Abstract
Background
Vascular aging is closely associated with increased vascular stiffness. It has recently been demonstrated that decreased nitric oxide (NO)‐induced S‐nitrosylation of tissue transglutaminase (TG2) contributes to age‐related vascular stiffness. In the current study, we tested the hypothesis that exercise restores NO signaling and attenuates vascular stiffness by decreasing TG2 activity and cross‐linking in an aging rat model.
Methods and Results
Rats were subjected to 12 weeks of moderate aerobic exercise. Aging was associated with diminished phosphorylated endothelial nitric oxide synthase and phosphorylated vasodilator‐stimulated phosphoprotein abundance, suggesting reduced NO signaling. TG2 cross‐linking activity was significantly increased in old animals, whereas TG2 abundance remained unchanged. These alterations were attenuated in the exercise cohort. Simultaneous measurement of blood pressure and pulse wave velocity (PWV) demonstrated increased aortic stiffness in old rats, compared to young, at all values of mean arterial pressure (MAP). The PWV‐MAP correlation in the old sedentary and old exercise cohorts was similar. Tensile testing of the vessels showed increased stiffness of the aorta in the old phenotype with a modest restoration of mechanical properties toward the young phenotype with exercise.
Conclusions
Increased vascular stiffness during aging is associated with decreased TG2 S‐nitrosylation, increased TG2 cross‐linking activity, and increased vascular stiffness likely the result of decreased NO bioavailability. In this study, a brief period of moderate aerobic exercise enhanced NO signaling, attenuated TG cross‐linking activity, and reduced ex vivo tensile properties, but failed to reverse functional vascular stiffness in vivo, as measured by PWV.
Keywords: aging, exercise, NO, pulse wave velocity, PWV, TG2, tTG, vascular stiffness
Introduction
Cardiovascular disease remains a leading cause of morbidity and mortality in both industrialized and developing countries, despite effective treatments that target established cardiovascular risk factors. However, the most important predictor of cardiovascular disease, age itself, evades specific interventions. Aging leads to a multitude of changes in the vasculature.1–3 Morphological changes include dilation of the central aorta3 and increased arterial wall thickness, even in the absence of atherosclerotic disease,4 mainly as a result of intimal thickening.5 Moreover, aging is associated with increased collagen deposition and elastin fracture/fragmentation in the extracellular matrix (ECM).6 These alterations lead to an increase in vascular stiffness,7 which is manifest as central pressure augmentation,8 systolic hypertension, increased pulse pressure,9 and a higher central blood pressure (BP) for any given peripheral (eg, brachial) BP.10 Assessments of arterial stiffness, such as pulse wave velocity (PWV), indices of wave reflection, and augmentation of central aortic BP, are increasingly being recognized as critical indicators to predict cardiovascular morbidity and mortality in the clinical setting11 and are associated with other risk factors, such as renal failure,12 stroke,13 coronary artery disease,14 impaired glucose metabolism,15 chronic inflammatory disease,16 smoking, and caffeine use.17 PWV, the speed at which the arterial pulse waveform propagates along the vascular tree, has gained particular interest in recent years and is increasingly utilized as a marker of cardiovascular disease and morbidity.18 PWV is directly related to stiffness, as defined by the Moens‐Korteweg equation: PWV=√(Eh/2pR), where E is Young's modulus of the arterial wall, h is wall thickness, R is arterial radius, and p is blood density. Aging is associated with increased PWV.19–20 At the cellular level, endothelial dysfunction and alterations in vascular smooth muscle function are well documented in the aging vasculature.21–22 However, the molecular mechanisms underlying these dynamic (eg, cell function) and structural (eg, elastin/collagen content) changes in age‐related vascular stiffness remain poorly understood. Therefore, targeted therapy remains elusive. Aging is accompanied by an altered redox milieu and decreased nitric oxide (NO) bioavailability, which accentuates vascular injury and/or impairs vascular repair. One of the enzymes intimately regulated by NO, and therefore affected by decreased NO levels with aging, is tissue transglutaminase (TG2).23 TG2 is expressed in the vascular endothelium, smooth muscle cells, and fibroblasts.24 TG2 is secreted to the ECM, where it catalyzes cross‐linking of ECM proteins, such as collagen, in a Ca2+‐dependent manner.25 We have recently demonstrated, in young rat aorta, that TG2 secretion and cross‐linking function are inhibited by S‐nitrosylation, a redox‐sensitive post‐translational modification of cysteine residues by NO.23 Loss of NO bioavailability in old rats leads to decreased TG2 S‐nitrosylation, increased TG2 in the vascular matrix, and increased TG2 cross‐linking function. Inhibition of TG2 in 15‐month‐old rats attenuated the age‐associated increase in PWV.23,26–27 The role of endothelial nitric oxide synthase (eNOS)‐dependent NO in regulating TG2 function is also evident as TG2 secretion and cross‐linking activity are higher in eNOS−/− mice, compared to wild type (WT), which further coincides with increased vascular stiffness in eNOS−/− mice.28 Therefore, it is of interest to further consider the role of TG2 in vascular stiffness to determine its therapeutic potential.
In the absence of well‐established therapeutic targets, lifestyle modifications, such as diet and moderate exercise, constitute the mainstay of therapy. Exercise, in particular, has been shown to broadly influence age‐related vascular stiffness by decreasing BP and improving endothelial function (and therefore NO bioavailability), among others.29–33 In this study, we tested the hypothesis that exercise‐mediated upregulation of NO decreases TG2 activity and thereby improves vascular stiffness in the commonly used Fischer 344 rat model of aging.34
Methods
Animals and Study Design
Male Fischer 344 rats were used in compliance with federal, state, local, and National Research Council guidelines. TG2−/− and WT mice were bred in‐house. Animals were fed and watered ad libitum. All surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee, which is accredited by the American Association for Accreditation in Laboratory Animal Care. First, the relationship between PWV and BP in Fischer 344 rats at different age groups (n=49) was evaluated. Next, the influence of 4 weeks of moderate exercise in 20‐month‐old animals (n=4), compared to age‐matched sedentary controls (n=10) and a young control group (6 months old; n=10) was examined. Finally, the effect of 14 weeks of moderate exercise was studied in middle‐aged rats (11 months old; n=8, exercise group) and compared to an age‐matched sedentary control group (old control group; n=7) and a young control group (6 months old; young control group, n=13).
