Abstract
Background
Metabolic disorders are relatively uncommon in young women, but may increase with obesity. The associations between body mass index (BMI) and risks of diabetes, hypertension, and dyslipidemia in apparently healthy, young women have been insufficiently investigated, and are the aims of this study.
Methods and Results
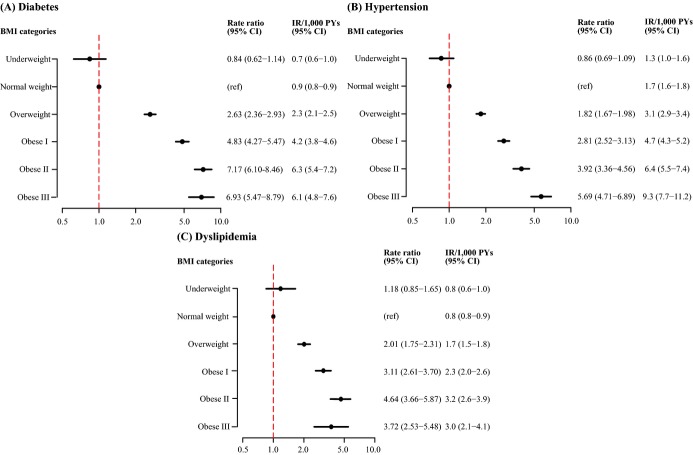
Women giving birth during the years 2004–2009, with no history of cardiovascular disease, renal insufficiency, pregnancy‐associated metabolic disorders, diabetes, hypertension, or dyslipidemia were identified in nationwide registers. Women were categorized as underweight (BMI<18.5 kg/m2), normal weight (BMI=18.5 to <25 kg/m2), overweight (BMI=25 to <30 kg/m2), obese‐I (BMI=30 to <35 kg/m2), obese‐II (BMI=35 to <40 kg/m2), and obese‐III (BMI≥40 kg/m2). We assessed risks by Poisson regression models (adjusted for age, calendar year; reference=normal weight). The cohort comprised 252 472 women with a median age of 30.4 years (IQR=27.2;33.7) and a median follow‐up of 5.5 years (IQR=3.9;6.8). In total, 2029 women developed diabetes, 3133 women developed hypertension, and 1549 women developed dyslipidemia. Rate ratios (RRs) of diabetes were: 0.84 (95% confidence interval [CI]=0.62 to 1.14) for underweight, 2.63 (CI=2.36 to 2.93) for overweight, 4.83 (CI=4.27 to 5.47) for obese grade‐I, 7.17 (CI=6.10 to 8.48) for obese grade‐II, and 6.93 (CI=5.47 to 8.79) for obese grade‐III women. For hypertension, corresponding RRs were 0.86 (CI=0.69 to 1.09), 1.82 (CI=1.67 to 1.98), 2.81 (CI=2.52 to 3.13), 3.92 (CI=3.36 to 4.56), and 5.69 (CI=4.71 to 6.89), and for dyslipidemia, RRs were 1.18 (CI=0.85 to 1.65), 2.01 (CI=1.75 to 2.31), 3.11 (CI=2.61 to 3.70), 4.64 (CI=3.66 to 5.87), and 3.72 (CI=2.53 to 5.48).
Conclusions
In this nationwide study of fertile, apparently healthy women, pre‐pregnancy BMI was strongly associated with an increased risk of diabetes, hypertension, and dyslipidemia within 5.5 years following childbirth.
Keywords: body mass index, diabetes, hypercholesterolemia, hypertension, women
Introduction
Although now presumably stabilizing,1 the prevalence of obesity has been increasing at an alarming rate over the last 4 decades,2 especially among the young.3 The cardiovascular consequences of obesity have a greater deleterious impact in women ≤65 years of age.4 However, the metabolically healthy but obese individuals have been shown to carry the same risk of cardiovascular disease as metabolically healthy and normal weight individuals, thus obesity is not simply obesity.5 Considering the difficulties in identifying these women and the potential benefit of keeping them metabolically healthy, it is important to clarify the extent to which obesity relates to metabolic disorders in young women. The matter is of crucial importance; especially after a recent meta‐analysis found overweight individuals to carry the lowest age‐, year‐, and smoking‐adjusted risk of all‐cause mortality.6 Consequently, confusion may arise as to whether clinicians should advise their overweight or obese patients, healthy or sick, to lose weight.
The aim of this nationwide study was to assess the associations between body mass index (BMI) and the risk of developing the well‐known cardiovascular risk factors: diabetes, hypertension, and dyslipidemia in young women. Secondarily, we sought to investigate the associations between BMI and the cumulative metabolic risk, thus the risk of developing at least 1, 2, or 3 metabolic disorders.
Methods
Data Sources and Definitions
From the Danish Medical Birth Register we identified women giving birth during the years 2004‐2009, with no previous history of cardiovascular disease, renal insufficiency, gestational diabetes, gestational hypertension, preeclampsia, diabetes, hypertension, or dyslipidemia, thus no prior cardiovascular disease or treated metabolic disorders. Detailed information on all women giving birth in Denmark is registered in the Medical Birth Register together with systematic registration of height and pre‐pregnancy weight since 2004. The height and measured pre‐pregnancy weight are mandatorily reported to the Medical Birth Register at the time of birth, but obtained at the first antenatal appointment at the general practitioner in gestation week 6 to 10. These antenatal routine appointments are governmentally financed and offered to all pregnant women independently of socioeconomic or employee status, thus ensuring a high rate of adherence as the general practitioners are also responsible for the referral to the obstetrician in the hospital setting. Furthermore, information on smoking during pregnancy, gestational hypertension, preeclampsia, and eclampsia was obtained from the Medical Birth Register.
