Abstract
Background
In 2011, the Brain Attack Coalition proposed door‐to‐treatment times of 2 hours as a benchmark for patients undergoing intra‐arterial therapy (IAT). We designed the Rapid Reperfusion Registry to capture the percentage of stroke patients who meet the target and its impact on outcomes.
Methods and Results
This is a retrospective analysis of anterior circulation patients treated with IAT within 9 hours of symptom onset. Data was collected from December 31, 2011 to December 31, 2012 at 2 centers and from July 1, 2012 to December 31, 2012 at 7 centers. Short “Door‐to‐Puncture” (D2P) time was hypothesized to be associated with good patient outcomes. A total of 478 patients with a mean age of 68±14 years and median National Institutes of Health Stroke Scale (NIHSS) of 18 (IQR 14 to 21) were analyzed. The median times for IAT delivery were 234 minutes (IQR 163 to 304) for “last known normal‐to‐groin puncture” time (LKN‐to‐GP) and 112 minutes (IQR 68 to 176) for D2P time. The overall good outcome rate was 39.7% for the entire cohort. In a multivariable model adjusting for age, NIHSS, hypertension, diabetes, reperfusion status, and symptomatic hemorrhage, both short LKN‐to‐GP (OR 0.996; 95% CI [0.993 to 0.998]; P<0.001) and short D2P times (OR 0.993, 95% CI [0.990 to 0.996]; P<0.001) were associated with good outcomes. Only 52% of all patients in the registry achieved the targeted D2P time of 2 hours.
Conclusions
The time interval of D2P presents a clinically relevant time frame by which system processes can be targeted to streamline the delivery of IAT care nationally. At present, there is much opportunity to enhance outcomes through reducing D2P.
Keywords: endovascular treatment, ischemic, stroke, stroke delivery, stroke management
Introduction
he NCDR CathPCI registry was first implemented in 1998 as a central repository for capturing real‐life data on patient profiles, clinical outcomes, and system processes associated with percutaneous coronary intervention (PCI).1 Beginning with <200 participating centers in 1999, the registry has grown to include >1500 US hospitals and >12 million patient records by 2012.2 Results from the CathPCI registry have subsequently been used to demonstrate the importance of “Door‐to‐Balloon” (D2B) times on patient mortality,3 while spearheading quality assurance initiatives aimed at enhancing the delivery of PCI care. Between 2005 and 2008, the number of hospitals in the registry that achieved D2B times of <90 minutes increased from 52% to 76%,4 reflecting successful implementation of the best practices associated with systems of care for STEMI patients.
Although multicenter registries have already been reported for endovascular therapies (IAT) in stroke5–7 the focus has been predicated on the intra‐procedural aspects of treatment rather than the pre‐procedural processes. A recent study demonstrated that >three‐quarters of the delays to reperfusion are comprised of inefficiencies preceding the procedure itself, which correlate with poorer outcomes following IAT.8 Unfortunately, data capture of system processes prior to IAT have yet to be standardized, thereby limiting the ability of hospitals and institutions to define bottlenecks in times to treatment.
To address these issues, we developed the Rapid Reperfusion Registry as a tool for collating multicenter data on patients treated with IAT, with the goal of standardizing data collection as well as providing a real‐time assessment on the pre‐procedural metrics of “last known normal‐to‐puncture” and “Door‐to‐Puncture” (D2P). We hypothesized that both time intervals would not only be associated with patient mortality and outcomes, but would also provide a standardized template for future data collection from which bottlenecks may be identified. The results from this study have subsequently set the foundation for the second phase of the registry, in which segmentation of D2P times will be prospectively collected following the implementation of evidence‐based strategies aimed at reducing treatment times, and ultimately, improving patient outcomes.
Methods
IRB approval was granted at Emory University to initiate a multicenter registry to capture system processes associated with endovascular therapy for ischemic stroke patients. Each contributing hospital obtained local IRB approval to submit their de‐identified patient information on a pre‐set template, which was collected and stored at the coordinating center (Grady Memorial Hospital). All acute ischemic stroke patients (AIS) treated with IAT between June 31, 2012 to December 31, 2012 were retrospectively reviewed and considered for inclusion in the analysis. Two institutions provided patient records over a 12‐month period between December 31, 2011 to December 31, 2012 (Grady Memorial Hospital and Vall d'Hebron Hospital).
Data Collection
Data pertaining to baseline characteristics (ie, age, gender, hypertension, diabetes mellitus, atrial fibrillation), initial stroke severity (NIHSS), prior IV‐tPA delivery, and clot location on angiography were collected in the registry. Significant time points in the work‐up of acute stroke patients were documented including: last known normal, arrival to first hospital, time of arterial access, time of reperfusion success, and in certain cases, time of initial CT imaging. Door‐to‐Puncture times were calculated as the interval between first hospital admission and arterial access, irrespective of transfer status. When possible, all pretreatment CT images were evaluated for the Alberta Stroke Program Early CT score as a surrogate for pretreatment infarct size.9 Symptomatic hemorrhages (sICH) were defined according to the ECASS definition as parenchymal hematomas type I or type II found of postprocedural CT images within 24 hours of treatment.10 Deterioration of NIHSS scores could not be accurately documented given the retrospective nature of the study, and thus, were excluded from the sICH definition. Successful reperfusion was classified as Thrombolysis in Cerebral Infarction (TICI) scores of 2b or greater on immediate angiographic imaging, which correlates to partial or complete reperfusions of the vascular territory involved.
