Abstract
Background
Patients with chronic kidney disease have an increased risk of death after myocardial infarction, coronary artery bypass graft surgery (CABG), and percutaneous coronary intervention. We sought to describe the association between chronic kidney disease and long‐term cardiovascular outcomes and death in patients who underwent CABG for acute coronary syndromes.
Methods and Results
All patients (N=12 956) from the Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry who underwent CABG for acute coronary syndromes in Sweden between 2000 and 2008 with complete information on preoperative serum creatinine values were included. Estimated glomerular filtration rates (eGFRs) were obtained, and hazard ratios with 95% CIs were calculated for the composite end point of myocardial infarction, heart failure, stroke, or death in relation to eGFR. During a mean follow‐up of 3.5 years, there were in total 2844 (22%) cardiovascular events and 1340 (10%) deaths. In patients with eGFR >60 mL/min per 1.73 m2, 45 to 60 mL/min per 1.73 m2, and 15 to 45 mL/min per 1.73 m2, there were 2896 (28%), 882 (43%), and 407 (61%) cardiovascular events or deaths, respectively. Hazard ratios with 95% CIs for death or any cardiovascular event in patients with eGFR 45 to 60 mL/min per 1.73 m2, and 15 to 45 mL/min per 1.73 m2 were 1.07 (0.98 to 1.15) and 1.36 (1.22 to 1.53) respectively, after multivariable adjustment. The corresponding figures for any cardiovascular event were 1.08 (0.98 to 1.19), and 1.24 (1.08 to 1.43).
Conclusions
Severe, but not moderate, renal dysfunction was independently associated with an increased risk of long‐term cardiovascular events and death in patients undergoing CABG for acute coronary syndromes.
Keywords: acute coronary syndromes, CABG, cardiovascular outcomes, chronic kidney disease
Introduction
Acute coronary syndromes (ACS) are common and affect millions of people each year globally.1 In addition to pharmacological treatment, patients with ACS commonly undergo early revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG). Early revascularization provides a better prognosis after ACS than does medical treatment only.2 However, patients with chronic kidney disease (CKD) and ACS are less likely to undergo early revascularization with CABG or PCI than are patients with no CKD.3
With increasing age and prevalence of diabetes mellitus in the general population, CKD is a growing health issue.4 Patients with CKD have a worse prognosis after myocardial infarction than do patients with normal renal function.5 Also, patients with CKD who undergo PCI after ACS have an increased risk of adverse outcome compared with patients with normal renal function.6 In addition, early revascularization with PCI or CABG in patients with moderate renal dysfunction improves prognosis to the same extent as for patients with normal renal function.7 One recent meta‐analyses found that even patients with severe renal dysfunction benefit from early revascularization.8
CABG is the most common cardiac surgical procedure, with 1 CABG performed per 1000 individuals in the United States.9 Renal dysfunction affects between 20% and 40% of patients who undergo CABG and increases the risk of early10 and late death,11–12 as well as the risks of myocardial infarction,13 stroke,14 or heart failure.15 Also, renal dysfunction increases the risk of postoperative acute kidney injury, which increases the risk of death and incident heart failure and a decline in renal function independent of preexisting CKD.16–17 Previous studies on prognosis in relation to preoperative renal function after CABG have often included a mix of patients undergoing elective, or emergency surgery for ACS.10–13 Other studies have only included elective patients, thus excluding patients with recent myocardial infarction.14–15 The aim of our study was to investigate the importance of CKD in the long‐term risk of cardiovascular events and death in patients who underwent CABG for ACS.
Methods
Study Population
We identified patients who underwent CABG between January 1, 2000, and December 31, 2008, and who were eligible for this study from the Swedish Web System for Enhancement and Development of Evidence‐Based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, in which all patients undergoing cardiac surgery in Sweden have been registered since 1992. This registry has been described in detail elsewhere.18 We excluded patients if they had undergone previous cardiac surgery, underwent surgery >10 days after the decision to operate, underwent other than isolated CABG during the operation, had missing preoperative serum creatinine (SCr) values, underwent CABG within 24 hours from decision (emergency), or had an estimated glomerular filtration rate (eGFR) <15 mL/min per 1.73 m2. The numbers of patients excluded are presented in Figure 1. The study complied with the Declaration of Helsinki and was approved by the Regional Ethics Committee in Stockholm, Sweden.