We calculated BMI (weight in kilograms divided by the square of the height in meters, kg/m2), and divided the population in 6 BMI categories according to the World Health Organization (WHO) classification7: underweight (BMI<18.5 kg/m2), normal weight (BMI=18.5 to <25 kg/m2), overweight (BMI=25 to <30 kg/m2), obese grade‐I (BMI=30 to <35 kg/m2), obese grade‐II (BMI=35 to <40 kg/m2), and obese grade‐III (BMI≥40 kg/m2).7
Cross‐linkage of national registers at an individual level is possible in Denmark due to each citizen's personal and permanent identification number. From the Danish Register of Medicinal Product Statistics we obtained data on pharmaceutical treatment in the 90 days prior to pregnancy (180 days for lipid‐lowering drugs8). The register has been shown to be accurate,9 and contains all prescriptions dispensed from Danish pharmacies according to the International Anatomical Therapeutic Chemical (ATC) classification system.
Diabetes prior to pregnancy was defined as either prescribed medications of glucose‐lowering agents (ATC A10) in 1 year prior to index pregnancy, or appearance in the National Patient Register prior to the index pregnancy (International Classification of Diseases [ICD]‐10 codes=DE10‐DE14, DH360, DO24 [excluding DO244]). Incident diabetes was defined as either (1) 2 prescription claims for glucose‐lowering agents within 6 months (date of second prescription claim defined as date of event), or (2) registration in the National Patient Register with a diagnosis of diabetes. Due to the risk of including events of gestational diabetes, we excluded prescriptions claimed during pregnancies and excluded prescriptions and diagnoses codes registered within 1 year following gestational diabetes (ICD 10=DO244). These definitions have been validated.10 Hypertension was defined as either (1) prescription claims of at least 2 antihypertensive agents within 90 days11 or, (2) a diagnosis of hypertension in the National Patient Register (outpatient visits and hospital admissions) with prescription of at least 1 antihypertensive agent within 90 days. To avoid including use of antihypertensive medication due to gestational hypertension or preeclampsia, we excluded prescriptions collected during pregnancies or within 1 year following a pregnancy‐induced hypertensive disorder. We considered this approach as conservative, but acknowledge that it may potentially delay the diagnosis of diabetes or hypertension for up to 1 year for women who experienced a pregnancy‐associated metabolic disorder and later developed a metabolic disorder during follow‐up. Dyslipidemia was defined as claimed prescriptions of lipid‐lowering medications (ATC C10A). We only had access to data from the Medical Birth Register until 2010, but achieved information on births in 2011 from the National Patient Register (ICD‐10=DO80‐DO84); we used the discharge diagnosis as date of birth and beginning of pregnancy as 42 weeks prior to date of birth.
Pregnancy‐induced metabolic disorders at baseline were defined as a discharge diagnosis from the National Patient Register or the Medical Birth Register in previous pregnancies or in the index pregnancy, or prescribed glucose‐lowering agents in index pregnancy, ie, gestational hypertension (ICD 10=DO13), preeclampsia (ICD‐10=DO14), eclampsia (ICD‐10=DO15), or gestational diabetes (ICD 10=DO244). We combined all pregnancy‐induced hypertensive disorders into one group comprising preeclampsia, eclampsia, and gestational hypertension.
Outcomes
The primary outcomes were diabetes, hypertension, and dyslipidemia (ATC C10A), and the secondary outcome was the risk of developing at least 1, 2, or 3 metabolic disorders in each individual.
Ethics
In Denmark, no ethics approval is needed for retrospective register studies. This study was approved by the Danish Data Protection Agency (2007‐58‐0015).
Statistical Analyses
We defined the index date of inclusion as the first date of childbirth between January 1, 2004 and December 31, 2009. All women were followed until outcome event, emigration, death, or December 31, 2011, whichever came first.
Cumulative risk curves were based on competing risk models, ie, Kaplan‐Meier curves of the absolute incidences of the outcomes in which the competing risk of death was taken into account. Time‐dependent multivariable Poisson regression models were used to assess the associations between pre‐pregnancy BMI and the risks of diabetes, hypertension, and dyslipidemia, and the number of metabolic disorders in 2 steps. The main analyses were only adjusted for age and calendar year (both variables updated yearly since index date), ie, not adjusted for any potential intermediaries. A second model was additionally adjusted for number of parities and pregnancy‐associated complications during follow‐up (gestational hypertension, pre‐eclampsia, and gestational diabetes). Further, we assessed the impact of change in BMI over time on our results in a subanalysis including women who gave birth during follow‐up, had no history of metabolic disorders at date of first birth during follow‐up, and available data on BMI. The method has been described in detail previously.12 The model assumptions of proportionality of rate ratios over time (by use of log(−log(survival)) plots), lack of interactions and linearity of continuous variables were tested and found valid unless otherwise indicated. All statistical calculations were performed using SAS for Windows, version 9.2 (SAS Institute Inc).
Results
From the Medical Birth Register we identified 292 847 women who gave birth between the years of 2004‐2009, excluding women with prior history of cardiovascular disease or renal insufficiency (n excluded=564); missing or incomplete registration of BMI (n excluded=18 907); incorrect vital status (n excluded=29); missing or extreme gestational age of foetus (n excluded=246); diabetes, hypertension, or dyslipidemia at baseline (n=5185); and gestational diabetes, gestational hypertension, or preeclampsia (n=15 444).
