Abstract
Background
Identifying the best markers to judge the adequacy of lipid‐lowering treatment is increasingly important for coronary heart disease (CHD) prevention given that several novel, potent lipid‐lowering therapies are in development. Reductions in LDL‐C, non‐HDL‐C, or apoB can all be used but which most closely relates to benefit, as defined by the reduction in events on statin treatment, is not established.
Methods and Results
We performed a random‐effects frequentist and Bayesian meta‐analysis of 7 placebo‐controlled statin trials in which LDL‐C, non‐HDL‐C, and apoB values were available at baseline and at 1‐year follow‐up. Summary level data for change in LDL‐C, non‐HDL‐C, and apoB were related to the relative risk reduction from statin therapy in each trial. In frequentist meta‐analyses, the mean CHD risk reduction (95% CI) per standard deviation decrease in each marker across these 7 trials were 20.1% (15.6%, 24.3%) for LDL‐C; 20.0% (15.2%, 24.7%) for non‐HDL‐C; and 24.4% (19.2%, 29.2%) for apoB. Compared within each trial, risk reduction per change in apoB averaged 21.6% (12.0%, 31.2%) greater than changes in LDL‐C (P<0.001) and 24.3% (22.4%, 26.2%) greater than changes in non‐HDL‐C (P<0.001). Similarly, in Bayesian meta‐analyses using various prior distributions, Bayes factors (BFs) favored reduction in apoB as more closely related to risk reduction from statins compared with LDL‐C or non‐HDL‐C (BFs ranging from 484 to 2380).
Conclusions
Using both a frequentist and Bayesian approach, relative risk reduction across 7 major placebo‐controlled statin trials was more closely related to reductions in apoB than to reductions in either non‐HDL‐C or LDL‐C.
Keywords: apoB, LDL‐C, meta‐analysis, non‐HDL‐C, statins
Introduction
Identifying the best marker to judge the adequacy of lipid‐lowering treatment for coronary heart disease prevention is increasingly important given that several novel potent lipid‐lowering therapies are being developed. Although recent guidelines1–3 differ as to the importance of targets, all emphasize the importance of LDL lowering with statins to prevent cardiovascular events. The European and Canadian guidelines relate the intensity of statin therapy to the decrease in LDL obtained with statins whereas the American Heart Association/American College of Cardiology Guidelines emphasize the statin regimens used in the clinical trials. Moreover, even guidelines that recommend statin targets do not clearly state on what evidence their choice of target is determined. Thus, changes in LDL‐C, non‐HDL‐C, or apolipoprotein B (apoB) could all be used to assess the response to statin therapy but which most closely relates to risk reduction on statin treatment, has not been established.
Although, Boekholdt et al reported that non‐HDL‐C was marginally superior to LDL‐C and apoB as a marker of future events on treatment,4 the differences were clinically not significant. Similarly, in the Heart Protection study, all 3 equally predicted the risk of future events on treatment.5 However, given that the on‐treatment level of LDL is low in most of these patients 6–8 and that event rates are also low, accurately determining the relative precision of these highly correlated markers for future events is challenging.