Outcome Measures
The primary endpoint of “good outcomes” was defined as a modified Rankin Scale score of 0 to 2 at 90±14 days, which was determined by follow‐up exams or phone interviews conducted by certified examiners blinded to the treatment times. The secondary endpoint of all‐cause mortality at 90 days was also obtained during this time.
All data pertaining to pretreatment imaging, reperfusion success, postoperative hemorrhages, and 90‐day outcomes were locally adjudicated at each center, with time metrics captured based on the official time stamps from each patient's electronic medical record.
Patient Selection
All consecutive patients treated with endovascular therapy were included with large‐vessel occlusions involving the anterior circulation (ie, ICA‐T/M1/M2) and treatment within 9 hours of last known normal.
Statistical Analysis
Baseline patient characteristics were compared between all contributing centers in the Rapid Reperfusion Registry. A univariate analysis was performed to determine the variables associated with favorable outcomes at 90 days. Each independent predictor with P<0.10 was subsequently adjusted in a binary logistic regression model, with odds ratios and 95% confidence intervals reported. The goodness‐of‐fit of the model was confirmed by Hosmer‐Lemeshow testing. “LKN‐to‐GP” times were subsequently quintiled to determine the pattern of association between time and 90‐day outcomes. The lowest quintile was used as the reference point for comparison with the latter time groups. A separate binary logistic regression model was also generated to determine the relationship between good outcomes and a narrower metric of D2P. All analyses were performed using IBM SPSS v.20.
Results
A total of 629 patients met inclusion criteria of which 75 (12%) did not have documented groin puncture times and 76 (12%) did not have a 90‐day mRS. Thus, a total of 478 (76%) patients were analyzed. The mean age and median NIHSS score for the entire cohort was 68.2±14.2 and 18 (IQR 14 to 21), respectively. Overall, 320 patients (68%) had successful reperfusion, 268 patients (56%) received IV t‐PA prior to IAT, and 58 patients (12.4%) developed parenchymal hematomas post procedure. The median times for IAT delivery corresponded to an LKN‐to‐GP time of 234 minutes (IQR 163 to 304) and a D2P time of 112 minutes (IQR 68 to 176). At 3 months, all‐cause mortality was reported in 111 patients (27.2%), while good outcomes were observed in 234 patients (39.7%). The differences in patient characteristics between the study centers are described in Table 1.
Table 1.
Baseline Patient Characteristics
| Study Site 1 | Study Site 2 | Study Site 3 | Study Site 4 | Study Site 5 | Study Site 6 | Other Sites (7 to 9) | |
|---|---|---|---|---|---|---|---|
| Recruitment Period | Dec 2012–Dec 2013 | Dec 2012–Dec 2013 | July 2013–Dec 2013 | July 2013–Dec 2013 | July 2013–Dec 2013 | July 2013–Dec 2013 | July 2013–Dec 2013 |
| Demographics | |||||||
| Number of patients | n=164 | n=142 | n=91 | n=27 | n=20 | n=15 | n=19 |
| Age, mean (STD) | 70.6 (13.5) | 65.6 (15.0) | 67.6 (13.4) | 67.2 (15.3) | 71.2 (11.6) | 62.3 (15.5) | 70.5 (14.0) |
| Male gender, no. (%) | 81 (49) | 76 (54) | 45 (50) | 13 (48) | 12 (60) | 3 (20) | 9 (47) |
| Hypertension, no. (%) | 105 (64) | 108 (76) | 45 (50) | 20 (74) | 13 (65) | 10 (67) | 14 (74) |
| Atrial fibrillation, no. (%) | 69 (42) | 54 (38) | 29 (32) | 14 (52) | 9 (45) | 3 (20) | 8 (42) |
| Diabetes, no. (%) | 32 (20) | 40 (28) | 15 (16) | 5 (19) | 4 (20) | 6 (40) | 6 (32) |
| Neurological severity | |||||||
| Pre‐treatment NIHSS, median (IQR) | 18.5 (17 to 21) | 18 (14 to 23) | 17 (13 to 21) | 14 (11 to 18) | 16 (14 to 18) | 19 (16 to 23) | 19 (15 to 22) |
| IV tPA given, no. (%) | 101 (62) | 79 (56) | 48 (53) | 10 (37) | 11 (55) | 7 (47) | 12 (63) |
| Radiographic assessment | |||||||
| ASPECTS, median (IQR) * | 10 (9 to 10) | 8 (7 to 9) | 8 (7 to 9) | 9 (7 to 10) | N/A | N/A | 8 (6 to 9) |