Exercise Regimen
Animals in the exercise group were exercised daily on a rodent treadmill (Columbus Instruments, Columbus, OH) for 40 min/day at approximately 50% of maximal exercise tolerance.35 Rats were acclimatized to the treadmill for 1 week by walking daily for 40 minutes starting at a speed of 5 m/min with increments of 1 m/min per day. After this period of acclimatization, rats were exercised daily for either 4 weeks (20 months old; see Figure 1) or for 14 weeks (11 months old; see Figures 2 through 6) at 12 m/min at an incline of 5° for the period of the study.
Figure 1.
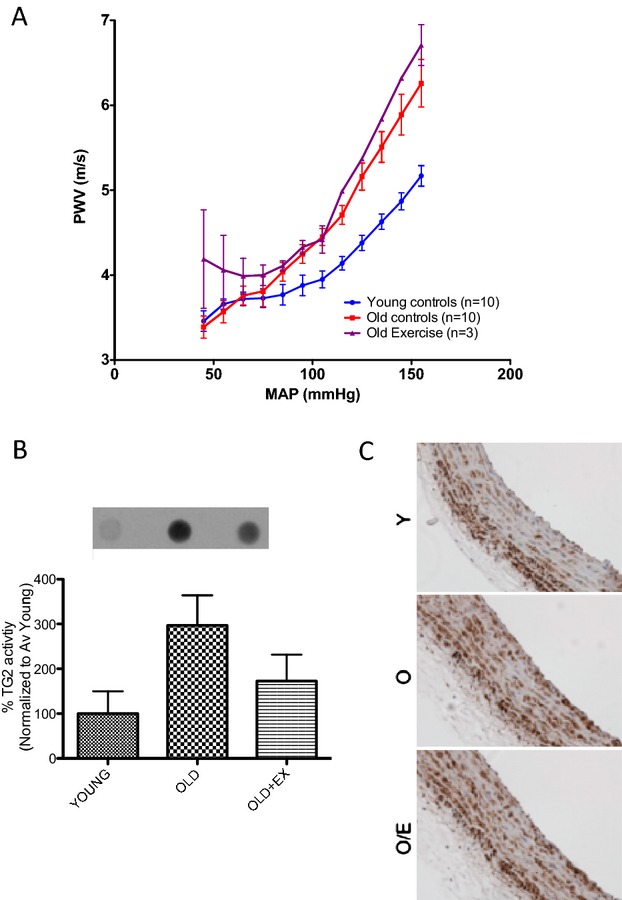
Effect of age and exercise in old rats. Old rats (20 months old) and young rats (6 months old) were examined. A, Aging is associated with increased vascular stiffness independent of exercise; B, TG activity increases with age in sedentary, but not in exercised, rats; and C, TG2 abundance remained unchanged. MAP indicates mean arterial pressure; PWV, pulse wave velocity; TG, transglutaminase.
Figure 6.
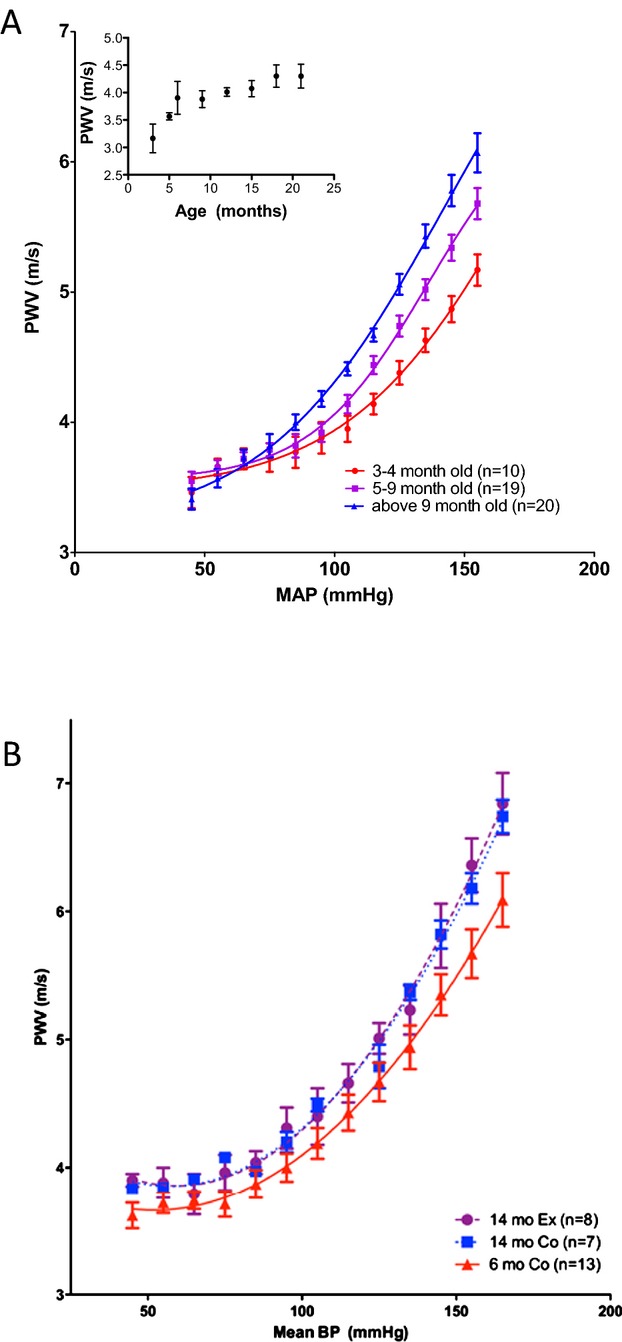
PWV‐MAP profiles change with age, but not with exercise. A, PWV increases exponentially with rising MAP. This increase is exaggerated with aging; Inset: PWV measured at the initial blood pressure increases with age in the Fischer 344 rat model; B, PWV‐MAP correlation in the exercise cohort is not altered by exercise in old animals. BP indicates blood pressure; MAP, mean arterial pressure; PWV, pulse wave velocity.