An alternative, more direct, and therefore potentially more informative approach is to evaluate the relation between the reduction in these markers and the observed benefit produced by statin therapy. The marker whose reduction relates most directly to benefit should also be the marker that is best to identify those whose outcome might be improved by further lipid lowering. Although change in apoB was more closely associated with benefit among statin trials in a recent study, the differences between markers using a Bayesian approach were modest due to methodological limitations.9
Accordingly, we conducted both a frequentist and a Bayesian meta‐analysis of the relations of the statin‐induced changes in LDL‐C, non‐HDL‐C, and apoB to the clinical benefit, as defined by the reduction in risk, observed in the 7 placebo controlled statin trials.5,10–15
Methods
Data Sources
We searched the literature using PubMed from inception to January 1, 2013 to identify all placebo‐controlled RCTs of statin therapy using the following search strategy adapted from Boekholdt et al4: hydroxymethylglutaryl coenzyme‐A reductase inhibitor, statin, atorvastatin, fluvastatin, lovastatin, simvastatin, pravastatin, rosuvastatin, apolipoprotein, cholesterol, coronary heart disease, and cardiovascular disease. We included only trials that reported 1‐year changes from baseline in LDL‐C, non‐HDL‐C, and apoB during statin therapy. We also excluded trials with a short duration (<2 years) of follow‐up or with fewer than 1000 participants enrolled. Two authors (G.T. and K.W.) screened and abstracted reported baseline and 1‐year levels of cholesterol and apolipoprotein values (or change at 1 year, if reported) as well as reported relative risk reductions for all cardiovascular endpoints. Any discordance in abstracted data was resolved by consensus (G.T., K.W., A.D.S.). Amongst the statin trials, we excluded those such as Post‐CABG 16 in which multiple drugs were allowed to reach the target that had been selected. We also excluded statin trials that compared doses of statins, because the greatest benefit and the greatest reductions occur with the initial dose. The between‐group differences both of change in markers and relative risk reduction will be much smaller and therefore less reliable than those observed in the statin vs placebo‐design trials. For each included trial, we selected the most comprehensive published coronary heart disease (CHD) composite outcome for the meta‐analysis. The composite coronary heart disease (CHD) outcome selected from each trial's report included myocardial infarction and coronary death. 4S,10 CARDS,14 and SPARCL15 also included resuscitated cardiac arrest. In AFCAPS/TexCAPS,12 JUPITER,13 CARDS,14 and SPARCL15 unstable angina was also included whereas in JUPITER,13 CARDS,15 and SPARCL14 revascularization was included in the CHD outcome. We selected CHD rather than total cardiovascular disease (CVD) outcomes because ischemic and hemorrhagic strokes were not always adequately differentiated and LDL is more closely related to the pathophysiology of ischemic strokes as opposed to hemorrhagic strokes. To calculate the reductions in lipid markers on statin therapy, we calculated the difference in lipid markers from baseline to 1 year in the treatment arm and corrected this difference for the difference in the lipid markers at baseline to 1 year in the control arm to limit regression‐dilution bias.
Statistical Analyses
The objective of this study was to relate changes in the plasma levels of the 3 markers by statins to the relative risk reduction observed with statin therapy from clinical trials. To calculate the risk reduction (and 95% CI) from statins per change in each lipid marker within each trial, we divided the logarithm of overall treatment HR (95% CI) in each trial by the difference between treatment and control group marker levels in SDs from that trial. For comparability, the standard deviations reported by Boekholdt4 (32 mg/dL for LDL‐C, 36 mg/dL for non‐HDL‐C, and 27 mg/dL for apoB) were used in each calculation. Standard errors for each point estimate were calculated from the confidence intervals. The results are presented as relative risk reduction per SD decrease in the marker (ie, 1 − HR).
Calculation of Variance
In clinical trials, the before‐ and after‐treatment values from which the changes in markers are estimated are derived from the same individuals. Thus, the differences we modeled were between paired samples. Moreover, LDL‐C, non‐HDL‐C, and apoB are all highly intercorrelated because they are metabolically tied to each other. Accordingly, their changes with therapy are not entirely independent of each other and the degree of linkage is expressed by the correlation coefficient, which we used in calculating the statistical significance of differences between marker changes versus benefit. Without individual level data, we estimated the standard errors for within‐trial “head‐to‐head” differences using the standard formula for differences between log HRs matched by trial.17 In the absence of correlation coefficients reported in the selected trials to compute standard errors, we used correlation coefficients calculated from NHANES 2005‐2010 18 representative of adult residents of the United States: 0.94 for LDL‐C versus non‐HDL‐C, 0.93 for non‐HDL‐C versus apoB, and 0.89 for LDL‐C versus apoB. Power to detect smaller differences in means pre‐ and post‐treatment will be greater in paired versus unpaired analyses when there is positive correlation among the samples.
Frequentist Meta‐Analysis
Frequentist meta‐analyses were performed as recommended by Borenstein et al 17 using Comprehensive Meta‐Analysis software (Biostat) assuming a random effects model. We chose random effects rather than a fixed‐effect model given the differences in study design and statin studied. We performed 6 frequentist meta‐analyses including 1 for each marker's HR calculation (per SD decrement) and 1 “head‐to‐head” comparison of within trial differences between each combination of log HRs. Point estimates of the benefits associated with decrements were calculated by exponentiation of the trial HR by each marker's decrement in standard deviations. Heterogeneity was assessed by the Q statistic. The potential for selection bias was assessed by examining funnel plots with imputed studies and Orwin's fail‐safe N statistics, which determines the number of additional studies with a mean effect of 0 (ie, HR=1.0 or 0% difference in HRs), which would be required to bring the overall mean point estimate below a level we preselected as not clinically meaningful (HR<1.09 or a difference between any pair of HRs, of less than 2%).