The study population comprised 252 472 women with a median age of 30.4 years (interquartile range 27.2 to 33.7). There was a somewhat U‐shaped relation between BMI‐category and active smoking during pregnancy, with the highest frequency of active smokers among underweight and obese women, and further, the number of parities increased with increasing BMI (Table 1).
Table 1.
Baseline Characteristics According to BMI‐Categories for 252 472 Women, Who Have Given Birth
| Variables | BMI‐Category (kg/m2) | |||||
|---|---|---|---|---|---|---|
| <18.5 (n=11 201) | 18.5 to <25.0 (n=165 933) | 25.0 to <30.0 (n=50 358) | 30.0 to <35.0 (n=17 012) | 35.0 to <40.0 (n=5598) | ≥40.0 (n=2370) | |
| Median age, IQR | 29.0 (25.2;32.5) | 30.5 (27.4;33.8) | 30.4 (27.1;33.9) | 30.1 (26.7;33.6) | 29.9 (26.4;33.4) | 30.0 (26.6;33.4) |
| Median follow‐up in years, IQR | 5.4 (3.8;6.8) | 5.5 (3.8;6.8) | 5.6 (3.9;6.8) | 5.6 (3.9;6.8) | 5.5 (3.9;6.8) | 5.5 (3.9;6.8) |
| Median pre‐pregnancy BMI, IQR | 17.9 (17.4;18.3) | 21.8 (20.4;23.2) | 26.8 (25.8;28.1) | 31.8 (30.8;31.1) | 36.8 (35.8;38.1) | 42.5 (41.0;45.0) |
| Smoking during pregnancy* | ||||||
| Never or ceased in pregnancy | 8500 (77.3%) | 142 371 (87.5%) | 42 021 (85.2%) | 13 794 (82.9%) | 4449 (81.2%) | 1898 (82.2%) |
| Active in pregnancy | 2494 (22.7%) | 20 354 (12.5%) | 7289 (14.8%) | 2925 (17.1%) | 1031 (18.8%) | 411 (17.8%) |
| Ethnicity* | ||||||
| Danish | 9032 (82.6%) | 143 477 (86.5%) | 43 482 (86.4%) | 14 954 (87.9%) | 5074 (90.7%) | 2214 (93.4%) |
| Immigrants | 1930 (17.2%) | 12 398 (12.3%) | 6298 (12.5%) | 1895 (11.1%) | 466 (8.3%) | 136 (5.7%) |
| Offspring of first generation immigrants | 238 (2.1%) | 2034 (1.2%) | 574 (1.1%) | 165 (1.0%) | 57 (1.0%) | 20 (0.8%) |
| Parity including index pregnancy* | ||||||
| 1 | 6744 (60.9%) | 94 945 (57.8%) | 25 915 (52.0%) | 8384 (49.8%) | 2676 (48.3%) | 1137 (48.6%) |
| 2 | 2907 (26.2%) | 44 903 (27.3%) | 14 378 (28.8%) | 4992 (29.7%) | 1719 (31.0%) | 707 (30.2%) |
| 3 | 11 081 (9.8%) | 19 275 (11.7%) | 6889 (13.8%) | 2345 (13.9%) | 768 (13.9%) | 338 (14.4%) |
| ≥4 | 345 (3.1%) | 5282 (3.2%) | 2684 (5.4%) | 1116 (6.6%) | 377 (6.8%) | 159 (6.8%) |
P value was <0.001 for all baseline characteristics unless otherwise indicated. BMI indicates body mass index; IQR, interquartile range.
Data missing for 5014 women (2.0%).
Data missing for 32 women (0.01%).
Data missing for 2406 women (0.9%).
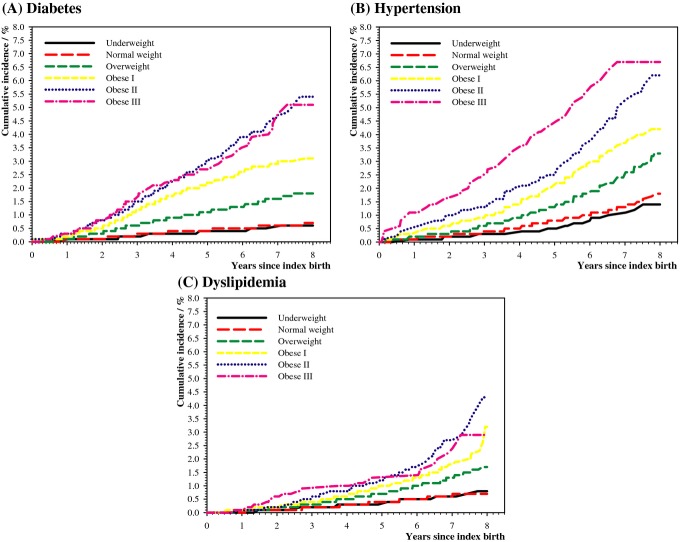
During a median follow‐up of 5.5 years (interquartile range 3.9 to 6.8), a total number of 2029 women (0.8%, 1.5/1000 person years) developed diabetes, 3133 women (1.2%, 2.4/1000 person years) developed hypertension, and 1549 women (0.6%, 1.2/1000 person years) developed dyslipidemia. Increasing pre‐pregnancy BMI was strongly associated with increasing risk of diabetes, hypertension, and dyslipidemia as shown in the incidence curves (Figure 1), and forest plots illustrating the rate ratios (RRs) and absolute incidence rates of diabetes, hypertension, and dyslipidemia adjusted for age and calendar year (Figure 2). The results were largely unchanged following adjustment for age; calendar year; smoking; and as time‐dependent variables: number of parities; gestational diabetes; pregnancy‐induced hypertensive disorder; diabetes; hypertension; and dyslipidemia (Table 2). Due to missing data, the multivariable models only included 245 105 women. Results were similar when women with missing data were included in the analyses using separate categories.
