire in 2007; 152 subjects reported the use of antihypertensive medication, 46 subjects used cholesterol‐lowering medication, 14 subjects used insulin, and 11 subjects used oral antidiabetic medication.
Venous blood samples were drawn after an overnight fast. All lipid and lipoprotein determinations were performed using standard methods, as described previously.26–27 Serum low‐density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula28 in subjects with triglycerides <4 mmol/L. Serum insulin was measured by microparticle enzyme immunoassay kit (Abbott Laboratories, Diagnostic Division, Dainabot). Serum glucose concentrations were analyzed enzymatically (Olympus Diagnostica GmbH).
Statistical Methods
Sex difference in the variables was studied by using t test, nonparametric median test or χ2 test, as appropriate. Due to the discontinuity of the 1986 MET‐index, it could not be normalized; therefore, it was categorized into tertiles that reflect meaningful differences in activity levels to ease the interpretation of the regression coefficients.10 However, similar relations were obtained when the continued MET‐index was used in the regression models in place of the categorized index. The tertile cut‐points were 3 MET h/wk and 12 MET h/wk―3 MET h/wk corresponds to moderate‐intensity physical activity for 60 minutes once a week, such as walking for 1 hour at the speed of 4 km/h, and 12 MET h/wk corresponds to 4 h/wk of moderate intensity physical activity or 1 to 2 h/wk of vigorous physical activity (eg, running for 1.5 hour at the speed of 8 km/h). Males and females aged 9 to 15 years were analyzed separately because there was an interaction between sex and MET‐index tertiles when Cdist measured in 2007 (P=0.047) was the explanatory variable. In young adults, there was no sex×MET‐index interaction (P=0.23), and, thus, males and females were analyzed combined. The association between 1986 MET‐index tertiles and carotid artery elasticity indices and IMT were examined separately for children (aged 9 to 15 years) and young adults (aged 18 to 24 years) using multivariate regression analysis. Age in 2007, sex, BMI in 1986 and in 2007, SBP in 1986 and in 2007, smoking in 1986 and in 2007, high‐density lipoprotein (HDL) cholesterol in 1986 and in 2007, LDL cholesterol in 1986 and in 2007, triglycerides in 1986 and in 2007, insulin in 1986 and in 2007, glucose in 1986 and in 2007, 21‐year change in MET‐indexes (ΔMET), and MET‐index in 2007 were used to adjust the analyses. We calculated 6‐year change in elasticity indices (ΔCdist, ΔYEM, and ΔSI). We also used mean arterial pressure, alcohol intake, pack‐years of smoking, total energy intake, socioeconomic status, antihypertensive medication, lipid‐lowering medication, insulin, and oral antidiabetic medication as covariates and covariates from 1986 only, but this did not change the results (data not shown). We also calculated the 21‐year change in BMI, SBP, smoking, HDL cholesterol, LDL cholesterol, triglycerides, insulin, and glucose and used these as covariates, but this did not change the results (data not shown). Due to skewness of triglyceride and insulin levels, YEM and SI, a natural logarithm transformation was calculated. To illustrate the associations between MET‐index tertiles and Cdist 2007 (Figures 1 and 2), we calculated the adjusted means (SE) by using ANCOVA with Tukey–Kramer adjusted multiple comparison. We additionally explored the possibility of a nonlinear relation between vascular measurements and MET‐index tertiles with multivariate models that included vascular measurement as the dependent variable and MET‐index tertile, age, and higher‐order MET‐index tertile terms as independent variables but did not find consistent evidence to support nonlinear relations (data not shown). These analyses were performed separately for males and females among 9‐ to 15‐year‐olds and 18‐ to 24‐year‐olds.
Figure 1.
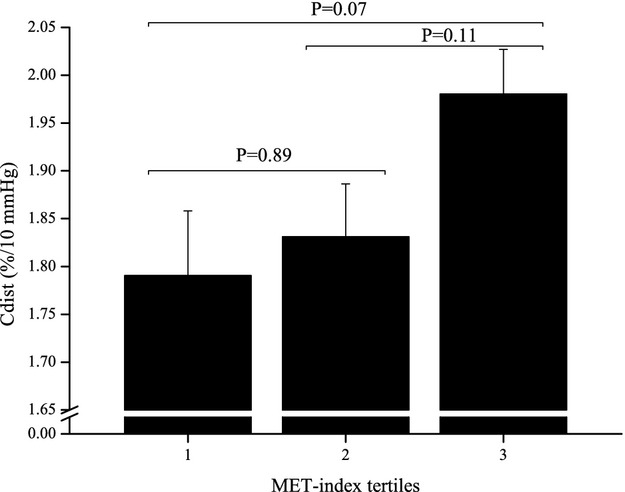
Association of carotid artery distensibility (Cdist) with metabolic equivalent (MET) index tertiles in males aged 9 to 15 years in 1986. P‐values shown are from ANCOVA with Tukey–Kramer multiple comparison. Descriptive data are given as estimated means (SE) adjusted for age in 2007, BMI in 1986 and in 2007, SBP in 1986 and in 2007, smoking in 1986 and in 2007, HDL cholesterol measured in 1986 and in 2007, LDL cholesterol measured in 1986 and in 2007, triglycerides measured in 1986 and in 2007, insulin measured in 1986 and in 2007, glucose measured in 1986 and in 2007, and ΔMET. BMI indicates body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Figure 2.