We repeated the key apoB versus non‐HDL‐C “head‐to‐head” comparison removing each trial 1 at a time to provide evidence to assess whether the inclusion of any 1 trial drove the overall results. We also conducted a subgroup analysis for this comparison whenever 2 groups with different attributes were identified with more than 1 trial in each group.
Bayesian Meta‐Analysis
We performed 3 different Bayesian meta‐analyses, 1 for each of the within‐trial differences in log HRs for each 2‐marker combination. For each analysis we developed stochastic results from Markov chain Monte Carlo (MCMC) simulation with 5000 burn‐ins and 50 000 generated posterior deviates based on a non‐informative prior distribution for the difference in log HRs and 3 different priors for the correlation between log HRs ranging from non‐informative to 1 reflecting published marker correlations. Conclusions in the results are based on the Bayes factor concept, which is the ratio of the posterior odds of the hypotheses A against B to the prior odds of hypotheses A against B. In our analyses the prior odds=1.0 assuming the HRs are equal and the posterior odds=the estimated probability that newer marker's HR > the older marker's divided by the probability that the older marker's HR > the newer marker's. The larger the Bayes factor is, the stronger evidence is towards hypothesis A with 1<BF<3 providing slight (“barely worth mentioning”) support for hypothesis A, 3<BF<15 providing substantial support,9 and BF>15 providing, in our view, definitive support. Conversely larger values of the reciprocal of the BF provide stronger evidence towards hypothesis B.
We also replicated the Bayesian analyses published by Robinson 9 and colleagues using their data and their model, which included intercept, marker change, and trial duration terms. For comparison we applied their approach to their data using our model, which included only a marker change term assuming intercept=0 (no reduction in risk if LDL is not lowered) and no association with duration (ie, proportional hazards over time). We compared these models in all 24 trials with CHD events, in the 12 statin trials, which included 5 with control groups receiving less intensive treatment, and in the 7 placebo‐controlled statin trials.
Results
Trial Statistics Used in the Meta‐Analysis
The 7 clinical trials described in Table 1 represent all published, placebo‐controlled studies, which have reported baseline and on‐treatment levels of LDL‐ C, non‐HDL‐C, and apoB. 5,10–15 The results of each trial in terms of HRs indicating the various reductions in risk of major coronary events and in terms of the reductions achieved in LDL marker levels are also shown in Table 1.
Table 1.
Study Characteristics Included in the Risk Associations Considered in Meta‐Analysis
| Trial | Year Published | Baseline CVD (%) | CHD Events* HR (95% CI) | 1‐Year on Trial Levels (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Treatment Group | Difference (SDs*) | ||||||||||
| LDLC | Non HDLC | apoB | LDLC | Non HDLC | apoB | LDLC | Non HDLC | apoB | ||||
| 4S | 1998 | 100 | 0.66 (0.59, 0.75) | 190 | 216 | 117 | 118 | 140 | 81 | 2.25 | 2.11 | 1.33 |
| LIPID | 1998 | 100 | 0.76 (0.68, 0.85) | 150 | 181 | 134 | 108 | 136 | 104 | 1.31 | 1.25 | 1.11 |
| AF/TexCAPS | 1998 | 0 | 0.63 (0.50, 0.79) | 156 | 190 | 123 | 115 | 144 | 96 | 1.28 | 1.28 | 1.00 |
| JUPITER | 2008 | 0 | 0.56 (0.46, 0.69) | 109 | 137 | 105 | 62 | 84 | 71 | 1.47 | 1.47 | 1.26 |
| SPARCL | 2006 | 100 | 0.80 (0.69, 0.92) | 132 | 160 | 130 | 70 | 92 | 81 | 1.94 | 1.89 | 1.81 |
| CARDS | 2004 | 0 | 0.63 (0.48, 0.83) | 120 | 158 | 111 | 72 | 100 | 80 | 1.50 | 1.60 | 1.15 |
| HPS | 2002/12 | 65 | 0.76 (0.72, 0.81) | 124 | 178 | 120 | 74 | 113 | 84 | 1.57 | 1.80 | 1.33 |
CVD indicates cardiovascular disease; HPS, heart protection study; HDL‐C, high‐density lipoprotein cholesterol; LDLC, low‐density lipoprotein cholesterol.