Figure 1.
Cumulative incidence of diabetes (A), hypertension (B), and dyslipidemia (C) according to BMI categories. BMI indicates body mass index.
Figure 2.
Rate ratios of diabetes (A), hypertension (B), and dyslipidemia (C) according to BMI‐categories and adjusted for age and calendar year using the normal weight women as a reference. BMI indicates body mass index; CI, 95% confidence interval; IR, incidence rate; RR, rate ratio.
Table 2.
Multivariable Poisson Regression Analyses of the Associations Between BMI‐Categories and Risks of Diabetes, Hypertension and Dyslipidemia in 245 105* Fertile Women
| Diabetes | Hypertension | Dyslipidemia | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| BMI‐category | ||||||
| Underweight | 0.74 | 0.51 to 1.08 | 0.83 | 0.66 to 1.06 | 1.09 | 0.78 to 1.53 |
| Normal weight | (ref) | NA | (ref) | NA | (ref) | NA |
| Overweight | 2.70 | 2.38 to 3.07 | 1.72 | 1.57 to 1.87 | 1.83 | 1.59 to 2.11 |
| Obese grade‐I | 4.85 | 4.19 to 5.61 | 2.61 | 2.34 to 2.92 | 2.45 | 2.05 to 2.94 |
| Obese grade‐II | 7.56 | 6.26 to 9.12 | 3.54 | 3.03 to 4.13 | 3.41 | 2.67 to 4.35 |
| Obese grade‐III | 7.36 | 5.62 to 9.64 | 4.98 | 4.10 to 6.05 | 2.11 | 1.40 to 3.18 |
| Age (continuous variable) | 0.99 | 0.98 to 1.00 | 1.10 | 1.09 to 1.11 | 1.09 | 1.08 to 1.11 |
| Active smoking | 0.94 | 0.82 to 1.09 | 1.22 | 1.11 to 1.34 | 2.46 | 2.16 to 2.80 |
| Calendar year | ||||||
| 2004–2005 | (ref) | NA | (ref) | NA | (ref) | NA |
| 2006–2007 | 1.42 | 1.16 to 1.73 | 1.05 | 0.90 to 1.21 | 1.76 | 1.32 to 2.35 |
| 2008–2009 | 1.77 | 1.47 to 2.12 | 1.12 | 0.98 to 1.30 | 2.09 | 1.60 to 2.74 |
| 2010–2011 | 1.97 | 1.65 to 2.35 | 1.26 | 1.09 to 1.45 | 2.42 | 1.86 to 3.13 |
| Total number of parities | ||||||
| 1 | (ref) | NA | (ref) | NA | (ref) | NA |
| 2 | 0.40 | 0.35 to 0.45 | 1.31 | 1.19 to 1.45 | 1.07 | 0.92 to 1.26 |
| 3 | 0.37 | 0.31 to 0.44 | 1.45 | 1.30 to 1.63 | 1.16 | 0.96 to 1.39 |
| 4 | 0.49 | 0.39 to 0.62 | 1.67 | 1.45 to 1.92 | 1.51 | 1.22 to 1.87 |
| Gestational diabetes | 28.09 | 17.72 to 44.55 | 1.04 | 0.26 to 4.15 | 2.24 | 0.31 to 15.90 |
| Pregnancy‐induced hypertensive disorder* | 2.16 | 1.16 to 4.05 | 6.19 | 4.88 to 7.87 | 1.11 | 0.46 to 2.67 |
| Diabetes | NA | NA | 1.92 | 1.45 to 2.54 | 10.02 | 7.92 to 12.71 |
| Hypertension | 1.78 | 1.24 to 2.58 | NA | NA | 6.93 | 5.59 to 8.59 |
| Dyslipidemia | 9.76 | 7.37 to 12.92 | 7.90 | 6.47 to 9.65 | NA | NA |
For absolute incidence rates please see Figure 2. 95% CI indicates 95% confidence interval; BMI, body mass index; NA, not applicable; RR, rate ratio.
Due to missing data.
Gestational hypertension. preeclampsia or eclampsia.
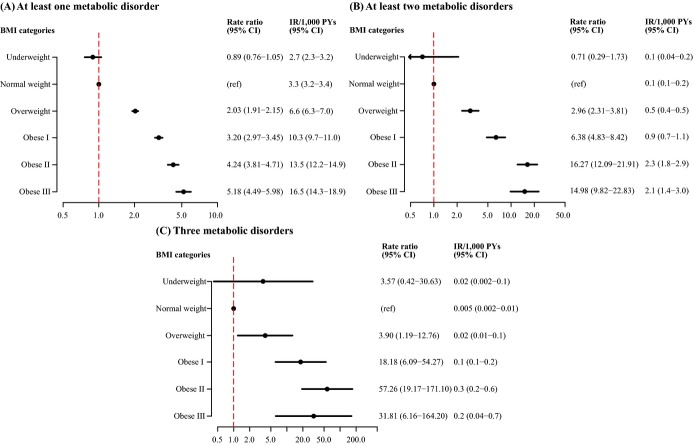
A total of 6242 women (2.5%) developed at least 1 metabolic disorder during follow‐up, 437 women (0.2%) developed at least 2, and 32 women (0.01%) developed 3 metabolic disorders during the median 5.5 years of follow‐up. Compared with the risk in normal weight women and adjusted for age and calendar year, obesity grade II and grade III were associated with a 4‐fold, 15‐fold, and a 30‐ to 60‐fold increased RR of developing at least 1, 2, and 3 metabolic disorders (Figure 3).