Figure 1.

Selection criteria for the study population consisting of 12 956 patients undergoing primary coronary artery bypass graft surgery (CABG) for acute coronary syndromes in Sweden between 2000 and 2008. eGFR indicates estimated glomerular filtration rate.
Definitions
The preoperative SCr value, which in clinical practice in general was measured within 24 hours before surgery, was used to obtain eGFRs by use of the simplified Modification of Diet in Renal Disease (MDRD) equation to estimate GFR: eGFR=186×(serum creatinine)−1.154×(age)−0.203×(0.742 if female). For the purpose of sensitivity analyses, we also calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.19 Renal function was divided into 3 categories: normal renal function (>60 mL/min per 1.73 m2), moderately reduced eGFR (45 to 60 mL/min per 1.73 m2), and severely reduced eGFR (15 to 45 mL/min per 1.73 m2). Acute kidney injury was classified according to the Acute Kidney Injury Network classification: stage 1, 0.3 to 0.5 mg/dL or 50% to 100% increase; stage 2, 0.5 to 1 mg/dL or 100% to 200% increase; and stage 3, >1 mg/dL or >200% increase. The highest postoperative SCr value during the entire hospitalization was used to calculate the difference between preoperative and postoperative SCr values. Diagnoses of hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes mellitus were made on the basis of patients' ongoing pharmacological treatment for these illnesses. Peripheral vascular disease was defined as previous surgery to the abdominal aorta, iliac artery, or carotid artery or the presence of claudication. Left ventricular function was assessed with echocardiography before surgery and categorized as normal (ejection fraction >50%), reduced (ejection fraction 30% to 50%), or severely reduced (ejection fraction <30%).
Outcome
The primary study outcome was death from any cause or first hospitalization for myocardial infarction, heart failure, or stroke with any of these diagnoses being the primary discharge diagnosis in the Swedish National Inpatient Registry, in which patients hospitalized in Sweden have been registered since 1964. Also, this registry was used to ascertain information about illnesses before surgery. The National Inpatient Registry has been described in detail previously, and all the outcomes under study have a high validity in this registry.20 The secondary outcome was defined as hospitalization for heart failure, stroke, or myocardial infarction. The date of death was obtained from the Swedish Total Population Register (Statistics Sweden), in which all deaths of persons residing in Sweden are registered. A fatal cardiovascular event was defined as a hospitalization for heart failure, myocardial infarction, or stroke, occurring within 1 month of death. Follow‐up began the first day postoperatively and ended at first hospitalization for any cardiovascular event, death, or December 31, 2008, whichever came first.
Statistical Analysis
Patient characteristics are described using frequencies and percentages for categorical variables and means and SDs for continuous variables. We used the Kaplan–Meier method to calculate the cumulative incidence of first hospitalization for heart failure, stroke, myocardial infarction, or death and for the secondary endpoint first hospitalization for heart failure, stroke, or myocardial infarction. We used Cox proportional hazards regression models to study the unadjusted and adjusted associations between renal function and primary and secondary end points. The relative risk of heart failure, stroke, myocardial infarction, or death was estimated using the hazard ratio (HR) and 95% CI for each stage of renal function, with eGFR >60 mL/min per 1.73 m2 as the reference category. Furthermore, because death could be considered a competing event, impeding the events of interest (ie, first hospitalization for heart failure, stroke, or myocardial infarction), we also used competing risks regression to calculate adjusted subhazard ratios and 95% CIs for the association between renal function and first hospitalization for heart failure, stroke, or myocardial infarction.21 We tested primary interactions, and we found no evidence for an interaction between renal function and sex, diabetes, or left ventricular ejection fraction.