Invasive PWV Measurements
All animals were anesthetized in a closed chamber with isoflurane. After supine positioning on a temperature‐controlled surface (Cole‐Parmer, Vernon Hills, IL) with fore‐ and hindpaws taped, anesthesia was maintained with 1.5% isoflurane (in 100% O2) by mask. Body temperature was maintained at 37°C and a 3‐electrode ECG was continuously recorded (ADInstruments, Colorado Springs, CO). Additionally, local anesthesia was provided with 2% lidocaine. A cervical midline excision was performed and the left carotid artery was exposed using a blunt dissection technique. A 1.6F dual pressure catheter (Scisence Inc, London, Ontario, Canada) was inserted into the thoracic aorta with the distal tip located just above the diaphragm and the proximal tip below the aortic arch to continuously measure BP and pulse transit time (LabChart 6; ADInstruments). In order to study the relationship between PWV and BP over a wide range of BPs, a left jugular venous catheter was placed and a phenyleprhine infusion was titrated to a systolic BP of 200 mm Hg. The infusion was then stopped, allowing the BP to return to baseline. Once baseline pressure was restored, sodium nitroprusside was infused to lower the systolic BP to 50 mm Hg, at which point the infusion was stopped, again allowing the BP to return to baseline. PWV was calculated after each infusion was discontinued (ie, during the period in which the BP returned to baseline). PWV was calculated using a macro written for LabChart 6 (ADInstruments). Pulse transit time was obtained from the pulse foot‐to‐foot delay, where a fiducial point was determined using the time of the peak of the second time differential of the pressure pulse. Pulse transit time for each pulse was obtained by gating using the ECG R wave. PWV was then calculated as distance between the 2 transducers (2 cm) divided by transit time (0.02 m/transit time [sec]). The PWV value obtained was transferred to a spreadsheet and grouped with a precision of 10 mm Hg. Data were graphed and analyzed using Prism 5 (GraphPad Software Inc., La Jolla, CA). Curve fitting was performed using sigmoidal dose response (variable slope method) with an ordinal x‐axis.
Tensile Testing
Rat aorta was harvested, cut into 2‐mm rings, and mounted onto the pins of an electromechanical puller (DMT560; Danish Myo Technology A/S, Aarhus, Denmark). After calibration and alignment, the pins were slowly moved apart using an electromotor at a rate of 50 μm/sec to apply radial stress on the specimen until breakage. Displacement and force were recorded continuously. A 1‐mm segment proximal to the ring was imaged at ×10 magnification along with a graticule. Vessel inner (Di) and outer diameters (Do) were measured at 4 different locations with ImageJ software (National Institutes of Health [NIH], Bethesda, MD). Average Di and Do values were used to calculate sample thickness. Engineering stress (S) was calculated by normalizing force (F) to the initial stress‐free area of the specimen (S=F/2t×l; where t=thickness and l=length of the sample). Engineering strain (λ) was calculated as the ratio of displacement to the initial stress‐free diameter. The stress‐strain relationship was represented by the equation S=α exp (βλ), where α and β are constants. α and β were determined by nonlinear regression for each sample and used to generate Figure 3 by treating the x‐axis as a continuous variable.
Figure 3.
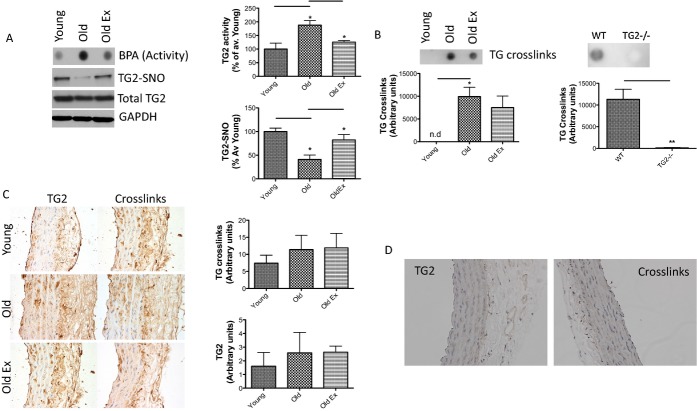
Exercise attenuates age‐associated changes in TG activity and S‐nitrosylation in old animals. A, TG activity increases and TG2‐nitrosylation decreases with age in sedentary rats, but not in rats subjected to 14 weeks of moderate aerobic exercise; TG2 abundance is not affected by age and exercise; bar graphs of densitometry analysis are shown on the right (n=8 in each group; TG2SNO, P=0.017 by 1‐way ANOVA; *P<0.05 young vs old and old vs old ex by Tukey's post‐hoc test; TG2 activity, P=0.0005 by 1‐way ANOVA; *P<0.05 young vs old and old vs old ex by Tukey's post‐hoc test). B, TG cross‐links (examined by digestion of vascular matrix followed by dot blotting with cross‐links antibody 81D4) increase with age independent of exercise in the rat aorta (n=4 in each group; P=0.01); WT and TG2−/− mouse aorta were used to confirm specificity of the method (n=4 in each group); (C), Immunohistochemical analysis of TG2 abundance (left) and TG‐specific cross‐links (right); IHC scores are represented in the bar graphs on the right (n=4 in each group); (D) Negative controls for IHC lacking TG2 primary antibody (left) or 81D4 antibody for cross‐links (right). IHC indicates immunohistochemistry; TG, transglutaminase; TG2‐SNO, S‐nitrosylated TG2; WT, wild type.
Western Blotting
Samples were homogenized in 1× radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors, centrifuged at 13 500g for 5 minutes at 4°C, and total protein concentration in the supernatants was determined (Bio‐Rad Protein Assay Reagent; Bio‐Rad, Hercules, CA). Duplicate gels containing homogenized samples (10 to 25 μg) were resolved by SDS‐PAGE and electrotransferred to a nitrocellulose membrane. After blocking (3% nonfat dry milk, 1 hour at room temperature), the membranes were incubated with primary antibody for 1 hour at room temperature, rinsed, and incubated with horseradish peroxidase (HRP)‐conjugated secondary antibody (Bio‐Rad). The first membrane was used to determine phosphorylated vasodilator‐stimulated phosphoprotein (pVASP) and phosphorylated eNOS (peNOS) and was subsequently stripped with Restore Plus stripping buffer (Pierce, Rockford, IL) and reused to determine total VASP and eNOS abundance. The second membrane was used to determine TG2 and GAPDH abundance. Rabbit pVASP Ser239 and mouse VASP were from Cell Signaling (1:1000; Danvers, MA), rabbit peNOS and mouse eNOS were from BD Bioscience (1:1000; San Jose, CA), mouse GAPDH was from Novus Biologicals (1:1000; Littleton, CO), rabbit tissue transglutaminase was from NeoMarkers (1:1000; Fremont, CA), transglutaminase 1 was from Abcam (1:1000; Cambridge, MA), and transglutaminase 4 was from Santa Cruz Biotechnology (1:1000; Santa Cruz, CA). Blots were developed using enhanced chemiluminescence and quantitated using ImageJ software (NIH). Four biological replicates were examined from each group on the same gel (Bio‐Rad Criterion 12+2 well gel; Bio‐Rad). The average density of bands from the young cohort was used to normalize the data. Homogenate of rat prostate sample was used as a positive control for TG4 and human skin sample was used as a positive control for TG1 expression.