The composite coronary heart disease (CHD) outcome selected from each trial's report included myocardial infarction and coronary death. 4S, CARDS, and SPARCL included resuscitated cardiac arrest. AF/TexCAPS, CARDS, SPARCL, and JUPITER included unstable angina. CARDS, SPARCL, and JUPITER included revascularization.
The difference between the treatment and control groups' means divided by the standard deviations reported by Boekholdt et al4 (32 mg/dL for LDL‐C, 36 mg/dL for non‐HDL‐C, and 27 mg/dL for apoB).
Frequentist Analysis of Risk Reduction Per SD Change in Each Marker
The forest plots of the benefits (95% CIs) per SD decrement from these studies for each of the 3 indices—LDL‐C, non‐HDL‐C, and apoB— are shown in Figure 1 (additional details are displayed in Figures S1 through S3). The mean relative risk reductions per SD change in lipid marker (95% CI) among these 7 trials were 20.1% (15.6%, 24.3%) for LDL‐C; 20.0% (15.2%, 24.7%) for non‐HDL‐C; and 24.4% (19.2%, 29.2%) for apoB. Each of the individual risk reductions was significantly (P<0.05) greater than 0%. The “head‐to‐head” comparisons are shown in more detail in Figures S4 through S6.1 and S6.4. The overall within‐trial difference between the reduction in non‐HDL‐C and LDL‐C with respect to risk reduction did not differ significantly from zero (2.4% [−3.6%, 8.4%] favoring LDL‐C, P=0.445). However, the risk reduction from statins per reductions in apoB averaged 21.6% (12.0%, 31.2%) greater than reductions in LDL‐C (P<0.001) and 24.3% (22.4%, 26.2%) greater than reduction in non‐HDL‐C (P<0.001).
Figure 1.
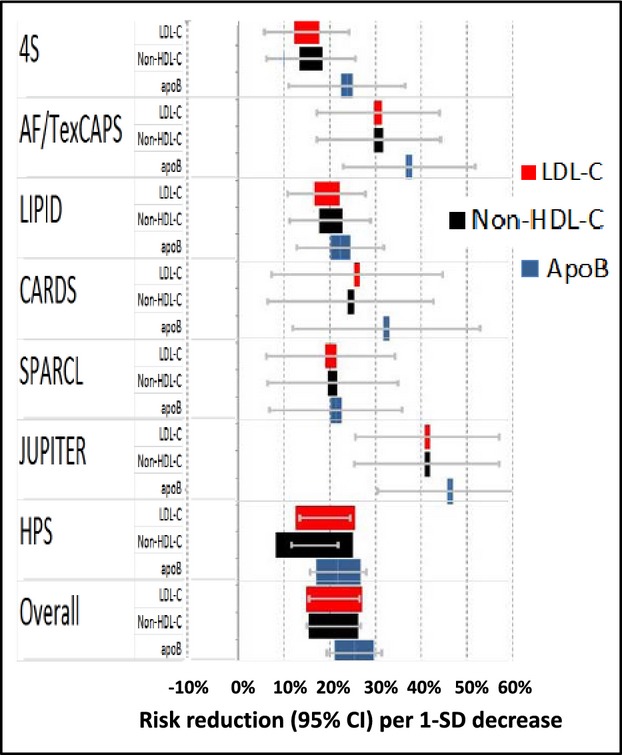
Random effects meta‐analysis results with marker sizes proportional to the precision of each estimate for the estimated reduction in risk per 1‐SD decrease of each marker. Overall LDL‐C (red): 20.1% (15.6%, 24.3%); Non‐HDL‐C (black): 20.0% (15.2%, 24.7%); ApoB (blue): 24.4% (19.2%, 29.2%). Summary of within trial “head‐to‐head” comparisons: LDL‐C was 2.4% (−3.6%, 8.4%) > non‐HDL‐C (P=0.445); apoB was 21.6% (12.0%, 31.2%) > than LDL‐C (P<0.001) and 24.3% (22.4%, 26.2%) > non‐HDL‐C (P<0.001). LDL‐C indicates low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol.