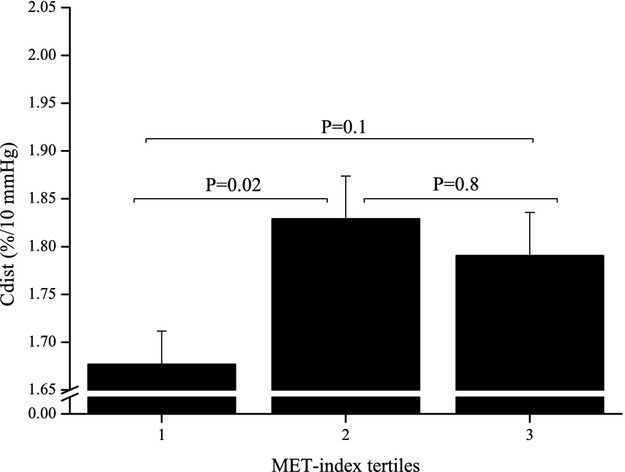
Association of carotid artery distensibility (Cdist) with metabolic equivalent (MET) index tertiles in young adults aged 18 to 24 years in 1986. P‐values shown are from ANCOVA with Tukey–Kramer multiple comparison. Descriptive data are given as estimated marginal means (SE) adjusted for age in 2007, sex, BMI in 1986 and in 2007, SBP in 1986 and in 2007, smoking in 1986 and in 2007, HDL cholesterol measured in 1986 and in 2007, LDL cholesterol measured in 1986 and in 2007, triglycerides measured in 1986 and in 2007, insulin measured in 1986 and in 2007, glucose measured in 1986 and in 2007, and ΔMET. BMI indicates body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
As a secondary analysis, we used longitudinal mixed modeling. As the exposure variable, we used a continuous physical activity index that has been collected using with similar questions in 1980, 1983, and 1986 in all participants aged ≥9 years. This index correlates strongly (r≈0.8) with the MET‐index that was available from study year 1986 and was used in the primary analysis. The mean exposure to physical activity in childhood and early adulthood during in 1980, 1983, and 1986 was calculated for each participant aged ≥9 years. As the outcome variable, we modeled adulthood carotid artery elasticity measured at 2 time points in 2001 and 2007. The covariates included age, sex, serum lipids, BMI, and insulin measured at each follow‐up, as well as physical activity index in adulthood.
Statistical analyses were made using the Statistical Analysis System software version 9.2, and statistical significance was inferred at a 2‐tailed probability value <0.05.
Results
Participant Characteristics
Participant characteristics in 1986 and 2007 are shown in Table 1 for those aged 9 to 15 years at baseline and in Table 2 for those aged 18 to 24 years at baseline. Among those aged 9 to 15 years at baseline, males were physically more active than females in 1986 (P<0.0001) but not in 2007 (P=0.4). Among those aged 18 to 24 years at baseline, males and females were equally active in both study years (P=0.5 and P=0.4). With the exception of age in 1986 and 2007, BMI in 1986, HDL cholesterol in 1986, and insulin levels in 2007, the remaining variables differed significantly between males and females (Table 1). There was a significant sex difference among those aged 18 to 24 years for all variables except age in 1986 and 2007, LDL cholesterol in 1986, triglycerides in 1986, and insulin levels in 1986 and 2007 (Table 2).
Table 1.
Characteristics of Study Participants at Baseline (1986) and Follow‐up (2007) in Females and Males Aged 9 to 15 Years
| Females | Males | |||
|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | |
| 1986 | ||||
| MET‐index (MET h/wk) (median, interquartile range) | 12 (3 to 18) | 719 | 12 (3 to 21) | 698 |
| Age, y | 12.0 (2.5) | 744 | 12.0 (2.5) | 731 |
| BMI, kg/m2 | 18.6 (3.0) | 734 | 18.5 (3.2) | 717 |
| SBP, mm Hg | 108 (10) | 734 | 109 (12) | 717 |
| DBP, mm Hg | 62 (10) | 731 | 61 (10) | 715 |
| Daily smokers, % | 2 (–) | 1218 | 4 (–) | 1274 |
| HDL‐chol, mmol/L | 1.5 (0.2) | 726 | 1.5 (0.3) | 715 |
| LDL‐chol, mmol/L | 3.0 (0.8) | 717 | 2.9 (0.7) | 705 |
| Triglycerides, mmol/L | 0.8 (0.3) | 725 | 0.8 (0.4) | 714 |
| Insulin, mU/L | 10.9 (10.2) | 724 | 9.6 (6.7) | 720 |
| Glucose, mmol/L | 4.6 (1.2) | 710 | 4.6 (0.5) | 689 |
| 2007 | ||||
| MET‐index, MET h/wk (median, interquartile range) | 8 (3 to 20) | 737 | 8 (1 to 31) | 629 |
| Cdist, %/10 mm Hg | 2.1 (0.7) | 735 | 1.8 (0.6) | 632 |
| YEM, kPa | 386 (1299) | 735 | 417 (246) | 632 |
| SI, unitless | 6.6 (22.4) | 735 | 6.2 (3.0) | 632 |
| Age, y | 35.4 (4.6) | 1218 | 35.7 (4.7) | 1274 |
| BMI, kg/m2 | 25.0 (5.1) | 717 | 26.6 (4.4) | 636 |
| SBP, mm Hg | 115 (13) | 735 | 125 (12) | 640 |
| DBP, mm Hg | 72 (11) | 735 | 78 (11) | 638 |
| Daily smokers, % | 16 (–) | 749 | 25 (–) | 643 |
| HDL‐chol, mmol/L | 1.5 (0.3) | 738 | 1.2 (0.3) | 635 |
| LDL‐chol, mmol/L | 2.9 (0.7) | 734 | 3.2 (0.8) | 617 |
| Triglycerides, mmol/L | 1.2 (0.7) | 739 | 1.6 (1.1) | 640 |
| Insulin, mU/L | 8.6 (8.5) | 739 | 9.2 (8.8) | 639 |
| Glucose, mmol/L | 5.1 (0.9) | 739 | 5.5 (1.0) | 640 |
All differences between sexes were significant (P<0.05) except age in 1986 and 2007, BMI in 1986, HDL levels in 1986, insulin levels in 2007, and MET‐index in 2007. Values are mean (SD) unless stated otherwise. MET indicates metabolic equivalent; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL‐chol, high‐density lipoprotein cholesterol; LDL‐chol, low‐density lipoprotein cholesterol; Cdist, carotid artery distensibility; YEM, Young's elastic modulus; SI, stiffness index.