TG Activity Assay
A dot blot assay was used to determine TG activity as previously described.23 Intact tissues were incubated with 0.1 mmol/L of 5‐(biotinamido)pentylamine (BPA) and 1 mmol/L Ca2+ at 37°C for 4 hours in culture media (phenol red‐free DMEM supplemented with 2% FBS and penicillin/streptomycin) and then rinsed to free it of unreacted BPA using PBS. Samples were then homogenized in 1× RIPA buffer containing protease and phosphatase inhibitors. Proteins (0.5 to 1 μg) were loaded onto nitrocellulose membrane (BioDot Dot Bolt apparatus; Bio‐Rad). The membrane was rinsed and blocked in 3% BSA overnight and probed with HRP‐conjugated streptavidin (1:10 000 dilution in 3% BSA; Amersham Bioscience, Foster City, CA) to determine BPA incorporation. Blots were stripped using Restore Plus stripping buffer (Pierce) and reprobed with GAPDH to determine protein loading. BPA incorporation and GAPDH levels were determined by densitometry analysis using ImageJ software. For each sample, activity was calculated as the ratio of BPA/GAPDH. The nonspecific TG inhibitor, cystamine, was from Sigma‐Aldrich (St. Louis, MO), and the nonpeptidyl active site‐directed specific TG2/Factor XIII inhibitor, L682.777, was from Zedira (Darmstadt, Germany).
S‐Nitrosylation Assay
TG2 S‐nitrosylation was determined using the biotin switch assay in tissue homogenates.23,26
Measurement of TG Cross‐links
The 81D4 antibody (Covalab, Villeurbanne, France) was used to detect Nε‐(γ‐glutamyl)‐lysine bonds in the aorta using an approach published by Thomas et al.,36 with some modifications. Aortic segments were crushed using a mortar and pestle and homogenized using RIPA buffer. Samples were centrifuged at 13 500g for 5 minutes in preweighed microcentrifuge tubes and the supernatant was removed. The resulting pellet was washed 2× in RIPA buffer and the tubes weighed again to obtain wet pellet weight. Pellets were then resuspended in solution of collagenase (50 units/mL each of collagenase 1, 2, 3, and 4; Calbiochem, San Diego, CA) and elastase (0.5 units/mL) and incubated for 3 days at 37°C. Samples were centrifuged at 13 500g for 5 minutes, the supernatant removed, and the tubes weighed again to obtain undigested pellet weight. An equivalent amount of digested pellet from each sample was loaded on a nitrocellulose membrane using the Bio‐Rad dot blot apparatus. Nε‐(γ‐glutamyl)‐lysine bonds were examined using the 81D4 antibody (1:250 in 3% nonfat dry milk, 4°C overnight; Covalab). Aorta from 12‐week‐old WT and TG2−/− mice were used to validate the dot blot approach to detect cross‐links.
IHC Staining for TG2 and TG‐Generated Cross‐links
Rat aortic tissue segments were fixed for 24 hours in 10% neutral formaldehyde. The tissue segments were embedded in paraffin, and 5‐μm transverse sections were cut using a Reichert‐Jung 2030 biocut rotary microtome. The tissue sections were mounted on positively charged aminopropyl‐triethoxysilane–coated glass slides and deparaffinized using successive washes with xylene/ethanol/water. Briefly, after deparaffinization and rehydration, slides were incubated with 3% H2O2 to block endogenous peroxidase activity. Antigen retrieval was performed using 0.01 mol/L of sodium citrate buffer in a microwave oven for 10 minutes. Before immunohistochemical (IHC) staining, the sections were blocked with 10% goat serum for 30 minutes. To detect TG2, the sections were incubated with 5 μg/mL of anti‐TG2 monoclonal antibody (mAb) CUB7402 (NeoMarkers) for 60 minutes at room temperature. To localize the TG2‐generated cross‐links, the sections were incubated with 10 μg/mL of mAb 81D4 specific for Nε‐(γ‐glutamyl)‐lysine isodipeptide cross‐links (Covalab) for 18 hours at 4°C. The slides were washed and incubated with secondary goat anti‐mouse immunoglobulin (Ig)G (for TG2) or anti‐mouse IgM (for cross‐links) conjugated with HRP for 30 minutes at room temperature. HRP activity was developed with 3,3′‐diaminobenzidine chromagenic substrate (Vector Laboratories, Burlingame, CA) for 5 minutes per the manufacturer's instructions. The sections were counterstained with hematoxylin to visualize nuclei before mounting with coverslips.
IHC Scoring
Digital images were taken using an Olympus DP20 camera system (Olympus, Tokyo, Japan) and white balanced using Photoshop CS2 (Adobe). An open‐source image analysis software program (FrIDA) was used to score individual images.37 Regions of interest (ROIs) were separately created for the media and adventitia of each vessel. Brown pixels were identified using a color‐picking algorithm that captured a user‐defined region of hue, saturation, and luminosity, and the percent of brown pixels to total pixels within each ROI was determined. For TG2, the media ROI was evaluated and for the cross‐links the adventitia ROI was used.
Statistical Analysis
All data are presented as mean±SEM. One‐way ANOVA, followed by a Tukey or Boneferroni post‐hoc analysis, was used, as indicated, for group comparisons. Two‐way ANOVA with Bonferroni multiple comparisons test was used for multiple comparisons. A value of P<0.05 was considered significant.
Results
Effect of Exercise on Vascular Stiffness in Old Rats
We initially tested the influence of aerobic exercise for 4 weeks on TG2 abundance, cross‐linking activity, and PWV in 20‐month‐old rats (Figure 1A). There was a significant increase in TG2 activity in old animals, as compared to young animals (Figure 1B), whereas TG2 abundance remained unchanged (Figure 1C). TG activity in the exercise cohort was comparable to young controls. However, PWV increased significantly in old animals, as compared to young controls, despite a brief period of aerobic exercise (4 weeks). Given this result, we hypothesized that initiating exercise at a younger age and extending the regimen for a longer period of time might prevent the NO‐dependent cross‐linking process. Therefore, the effect of 14 weeks of moderate‐intensity exercise in an 11‐month‐old cohort of rats (hereafter referred to as the “old” group) was examined in this study. Sedentary age‐matched animals and 6‐month‐old (young) rats were used as controls. Table demonstrates whole body weight, baseline BP, and heart rate in the 3 groups examined in this study.
Table 1.
Characteristics of Animals Used in this Study
| Young Animals (6 Months Old; n=13) | Sedentary Old (14 Months Old; n=7) | Old Exercise (14 Months Old; n=8) | |
|---|---|---|---|
| Whole body weight, g | 409±17 | 473±30 | 431±8 |
| Mean BP, mm Hg | 101±5.76 | 96.7±9.93 | 100±8.04 |
| Heart rate, bpm | 293±35 | 290±22 | 270±30 |
BP indicates blood pressure.