| Multimodal imaging, no. (%)* | 38 (47) | 78 (55) | 91 (100) | 16 (59) | 20 (100) | 14 (93) | 19 (100) |
| Clot location | |||||||
| ICA, no (%) | 61 (37) | 29 (20) | 24 (26) | 7 (26) | 6 (30) | 4 (27) | 9 (47) |
| MCA, no (%) | 103 (63) | 113 (80) | 67 (74) | 20 (74) | 14 (70) | 11 (73) | 10 (53) |
| Successful reperfusion, no. (%)* | 77 (49) | 111 (79) | 74 (81) | 24 (89) | 12 (60) | 11 (73) | 11 (58) |
| PH1/PH2 hemorrhage, no. (%) | 18 (11) | 9 (6) | 16 (17) | 6 (22) | 2 (10) | 2 (13) | 5 (26) |
| Time metrics (in minutes) | |||||||
| LKN‐to‐GP, median (IQR) | 210 (150 to 270) | 261 (195 to 340) | 177 (124 to 254) | 290 (250 to 354) | 288 (209 to 328) | 330 (193 to 422) | 266 (230 to 350) |
| Door‐to‐GP, median (IQR) | 75 (50 to 128) | 164 (129 to 233) | 84 (59 to 104) | 90 (37 to 120) | 259 (213 to 311) | 214 (105 to 251) | 200 (146 to 221) |
| Procedure time, median (IQR) | 83 (54 to 131) | 64 (41 to 95) | 43 (27 to 75) | 56 (41 to 68) | 69 (51 to 96) | 57 (40 to 67) | 84 (71 to 150) |
| Transfer from PSC, no. (%)* | 56 (37) | 98 (69) | 0 (0) | N/A | 12 (60) | 10 (67) | 13 (68) |
| Outcome measures | |||||||
| 90‐day mortality rate, no. (%) | 52 (32) | 37 (26) | 20 (22) | 8 (30) | 5 (25) | 2 (13) | 6 (32) |
| Good outcomes, no. (%) | 57 (35) | 51 (36) | 54 (59) | 12 (44) | 6 (30) | 6 (40) | 4 (21) |
| Acceptable outcomes, no. (%) | 74 (45) | 66 (46) | 62 (68) | 15 (56) | 8 (40) | 8 (53) | 6 (32) |
ASPECTS indicates Alberta Stroke Program Early CT Score; GP, groin puncture; ICA, internal carotid artery; IQR, interquartile range; LKN, last known normal; MCA, middle cerebral artery; NIHSS, NIH Stroke Scale; PH, parenchymal hematoma; PSC, primary stroke center; t‐PA, tissue plasminogen activator.
ASPECTS scores were not evaluated at Sites 5 and 6.
Multimodal imaging status was not available for every patient at Site 1.
Reperfusion status was not available for every patient at Site 1.
PSC transfer data was not available at Site 4.
LKN‐to‐GP and Patient Outcomes
In a binary logistic regression model adjusting for age, NIHSS, hypertension, diabetes mellitus, IV t‐PA delivery, reperfusion status, and symptomatic hemorrhage, the pre‐procedural time frame of LKN‐to‐GP was directly associated with 90‐day good outcomes (OR 0.996; 95% CI [0.993 to 0.998]; P<0.001) (Table 2). This relationship also held true in a secondary model adjusting for pretreatment ASPECTS (OR 0.996; 95% CI [0.994 to 0.999]; P=0.003). As shown in Figure 1, 53% of the patients had a good outcome when treated within 150 minutes from LKN, which declined to 39% at 208 to 259 minutes and 27% at 330 minutes or greater. The odds ratio for a good outcome declined at each interval beyond the reference point (LKN‐to‐GP<150 minutes) with significance achieved beyond 260 minutes: LKN‐to‐GP 260 to 329 minutes: (OR 0.43; 95% CI [0.20 to 0.89]; P=0.023; LKN‐to‐GP ≥330 minutes: (OR 0.30; 95% CI [0.14 to 0.65]; P=0.002).
Table 2.
Patient Outcomes Stratified by LKN‐to‐Puncture
| LKN‐to‐Puncture | Good Outcome/Total | %Good Outcome | Unadjusted Odds Ratio for Good Outcome | Adjusted Odds Ratio for Good Outcome* (Model 1) | Adjusted Odds Ratio for Good Outcome* (Model 2) |
|---|---|---|---|---|---|
| <150 minutes | 49/92 | 53.3 | Reference | Reference | Reference |
| 150 to 207 minutes | 48/99 | 48.5 | 0.83 (0.47 to 1.46); P=0.51 | 0.96 (0.46 to 1.97); P=0.90 | 1.02 (0.47 to 2.19); P=0.97 |
| 208 to 259 minutes | 37/95 | 38.9 | 0.56 (0.31 to 1.00); P=0.05 | 0.83 (0.40 to 1.72); P=0.61 | 0.83 (0.38 to 1.81); P=0.64 |
| 260 to 329 minutes | 30/95 | 31.6 | 0.41 (0.22 to 0.74); P=0.003 | 0.43 (0.20 to 0.89); P=0.023 | 0.53 (0.24 to 1.17); P=0.12 |
| ≥330 minutes | 26/97 | 26.8 | 0.32 (0.18 to 0.59); P<0.001 | 0.30 (0.14 to 0.65); P=0.002 | 0.31 (0.13 to 0.74); P=0.008 |
| All | 190/478 | 39.7 | 0.996 (0.994 to 0.998); P<0.001 | 0.996 (0.993 to 0.998); P<0.001 | 0.996 (0.994 to 0.999); P=0.003 |
ASPECTS indicates Alberta Stroke Program Early CT Score; LKN, last known normal; NIHSS, NIH Stroke Scale; tPA, tissue plasminogen activator.