Table 2.
Characteristics of Study Participants at Baseline (1986) and Follow‐up (2007) in Females and Males Aged 18 to 24 Years
| Females | Males | |||
|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | |
| 1986 | ||||
| MET‐index (MET h/wk) (median, interquartile range) | 5 (2 to 12) | 561 | 5 (2 to 20) | 438 |
| Age, y | 20.7 (2.4) | 614 | 20.6 (2.4) | 490 |
| BMI, kg/m2 | 21.9 (2.8) | 581 | 22.3 (2.8) | 468 |
| SBP, mm Hg | 116 (11) | 581 | 126 (11) | 468 |
| DBP, mm Hg | 68 (9) | 581 | 71 (10) | 467 |
| Daily smokers, % | 22 (–) | 614 | 31 (–) | 490 |
| HDL‐chol, mmol/L | 1.5 (0.3) | 581 | 1.3 (0.2) | 467 |
| LDL‐chol, mmol/L | 3.0 (0.9) | 577 | 2.9 (0.9) | 461 |
| Triglycerides, mmol/L | 0.9 (0.4) | 582 | 1 (0.5) | 465 |
| Insulin, mU/L | 9.9 (5.2) | 586 | 9.6 (5.9) | 475 |
| Glucose, mmol/L | 4.3 (0.4) | 569 | 4.6 (0.7) | 454 |
| 2007 | ||||
| MET‐index (MET h/wk) (median, interquartile range) | 12 (3 to 20) | 469 | 8 (1 to 21) | 344 |
| Cdist, %/10 mm Hg | 1.8 (0.7) | 470 | 1.6 (0.5) | 350 |
| YEM, kPa | 368 (222) | 470 | 446 (299) | 350 |
| SI, unitless | 6.5 (3.8) | 470 | 7.0 (3.8) | 350 |
| Age, y | 41.7 (2.4) | 614 | 41.6 (2.4) | 490 |
| BMI, kg/m2 | 26 (5) | 466 | 27 (4) | 351 |
| SBP, mm Hg | 119 (15) | 472 | 127 (14) | 352 |
| DBP, mm Hg | 75 (11) | 472 | 81 (11) | 352 |
| Daily smokers, % | 13 (–) | 476 | 19 (–) | 356 |
| HDL‐chol, mmol/L | 1.4 (0.3) | 471 | 1.2 (0.3) | 349 |
| LDL‐chol, mmol/L | 3.0 (0.8) | 469 | 3.4 (0.8) | 338 |
| Triglycerides, mmol/L | 1.2 (0.6) | 471 | 1.7 (1.2) | 354 |
| Insulin, mU/L | 8.5 (6.3) | 471 | 10.2 (11.4) | 354 |
| Glucose, mmol/L | 5.2 (0.7) | 471 | 5.6 (0.9) | 354 |
All differences between sexes were significant (P<0.05) except age in 1986 and 2007, LDL levels in 1986, triglycerides levels in 1986, insulin levels in 1986 and 2007, and MET‐index in 1986 and 2007. Values are mean (SD) unless stated otherwise. MET indicates metabolic equivalent; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL‐chol, high‐density lipoprotein cholesterol; LDL‐chol, low‐density lipoprotein cholesterol; Cdist, carotid artery distensibility; YEM, Young's elastic modulus; SI, stiffness index.
Association Between Physical Activity in Childhood and Adult Arterial Elasticity Measured 21 Years Later
Among 9‐ to 15‐year‐old males, physical activity was directly associated with adult Cdist (β=0.097 per tertile increase in MET‐index, SE=0.038, P=0.010) and inversely with adult YEM (β=−0.060, SE=0.027, P=0.028) and SI (β=−0.062, SE=0.023, P=0.007). In multivariate analyses, physical activity remained a significant determinant of adult Cdist, YEM, and SI after adjustments for covariates (Table 3) (Figure 1). Adult Cdist, YEM, and SI were measured in 2007.