NO Signaling During Aging and Exercise
Phosphorylation of eNOS at Ser1177 in response to various stimuli is known to activate NO synthesis. Moreover, VASP is phosphorylated at Ser239 by the cGMP‐dependent kinase, PKG. Thus, peNOS Ser1177 was determined as a measure of active eNOS in the rat aorta, and pVASP Ser239 levels were used to examine NO/cGMP signaling.38–39 Both peNOS/NOS and pVASP/VASP ratios declined significantly in the sedentary old rats (Figure 2), compared to young. These ratios were similar to the young cohort in the old exercise group and significantly higher compared to the old sedentary animals. GAPDH was used as the loading control.
Figure 2.
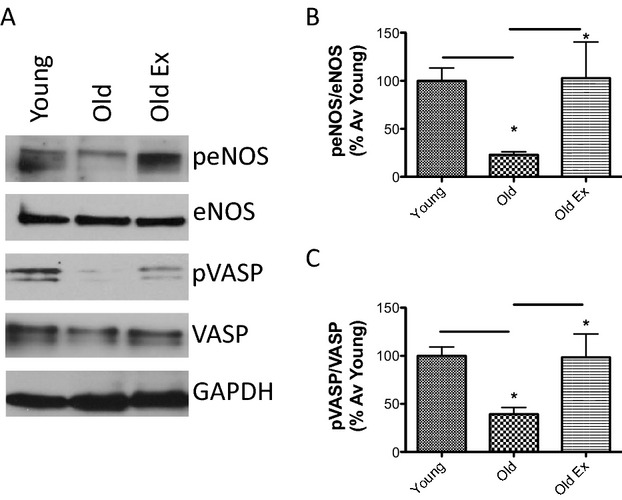
Nitric oxide signaling in aging and exercise. A, Old animals (old; 14 months old) have significantly reduced peNOS/eNOS and pVASP/VASP ratios, compared to young animals (young controls; 6 months old). A 14‐week period of exercise (old ex; 14 months old) partially restores these ratios toward the young phenotype. B, Bar graphs of densitometry analysis of peNOS/eNOS (n=12 in each group; P=0.0220 by 1‐way ANOVA; *P<0.05 young vs old and *P<0.05 old vs old ex by Tukey's post‐hoc test). C, Bar graphs of densitometry analysis of pVASP/VASP (n=12 in each group; P=0.0127 by 1‐way ANOVA; *P<0.05 young vs old and *P<0.05 old vs old ex by Tukey's post‐hoc test). VASP indicates vasodilator‐stimulated phosphoprotein, eNOS indicates endothelial nitric oxide synthase, GAPDH indicates glyceraldehyde 3‐phosphage dehydrogenase.
TG2 Function During Aging and Exercise
The effect of age and exercise on TG cross‐linking activity was examined. There was significant increase in TG activity (measured by BPA incorporation) in old sedentary animals, but not in the old exercise group, relative to the young controls (Figure 3A). TG2 S‐nitrosylation diminished with age in the sedentary, but not in the exercise, group (Figure 3A), compared to young controls. TG2 abundance remained unchanged in the 3 groups as determined by western blotting (Figure 3A). TG cross‐links (as examined by digesting the vascular matrix followed by a dot blot using the 81D4 antibody) increased significantly with age and were not altered by exercise (Figure 3B). The specificity of the method was confirmed by examining TG cross‐links in digests of WT mouse aorta with TG2−/− mouse aorta as negative control (Figure 3B, right panel). TG2 abundance and TG cross‐links were also determined by IHC (Figure 3C; negative controls are shown in Figure 3D). There was a modest increase in TG cross‐links in both the sedentary and exercise groups, compared to young, but the data did not reach statistical significance. In addition to TG2, TG4 was detected in the rat aorta (prostate sample used as positive control; Figure 4A) and its abundance decreased with age, whereas TG1 was not detected (skin sample used as positive control; Figure 4B). FITC cadaverine was incorporated in the ECM in the vasculature (Figure 4C) in a TG2‐dependent manner, and L682.777 (10 μmol/L), a specific inhibitor to TG2 and Factor XIII,40 inhibited over 75% of TG activity in both young and old rat aorta (Figure 4C and 4D). The nonspecific TG inhibitor, cystamine (200 μmol/L), yielded similar results (Figure 4D).
Figure 4.
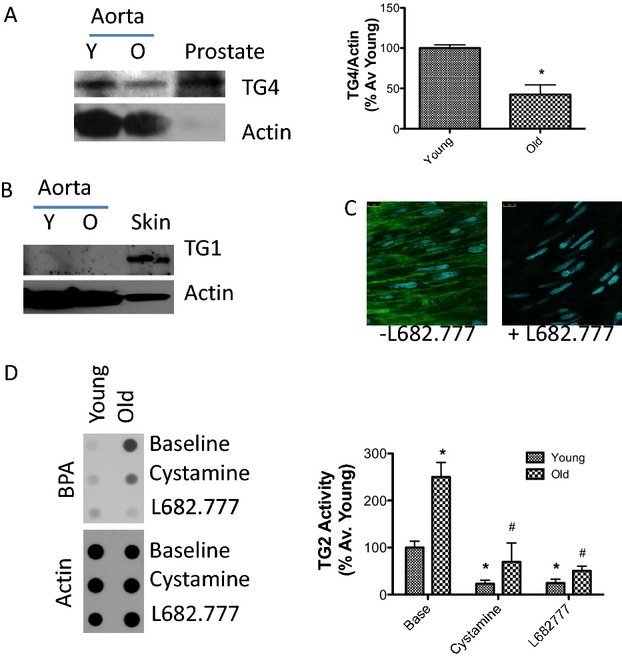
TGases in the rat vasculature. A, TG4 is expressed in the rat aorta and its abundance decreases with age (n=3 in each group; rat prostate used as positive control; actin used as loading control; bar graph of densitometry is shown on the right; P=0.01 young vs old by Student t test); B, TG1 expression was not detected in the rat aorta (n=3 in each group; skin sample used as positive control; actin used as loading control); C, TG2 inhibitor L682.777 (10 μmol/L) markedly inhibits FITC cadaverine incorporation in the ECM of the rat aorta (n=3 in each group); D, L682.777 (10 μmol/L) and cystamine (200 μmol/L) inhibit >75% of TG activity in young and old rat aorta; bar graph of densitometry analysis shown on the right (n=4 in each group; *P=0.0004 for age effects; #P<0.0001 for drug effects by 2‐way ANOVA with Bonferroni multiple comparisons). BPA indicates 5‐(biotinamido)pentylamine; ECM, extracellular matrix; FITC, fluorescein isothiocyanate; TG, transglutaminase.