Model 1: Corrected for age, NIHSS, hypertension, diabetes, IV tPA delivery, reperfusion status, and symptomatic hemorrhage.
Model 2: Corrected for age, NIHSS, hypertension, diabetes, IV tPA delivery, reperfusion status, symptomatic hemorrhage, and ASPECTS scores (excluding Sites 5 and 6).
Figure 1.
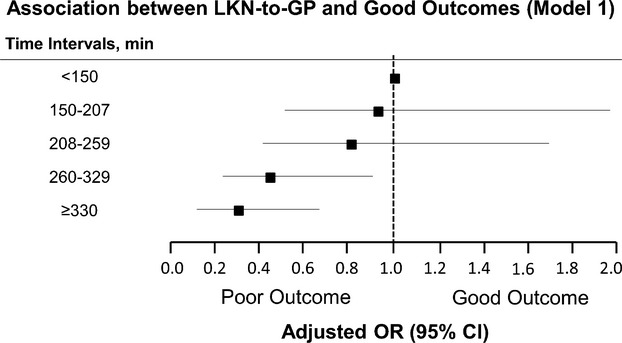
Association between LKN‐to‐GP and good outcomes. GP indicates groin puncture; LKN, last known normal; OR, odds ratio; Hosmer Lemeshow test confirms good‐of‐fit of the model (P>0.05).
Door‐to‐GP and Patient Outcomes
The direct relationship between time and patient outcomes was also noted for D2P. As shown in Figure 2, the probability for a good outcome declined at each subsequent interval from the initial reference group of “D2P<60 minutes.” Of note, the odds for good outcomes significantly decreased to 0.40 (95% CI 0.17 to 0.93; P=0.034) and 0.17 (95% CI 0.07 to 0.43; P<0.001) at “D2P” intervals of 136 to 205 minutes and ≥206 minutes, respectively (Table 3). This relationship between “D2P” times and outcome rates remained significant in all models, either adjusting for ASPECTS (Model 2: OR 0.995, 95% [0.991 to 0.999]; P=0.012), adjusting without ASPECTS (Model 1: OR 0.993, 95% CI [0.990 to 0.996]; P<0.001), or including “site” as an independent predictor (Table 4: OR 0.994, 95% CI [0.990 to 0.998]; P=0.007). In total, only 211 patients (52%) met the AHA guideline of arrival‐to‐treatment within 2 hours.
Figure 2.
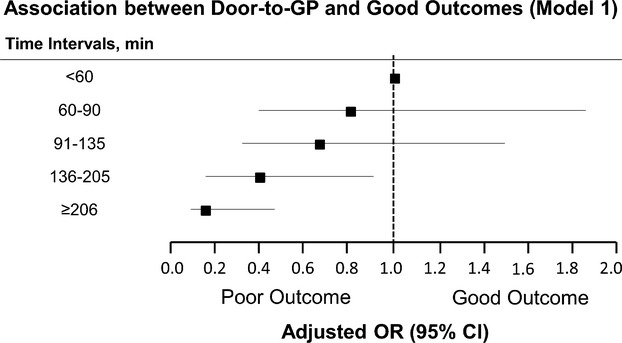
Association between door‐to‐puncture and good outcomes. GP indicates groin puncture; LKN, last known normal; OR, odds ratio; Hosmer‐Lemeshow test confirms good‐of‐fit of the model (P>0.05).
Table 3.
Patient Outcomes Stratified by Door‐to‐Puncture
| Door‐to‐Puncture | Good Outcome/Total | %Good Outcome | Unadjusted Odds Ratio for Good Outcome | Adjusted Odds Ratio for Good Outcome* (Model 1) | Adjusted Odds Ratio for Good Outcome* (Model 2) |
|---|---|---|---|---|---|
| <60 minutes | 36/77 | 46.8 | Reference | Reference | Reference |
| 60 to 90 minutes | 41/85 | 48.2 | 1.06 (0.57 to 1.97); P=0.85 | 0.83 (0.37 to 1.85); P=0.64 | 0.72 (0.31 to 1.66); P=0.44 |
| 91 to 135 minutes | 44/83 | 53.0 | 1.28 (0.69 to 2.39); P=0.43 | 0.65 (0.28 to 1.50); P=0.32 | 0.64 (0.27 to 1.53); P=0.32 |
| 136 to 205 minutes | 27/82 | 33.0 | 0.56 (0.29 to 1.06); P=0.076 | 0.40 (0.17 to 0.93); P=0.034 | 0.47 (0.19 to 1.21); P=0.12 |
| ≥206 minutes | 16/79 | 20.2 | 0.29 (0.14 to 0.59); P=0.001 | 0.17 (0.07 to 0.43); P<0.001 | 0.24 (0.09 to 0.67); P=0.006 |
| All (per minute) | 164/406* | 40.4 | 0.995 (0.992 to 0.997); P<0.001 | 0.993 (0.990 to 0.996); P<0.001 | 0.995 (0.991 to 0.999); P=0.012 |
Model 1: Corrected for age, NIHSS, hypertension, diabetes, IV tPA delivery, reperfusion status, and symptomatic hemorrhage.