Figure 3.
Rate ratios of developing at least 1 metabolic disorder (A), at least 2 metabolic disorders (B), or 3 metabolic disorders (C) according to BMI‐categories and adjusted for age and calendar year using the normal weight women as a reference. BMI indicates body mass index; CI, 95% confidence interval; IR, incidence rate; RR, rate ratio.
Sensitivity Analyses and Exploratory Analyses
Prescription of antihypertensive drugs during follow‐up could be due to postpartum use or due to preeclampsia during a future pregnancy. Likewise, use of glucose‐lowering agents could be due to future gestational diabetes. We therefore conducted sensitivity analyses, in which women were censored at the first day of a pregnancy during follow‐up, and including only first‐time pregnancies. The overall results were unaltered with RRs for obese grade‐I women of 4.85 (CI=4.08 to 5.76) for diabetes, 2.95 (CI=2.43 to 3.58) for hypertension, and 3.27 (CI=2.83 to 3.78) for dyslipidemia. Furthermore, for hypertension we restricted follow‐up to begin after 45 days of follow‐up (index date+45 days), which did not change our results (RR for obese grade‐I women=2.84 [CI=2.55 to 3.17]).
Biguanides, ie, metformin, are increasingly used to treat polycystic ovary syndrome, and we therefore conducted a sensitivity analysis excluding use of oral glucose‐lowering agents to identify individuals with diabetes. Despite a substantial decrease in the number of incident cases of diabetes (n=974 versus n=2029), the RRs did not change substantially: 1.01 (CI=0.69 to 1.48) for underweight, 2.22 (CI=1.91 to 2.59) for overweight, 3.23 (CI=2.67 to 3.92) for obese grade‐I, 5.88 (CI=4.64 to 7.45) for obese grade‐II, and 6.42 (CI=4.60 to 8.95) for obese grade‐III women.
A subanalysis evaluating associations between weight change and metabolic risk included 86 758 women who gave birth during follow‐up (n=108 034 women), with no history of metabolic disorders at date of first birth during follow‐up (n excluded=3133), and available data on pre‐pregnancy BMI for the birth during follow‐up (n excluded=18 143). Although weight gain was associated with increased risk of metabolic disorders in unadjusted analyses, these associations were attenuated following adjustment for baseline BMI category, whereas the associations between BMI category and the metabolic disorders were similar to our main results (Table 3).
Table 3.
Associations Between Weight Change in Between Pregnancies and Risks of Diabetes, Hypertension and Dyslipidemia for 86 758 Women Free of Metabolic Disorders Who Gave Birth During Follow‐Up
| Unadjusted | Diabetes | Hypertension | Dyslipidemia | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | IR/1000 PYs | RR (95% CI) | IR/1000 PYs | RR (95% CI) | IR/1000 PYs | |
| Change in BMI | ||||||
| Weight loss, >1 kg/m2 (n=13 454; 15.5%) | 1.40 (0.98 to 2.02) | 1.0 | 1.28 (0.99 to 1.64) | 0.8 | 1.40 (0.98 to 2.02) | 1.0 |
| Stable BMI, ±1 kg/m2 (n=44 277; 51.0%) | (ref) | 0.7 | (ref) | 0.6 | (ref) | 0.7 |
| Minor weight gain, >1 to 2 kg/m2 (n=13 414; 15.5%) | 1.76 (1.26 to 2.48) | 1.2 | 1.32 (1.02 to 1.70) | 0.8 | 1.21 (0.82 to 1.78) | 0.8 |
| Major weight gain, >2 kg/m2 (n=15 643; 18.0%) | 2.10 (1.54 to 2.85) | 1.4 | 1.84 (1.48 to 2.29) | 1.1 | 1.92 (1.40 to 2.63) | 1.3 |
| Multivariable analysis adjusted for BMI‐category, change in BMI, years between index‐pregnancy and first pregnancy during follow‐up, age and calendar year | |||
|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| BMI‐category | |||
| Underweight | 1.01 (0.47 to 2.18) | 1.03 (0.60 to 1.76) | 1.32 (0.68 to 2.57) |
| Normal weight | (ref) | (ref) | (ref) |
| Overweight | 2.20 (1.54 to 3.15) | 1.72 (1.35 to 2.19) | 1.55 (1.09 to 2.20) |
| Obese grade‐I | 3.66 (2.38 to 5.63) | 2.36 (1.71 to 3.24) | 2.69 (1.75 to 4.12) |
| Obese grade‐II | 7.14 (4.33 to 11.79) | 2.95 (1.87 to 4.64) | 2.62 (1.36 to 5.02) |
| Obese grade‐III | 6.40 (3.10 to 13.22) | 4.21 (2.36 to 7.51) | 1.15 (0.28 to 4.72) |
| Change in BMI | |||
| Weight loss, >1 kg/m2 | 0.85 (0.57 to 1.27) | 1.12 (0.80 to 1.44) | 1.06 (0.71 to 1.60) |
| Stable BMI, ±1 kg/m2 | (ref) | (ref) | (ref) |
| Minor weight gain, >1 to 2 kg/m2 | 1.43 (0.98 to 2.09) | 1.24 (0.93 to 1.66) | 1.03 (0.68 to 1.58) |
| Major weight gain, >2 kg/m2 | 1.38 (0.97 to 1.95) | 1.66 (1.26 to 2.11) | 1.50 (1.04 to 2.15) |
95% CI indicates 95% confidence interval; BMI, body mass index; IR, incidence rate; PYs, person years; RR, rate ratio.