Mechanical Properties of Aorta During Aging and Exercise
Next, ex vivo tensile tests were performed using an electromechanical puller to evaluate the mechanical properties of the thoracic aorta. Aortic samples from young rats were more elastic, compared to old rats (both sedentary and exercise groups), as demonstrated by an up‐ and leftward shift of the stress‐strain curve (Figure 5A). The aortas from the exercise group were statistically significantly more elastic than the sedentary group, but demonstrated tensile properties that were more similar to the sedentary old rats than to the young rats. Collagen content (IHC, Figure 5B and hydroxyproline assay, Figure 5C) trended toward an increase in the sedentary old rats, but did not reach statistical significance. Elastin content (Figure 5B) was similar in all 3 groups. MMP activity was significantly higher in the sedentary old rats, compared to young controls (Figure 5D).
Figure 5.
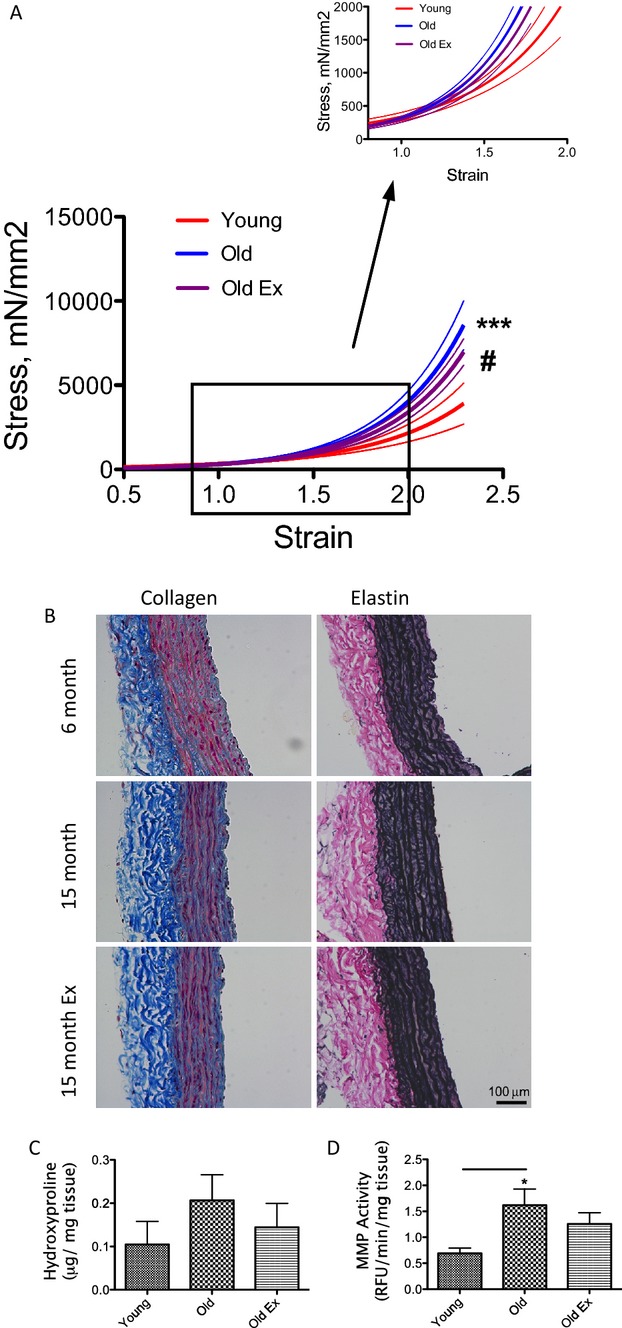
Elasticity and composition of the vascular matrix during aging and exercise. A, Aortic samples from old sedentary animals (blue) are significantly stiffer than young control aortas (red), as compared by tensile testing. Fourteen weeks of moderate exercise leads to a small, but statistically significant, shift in the tensile properties toward the young phenotype (purple). Solid lines represent the average values (n=7); dotted lines represent SE (P=0.001 by 1‐way ANOVA; *P<0.05 old vs young; #P<0.05 old vs old exercise by Bonferroni post‐hoc analysis); B, IHC analysis of collagen (Masson Trichrome) and elastin (VVG) in the rat aorta; C, Hydroxyproline assay was used to determine collagen crosslinks; and D, MMP activity was measured in the 3 groups examined in this study (n=4 in each group; *P<0.05). IHC indicates immunohistochemistry; MMP, matrix metalloprotease; VVG, Verhoeff‐Van Gieson stain.
Influence of Aging and Exercise on Vascular Stiffness
PWV measurements were used to assess in vivo vascular stiffness. Given the importance of pressure dependence of PWV, an invasive dual pressure catheter was used to determine pulse transit time and generate PWV‐MAP relationships over a wide range of blood pressures. First, the effect of age on the PWV‐MAP correlation was examined. PWV was dependent on BP and increased with rising MAP (Figure 6A). There was a significantly greater increase in PWV with MAP in the old phenotype, compared to young (Figure 6A). PWV measured at the initial pressure increased rapidly with age until 6 months and at a slower rate thereafter (Figure 6A, inset). The aerobic exercise regimen used in this study did not alter the PWV‐MAP curve in the exercise cohort, compared to age‐matched sedentary controls (Figure 6B).
Discussion
The benefits of regular exercise in preventing or reducing the deleterious effects of various cardiovascular pathologies, such as hypertension, coronary artery disease, atherosclerosis, and aging are well known.41 Seals et al.29 have shown that the increase in carotid artery stiffness that occurs with aging is largely absent in humans who are either habitually physically active or exercise trained. The mechanisms by which exercise exerts its beneficial effects are complex, but, in part, involve improved endothelial function.42 Regular exercise results in an increase in blood flow, thus exerting higher shear stress on endothelial cells. This, in turn, results in increased Akt‐dependent eNOS phosphorylation, and thus increased NO production, which contributes to the beneficial effects of exercise on vascular function.43 In addition to increased vasodilation through cGMP, NO can indirectly regulate vascular properties through protein S‐nitrosylation. We have previously shown that TG2 plays a role in vascular stiffening associated with aging, and that eNOS‐dependent NO regulates TG2 function.23,26–28 In this study, the effect of exercise mediated improvements in NO bioavailability on TG2 function, and consequently, the mechanical properties of the arterial vasculature were examined. Endothelial function was impaired with age (reduced peNOS and pVASP levels) in the sedentary group and was largely preserved in the exercise cohort, which is in agreement with previous studies.43 Moreover, TG2 S‐nitrosylation was maintained and its cross‐linking activity was suppressed in the exercise cohort, but not in the sedentary rats, compared to young controls. Thus, in this study, we demonstrate that increased NO resulting from a physiologic stimulus (ie, exercise) can alter protein function through S‐nitrosylation.