The apoB versus non‐HDL‐C comparison was not substantially altered with respect to statistical significance or magnitude of the effect size estimate by the exclusion of any 1 trial (Figure S6.2). In addition, although the funnel plot (Figure S6.3) showed some asymmetry indicative of potential selection bias, the inclusion of 2 trials imputed to make the funnel plot symmetric did not materially affect the findings.
Frequentist Meta‐Analysis Statistics
As shown in Table 2, heterogeneity was not statistically significant across all the frequentist meta‐analyses (each Q statistic P>0.14) perhaps as a result of the relatively small number of trials included. On the other hand, with the exception of the comparison of the 2 cholesterol markers, the I2 statistic ranged in the low or near the low to moderate range of 25% to 50%,17 thus indicating some of the variance in the trials' results may be attributable to real influences of differences in the trials' attributes. However, we estimate that at least 9 trials with no association would have to be added to the 7 included trials included to reduce the overall results to nonsignificance or to a magnitude deemed not clinically meaningful (ie, a HR≤1.09 or a difference≤2%) (Table 2). Taken altogether these statistics indicate that our findings of a difference between the relative risk reductions per SD decrement of apoB versus each cholesterol marker are robust.
Table 2.
Selected Statistics From Each Meta‐Analysis
| Marker(s) | Q* | P Value | I2 | Fail safe N* |
|---|---|---|---|---|
| LDL‐C | 9.5 | 0.147 | 37.0 | 9 |
| Non‐HDL‐C | 9.5 | 0.147 | 37.0 | 9 |
| ApoB | 8.7 | 0.190 | 31.2 | 13 |
| Non‐HDL‐C—LDL‐C | 7.3 | 0.292 | 18.1 | 0* |
| ApoB—LDL‐C | 5.4 | 0.497 | 0.0 | 11 |
| ApoB—non‐HDL‐C | 8.6 | 0.197 | 30.3 | 15 |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Higher Q statistic values indicate greater heterogeneity.
The number of trials with mean effect of 0 (ie, HR=1.0 or a 0% HR difference) which would have to be added in addition to the 7 trials observed to bring the overall HR within 1.0±9% or a log HR difference within ±1.8% which would be trivial in our judgment.
Fail safe N not calculated since the difference is already trivial.
Bayesian Meta‐Analysis
The Bayesian analysis is presented in detail in the appendix pages 11 to 21. If a noninformative prior for the correlation between log apoB HR and log non‐HDL‐C HR is assumed, the Bayes factor favoring log apoB HR over log non‐HDL‐C HR is 484 (Table S4). A similarly definitive Bayes factor (BF=1110) favoring apoB over non‐HDL‐C results from using a uniform prior distribution of correlation between 0.5 and 1.0 (Table S3). If the prior distribution is based on the published19–23 correlations (truncated normal with mean=0.87, and variance=0.172), the Bayes factor favoring log apoB HR over log non‐HDL‐C is 2380 (Table S2). With an informative prior distribution for the correlation between apoB and LDL‐C (truncated normal with mean=0.84, and variance=0.112) yields BF=188 favoring apoB. Similarly, an informative prior distribution for the correlation between non‐HDL‐C and LDL‐C (r=0.89, SD=0.06) yields a BF of 6.21 favoring LDL‐C, indicating there is no important advantage of non‐HDL‐C over LDL‐C in these data. The principal results of the Bayesian analysis are, therefore, consistent with the frequentist analysis.
Our replication of the Bayesian meta‐analyses conducted by Robinson et al9 presented in Figure S8 (and described on pages 19 to 21 of the appendix), indicated that the differences between their findings and ours are principally attributable to differences in analytical methods.
Implications for Assessing the Adequacy of the Response to Therapy
To illustrate how the choice of a marker could influence the assessment of the response to therapy and the potential benefits of uptitration of statin therapy based on various lipid markers, we examined lipid values from the NHANES 2005–2010 survey. The corresponding population values of non‐HDL‐C and apoB that are equivalent to the percentile values of an LDL‐C of 70 mg/dL would be 90 mg/dL, and 65 mg/dL, respectively. To estimate the additional benefits that might accrue if LDL‐lowering therapy were based on either non‐HDL‐C or apoB rather than LDL‐C, the decrease in events that would be predicted to occur with reductions of LDL‐C, non‐HDL‐C, and apoB to these equivalent levels were calculated for 3 treatment scenarios. The results are presented in Table 3 and are based on different baseline marker levels representing: (1) the mean of the achieved marker levels across the 7 trials; (2) a 1‐standard deviation above the mean achieved; and (3) a 2‐standard deviation above the mean achieved. As listed in each scenario, the relative percent reduction in the 3 markers would be the same. However, as expected, the risk reduction would differ with the greatest benefit achieved at the highest baseline levels (Figure 2). More importantly, in each scenario, benefit would be substantially greater if therapy were targeted at apoB rather than at LDL‐C or non‐HDL‐C.