We considered all variables listed in Table 1 for inclusion in the final statistical model and reached a final parsimonious model, including the following variables: age (continuous), sex (male vs female), left ventricular ejection fraction (3 categories: ejection fraction >50% [normal], 30% to 50% [moderate], or <30% [poor]), atrial fibrillation (yes/no), diabetes mellitus (yes/no), peripheral vascular disease (yes/no), chronic obstructive pulmonary disease (yes/no), prior myocardial infarction (yes/no), prior heart failure (yes/no), and history of stroke (yes/no). In a final step, we adjusted for perioperative acute kidney injury categorized according to Acute Kidney Injury Network criteria.
Table 1.
Characteristics of the Study Population in Relation to Glomerular Filtration Rates Estimated by Using the MDRD Study Equation
| All Patients | Estimated GFR, mL/min per 1.73 m2 | |||
|---|---|---|---|---|
| >60 | 45 to 60 | 15 to 45 | ||
| No. of patients | 12 956 | 10 252 | 2041 | 663 |
| Percent of study population | 100 | 79 | 16 | 5 |
| Age, y | 67.5 (9.6) | 66.1 (9.5) | 72.3 (7.8) | 73.3 (8.2) |
| Female sex, % | 23 | 19 | 35 | 44 |
| Estimated GFR (mL/min per 1.73 m2), MDRD | 77 (21) | 84 (17) | 54 (4) | 37 (6) |
| Estimated GFR (mL/min per 1.73 m2), CKD‐EPI | 73 (18) | 80 (13) | 51 (5) | 35 (6) |
| Serum creatinine, μmol/L | 92 (25) | 83 (15) | 113 (15) | 157 (40) |
| Serum creatinine, mg/dL | 1.0 (0.3) | 0.9 (0.2) | 1.3 (0.2) | 1.8 (0.5) |
| EuroSCORE | 4.4 (2.6) | 4.1 (2.5) | 5.6 (2.4) | 6.5 (2.6) |
| Diabetes mellitus, % | 23 | 21 | 27 | 37 |
| Atrial fibrillation, % | 25 | 23 | 33 | 32 |
| Hypertension, % | 54 | 52 | 64 | 74 |
| Hyperlipidemia, % | 54 | 52 | 56 | 73 |
| Peripheral vascular disease, % | 8 | 7 | 11 | 19 |
| Current smoking, % | 20 | 21 | 14 | 15 |
| COPD, % | 6 | 6 | 7 | 9 |
| Prior myocardial infarction, % | 56 | 54 | 60 | 65 |
| Prior heart failure, % | 3 | 2 | 6 | 10 |
| Prior stroke, % | 5 | 4 | 6 | 13 |
| Left ventricular function | ||||
| Ejection fraction >50% (%) | 67 | 69 | 62 | 51 |
| Ejection fraction 30% to 50% (%) | 29 | 28 | 33 | 44 |
| Ejection fraction <30% (%) | 3 | 3 | 5 | 5 |
| Internal thoracic artery use, % | 95 | 95 | 94 | 93 |
| CABG without cardiopulmonary bypass, % | 5 | 4 | 5 | 6 |
Age, GFR, creatinine values, and EuroSCORE (European System for Cardiac Operative Risk Evaluation) are given as means with SDs. Acute kidney injury was defined as >0.3 mg/dL (26 μmol/L) increase in postoperative creatinine values. CABG indicates coronary artery bypass grafting; CKD EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease. Age, eGFR, serum creatinine, and EuroSCORE is given with standard deviations in parantheses.
Some data were missing in the study dataset (left ventricular ejection fraction, 11%; diabetes mellitus, 15%; peripheral vascular disease, 11%; postoperative creatinine, 11%), and multiple imputation by chained equations was used to handle missing data. Fifty datasets were imputed, and estimates from these datasets were combined. In addition to the main analysis of the multiple imputed dataset, we performed a complete‐case analysis where only observations without missing values for model covariates were included (n=9447). The results from the complete‐case analyses were consistent with the main analyses and were therefore not reported. Stata version 13.0 (StataCorp LP) was used for data management and all statistical analyses.