PWV, as an indicator of vascular stiffness, has been shown to correlate with morbidity and mortality in the clinical setting.18 Traditionally, PWV is measured noninvasively as a single‐point measurement. However, wall stiffness depends on the distending pressure resulting from the elastic nature of arteries, which, in turn, is related to the composition and structure of the wall and its load‐bearing components (mainly elastin and collagen) in the ECM. Hence, changes in PWV must be adjusted for arterial pressure in order to accurately reflect changes in arterial stiffness.44 We therefore measured PWV invasively over a wide range of blood pressures. As predicted, PWV varied greatly over the range of MAPs examined in this study, almost doubling at higher MAPs, compared to resting BP (Figure 6). This is in agreement with previous work by Ng et al., who have demonstrated the interaction of PWV and BP in a model of chronic kidney disease.45 As shown in this study, aging resulted in effects similar to those observed in vascular calcification. Though exercise led to increased TG2 S‐nitrosylation and decreased TG2 activity along with improved vascular elasticity ex vivo, this did not translate to improvement in aortic stiffness determined by PWV‐MAP measurements. This dichotomy might be a result of alterations to the ECM preceding the initiation of the exercise regimen in this study at 11 months of age. It is well established that cross‐linked collagen has a relatively long half‐life in vivo. Thus, though exercise may suppress further alterations to the vascular matrix, it cannot reverse changes that have accumulated between 6 and 11 months of age, and therefore may not significantly impact arterial stiffness and thus the PWV‐MAP curve. Together, these data suggest that the lower single‐point PWV observed with exercise in other rat studies may be the result of decreased MAP and/or lower resting heart rate and not the result of altered intrinsic stiffness of the vessel wall. Thus, in this study, the benefits of exercise to the vasculature included improved NO bioavailability and reduced oxidative stress, as well as reduced TG2 cross‐linking function, but not a direct effect on vascular stiffness.
Limitations
There is ongoing debate on the impact of exercise intensity on improving cardiovascular status.35,46–47 We chose a model of continuous moderate exercise to resemble the regimen commonly practiced and recommended for adults to improve cardiovascular function.47 However, high‐intensity aerobic interval training might be more efficient at improving vascular function.35,46 The effect of high‐intensity aerobic exercise on the PWV‐MAP curves remains to be elucidated and is the focus of ongoing studies in our laboratory. Furthermore, the possibility of a longer period of exercise maintaining the PWV‐MAP relationship similar to the young phenotype cannot be excluded. Also, the effects of aging and exercise may be gender specific. In a recent study on a human cohort of seniors, male participants in an exercise group did not demonstrate improvements in arterial stiffness, whereas female participants did.48 The effect of exercise on female animals was not examined in this study, and this difference remains the focus of ongoing experiments. Whereas the well‐established, commonly used Fischer 344 rat model of aging was examined in this study, strain differences have been described for a variety of vascular processes and the applicability of animal studies to humans is always of concern. Indeed, Fischer 344 rats have an early rapid increase in PWV (3 to 6 months; corresponding to ≈18‐year‐old human adults), with a smaller rate of increase in PWV thereafter. However, PWV increases more rapidly in middle‐aged and elderly humans,19–20 compared to young adults. It would therefore be of interest to investigate the effect of exercise in younger rats (ie, initiate the exercise regimen at 3 months for a total of 12 weeks or longer). Furthermore, though there is a clear relationship between eNOS‐dependent NO production and TG2, as demonstrated previously,23,27–28,26 and it has been well established that exercise influences eNOS‐dependent NO,42,49–50 we do not provide a direct mechanistic relationship, but rather an association between exercise‐mediated improvement in eNOS function and TG2 activity, in this study.
The arterial pressure at the time of surgery was recorded in anesthetized rats; BP in conscious animals was not measured in this study. Also, the vasoactive drugs, phenylephrine and sodium nitroprusside, were used to vary BP in the invasive PWV‐MAP measurements. It is assumed that the main site of action of these drugs is in the resistance arteries, although it is possible that they may also affect the tone of the smooth muscle cells in the aortic wall. By nonpharmacological manipulation of arterial pressure using maneuvers that affect venous return, we have verified that changes in PWV during the drug infusions are the result of pressure changes associated with altered resistance (Alberto Avolio and Mark Butlin, unpublished observations). Furthermore, because phenylephrine and sodium nitroprusside were administered equally to all treatment groups, any possible confounding effects, although minor, would apply equally to all groups, hence not affecting comparisons across groups.
In this study, while a brief period of moderate aerobic exercise did not prevent age‐associated increase PWV, it successfully maintained NO signaling in aging rats.
Acknowledgment
The authors thank Koenraad Vandergaer for his technical assistance.
Authors Contributions
Steppan, Santhanam: study design, performed experiments, data analysis, data interpretation, writing of the manuscript; Belkin, Jandu: performed experiments; Sikka, Barodka, Halushka, Butlin: data interpretation, writing of the manuscript; Nyhan, Avolio, Berkowitz: study design, data interpretation, writing of the manuscript.
Sources of Funding
This work was supported by American Heart Association grants (09BGIA2220181 [to Santhanam] and 11GRNT720007 [to Belkin], a National Institutes of Health grant (1R01‐HL105296‐01; to Berkowitz), and an Australian Research Council Discovery grant (DP110101134; to Avolio).
Disclosures
None.