Model 2: Corrected for age, NIHSS, hypertension, diabetes, IV tPA delivery, reperfusion status, symptomatic hemorrhage, and ASPECTS scores (excluding Sites 5 and 6).
Door‐to‐Puncture times were not available for 72 patients. ASPECTS indicates Alberta Stroke Program Early CT Score; NIHSS, NIH Stroke Scale; tPA, tissue plasminogen activator.
Table 4.
Binary Logistic Regression Model Identifying Variables Associated With Good Outcome After Intra‐arterial Therapy for Acute Ischemic Stroke
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age | 0.96 (0.94 to 0.98) | <0.001 |
| Hypertension | 0.69 (0.38 to 1.23) | 0.229 |
| NIHSS | 0.84 (0.79 to 0.89) | <0.001 |
| Diabetes | 0.66 (0.33 to 1.30) | 0.225 |
| IV tPA given | 1.68 (0.98 to 2.89) | 0.062 |
| Successful reperfusion | 9.33 (4.69 to 18.57) | <0.001 |
| Symptomatic hemorrhage | 0.40 (0.16 to 1.03) | 0.059 |
| Door‐to‐puncture (min) | 0.994 (0.990 to 0.998) | 0.007 |
| Site no. | Site 1 (reference) | 0.460 |
| Site 2 | 0.68 (0.32 to 1.47) | 0.329 |
| Site 3 | 1.42 (0.65 to 3.08) | 0.377 |
| Site 4 | 0.35 (0.10 to 1.20) | 0.095 |
| Site 5 | 0.60 (0.12 to 2.95) | 0.524 |
| Site 6 | 0.70 (0.09 to 5.59) | 0.735 |
| Other sites | 0.53 (0.06 to 4.58) | 0.560 |
CI indicates confidence interval; NIHSS, NIH Stroke Scale; OR, odds ratio; Homser‐Lemeshow test depicts goodness of fit to the model (P>0.05); t‐PA, tissue plasminogen activator.
In a secondary analysis, imputation of “poor outcomes” (ie, mRS=6) was applied to all patients with missing 90‐day mRS scores (n=76) and adjusted accordingly to Model 1. In this scenario, both “LKN‐to‐GP” (OR 0.996; 95% CI 0.994 to 0.998; P<0.001) and “Door‐to‐Puncture” times (OR 0.993; 95% CI 0.990 to 0.996; P<0.001) remained independently associated with poor patient outcomes for every minute delay to puncture, thereby supporting the results of the initial models.
“D2P” times were also associated with all‐cause mortality at 90 days (OR 1.004; 95% CI [1.001 to 1.007]; P=0.018) when correcting for age, NIHSS, hypertension, diabetes mellitus, reperfusion status, and hemorrhage rates. Patients presenting within 60 minutes of “D2P” had a 24.7% all‐cause mortality rate (reference), as compared with 31.7% (OR 1.70, 95% CI [0.69 to 4.2]; P=0.24) and 39.2% (OR 2.80, 95% CI [1.16 to 6.74]; P=0.022) at intervals of 136 to 205 minutes and ≥206 minutes, respectively.
Discussion
Performance metrics for standardizing data collection involving IAT at Comprehensive Stroke Centers have previously been described in several consensus statements11–12 and retrospective studies.8,13 As recently as 2011, the AHA proposed the use of an “Arrival‐to‐Treatment” metric of <2 hours for all endovascular interventions. Real world data, however, has yet to provide clinical significance for the implementation of this metric and its corresponding parameters.
More recently, the term “onset‐to‐reperfusion time” (ORT) has been proposed as an ideal marker for performance, in which shorter ORTs directly correlate with improved patient outcomes and lower mortality rates at 90 days.14 Although this metric may hold the most physiological relevance with regard to ischemic burden, certain variables within the overall time frame may be difficult to modify at an institutional level. For example, the time from onset‐to‐hospital arrival is contingent on patient awareness of stroke symptoms and the infrastructure of EMS transportation to CSCs. Conversely, the procedure time may rely heavily on the experience of the personnel in the angiosuite, the types of devices used during the operation, and the interpretation of reperfusion status, all of which are more difficult to homogenize in establishing standards. As such, the AHA‐proposed “Arrival‐to‐Treatment” time (which we define as “Door‐to‐Puncture”) may present a more “actionable” time frame by which strategies can be implemented at an institutional level to effect change.