Results
We included 12 956 patients with a mean age of 67.5 years, of whom 23% were women. Patients' characteristics are presented in Table 1. The overall prevalence of CKD was 21%. Moderately and severely reduced eGFR was present in 16% and 5% of the study cohort, respectively. Patients with CKD were more likely to be older, having more comorbidities including a reduced left ventricular ejection fraction, compared with patients without CKD. The median duration of hospital stay in the department of cardiothoracic surgery was 6 and 7 days in patients with moderate or severe renal dysfunction, respectively, and 6 days in patients with no CKD. The incidence of acute kidney injury was 13% in the study population.
Hospitalization for Cardiovascular Events
The HR for the primary outcome of death from any cause or hospitalization for cardiovascular disease increased with the severity of CKD in both unadjusted and multivariable adjusted analyses (Table 2). In unadjusted analysis, the risk of hospitalization for cardiovascular disease or death was increased by 50% in patients with moderate and more than doubled in patients with severe renal dysfunction (HR [95% CI] 1.50 [1.39 to 1.62] and 2.49 [2.24 to 2.76], respectively) compared with patients with no renal dysfunction. After adjustment for confounders, only severe renal dysfunction remained significantly associated with an increased risk of cardiovascular events or death by 36% (1.36 [1.22 to 1.53]) (Table 2). For the secondary outcome of hospitalization for cardiovascular disease, unadjusted risks in patients with moderate and severe renal dysfunction were significantly increased, but after adjustment for confounders, only severe renal dysfunction was associated with an increased risk of hospitalization for cardiovascular disease by 24% (1.24 [1.08 to 1.43]) (Table 2). The analysis was repeated using competing risk regression, with death as the competing event, and the results were similar.
Table 2.
Hazard Ratios and 95% CIs for the Primary and Secondary Outcomes in Relation to Preoperative Glomerular Filtration Rate Estimated by Using the MDRD Equation in 12 956 Patients Undergoing CABG for Acute Coronary Syndromes Between 2000 and 2008 in Sweden
| Estimated GFR, mL/min per 1.73 m2 | |||||
|---|---|---|---|---|---|
| >60* | 45 to 60 | P Value | 45 to 15 | P Value | |
| No. of patients, % | 10 252 (79) | 2041 (16) | 663 (5) | ||
| Death or rehospitalization | |||||
| No. of events, % | 2896 (28) | 882 (43) | 407 (61) | ||
| Crude | 1.00 | 1.50 (1.39 to 1.62) | <0.001 | 2.49 (2.24 to 2.76) | <0.001 |
| Adjustment for age and sex | 1.00 | 1.20 (1.11 to 1.29) | <0.001 | 1.88 (1.68 to 2.09) | <0.001 |
| Multivariable adjustment* | 1.00 | 1.08 (1.00 to 1.17) | 0.044 | 1.47 (1.32 to 1.64) | <0.001 |
| Multivariable adjustment*+AKI | 1.00 | 1.07 (0.98 to 1.15) | 0.117 | 1.36 (1.22 to 1.53) | <0.001 |
| Rehospitalization for myocardial infarction, heart failure, or stroke | |||||
| No. of events (%) | 2005 (20) | 588 (29) | 251 (38) | ||
| Crude | 1.00 | 1.46 (1.33 to 1.60) | <0.001 | 2.15 (1.89 to 2.46) | <0.001 |
| Adjustment for age and sex | 1.00 | 1.23 (1.12 to 1.35) | <0.001 | 1.74 (1.52 to 2.00) | <0.001 |
| Multivariable adjustment* | 1.00 | 1.10 (1.00 to 1.21) | 0.062 | 1.33 (1.16 to 1.53) | <0.001 |
| Multivariable adjustment*+AKI | 1.00 | 1.08 (0.98 to 1.19) | 0.130 | 1.24 (1.08 to 1.43) | 0.003 |
MDRD indicates Modification of Diet in Renal Disease; AKI, acute kidney injury; CABG, coronary artery bypass graft surgery; GFR, glomerular filtration rate.