Table 3.
Association Between Physical Activity in Males Aged 9 to 15 Years and Carotid Artery Elasticity Indices in Adulthood
| Model | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cdist* | lnYEM* | lnSI* | |||||||
| β | SE | P Value | β | SE | P Value | β | SE | P Value | |
| A: Age adjusted 1986 MET‐index tertiles | 0.091 | 0.038 | 0.02 | −0.059 | 0.027 | 0.03 | −0.060 | 0.023 | 0.010 |
| B: A+BMI* in 1986 and in 2007 | 0.084 | 0.036 | 0.02 | −0.054 | 0.027 | 0.05 | −0.058 | 0.023 | 0.01 |
| C: B+SBP in 1986 and in 2007 | 0.088 | 0.036 | 0.02 | −0.056 | 0.027 | 0.04 | −0.058 | 0.023 | 0.01 |
| D: C+smoking in 1986 and in 2007 | 0.090 | 0.036 | 0.01 | −0.061 | 0.027 | 0.02 | −0.061 | 0.022 | 0.007 |
| E: D+HDL‐chol in 1986 and in 2007 | 0.091 | 0.037 | 0.01 | −0.063 | 0.027 | 0.02 | −0.062 | 0.023 | 0.007 |
| F: E+LDL‐chol in 1986 and in 2007 | 0.095 | 0.037 | 0.01 | −0.064 | 0.027 | 0.02 | −0.063 | 0.023 | 0.006 |
| G: F+triglycerides in 1986 and in 2007 | 0.103 | 0.038 | 0.007 | −0.069 | 0.027 | 0.01 | −0.070 | 0.023 | 0.003 |
| H: G+insulin in 1986 and in 2007 | 0.097 | 0.038 | 0.01 | −0.066 | 0.027 | 0.02 | −0.068 | 0.023 | 0.004 |
| I: H+glucose in 1986 and in 2007 | 0.092 | 0.039 | 0.02 | −0.061 | 0.028 | 0.03 | −0.066 | 0.024 | 0.007 |
| J: I+ΔMET | 0.102 | 0.042 | 0.02 | −0.076 | 0.030 | 0.01 | −0.075 | 0.026 | 0.005 |
| K: I+MET‐index in 2007 | 0.087 | 0.040 | 0.03 | −0.052 | 0.029 | 0.07 | −0.061 | 0.025 | 0.01 |
Cdist, YEM, and SI were measured in 2007. β and SE are for a 1‐tertile increase in 1986 MET‐index. Cdist indicates carotid artery distensibility; ln, natural logarithm; YEM, Young's elastic modulus; SI, stiffness index; β, regression coefficient; MET, metabolic equivalent; BMI, body mass index; SBP, systolic blood pressure; HDL‐chol, high‐density lipoprotein cholesterol; LDL‐chol, low‐density lipoprotein cholesterol; ΔMET, 21‐year change in MET‐indexes.
N varies between 450 and 387.
The result remained similar when weight or height were separately used as a covariate in place of BMI.
Among 9‐ to 15‐year‐old females, we found no association between physical activity in childhood and adult Cdist (β=−0.016, SE=0.042, P=0.70), YEM (β=0.005, SE=0.026, P=0.85), or SI (β=−0.003, SE=0.024, P=0.91). We did not find an association between physical activity and carotid artery elasticity indices measured in 2001 (Cdist [β=−0.00002, SE=0.00003, P=0.47], YEM [β=0.02, SE=0.02, P=0.28], or SI [β=0.007, SE=0.01, P=0.57]) or between physical activity and ΔCdist (β=0.03, SE=0.03, P=0.39) or ΔYEM (β=−13.07, SE=9.72, P=0.18) or ΔSI (β=−0.22, SE=0.13, P=0.09). Therefore, these outcomes were not further examined.
Association Between Young Adult Physical Activity and Arterial Elasticity Measured 21 Years Later
Physical activity of young adults aged 18 to 24 years was directly associated with future Cdist (β=0.068, SE=0.027, P=0.014) and inversely with YEM (β=−0.057, SE=0.020, P=0.004) and SI (β=−0.050, SE=0.017, P=0.003). In multivariable analyses, physical activity in 1986 remained a significant determinant of Cdist, YEM, and SI 21 years later after adjustments for covariates (Table 4, Figure 2). Adult Cdist, YEM, and SI were measured in 2007. We did not find association between physical activity and carotid artery indices measured in 2001 (Cdist [β=−0.00003, SE=0.00003, P=0.29], YEM [β=0.02, SE=0.02, P=0.29], or SI [β=0.02, SE=0.01, P=0.14]. Therefore, these outcomes were not further examined. Physical activity was directly associated with ΔCdist (β=0.084, SE=0.028, P=0.0032) and inversely with ΔYEM (β=−53.41, SE=13.34, P<0.0001) and ΔSI (β=−0.79, SE=0.19, P<0.0001). These results remained significant after adjustments for covariates (data not shown).
Table 4.