Our overall results were unaltered following adjustment for educational attainment with RRs for obese grade‐I women of 4.86 (95% CI=4.27 to 5.53) for diabetes, 2.52 (95% CI=2.34 to 2.92) for hypertension, and 2.73 (95% CI=2.29 to 3.26) for dyslipidemia (Table 4). There was no interaction between age and highest attained educational level for hypertension (P for interaction=0.92) or for dyslipidemia (P for interaction=0.17), but only for diabetes (P for interaction< 0.001). We found no associations between educational attainment and diabetes for women <35 years of age. However, being ≥35 years old with only basic school education was associated with a higher risk of diabetes than in other age groups: RRs of diabetes were 0.63 (95% CI 0.43 to 0.92) for upper secondary, 0.74 (95% CI 0.58 to 0.95) for vocational, 0.57 (95% CI 0.44 to 0.74) for short or medium length, and 0.55 (95% CI 0.38 to 0.79) for a master's degree or PhD as highest attained education. The associations between BMI categories and risk of diabetes were nonetheless unaffected.
Table 4.
Multivariable Poisson Regression Analyses of the Associations Between BMI Categories and Risks of Diabetes, Hypertension, and Dyslipidemia in Fertile Women Adjusted for Age, Calendar Year, and Educational Attainment
| Diabetes | Hypertension | Dyslipidemia | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| BMI‐category | ||||||
| Underweight | 0.75 | 0.52 to 1.08 | 0.83 | 0.66 to 1.04 | 1.12 | 0.80 to 1.66 |
| Normal weight | (ref) | NA | (ref) | NA | (ref) | NA |
| Overweight | 2.69 | 2.37 to 3.05 | 1.71 | 1.57 to 1.86 | 1.87 | 1.63 to 2.16 |
| Obese grade‐I | 4.83 | 4.18 to 5.59 | 2.52 | 2.34 to 2.92 | 2.73 | 2.29 to 3.26 |
| Obese grade‐II | 7.42 | 6.16 to 8.94 | 3.44 | 2.95 to 4.01 | 3.96 | 3.12 to 5.03 |
| Obese grade‐III | 7.23 | 5.52 to 9.47 | 4.87 | 4.02 to 5.89 | 3.04 | 2.06 to 4.48 |
| Age (continuous variable) | 0.98 | 0.97 to 0.99 | 1.12 | 1.12 to 1.13 | 1.12 | 1.11 to 1.13 |
| Calendar year | ||||||
| 2004–2005 | (ref) | NA | (ref) | NA | (ref) | NA |
| 2006–2007 | 1.43 | 1.18 to 1.74 | 0.93 | 0.87 to 1.00 | 1.82 | 1.37 to 2.41 |
| 2008–2009 | 1.78 | 1.49 to 2.14 | 1.03 | 0.97 to 1.10 | 2.17 | 1.66 to 2.83 |
| 2010–2011 | 1.91 | 1.60 to 2.27 | 1.08 | 1.11 to 1.85 | 2.63 | 2.03 to 3.39 |
| Educational attainment* | ||||||
| (1) Basic school (n=45 750, 18.1%) | (ref) | NA | (ref) | NA | (ref) | NA |
| (2) Upper secondary (n=30 239, 12.0%) | 0.99 | 0.84 to 1.18 | 0.67 | 0.58 to 0.77 | 0.55 | 0.45 to 0.69 |
| (3) Vocational (n=69 837, 27.7%) | 0.83 | 0.72 to 0.96 | 0.83 | 0.75 to 0.91 | 0.62 | 0.53 to 0.72 |
| (4) Short or medium length higher education (n=68 651, 27.2%) | 0.81 | 0.69 to 0.95 | 0.58 | 0.52 to 0.64 | 0.38 | 0.32 to 0.46 |
| (5) master's degree or PhD (n=23 141, 9.2%) | 0.75 | 0.58 to 0.97 | 0.40 | 0.34 to 0.48 | 0.36 | 0.28 to 0.47 |
| (6) Unknown (n=14 854, 5.9%) | 1.23 | 0.98 to 1.54 | 0.60 | 0.48 to 0.74 | 0.56 | 0.41 to 0.76 |
95% CI indicates 95% confidence interval; BMI, body mass index; NA, not applicable; RR, rate ratio.
(1) Basic school (primary, lower secondary); (2) upper secondary (general secondary, technical secondary; “high‐school”); (3) vocational (eg, electrician or chef); (4) short or medium length higher education (academy professional degree, professional bachelor's degree, university bachelor's degree; 2 to 4 years following upper secondary); (5) master's degree or PhD; (6) unknown.