Table 3.
Illustrative Calculations Using Parameter Estimates From the Present Analysis
| Scenario From | Marker | Marker Level (mg/dL) | Decrement | Risk% Reduction | %>LDL‐C | ||
|---|---|---|---|---|---|---|---|
| mg/dL | % | SDs | |||||
| Mean | LDL‐C | 88 | 18 | 20% | 0.56 | 12% | |
| Non‐HDL‐C | 116 | 26 | 22% | 0.72 | 15% | 26% | |
| apoB | 85 | 20 | 24% | 0.74 | 19% | 58% | |
| Mean+1 SD | LDL‐C | 120 | 50 | 42% | 1.56 | 30%* | |
| Non‐HDL‐C | 152 | 62 | 41% | 1.72 | 32% | 8% | |
| apoB | 112 | 47 | 42% | 1.74 | 39%* | 30% | |
| Mean+2 SD | LDL‐C | 152 | 82 | 54% | 2.56 | 44% | |
| Non‐HDL‐C | 188 | 98 | 52% | 2.72 | 46% | 4% | |
| apoB | 139 | 74 | 53% | 2.74 | 54% | 22% | |
Each scenario involves reduction to the NHANES 2005‐2010 8th percentile of each marker: 70 mg/dL of LDL‐C, 90 mg/dL of non‐HDL‐C, and 65 mg/dL of apoB. Mean levels are the simple average treatment group on treatment levels across the 7 placebo‐controlled trials. Risk reductions are estimated from the point estimate of each marker's overall HR per SD from Figure 1: 0.799 per 32 mg/dL of LDL‐C, 0.800 per 36 mg/dL of non‐HDL‐C, and 0.756 per 27 mg/dL of apoB. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Illustrated in Figure 2.
Figure 2.
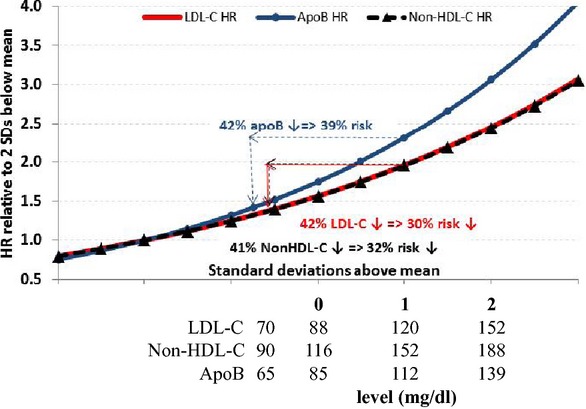
Illustration of the implications of the finding of statistically significant difference in different markers' hazard ratios per standard deviation decrements. The population represented by the gray bell‐shaped curve includes subjects eligible for, and treated under, the 7 placebo‐controlled statin trials. The curves represent the hazard ratios (HRs) relative to 2 standard deviations (SDs) below the mean of this population using the point estimate for each marker's HR per SD (0.799 for LDL‐C, 0.800 for non‐HDL‐C, and 0.756 for apoB). The sample calculations use these parameters to estimate risk reductions if individuals with marker levels 1 SD above the mean are reduced to the 8th NHANES 2005–2010 percentile level: 70 mg/dL for LDL‐C, 65 mg/dL for apoB, and 90 mg/dL for non‐HDL‐C. A, 50 mg/dL (42%) reduction in LDL‐C from 120 to 70 mg/dL would be expected to reduce CHD risk by 30%. Similar decreases to equivalent target levels of non‐HDL‐C and apoB would yield expected risk decreases of 32% and 39%, respectively. CHD indicates coronary heart disease; LDL‐C indicates low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol
Discussion
Using both a frequentist and Bayesian approach, we demonstrate that relative risk reduction from statin therapy in the 7 major placebo‐controlled statin trials was more closely related to reductions in apoB than to reductions in either non‐HDL‐C or LDL‐C. Our results point to a hierarchy of accuracy amongst the 3 markers with reductions in apoB being associated with the greatest risk reduction from statin therapy whereas changes in non‐HDL‐C and LDL‐C appeared to be statistically indistinguishable with respect to risk reduction of statin therapy. These findings should be expected given that statins lower LDL‐C and non‐HDL‐C more than they lower apoB.24 Accordingly, these results indicate that reductions in apoB are more closely related to benefit from statin therapy than are changes in the cholesterol markers and support apoB as the most informative marker of the adequacy of statin therapy. These findings also extend our prior meta‐analysis demonstrating that apoB was superior to LDL‐C or non‐HDL‐C in predicting cardiovascular events.25