Association Between Physical Activity in Young Adults Aged 18 to 24 Years and Carotid Artery Elasticity Indices in Adulthood
| Model | Young Adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cdist* | lnYEM* | lnSI* | |||||||
| β | SE | P Value | β | SE | P Value | β | SE | P Value | |
| A: Age‐ and sex‐adjusted 1986 MET‐index tertiles | 0.076 | 0.027 | 0.005 | −0.065 | 0.019 | 0.001 | −0.053 | 0.017 | 0.002 |
| B: A+BMI* in 1986 and in 2007 | 0.056 | 0.026 | 0.03 | −0.053 | 0.019 | 0.005 | −0.044 | 0.017 | 0.008 |
| C: B+SBP in 1986 and in 2007 | 0.055 | 0.026 | 0.03 | −0.052 | 0.019 | 0.006 | −0.044 | 0.017 | 0.008 |
| D: C+smoking in 1986 and in 2007 | 0.064 | 0.026 | 0.01 | −0.059 | 0.019 | 0.002 | −0.051 | 0.017 | 0.003 |
| E: D+HDL‐chol in 1986 and in 2007 | 0.061 | 0.026 | 0.02 | −0.060 | 0.019 | 0.002 | −0.052 | 0.017 | 0.003 |
| F: E+LDL‐chol in 1986 and in 2007 | 0.062 | 0.027 | 0.02 | −0.059 | 0.020 | 0.003 | −0.053 | 0.018 | 0.003 |
| G: F+triglycerides in 1986 and in 2007 | 0.061 | 0.027 | 0.02 | −0.056 | 0.020 | 0.004 | −0.052 | 0.018 | 0.003 |
| H: G+insulin in 1986 and in 2007 | 0.063 | 0.027 | 0.02 | −0.057 | 0.020 | 0.004 | −0.053 | 0.018 | 0.003 |
| I: H+glucose in 1986 and in 2007 | 0.063 | 0.027 | 0.02 | −0.058 | 0.020 | 0.004 | −0.053 | 0.018 | 0.003 |
| J: I+ΔMET | 0.065 | 0.030 | 0.03 | −0.054 | 0.022 | 0.01 | −0.054 | 0.020 | 0.006 |
| K: I+MET‐index in 2007 | 0.064 | 0.028 | 0.02 | −0.062 | 0.021 | 0.003 | −0.054 | 0.019 | 0.004 |
Cdist, YEM, and SI were measured in 2007. β and SE are for a 1‐tertile increase in 1986 MET‐index. Cdist indicates carotid artery distensibility; ln, natural logarithm; YEM, Young's elastic modulus; SI, stiffness index; β, regression coefficient; MET, metabolic equivalent; BMI, body mass index; SBP, systolic blood pressure; HDL‐chol, high‐density lipoprotein cholesterol; LDL‐chol, low‐density lipoprotein cholesterol; ΔMET, 21‐year change in MET‐indexes.
N varies between 746 and 655.
The result remained similar when weight or height were separately used as a covariate in place of BMI.
Association Between Physical Activity and Carotid Artery IMT Measured 21 Years Later
Among 9‐ to 15‐year‐old males, physical activity was not associated with adult IMT (β=0.0007 per tertile increase in MET‐index, SE=0.006, P=0.9). The same was true among 9‐ to 15‐year‐old females and 18‐ to 24‐year‐old young adults (β=−0.007, SE=0.004, P=0.1, and β=0.004, SE=0.004, P=0.4, respectively).
Secondary Analyses Using Longitudinal Modeling
A general physical activity index available from participants aged ≥9 years from study years 1980, 1983, and 1986 was used as a exposure variable in longitudinal model with the adulthood carotid artery elasticity measured at 2 time points in 2001 and 2007 as the outcome variable. This index has normal a distribution with a mean of 9.0, SD of 1.6, and range from 5 to 14. In the longitudinal mixed model including data from all individuals between ages 9 and 24 years, 1‐SD increase in the index was associated with 0.026‐unit (%/10 mm Hg) increase in Cdist in the model adjusted for age, sex, serum lipids, BMI, and insulin measured at each follow‐up, as well as physical activity index in adulthood (P=0.026). The effect was stronger in males (β=0.045, P=0.005) than in females (β=0.009, P=0.66), but no evidence for significant sex interaction was detected (interaction P=0.40).
Discussion
We observed in this prospective, longitudinal study that physical activity in children and young adults is associated with increased carotid artery elasticity, a marker of arterial health, at age 30 to 45 years. This favorable effect was independent of several other cardiometabolic risk factors.
Previous longitudinal studies on the association of physical activity and carotid artery elasticity are scarce. A study with 377 (196 females) participants aged 13 years at baseline showed that lifetime vigorous, but not light‐to‐moderate, intensity habitual physical activity is favorably associated with future carotid artery elasticity measured with the SI. This association persisted after adjustment for sex, height, time spent in physical activities, alcohol consumption, smoking, and energy intake.29 We did not find other publications of longitudinal studies on the association between physical activity and enhanced carotid artery elasticity.