Discussion
In this nationwide study of 252 472 fertile women with a median age of 30.4 years without prior cardiovascular disease, renal insufficiency, diabetes, hypertension, dyslipidemia or any pregnancy‐associated metabolic disorders, we found pre‐pregnancy BMI to be strongly associated with increased risk of developing diabetes, hypertension, and dyslipidemia requiring intervention within 5.5 years after childbirth. Additionally, a BMI≥35 kg/m2 was associated with a 4‐fold, 15‐fold, and a 30‐ to 60‐fold increased rate ratio of developing at least 1, 2, or 3 metabolic disorders compared with normal weight women. Due to the rarity of developing multiple disorders requiring intervention in this young, healthy population, the confidence intervals became increasingly wide but were highly significant, and the rate ratios were disturbingly high. Thus, the key message of this study is that despite young age, obesity is closely related to development of metabolic disorders requiring intervention in healthy, fertile women, even in the short term.
Our findings add knowledge to the debate of how to handle obesity. Evidence is increasing about the appropriateness of distinguishing between metabolically unhealthy obese, and metabolically healthy obese, the latter of which is found to have a cardiovascular risk that resembles the metabolically healthy normal weight individuals.5 These metabolic disorders are recognized cardiovascular risk factors, and with the early onset in our study of young women, these women can be assumed to be at higher risk of early‐onset cardiovascular disease. Young women especially are found to have a poorer prognosis following stroke and myocardial infarction,13–14 thus emphasizing the need to keep these women metabolically healthy. The high rate ratios of developing a metabolic disorder associated with overweight and obesity in our study of apparently healthy, young women, suggest the importance of keeping BMI within the normal range via physical activity and a healthy diet. Women, especially those who are obese, might also be strongly encouraged to optimize all cardiovascular risk factors, such as smoking cessation, in addition to striving to achieve normal weight. Using a different population and only discharge diagnoses for the definitions of diabetes and hypertension, a recent study examined the impact of obesity on the risks of type 2 diabetes, cardiovascular morbidity and death in young men <55 years of age over 33 years of follow‐up.15 The relative risks are comparable to ours, although the study might have underestimated the incidence of hypertension by only using the National Patient Register, which does not contain data from the general practitioner, ie, uncomplicated hypertension was not identified. The risk of type 2 diabetes strongly resembled the estimates in our female population. For hypertension, the relative risk in overweight men was also similar, whereas the relative association with obesity was approximately half. These results indicate that the relative risk of developing diabetes is increased for both obese men and obese women, whereas obese women might carry an excess risk of hypertension compared with men. Additionally, a previous case‐control study demonstrated a higher sex‐specific impact of hypertension and diabetes on future risk of coronary heart disease in women.4 If obesity is associated with a greater relative risk of hypertension in women, and hypertension is associated with a greater risk of coronary heart disease in women, sex‐specific strategies for prevention of hypertension and diabetes might be beneficial. An American study of secular trends in cardiovascular risk factors according to BMI categories found that the prevalence of hypertension and dyslipidemia had decreased partly due to increased use of antihypertensive and lipid‐lowering drugs, whereas the prevalence of diabetes had remained stable.16 However, as a consequence of the obesity epidemic, many individuals have shifted to heavier weight categories, thus increasing the overall burden on the health care system, including expenses of risk factor‐modifying medications, especially as our data support that overweight and obesity is associated with early‐age onset of the risk factors.
Although we had a shorter follow‐up period and a selected population of women who gave birth twice or more during follow‐up, we found a tendency towards increased risks of developing hypertension, diabetes, or dyslipidemia among those who experienced weight gain between pregnancies. The tendency towards increased risks associated with weight loss in unadjusted analyses seems contradictory. These findings may be a result of a higher age among the women experiencing weight loss or that the weight loss was motivated by, eg, borderline hypertension, thus that women experiencing weight loss had a higher a priori risk of developing metabolic disorders. Importantly, however, the associations between pre‐pregnancy BMI and risk of the disorders were largely unchanged.
Strengths and Limitations
While the epidemiological approach has limitations, this study provides several strengths: it is a nationwide study, which minimizes the risk of demographic selection bias; the comprehensive Danish registers ensure a minimal loss to follow‐up; and the large study population makes it possible to study relatively rare events and risk factors. We wished to study a young population in which metabolic disorders are uncommon, but where a high BMI may or may not display deleterious effects on metabolism despite a low baseline rate. The Medical Birth Register was ideal for our study aim, as glucose abnormalities and blood pressure are closely monitored throughout pregnancy.
The primary limitation of this study is the use of prescription claims and diagnoses from the hospitals and outpatient clinics to define diabetes, hypertension, and dyslipidemia, which could potentially underestimate the true incidence rates of especially dyslipidemia. Further, we cannot rule out a selection bias, whereby the clinician may be more likely to screen for metabolic disorders or perhaps even initiate treatment in obese women. A woman with a history of a metabolic disorder may also be more likely to be monitored for other cardiovascular risk factors, but it is also reasonable of the clinician to assume that if obesity increases the woman's risk in one aspect, other obesity‐related metabolic disorders are likely to follow.
Although we cannot preclude that some of the events of hypertension were due to postpartum treatment for preeclampsia, sensitivity analyses did not change our results. For diabetes events, some of these might be women receiving biguanides because of polycystic ovary syndrome, a syndrome that can be regarded as a metabolic disorder with a prevalence of 5% to 10% in Danish fertile women. It increases the risk of diabetes and cardiovascular disease, and because it is mostly diagnosed in the non‐hospital setting, it might not be possible to identify in the national registers. However, a sensitivity analysis excluding prescriptions of oral glucose‐lowering agents did not change the overall results.