In this report, we present preliminary data from the multicenter Rapid Reperfusion Registry that demonstrates the clinical significance of “D2P” times on patient outcomes, as well as establishes a benchmark for the current status of D2P times from 9 separate institutions. In particular, our data reveal that patients treated within 1 hour from first hospital arrival have the highest odds ratio for a good outcome, regardless of transfer status, which subsequently declines by 7% for every 10‐minute delay to treatment. This relationship similarly holds true when evaluating the broader metric of “LKN‐to‐puncture,” in which every 30‐minute delay to treatment from last known normal corresponds to a 12% reduction in the odds of a good outcome. This is consistent with the results from a previously published multicenter analysis.14
Regardless of the metric used, the timing to IAT delivery remains an important denominator to consider in light of the recent nonpositive trial results comparing IAT with medical therapy.15–17 Between 1999 and 2013, reperfusion rates and procedure times continued to improve with advancements in device technology,18–21 yet the timing from “LKN‐to‐treatment” has not substantially decreased from the initial 4‐ to 5‐hour time window described in PROACT II.22 From the current dataset of patients treated in 2012, the median time from “LKN‐to‐GP” remains at ≈4 hours (234 minutes), whereas the median time from “D2P” was nearly 2 hours (112 minutes), reiterating the notion that system processes in IAT have yet to improve in conjunction with device technologies.
In IMS III, the endovascular arm had a mean onset‐to‐groin puncture time of 208 minutes, with an average NIHSS of 17 and overall good outcome rate of 40.8%. Our dataset reveals a similar result, with an average “LKN‐to‐GP” of 234 minutes, median NIHSS of 18, and an overall good outcome rate of 39.7%. When comparing our registry data to the IV tPA arm in IMS III, which had an onset‐to‐treatment time of 121 minutes, the net difference in percent good outcomes was insignificant (RRR: 39.7% versus IMS III: 38.7%). However, if the IAT curve can be reduced by 60 minutes and shifted closer to the 3‐hour mark (ie, LKN‐to‐GP 150 to 207 minutes; NIHSS 19), as observed in the second quintile of our dataset, the overall good outcome rate approaches 48.5%. This would establish an absolute difference of 10% when compared to the IV tPA group in IMS III, which was the estimated difference in powering the original study (Table 5; Figure 3).
Table 5.
Comparison of Patient Outcomes in the RRR Database Versus Previously Published IAT and IV‐tPA Randomized Controlled Studies
| Study Group | LKN‐to‐Treatment, Mean Minutes (STD) | % Good Outcome Rate | Median NIHSS |
|---|---|---|---|
| RRR: IAT <150 minutes | 108 (28) | 53.5 | 17 |
| RRR: IAT 150 to 207 minutes | 178 (18) | 48.5 | 19 |
| RRR: IAT 208 to 259 minutes | 231 (16) | 38.9 | 18 |
| RRR: IAT 260 to 329 minutes | 290 (19) | 31.6 | 18 |
| RRR: IAT ≥330 minutes | 411 (63) | 26.8 | 17 |
| IMS III: IV tPA arm15 | 121 (34) | 38.7 | 16 |
| IMS III: IAT arm15 | 208 (47) | 40.8 | 17 |
| Lancet 2010: IV tPA pooled, <90 minutes group23 | 90 (N/A) | 48 | 11 |
IAT indicates intra‐arterial therapy; IMS, Interventional Management of Stroke; LKN, last known normal; N/A, not available; NIHSS, NIH Stroke Scale; RRR, Rapid Reperfusion Registry; t‐PA, tissue plasminogen activator.
Figure 3.
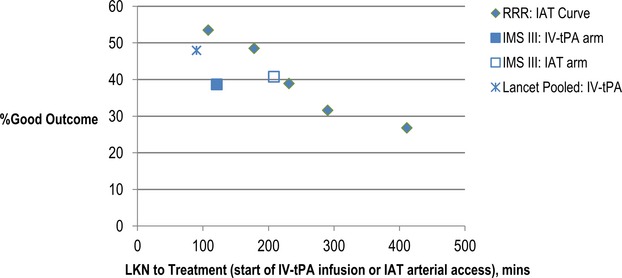
The impact of time on patients outcomes: RRR vs previous IAT/IV‐tPA studies. Median NIHSS in lancet analysis23 for <90 minutes group was 11. All other study groups depicted in the figure had a median NIHSS of 16 to 19. IAT indicates intra‐arterial therapy; IMS, Interventional Management of Stroke III trial; LKN, last known normal; NIHSS, NIH Stroke Scale; RRR, Rapid Reperfusion Registry; t‐PA, tissue plasminogen activator.
The current study suggests that any effort to improve the treatment efficacy of IAT must begin with reductions in “LKN‐to‐GP” times. This may be achieved by targeting the “actionable” time frame of D2P. Strategies including: direct activation of the IR lab by the ED physician; selection of patients for IAT without multimodal imaging (ie, ASPECTS scoring; thin‐cut reconstruction); and applying distance thresholds for inter‐facility transfers have all been previously described as measures to reduce the delays associated with IAT.8 It is also notable in the current registry that the target D2P of <2 hours, as proposed by the AHA, was achieved in only 52% of the patients. Indeed, this is a significant obstacle to any study evaluating the efficacy of IAT, as delays to treatment will always diminish the anticipated effect size of IAT. The AHA's proposal of 2 hours from “arrival‐to‐treatment” reflects more of an infrastructural limitation on the ability of hospitals to achieve IAT within 2 hours, rather than a definitive therapeutic benefit. As our data suggest, treatment with IAT at even earlier time points of <60 minutes may provide the greatest therapeutic benefit for patients undergoing endovascular therapies. The fact that only half of the patients in our registry were treated within 120 minutes highlights the importance for centers to improve their current performance standards and to implement new strategies for streamlining the delivery of IAT. Such measures have already been initiated in a study by Mehta et al, in which strategies for parallel processing and lean modeling have substantially reduced the delays from “door‐to‐puncture” by 36 minutes at a single center (B. Mehta, MD, et al, unpublished data, 2014). To our knowledge, this database is also the first pooled analysis of IAT treatment times to control for pretreatment ASPECTS as a potential confounder for initial stroke severity.