In a cross‐sectional study of 135 (67 females) healthy adults aged 20 to 40 years, sedentary individuals were characterized by reductions in arterial compliance relative to their endurance‐trained peers, independent of changes in conventional risk factors for cardiovascular disease.30 In another cross‐sectional study of 102 (59 females) children aged 8 to 11 years, physical activity was associated with small, but not large, artery compliance.31
There are several possible mechanisms that might explain the association of physical activity in children and young adults with enhanced carotid artery elasticity. In rats, physical activity increases the elastin content of the aortic wall and probably enhances arterial dilatation and distensibility.32 Sugawara et al found that endurance training enhances carotid artery compliance in middle‐aged and older adults and that the effect is mediated through reduction in α‐adrenergic receptor–mediated vascular tone (ie, sympathetic nervous system activity is reduced with endurance training).33 Moreover, aerobic physical activity training enhances the release of nitric oxide via increased shear stress during or immediately after physical activity bouts.34 It is also suggested that regular physical activity might delay the age‐associated reduction or fraction of elastic lamellae in the arterial wall.35 After 3 months of aerobic exercise training, common carotid artery compliance was enhanced and corresponded with a reduction in plasma endothelin‐1 concentration.36 Endothelin‐1 is the most potent vasoconstrictor peptide secreted by vascular endothelial cells37; therefore, its reduction may be an important factor for vascular elasticity.
The strengths of this study include a large number of participants followed for 21 years who have been well phenotyped using established methods since a young age. The study subjects have been closely followed with well‐established methods since a young age to adulthood. A limitation is that we did not use applanation tonometry, a putative gold standard, to measure aortic pulse wave velocity as a marker of arterial elasticity. Alternatively, we used ultrasound to measure changes in carotid artery diameter during the cardiac cycle and we calculated elasticity indices (distensibility). This method is another commonly used measure of arterial elasticity, and it shows similar relations with cardiovascular risk markers as the pulse‐wave velocity.38–39 A potential source of inaccuracy, however, in this method is that it uses peripheral blood pressure measurements in the calculation of distensibility. It would be ideal to study the pulse pressure from the artery in question, because the use of brachial pressures overestimates pulse pressure in central arteries.40 However, the difference between central and peripheral pulse pressures is likely to be similar between study subjects within a narrow age range, as in our study. Moreover, Borow and Newburger demonstrated a strong correlation between SBP (r=0.98) and DBP (r=0.97) measured in central and peripheral arteries.41
One limitation is that physical activity was measured using questionnaires and no objective measures, such as step‐counters, were available in childhood and early adulthood in the 1986 study. However, we have collected step data collected in adulthood in 2007 and have shown that we see similar highly significant correlations between questionnaire data and step data that have been reported in several other studies.10 Additionally, we have demonstrated that physical activity measured with our questionnaire shows significant relations between repeated measurements in childhood, adolescence, and early adulthood. Approximately half of the individuals classified as active or sedentary remained in the same category in a 6‐year follow‐up.20 We have also recently shown that a physically active lifestyle starts to develop early in childhood and that the stability of physical activity is moderate or high along the life‐course from youth to adulthood over period of 30 years.42 Therefore, the data suggest that the questionnaire method reasonably well characterizes the participants according to their long‐term activity level.
Although physical activity was related to arterial elasticity, we found no link between youth physical activity and adult carotid IMT, a structural marker of early atherosclerosis. We have previously observed an indirect association between physical activity and aortic IMT in adolescents participating in the Special Turku Coronary Risk Factor Intervention Project study. However, aortic IMT may be a more sensitive marker of early atherosclerosis than carotid IMT.43 In adults, the majority of cross‐sectional studies have reported inverse associations between physical inactivity and carotid IMT, but many studies have also failed to demonstrate this relation. For example, Kronenberg et al did not find an association between physical activity and carotid IMT in 1778 individuals participating the National Heart, Lung, and Blood Institute Family Heart Study.44 Tanaka et al found no difference in carotid IMT between sedentary and endurance‐trained subjects.45 A review by Kadoglou et al suggested that a possible explanation for the differing results derives from the wide variability of study populations. The influence of physical activity on carotid IMT seems especially inconsistent among healthy subjects.46
In our study, we did not find association in the younger age group of 9‐ to 15‐year‐old females between physical activity in childhood and adult carotid artery elasticity. Potential explanations may involve sex differences in the responsiveness to the exposure due to differences in physiology (eg, hormonal differences) and/or to the possible differences in the quality or quantity of the exposure dose, such as the amount of vigorous physical activity. For example, estrogen may protect arteries against atherogenesis.47 In 2007, the females in the younger age group were 30 to 36 years old and the protective effect of estrogen may have masked a weaker effect associated with early physical activity exposure. Other explanation might be that there is a difference in quality of physical activity in 9‐ to 15‐year‐old males and females. Finally, we were unable to control for the effect of youth waist circumference because this measurement was not available from 1986. Beneficial effects of physical activity on arterial elasticity may be mediated by changes adiposity that could potentially be better captured by waist circumference measurements than by BMI. However, adjustment for adult waist circumference did not dilute the effect of youth physical activity on elasticity.
Our data provide support for the potentially independent benefits of physical activity on future cardiovascular health. What our data add that is unique to previous work is that the potential benefit of physical activity to cardiovascular health may be maximized only if an individual is active from early life, extending even to childhood.
Sources of Funding
This study was financially supported by the Academy of Finland (grants 126925, 124282, 77841, 210283, 121584, and 34316), the Social Insurance Institution of Finland, the Turku University Foundation, the Juho Vainio Foundation, Emil Aaltonen Foundation (Dr Lehtimäki) Finnish Foundation for Cardiovascular Research (Dr Lehtimäki), research funds from the Tampere (9N035 for Dr Lehtimäki), and Turku University Hospitals, the Research Foundation of Orion Corporation, and the Finnish Cultural Foundation. Dr Magnussen holds a National Health and Medical Research Council Early Career Fellowship (Public Health Fellowship, APP1037559).