Only BMI was available from the Medical Birth Register, and although it would have been preferable to have other anthropometric measures of obesity and body fat distribution, the WHO has recommended BMI as a useful population‐level measure of obesity.17 BMI makes results between studies more comparable, and a review concluded that other anthropometric measures were not superior to BMI regarding a wide range of cardiovascular disease risk factors.18 Nevertheless, it is increasingly acknowledged that BMI must be regarded only as the first step in the overall risk assessment. Future risk assessment should preferably include evaluation of the woman's physical activity level or alcohol consumption, information that is not available from the Danish registers. Physical activity is known to improve especially glucose metabolism,19 although obesity is a stronger determinant of diabetes than physical activity.20 Socioeconomic status is known to be associated with obesity and lifestyle habits,21 but subanalyses including highest attained educational level left our overall findings unchanged. However, caution is warranted when interpreting the estimates, as many of the women are still undergoing education at the time of delivery. Although a teenage pregnancy potentially indicates lower socioeconomic status, highest attained level of education may not accurately reflect current socioeconomic status.
Running a prospective study with sufficient power to examine metabolic disorders in a female population as young as in our study is very unlikely, which makes these data unique and leaves the key message of our study unchanged: The health‐related consequences of obesity become apparent at an early age, which increases the burden on the health care system, increases the economic costs for the individual and increases the risks of cardiovascular disease.
In conclusion, in this nationwide study of fertile, apparently metabolically healthy women, increasing pre‐pregnancy BMI was strongly associated with increased risk of hypertension, dyslipidemia, and diabetes within only 5.5 years of childbirth. In addition, individuals with a BMI≥35 kg/m2 had a 15‐fold increased risk of developing at least 2 metabolic disorders and a 30‐ to 60‐fold increased risk of developing 3 metabolic disorders requiring intervention. BMI may thus be used to identify women for whom lifestyle intervention is important, and by referring to the short‐term consequences, our results might promote guidance and motivation for lifestyle changes in young, healthy, obese women.
Sources of Funding
The project was funded by a grant from the University of Copenhagen, Denmark. The University of Copenhagen had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
None of the authors have any conflicts of interest and all of the authors declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012; 307:491-497 [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002; 288:1723-1727 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002; 288:1728-1732 [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004; 364:937-952 [DOI] [PubMed] [Google Scholar]
- 5.Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013; 34:389-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013; 309:71-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Global database on body mass index Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed November 20, 2012.
- 8.Gislason GH, Rasmussen JN, Abildstrom SZ, Gadsboll N, Buch P, Friberg J, Rasmussen S, Kober L, Stender S, Madsen M, Torp‐Pedersen C. Long‐term compliance with beta‐blockers, angiotensin‐converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006; 27:1153-1158 [DOI] [PubMed] [Google Scholar]
- 9.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997; 44:445-448 [PubMed] [Google Scholar]
- 10.Carstensen B, Kristensen JK, Marcussen MM, Borch‐Johnsen K. The national diabetes register. Scand J Public Health. 2011; 39:58-61 [DOI] [PubMed] [Google Scholar]
- 11.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011; 342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson C, Vaag A, Selmer C, Schmiegelow M, Sorensen R, Lindhardsen J, Gislason GH, Kober L, Torp‐Pedersen C. Risk of cancer in patients using glucose‐lowering agents: a nationwide cohort study of 3.6 million people. BMJ Open. 2012; 2:e00043310.1136/bmjopen‐2011‐000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ. Association of age and sex with myocardial infarction symptom presentation and in‐hospital mortality. JAMA. 2012; 307:813-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez‐Sanchez P, Fuentes B, Fernandez‐Dominguez J, Ortega‐Casarrubios Mde L, Aguilar‐Amar MJ, Abenza‐Abildua MJ, Idrovo‐Freire L, Diez‐Tejedor E. Young women have poorer outcomes than men after stroke. Cerebrovasc Dis. 2011; 31:455-463 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Johannesdottir SA, Lemeshow S, Lash TL, Ulrichsen SP, Botker HE, Toft Sorensen H. Obesity in young men, and individual and combined risks of type 2 diabetes, cardiovascular morbidity and death before 55 years of age: a Danish 33‐year follow‐up study. BMJ Open. 2013; 3:e00269810.1136/bmjopen‐2013‐002698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005; 293:1868-1874 [DOI] [PubMed] [Google Scholar]
- 17.WHO. Obesity and overweight, fact sheet no 311 Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed May 30, 2013.
- 18.Taylor AE, Ebrahim S, Ben‐Shlomo Y, Martin RM, Whincup PH, Yarnell JW, Wannamethee SG, Lawlor DA. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all‐cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr. 2010; 91:547-556 [DOI] [PubMed] [Google Scholar]
- 19.Gibbs BB, Brancati FL, Chen H, Coday M, Jakicic JM, Lewis CE, Stewart KJ, Clark JM. Effect of improved fitness beyond weight loss on cardiovascular risk factors in individuals with type 2 diabetes in the Look AHEAD study. Eur J Prev Cardiol. 2012. 10.1177/2047487312462823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consortium I. Physical activity reduces the risk of incident type 2 diabetes in general and in abdominally lean and obese men and women: the EPIC‐InterAct Study. Diabetologia. 2012; 55:1944-1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TC, Glynn RJ, Pena JM, Paynter NP, Conen D, Ridker PM, Pradhan AD, Buring JE, Albert MA. Socioeconomic status and incident type 2 diabetes mellitus: data from the Women's Health Study. PLoS One. 2011; 6:e27670. [DOI] [PMC free article] [PubMed] [Google Scholar]