This current analysis has several limitations. First, we did not collate data on the intra‐procedural processes associated with endovascular reperfusion, and thus, cannot comment on its impact on patient outcomes. The type of thrombectomy device used, the number of passes attempted, the presence of adjuvant therapy, and the choice of anesthesia6,24–25 are all variables that cannot be presently accounted for, although the technologies employed are all from the post‐Merci retriever era. Nevertheless, our goal was to focus primarily on the pre‐procedural components of patient care, and to model our analysis after the successful “Door‐to‐Balloon” studies in cardiology, which emphasized the importance of times to catheterization rather than device strategy.26
Second, given the retrospective nature of the study, only 85% of the patients had documented “Door‐to‐Puncture” times, and thus, conclusions may not be applicable to all consecutive patients. Among the patients who received IV tPA, our current dataset cannot distinguish between those who received t‐PA in a “drip‐and‐ship” model versus those who received t‐PA at the CSC. In addition, the utilization of imaging parameters in the decision‐making for IAT (ie, baseline ASPECTS and/or multimodal imaging) was not standardized across all contributing centers, which adds to the level of heterogeneity. For example, at Study Site 5, all patients undergoing IAT were required to undergo multimodal imaging prior to intervention, whereas at Study Site 2, such imaging was only utilized in 55% of the patients.
Lastly, the Rapid Reperfusion Registry was designed as only an observational study. Definitive conclusions regarding the impact of time on outcomes cannot be proven without randomizing patients in a case‐control fashion to a “fast treated” group versus a “slow treated” group. The results from the study are also only reflective of the 9 contributing hospitals, and thus, are not representative of all Comprehensive Stroke Centers on a national level.
Nevertheless, our study demonstrates the importance of pre‐procedural treatment times in association with patient outcomes, as observed from a multicenter perspective. Our results have subsequently set the stage for the second phase of our registry, which will be designed to prospectively collect data pertaining to the interval processes involved in the work‐up of acute stroke patients designated for endovascular intervention. Time points including: initial EMS contact in the field; arrival to PSC/CSC; initial CT imaging; time of multimodal imaging; time of neurology consultation; time of tPA delivery; initial contact with neurointerventionists; acceptance to treatment facilities; and EMS transfer times for inter‐facility transfers will all be documented in a prospective manner. This will then allow us to provide granular detail regarding the system processes associated with delays to IAT, and thus, provide a framework by which strategies can be applied to minimize treatment times. These strategies will then be implemented in a subsequent phase with the goal of improving patient outcomes in endovascular therapy for stroke.
Disclosures
Sun, none; Ribo, recipient of a grant from Instituto de Salud Carlos III, Ministerio de Economía y Competitividad; Goyal, Institutional grant for ESCAPE trial from Covidien EV3, Covidien EV3 for help with design and execution of SWIFT PRIME; Yoo, receives research funding from the National Institutes of Health, Penumbra Inc, and Remedy Pharmaceuticals; Jovin, Silk Road Medical; Cronin, none; Zaidat, penumbra, stryker, microvention, EV3, codman; Nogueira, Scientific advisory board for Stryker Neurovascular, Covidien, CoAxia. Serves on the Data Safety Monitoring Board for Rapid Medical and Imaging Core Lab for Covidien and Reverse Medical, Editor of Interventional Neurology; Nguyen, none; Hussain, none; Menon, none; Mehta, none; Jindal, none; Horev, none; Norbash, none; Leslie‐Mazwi, none; Wisco, none; Gupta, scientific advisory board for Stryker Neurovascular, Covidien, CoAxia. Member of the Data Safety Monitoring Board for Reverse Medical and Rapid Medical; Associate Editor for the Journal of Neuroimaging, Associate Editor for Interventional Neurology, and Associate Editor for the Journal of Neurointerventional Surgery.