Reference category.
Multivariable adjustment was made for age, sex, diabetes, chronic obstructive pulmonary disease, peripheral vascular disease, left ventricular ejection fraction, atrial fibrillation, prior myocardial infarction, prior heart failure, and history of stroke.
The cumulative incidence of the composite end point of death or hospitalization for cardiovascular events is shown in Figure 2. After 1, 5, and 10 years of follow‐up, the overall survival was 97%, 88%, and 69%, respectively. In patients with normal renal function, survival at 1, 5, and 10 years was 98%, 90%, and 75%; in patients with moderate renal dysfunction, 95%, 82%, and 57%; and in patients with severe renal dysfunction, 89%, 70%, and 29%, respectively.
Figure 2.
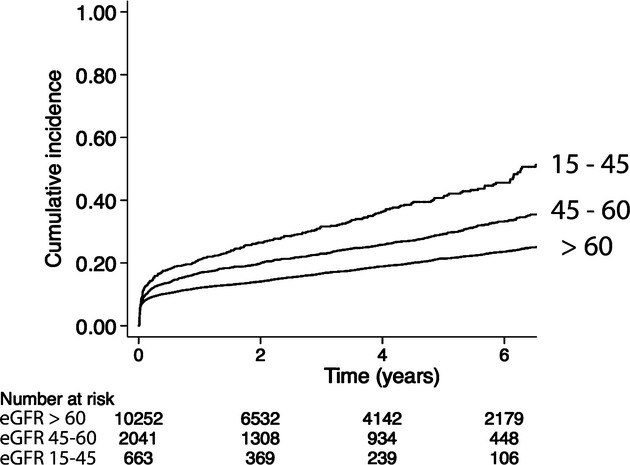
Unadjusted cumulative incidence of death or first rehospitalization for myocardial infarction, heart failure, or stroke in 12 956 patients following coronary artery bypass graft surgery for acute coronary syndromes in Sweden between 2000 and 2008. eGFR indicates estimated glomerular filtration rate.
During 448 456 person‐years and a mean of 3.5 years of follow‐up, a total of 2844 (22%) patients were hospitalized for cardiovascular events (1307 [46%] for myocardial infarction, 822 [29%] for heart failure, and 715 [25%] for stroke). The cardiovascular event was fatal in 187 (7%) patients (heart failure, 4%; myocardial infarction, 5%; and stroke, 11%). In patients with moderately and severely reduced eGFR, 29% and 38%, respectively, were hospitalized for cardiovascular events compared with 20% of patients without CKD. The cumulative incidence of first hospitalization for cardiovascular disease in relation to renal function is shown in Figure 3.
Figure 3.
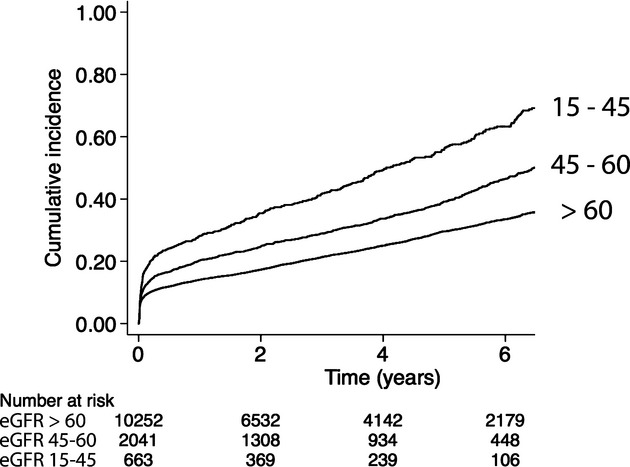
Unadjusted cumulative incidence of first rehospitalization for myocardial infarction, heart failure, or stroke in 12 956 patients following coronary artery bypass graft surgery for acute coronary syndromes in Sweden between 2000 and 2008. eGFR indicates estimated glomerular filtration rate.