Disclosures
None.
Acknowledgments
Irina Lisinen and Ville Aalto are acknowledged for skillful data management and analysis.
References
- 1.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993; 88:1456-1462 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, DeSouza CA, Seals DR. Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998; 18:127-132 [DOI] [PubMed] [Google Scholar]
- 3.Juonala M, Kähönen M, Laitinen T, Hutri‐Kähönen N, Jokinen E, Taittonen L, Pietikäinen M, Helenius H, Viikari JS, Raitakari OT. Effect of age and sex on carotid intima‐media thickness, elasticity and brachial endothelial function in healthy adults: the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008; 29:1198-1206 [DOI] [PubMed] [Google Scholar]
- 4.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994; 140:669-682 [DOI] [PubMed] [Google Scholar]
- 5.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age‐related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003; 57:861-868 [DOI] [PubMed] [Google Scholar]
- 6.Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011; 107:783-787 [DOI] [PubMed] [Google Scholar]
- 7.Gando Y, Yamamoto K, Murakami H, Ohmori Y, Kawakami R, Sanada K, Higuchi M, Tabata I, Miyachi M. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension. 2010; 56:540-546 [DOI] [PubMed] [Google Scholar]
- 8.van de Laar RJ, Ferreira I, van Mechelen W, Prins MH, Twisk JW, Stehouwer CD. Habitual physical activity and peripheral arterial compliance in young adults: The Amsterdam growth and health longitudinal study. Am J Hypertens. 2011; 24:200-208 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age‐ and sex‐related differences in vascular function and vascular response to mental stress. Longitudinal and cross‐sectional studies in a cohort of healthy children and adolescents. Atherosclerosis. 2012; 220:269-274 [DOI] [PubMed] [Google Scholar]
- 10.Mansikkaniemi K, Juonala M, Taimela S, Hirvensalo M, Telama R, Huupponen R, Saarikoski L, Hurme M, Mallat Z, Benessiano J, Jula A, Taittonen L, Marniemi J, Kähönen M, Lehtimäki T, Rönnemaa T, Viikari J, Raitakari OT. Cross‐sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2012; 44:733-744 [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Telama R, Hirvensalo M, Viikari JS, Raitakari OT. Sustained participation in youth sport decreases metabolic syndrome in adulthood. Int J Obes (Lond). 2009; 33:1219-1226 [DOI] [PubMed] [Google Scholar]
- 12.Raitakari OT, Taimela S, Porkka KV, Viikari JS. Effect of leisure‐time physical activity change on high‐density lipoprotein cholesterol in adolescents and young adults. Ann Med. 1996; 28:259-263 [DOI] [PubMed] [Google Scholar]
- 13.Rizzo NS, Ruiz JR, Oja L, Veidebaum T, Sjöström M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr. 2008; 87:586-592 [DOI] [PubMed] [Google Scholar]
- 14.Hardman AE. Physical activity, obesity and blood lipids. Int J Obes Relat Metab Disord. 1999; 23suppl 3:S64-S71 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt MD, Cleland VJ, Thomson RJ, Dwyer T, Venn AJ. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol. 2008; 18:378-386 [DOI] [PubMed] [Google Scholar]
- 16.Koivistoinen T, Hutri‐Kähönen N, Juonala M, Aatola H, Kööbi T, Lehtimäki T, Viikari JS, Raitakari OT, Kähönen M. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Med. 2011; 43:312-319 [DOI] [PubMed] [Google Scholar]
- 17.Mattsson N, Magnussen CG, Rönnemaa T, Mallat Z, Benessiano J, Jula A, Taittonen L, Kähönen M, Juonala M, Viikari JS, Raitakari OT. Metabolic syndrome and carotid intima‐media thickness in young adults: roles of apolipoprotein B, apolipoprotein A‐I, C‐reactive protein, and secretory phospholipase A2: the Cardiovascular Risk in Young Finns study. Arterioscler Thromb Vasc Biol. 2010; 30:1861-1866 [DOI] [PubMed] [Google Scholar]
- 18.Juonala M, Viikari JS, Kähönen M, Taittonen L, Laitinen T, Hutri‐Kähönen N, Lehtimäki T, Jula A, Pietikäinen M, Jokinen E, Telama R, Räsänen L, Mikkilä V, Helenius H, Kivimäki M, Raitakari OT. Life‐time risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur Heart J. 2010; 31:1745-1751 [DOI] [PubMed] [Google Scholar]
- 19.Raitakari OT, Juonala M, Rönnemaa T, Keltikangas‐Järvinen L, Räsänen L, Pietikäinen M, Hutri‐Kähönen N, Taittonen L, Jokinen E, Marniemi J, Jula A, Telama R, Kähönen M, Lehtimäki T, Åkerblom HK, Viikari JS. Cohort profile: the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008; 37:1220-1226 [DOI] [PubMed] [Google Scholar]
- 20.Raitakari O, Porkka K, Taimela S, Telama R, Räsänen L, Viikari J. Effects of persistent physical activity and in activity on coronary risk factors in children and young adults. Am J Epidemiol. 1994; 140:195-205 [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993; 25:71-80 [DOI] [PubMed] [Google Scholar]
- 22.Tudor‐Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: construct validity. Sports Med. 2004; 34:281-291 [DOI] [PubMed] [Google Scholar]