Acknowledgments
Sun and Gupta involved in writing of the manuscript. Sun, Gupta, Nogueira, Yoo, Mehta, and Mazwi involved in conception of research. Sun and Gupta performed statistical analysis. Ribo, Goyal, Yoo, Jovin, Cronin, Zaidat, Nogueira, Nguyen, Hussain, Menon, Mehta, Jindal, Horev, Norbash, Mazwi revised the manuscript. Ribo, Goyal, Yoo, Jovin, Cronin, Zaidat, Nogueira, Nguyen, Hussain, Menon, Mehta, Jindal, Horev, Norbash, Mazwi, Wisco, Sun, Gupta collected the data. Gupta and Sun have had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology‐National Cardiovascular Data Registry (ACC‐NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001; 37:2240-2245 [DOI] [PubMed] [Google Scholar]
- 2.Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013; 99:297-303 [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, Krumholz HMNational Cardiovascular Data Registry. Association of door‐to‐balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009; 338:b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley EH, Nallamothu BK, Herrin J, Ting HH, Stern AF, Nembhard IM, Yuan CT, Green JC, Kline‐Rogers E, Wang Y, Curtis JP, Webster TR, Masoudi FA, Fonarow GC, Brush JE, Jr, Krumholz HM. National efforts to improve door‐to‐balloon time results from the Door‐to‐Balloon Alliance. J Am Coll Cardiol. 2009; 54:2423-2429 [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Horev A, Nguyen T, Gandhi D, Wisco D, Glenn BA, Tayal AH, Ludwig B, Terry JB, Gershon RY, Jovin T, Clemmons PF, Frankel MR, Cronin CA, Anderson AM, Hussain MS, Sheth KN, Belagaje SR, Tian M, Nogueira RG. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg. 2013; 5:294-297 [DOI] [PubMed] [Google Scholar]
- 6.Abou‐Chebl A, Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller‐Kronast N, English JD, Linfante I, Dabus G, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Nogueira RG. North American SOLITAIRE Stent‐Retriever Acute Stroke Registry: Choice of Anesthesia and Outcomes. Stroke. 2014. Mar 25. [Ex2010pub ahead of print]. [DOI] [PubMed]
- 7.Singer OC, Haring HP, Trenkler J, Nolte CH, Bohner G, Neumann‐Haefelin T, Hofmann E, Reich A, Wiesmann M, Niederkorn K, Deutschmann H, Bussmeyer M, Mpotsaris A, Stoll A, Bormann A, Petzold GC, Urbach H, Jander S, Turowski B, Weimar C, Schlamann M, Gröschel K, Boor S, Berkefeld JENDOSTROKE Study Group. Periprocedural aspects in mechanical recanalization for acute stroke: data from the ENDOSTROKE registry. Neuroradiology. 2013; 55:1143-1151 [DOI] [PubMed] [Google Scholar]
- 8.Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, Camp D, Frankel MR, Belagaje SR, Anderson AM, Isakov AP, Gupta R. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013; 127:1139-1148 [DOI] [PubMed] [Google Scholar]
- 9.Barber PA, Demchuk A, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000; 355:1670-1674 [DOI] [PubMed] [Google Scholar]
- 10.Berger C, Fiorelli M, Steiner T, Schäbitz WR, Bozzao L, Bluhmki E, Hacke W, von Kummer R. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001; 32:1330-1335 [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, Black CM, Cognard C, Connors JJ, III, Frei D, Gupta R, Jovin TG, Kluck B, Meyers PM, Murphy KJ, Ramee S, Rüfenacht DA, Stallmeyer MJ, Vorwerk D. Multisociety consensus quality improvement guidelines for intraarterial catheter‐directed treatment of acute ischemic stroke. AJNR Am J Neuroradiol. 2013; 34:E0. [PMC free article] [PubMed] [Google Scholar]
- 12.Leifer D, Bravata DM, Connors JJ, III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz RAmerican Heart Association Special Writing Group of the Stroke Council; Atherosclerotic Peripheral Vascular Disease Working Group; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow‐up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:849-877 [DOI] [PubMed] [Google Scholar]
- 13.Khatri P, Abruzzo T, Yeatts S, Nichols C, Broderick JP, Tomsick TAIMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time‐dependent. Neurology. 2009; 73:1066-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazighi M, Chaudhry SA, Ribo M, Khatri P, Skoloudik D, Mokin M, Labreuche J, Meseguer E, Yeatts SD, Siddiqui AH, Broderick J, Molina CA, Qureshi AI, Amarenco P. Impact of onset‐to‐reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013; 127:1980-1985 [DOI] [PubMed] [Google Scholar]
- 15.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer R, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TAInterventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t‐PA versus t‐PA alone for stroke. N Engl J Med. 2013; 368:893-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi ESYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:904-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JLMR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914-923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MPMERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005; 36:1432-1438 [DOI] [PubMed] [Google Scholar]
- 19.Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009; 40:2761-2768 [DOI] [PubMed] [Google Scholar]
- 20.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WSTREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012; 380:1231-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OOSWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel‐group, non‐inferiority trial. Lancet. 2012; 380:1241-1249 [DOI] [PubMed] [Google Scholar]
- 22.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999; 282:2003-2011 [DOI] [PubMed] [Google Scholar]
- 23.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke WECASS, ATLANTIS, NINDS and EPITHET rt‐PA Study Group. Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010; 375:1695-1703 [DOI] [PubMed] [Google Scholar]
- 24.Whalin MK, Lopian S, Wyatt K, Sun CH, Nogueira RG, Glenn BA, Gershon RY, Gupta R. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014; 6:270-275 [DOI] [PubMed] [Google Scholar]
- 25.Abou‐Chebl A, Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller‐Kronast N, English JD, Linfante I, Dabus G, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Nogueira RG. North American SOLITAIRE Stent‐Retriever Acute Stroke Registry: Choice of Anesthesia and Outcomes. Stroke. 2014. ‐ [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom‐onset‐to‐balloon time and door‐to‐balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000; 283:2941-2947 [DOI] [PubMed] [Google Scholar]