The relationship between estimated GFR as a continuous variable and the primary outcome is shown in Figure 4.
Figure 4.
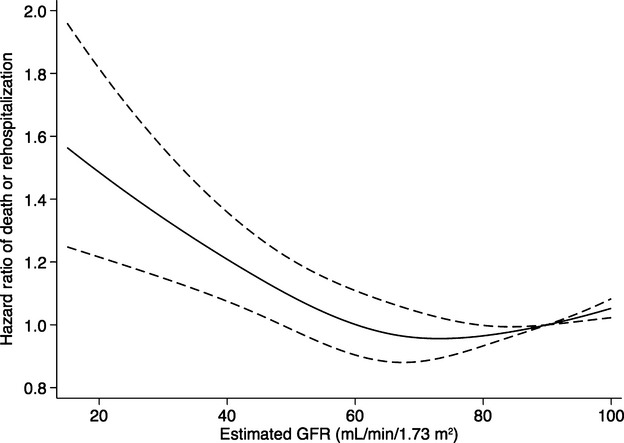
The graph shows the adjusted hazard ratio and 95% CI for the association between estimated glomerular filtration rates (GFR) and death or rehospitalization for cardiovascular events. Estimated GFR was modeled by restricted cubic splines with 4 knots (at 44, 67, 83, and 113) in a Cox regression model. The reference level was set at 90 mL/min per 1.73 m2 for the estimation of hazard ratios. The Cox model was adjusted for age, sex, diabetes, chronic obstructive pulmonary disease, peripheral vascular disease, left ventricular ejection fraction, atrial fibrillation, prior myocardial infarction, prior heart failure, history of stroke, and acute kidney injury classified according to the Acute Kidney Injury Network (AKIN) criteria.
Model Sensitivity to Estimation of Renal Function
We repeated the analyses using eGFR derived with the CKD‐EPI equation, and the main findings were similar, showing a significant association between severe renal dysfunction and increased risk for death or rehospitalization for cardiovascular events in our study population. The multivariable adjusted HRs for the primary outcome was 1.05 (95% CI 0.97 to 1.13; P=0.249) and 1.32 (95% CI 1.19 to 1.46; P<0.001), for moderate and severe renal dysfunction, respectively.
Discussion
In a nationwide study population who underwent CABG after ACS, we found that severe renal dysfunction was independently associated with death or hospitalization for cardiovascular events.
In previous studies, renal dysfunction has been associated with adverse outcome after myocardial infarction, PCI, or CABG.5–6,5–15 The associations found between renal dysfunction and cardiovascular disease or death have been strong, and in some cases, renal dysfunction has been associated with a several‐fold increased risk of death or cardiovascular events.10–13 Most of these studies have been single‐center studies limiting the external validity,10–11,13 and they have included a mix of elective and urgent patients. In 2 recent studies from the SWEDEHEART registry, we found that an eGFR between 45 and 60 mL/min per 1.73 m2 was independently associated with both first stroke and new onset of heart failure in patients who underwent elective CABG and who did not have a recent myocardial infarction.14–15 In the present study conducted in patients who underwent CABG within 10 days of decision to operate, in most cases because of ACS, we found no association between an eGFR between 45 and 60 mL/min per 1.73 m2 and long‐term risk of death or hospitalization for cardiovascular events. However, there was a 36% increased risk of death or hospitalization for cardiovascular disease in patients with eGFR between 15 and 45 mL/min per 1.73 m2. In contrast, our previous studies on patients who underwent elective CABG, we found that the long‐term risk of first stroke was increased by 52% and by more than 2‐fold for new‐onset heart failure at the same level of renal dysfunction.14–15
There may be several explanations to the differences in results seen between the present study and previous studies. One is that there may have been risk factors that were not taken into account in previous studies that had a relatively strong association with both the exposure and the outcome. More specifically, in most previous studies, acute kidney injury has not been accounted for in statistical analyses.10–13 Because acute kidney injury may be on the causal pathway between renal dysfunction and adverse outcome, this may have hampered the results in these studies. However, when we added acute kidney injury to our statistical model, it changed the point estimates to only a small degree. Thus, our findings could not be explained by acute kidney injury as a bridge between renal dysfunction and cardiovascular disease. Another possible explanation is that these patients were unstable in the respect that they underwent surgery soon after a myocardial infarction or because of unstable angina/ACS. This means that other characteristics that we could not control for in our statistical analyses, like anticoagulation or other medication given before surgery, blood transfusions, or other perioperative events, may have affected the association between renal dysfunction and outcome in the direction seen. Thus, there may have been residual confounding that changed our point estimates to the nonsignificant side explaining our findings.