- 23.Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki‐Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, Åkerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima‐media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003; 290:2277-2283 [DOI] [PubMed] [Google Scholar]
- 24.Juonala M, Järvisalo MJ, Mäki‐Torkko N, Kähönen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005; 112:1486-1493 [DOI] [PubMed] [Google Scholar]
- 25.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non‐insulin‐dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995; 91:1432-1443 [DOI] [PubMed] [Google Scholar]
- 26.Porkka KV, Raitakari OT, Leino A, Laitinen S, Räsänen L, Rönnemaa T, Marniemi J, Lehtimäki T, Taimela S, Dahl M, Uhari M, Åkerblom HK, Viikari JS. Trends in serum lipid levels during 1980‐1992 in children and young adults. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol. 1997; 146:64-77 [DOI] [PubMed] [Google Scholar]
- 27.Juonala M, Viikari JS, Hutri‐Kähönen N, Pietikäinen M, Jokinen E, Taittonen L, Marniemi J, Rönnemaa T, Raitakari OT. The 21‐year follow‐up of the Cardiovascular Risk in Young Finns Study: risk factor levels, secular trends and east‐west difference. J Intern Med. 2004; 255:457-468 [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502 [PubMed] [Google Scholar]
- 29.van de Laar RJ, Ferreira I, van Mechelen W, Prins MH, Twisk JW, Stehouwer CD. Lifetime vigorous but not light‐to‐moderate habitual physical activity impacts favorably on carotid stiffness in young adults: the Amsterdam growth and health longitudinal study. Hypertension. 2010; 55:33-39 [DOI] [PubMed] [Google Scholar]
- 30.McGavock JM, Anderson TJ, Lewanczuk RZ. Sedentary lifestyle and antecedents of cardiovascular disease in young adults. Am J Hypertens. 2006; 19:701-707 [DOI] [PubMed] [Google Scholar]
- 31.Nettlefold L, McKay HA, Naylor PJ, Bredin SS, Warburton DE. The relationship between objectively measured physical activity, sedentary time, and vascular health in children. Am J Hypertens. 2012; 25:914-919 [DOI] [PubMed] [Google Scholar]
- 32.Matsuda M, Nosaka T, Sato M, Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol. 1993; 66:122-126 [DOI] [PubMed] [Google Scholar]
- 33.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Reduction in alpha‐adrenergic receptor‐mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009; 135:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol. 1997; 272:H1070-H1077 [DOI] [PubMed] [Google Scholar]
- 35.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977; 39:13-20 [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, Sugawara J, Yoshizawa M, Otsuki T, Shimojo N, Jesmin S, Ajisaka R, Miyauchi T, Tanaka H. Involvement of endothelin‐1 in habitual exercise‐induced increase in arterial compliance. Acta Physiol (Oxf). 2009; 196:223-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988; 332:411-415 [DOI] [PubMed] [Google Scholar]
- 38.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large‐artery remodeling. Circulation. 1999; 100:1387-1393 [DOI] [PubMed] [Google Scholar]
- 39.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003; 23:554-566 [DOI] [PubMed] [Google Scholar]
- 40.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993; 14:160-167 [DOI] [PubMed] [Google Scholar]
- 41.Borow KM, Newburger JW. Noninvasive estimation of central aortic pressure using the oscillometric method for analyzing systemic artery pulsatile blood flow: comparative study of indirect systolic, diastolic, and mean brachial artery pressure with simultaneous direct ascending aortic pressure measurements. Am Heart J. 1982; 103:879-886 [DOI] [PubMed] [Google Scholar]
- 42.Telama R, Yang X, Leskinen E, Kankaanpää A, Hirvensalo M, Tammelin T, Viikari JS, Raitakari OT. Tracking of physical activity from early childhood through youth into adulthood. Med Sci Sports Exerc. 2013 [DOI] [PubMed] [Google Scholar]
- 43.Pahkala K, Heinonen OJ, Simell O, Viikari JS, Rönnemaa T, Niinikoski H, Raitakari OT. Association of physical activity with vascular endothelial function and intima‐media thickness. Circulation. 2011; 124:1956-1963 [DOI] [PubMed] [Google Scholar]
- 44.Kronenberg F, Pereira MA, Schmitz MK, Arnett DK, Evenson KR, Crapo RO, Jensen RL, Burke GL, Sholinsky P, Ellison RC, Hunt SC. Influence of leisure time physical activity and television watching on atherosclerosis risk factors in the NHLBI Family Heart Study. Atherosclerosis. 2000; 153:433-443 [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age‐related increase in carotid artery intima‐media thickness in healthy men. J Appl Physiol. 1985; 200292:1458-1464 [DOI] [PubMed] [Google Scholar]
- 46.Kadoglou NP, Iliadis F, Liapis CD. Exercise and carotid atherosclerosis. Eur J Vasc Endovasc Surg. 2008; 35:264-272 [DOI] [PubMed] [Google Scholar]
- 47.Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, Tsai B, Hile K, Brown JW, Meldrum DR. The role of estrogen in cardiovascular disease. J Surg Res. 2003; 115:325-344 [DOI] [PubMed] [Google Scholar]