References
- 1.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005; 46:454-462 [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003; 107:139-146 [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993; 73:413-467 [DOI] [PubMed] [Google Scholar]
- 4.Nagai R, Hoshino Y. Molecular genetics of cardiovascular diseases. Rinsho Byori. 1998; 46:249-257 [PubMed] [Google Scholar]
- 5.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O'Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991; 139:1119-1129 [PMC free article] [PubMed] [Google Scholar]
- 6.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005; 45:652-658 [DOI] [PubMed] [Google Scholar]
- 7.Vaikevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993; 88:1456-1462 [DOI] [PubMed] [Google Scholar]
- 8.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005; 18:3S-10S [DOI] [PubMed] [Google Scholar]
- 9.Franklin SS, Gustin W, IV, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 96:308-315 [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001; 38:1461-1466 [DOI] [PubMed] [Google Scholar]
- 11.Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011; 2011:263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronson S, Fontes ML, Miao Y, Mangano DTInvestigators of the Multicenter Study of Perioperative Ischemia Research G, Ischemia R, Education F. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007; 115:733-742 [DOI] [PubMed] [Google Scholar]
- 13.Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006; 113:657-663 [DOI] [PubMed] [Google Scholar]
- 14.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004; 109:184-189 [DOI] [PubMed] [Google Scholar]
- 15.Schram MT, Henry RMA, van Dijk RAJM, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CDA. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004; 43:176-181 [DOI] [PubMed] [Google Scholar]
- 16.Jung AS, Harrison R, Lee KH, Genut J, Nyhan D, Brooks‐Asplund EM, Shoukas AA, Hare JM, Berkowitz DE. Simulated microgravity produces attenuated baroreflex‐mediated pressor, chronotropic, and inotropic responses in mice. Am J Physiol Heart Circ Physiol. 2005; 289:H600-H607 [DOI] [PubMed] [Google Scholar]
- 17.Vlachopoulos C, Kosmopoulou F, Panagiotakos D, Ioakeimidis N, Alexopoulos N, Pitsavos C, Stefanadis C. Smoking and caffeine have a synergistic detrimental effect on aortic stiffness and wave reflections. J Am Coll Cardiol. 2004; 44:1911-1917 [DOI] [PubMed] [Google Scholar]
- 18.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010; 55:1318-1327 [DOI] [PubMed] [Google Scholar]
- 19.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983; 68:50-58 [DOI] [PubMed] [Google Scholar]
- 20.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008; 51:1377-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda N. Age‐related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012; 133:159-176 [DOI] [PubMed] [Google Scholar]
- 22.Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010; 10suppl 1:S213-S220 [DOI] [PubMed] [Google Scholar]
- 23.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S‐nitrosylation of tissue transglutaminase contributes to age‐related increases in vascular stiffness. Circ Res. 2010; 107:117-125 [DOI] [PubMed] [Google Scholar]
- 24.Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009; 89:991-1023 [DOI] [PubMed] [Google Scholar]
- 25.Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J. 2011; 278:4704-4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jandu SK, Webb AK, Pak A, Sevinc B, Nyhan D, Belkin AM, Flavahan NA, Berkowitz DE, Santhanam L. Nitric oxide regulates tissue transglutaminase localization and function in the vasculature. Amino Acids. 2013; 44:261-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santhanam L, Berkowitz DE, Belkin AM. Nitric oxide regulates non‐classical secretion of tissue transglutaminase. Commun Integr Biol. 2011; 4:584-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung SM, Jandu S, Steppan J, Belkin A, An SS, Pak A, Choi EY, Nyhan D, Butlin M, Viegas K, Avolio A, Berkowitz DE, Santhanam L. Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am J Physiol Heart Circ Physiol. 2013; 305:H803-H810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009; 587:5541-5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010; 65:1028-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagg U, Andersson I, Naylor AS, Gronros J, Jonsdottir IH, Bergstrom G, Gan LM. Voluntary physical exercise‐induced vascular effects in spontaneously hypertensive rats. Clin Sci. 2004; 107:571-581 [DOI] [PubMed] [Google Scholar]
- 32.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation. 2000; 102:1351-1357 [DOI] [PubMed] [Google Scholar]
- 33.Spier SA, Delp MD, Stallone JN, Dominguez JM, II, Muller‐Delp JM. Exercise training enhances flow‐induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2007; 292:H3119-H3127 [DOI] [PubMed] [Google Scholar]
- 34.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left ventricular pressure‐volume relationship in a rat model of advanced aging‐associated heart failure. Am J Physiol Heart Circ Physiol. 2004; 287:H2132-H2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007; 14:753-760 [DOI] [PubMed] [Google Scholar]
- 36.Thomas V, Fournet G, Simonet F, Roch AM, Ceylan I, El Alaouia S, Quash G. Definition of the fine specificity of the monoclonal antibody 81D4: its reactivity with lysine and polyamine isopeptide cross‐links. J Immunol Methods. 2004; 292:83-95 [DOI] [PubMed] [Google Scholar]
- 37.Selvin E, Najjar SS, Cornish TC, Halushka MK. A comprehensive histopathological evaluation of vascular medial fibrosis: insights into the pathophysiology of arterial stiffening. Atherosclerosis. 2010; 208:69-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator‐stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res. 2000; 87:999-1005 [DOI] [PubMed] [Google Scholar]
- 39.Lawrence DW, Pryzwansky KB. The vasodilator‐stimulated phosphoprotein is regulated by cyclic GMP‐dependent protein kinase during neutrophil spreading. J Immunol. 2001; 166:5550-5556 [DOI] [PubMed] [Google Scholar]
- 40.Freund KF, Doshi KP, Gaul SL, Claremon DA, Remy DC, Baldwin JJ, Pitzenberger SM, Stern AM. Transglutaminase inhibition by 2‐[(2‐oxopropyl)thio]imidazolium derivatives: mechanism of factor XIIIa inactivation. Biochemistry. 1994; 33:10109-10119 [DOI] [PubMed] [Google Scholar]
- 41.Kingwell BA. Nitric oxide‐mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J. 2000; 14:1685-1696 [DOI] [PubMed] [Google Scholar]
- 42.Gielen S, Sandri M, Erbs S, Adams V. Exercise‐induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol. 2011; 12:1375-1384 [DOI] [PubMed] [Google Scholar]
- 43.Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010; 459:807-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985; 71:202-210 [DOI] [PubMed] [Google Scholar]
- 45.Ng K, Hildreth CM, Phillips JK, Avolio AP. Aortic stiffness is associated with vascular calcification and remodeling in a chronic kidney disease rat model. Am J Physiol Renal Physiol. 2011; 300:F1431-F1436 [DOI] [PubMed] [Google Scholar]
- 46.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005; 67:161-172 [DOI] [PubMed] [Google Scholar]
- 47.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994; 266:H693-H701 [DOI] [PubMed] [Google Scholar]
- 48.Williams AD, Ahuja KD, Almond JB, Robertson IK, Ball MJ. Progressive resistance training might improve vascular function in older women but not in older men. J Sci Med Sport. 2013; 16(1):76-81 [DOI] [PubMed] [Google Scholar]
- 49.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium‐derived nitric oxide function in humans. J Physiol. 2004; 561:1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab. 2008; 33:173-178 [DOI] [PMC free article] [PubMed] [Google Scholar]