Our results may have been affected by misclassification of renal function. We used the simplified MDRD study equation, which has been validated in several previous studies and has been predictive for adverse outcomes in prior studies in the CABG population.11–12,11–15 We defined normal renal function as eGFR >60 mL/min per 1.73 m2, since the MDRD study equation underestimates eGFR in the normal or near‐normal range.22 In addition, our main analyses were repeated using the CKD‐EPI formula to estimate GFR and classify renal function. The findings were very similar for the association between a reduced eGFR and outcome.
Patients with CKD are less likely to be scheduled for invasive procedures after myocardial infarction.3 Yet, their benefit from PCI or CABG is similar to that for patients without renal dysfunction.7–8 Our results indicate that the prognosis of patients with moderate CKD following CABG for ACS is similar to those without CKD, when comorbidities and age has been taken into account. Thus, our results strengthen the concept that patients with CKD should be offered the same opportunity to undergo CABG or PCI after ACS as are patients without CKD.
The main strength of our study was the large cohort under study, which encompassed all patients who underwent CABG for ACS in Sweden during 9 years. There were a large number of events during follow‐up, which made our multivariable analysis robust. Even if it cannot be proved, we believe that the external validity of our data is good since our study was a nationwide population‐based investigation, including all patients who underwent CABG in a whole country, during a relatively long period (9 years). Thus, we believe that our results will be applicable to patients undergoing CAGB for ACS in other countries with a similar level of health care. Also, follow‐up was complete because of the national registries used, and the validity of the outcomes under study is very high.20 We do not think that there was any or very little misclassification of the heart failure, stroke, or myocardial infarction diagnosis. In case the cardiovascular diagnosis was misclassified, most likely the misclassification was not related to exposure (ie, nondifferential).
As in every cohort study performed from a registry, there was missing data. We used multiple imputation to account for missing data to reduce bias and not lose statistical power. Also, we did not have information on perioperative variables, such as cross‐clamp time, fluids, or diuretics given, that may have been related to both renal dysfunction and outcome. Information on cardiopulmonary bypass time was not available to us. However, we had information on number of bypassed vessels, which corresponds well with duration of CABG. In addition, we had no information on proteinuria, which in previous studies has been one of the most important predictors of adverse outcome in patients with CKD.23 The time period during which patients were included was rather long, 9 years, and there may have been changes in surgical techniques or perioperative management on which we had no information that may have affected outcome. Although in clinical practice our experience is that the SCr values registered in the SWEDEHEART registry almost always are taken within 24 hours of surgery, the exact proportion of SCr values that were not measured the day before or on the day of surgery was unknown to us. Thus, there were variables that we could not control for in our statistical models (ie, residual confounding), which may have affected our results.
In conclusion, we found that severe, but not moderate, renal dysfunction was independently associated with an increased risk of long‐term cardiovascular events and death in patients undergoing CABG for ACS.
Sources of Funding
This study was supported by research grants from the Swedish Foundation for Strategic Research (KF 10‐0024), The Swedish Society of Medicine (Drs Sartipy and Holzmann), and the Mats Kleberg Foundation (Dr Sartipy).
Disclosures
None.
Acknowledgments
The authors are thankful to all collaborators of SWEDEHEART for making research in the registry possible.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006; 367:1747-1757 [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, Widimsky P, McCullough PA, Hunt D, Braunwald E, Yusuf S. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta‐analysis of randomized trials. JAMA. 2005; 293:2908-2917 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD. Acute Coronary Treatment and Intervention Outcomes Network registry. Use of evidence‐based therapies in short‐term outcomes of ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010; 121:357-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens L, Manzi J, Kusek J, Eggers P, Van Lente F, Levey A. Prevalence of chronic kidney disease in the United States. JAMA. 2007; 298:2038-2047 [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004; 351:1285-1295 [DOI] [PubMed] [Google Scholar]
- 6.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002; 39:1113-1119 [DOI] [PubMed] [Google Scholar]
- 7.Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T. Influence of renal function on the effects of early revascularization in non‐ST‐elevation myocardial infarction: data from the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009; 120:851-858 [DOI] [PubMed] [Google Scholar]
- 8.Huang HD, Alam M, Hamzeh I, Virani S, Deswal A, Aguilar D, Rogers P, Kougias P, Birnbaum Y, Paniagua D, Kar B, Ballantyne C, Bozkurt B, Jneid H. Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int J Cardiol. 2013; 168:3741-3746 [DOI] [PubMed] [Google Scholar]
- 9.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001‐2008. JAMA. 2011; 305:1769-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzmann MJ, Ahnve S, Hammar N, Jorgensen L, Klerdal K, Pehrsson K, Ivert T. Creatinine clearance and risk of early mortality in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2005; 130:746-752 [DOI] [PubMed] [Google Scholar]
- 11.Hillis GS, Croal BL, Buchan KG, El‐Shafei H, Gibson G, Jeffrey RR, Millar CG, Prescott GJ, Cuthbertson BH. Renal function and outcome from coronary artery bypass grafting: Impact on mortality after a 2.3‐year follow‐up. Circulation. 2006; 113:1056-1062 [DOI] [PubMed] [Google Scholar]
- 12.Cooper WA, O'Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006; 113:1063-1070 [DOI] [PubMed] [Google Scholar]
- 13.Holzmann MJ, Hammar N, Ahnve S, Nordqvist T, Pehrsson K, Ivert T. Renal insufficiency and long‐term mortality and incidence of myocardial infarction in patients undergoing coronary artery bypass grafting. Eur Heart J. 2007; 28:865-871 [DOI] [PubMed] [Google Scholar]
- 14.Holzmann MJ, Ahlbäck E, Jeppsson A, Sartipy U. Renal dysfunction and long‐term risk of ischemic and hemorrhagic stroke following coronary artery bypass grafting. Int J Cardiol. 2013; 168:1137-1142 [DOI] [PubMed] [Google Scholar]
- 15.Holzmann MJ, Gardell C, Jeppsson A, Sartipy U. Renal dysfunction and long‐term risk of heart failure after coronary artery bypass grafting. Am Heart J. 2013; 166:142-149 [DOI] [PubMed] [Google Scholar]
- 16.James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011; 123:409-416 [DOI] [PubMed] [Google Scholar]
- 17.Olsson D, Sartipy U, Braunschweig F, Holzmann MJ. Acute kidney injury following coronary artery bypass surgery and long‐term risk of heart failure. Circ Heart Fail. 2013; 6:83-90 [DOI] [PubMed] [Google Scholar]
- 18.Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web‐system for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Heart. 2010; 96:1617-1621 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh JCKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J‐L, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011; 11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496-509 [Google Scholar]
- 22.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft‐Gault, MDRD, and new CKD‐EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010; 5:1003-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011; 154:12-21 [DOI] [PubMed] [Google Scholar]