By contrast, Boekholdt et al4 and Parish et al5 did not identify any superiority for apoB as a marker of residual risk in patients whose LDL had been substantially reduced by statin therapy. However, these findings do not conflict with ours since the issue of residual risk is different from the issue of benefit. The absolute level of LDL determines the absolute residual risk related to LDL, which, in most of the patients in these studies, was low following statin therapy. At the same time, there are multiple other determinants of cardiovascular events while on statin therapy such as extent of coronary artery disease, left ventricular function, renal function, blood pressure, HDL, diabetes, smoking, and age. Moreover, it is certainly possible that once arterial damage is sufficiently advanced the processes of inflammation and partial repair will continue to produce non‐LDL‐related events and these mechanisms might account for the majority of events in patients on statin therapy.
Robinson and colleagues 9 have previously compared the relations of the 3 markers, LDL‐C, non‐HDL‐C, and apoB, to risk reduction from statin therapy using a Bayesian meta‐analysis. While their primary comparison was based on multiple therapies, a number of which were unsuccessful in reducing LDL or clinical events, they also found that change in apoB was more closely related to the risk reduction in statin trials than changes in non‐HDL‐C or LDL‐C. However, the magnitude of their findings in favor of apoB was much less than in the present analysis. The differences between their results and ours appear to be due to differences in analytic methods. Robinson et al9 evaluated the 3 markers based on how well changes induced by medications fit a 3‐parameter simple linear model and did not focus primarily on the slope, which directly expresses the degree of change. Moreover, our Bayes factors were calculated accounting for the fact that the markers are highly intercorrelated and the changes induced by statins occur in the same individuals.
The HPS 6 and the CTT meta‐analysis7 demonstrated that a reduction in LDL‐C of 1 mmol/L was associated with a 20% reduction in clinical events. Indeed, the same character of relationship between clinical events and change in LDL is documented in this study expressed in different units. Although not widely appreciated, this means that the total risk reduction possible from statin therapy relates to the absolute level of LDL – the higher the level of LDL, the more units of decrease that are possible, and therefore, the greater the benefit possible. Thus, a patient with an LDL‐C of 5 mmol/L will benefit with successive reductions in events from 5 to 4 and 4 to 3 and 3 to 2 mmol/L LDL‐C. In accordance with the results of both the HPS and the CTT meta‐analysis, we demonstrate that the benefit of statin therapy is directly proportional to the baseline LDL.
All guidelines accept that the level of LDL is a major determinant of the risk of vascular disease and that lowering LDL is a potent intervention to reduce the risk of cardiovascular disease. That said, the recent Canadian (1) and European (2) guidelines remain focused on high baseline LDL and use specific targets for LDL‐C, non‐HDL‐C, and apoB for lipid lowering whereas the recent American guidelines (3) deemphasize the baseline LDL levels for statin initiation and the validity of targets for LDL‐lowering therapy. Although the differences with respect to targets should not be exaggerated since the actual recommendation of the AHA/ACC guidelines regarding targets is null – that is, they neither recommend for nor against targets – and they explicitly allow follow‐up testing to determine the adequacy of response. The AHA/ACC guidelines are correct to point out that there is no meaningful difference between, for example, an LDL‐C of 2.1 mmol/L and an LDL‐C of 2.0 mmol/L. Indeed, the relationship we have noted predicts progressively less absolute benefit as levels of LDL are lowered. More importantly, the results are also in keeping with the recent meta‐analysis of residual risk by Boekholdt et al demonstrating a relatively low level of residual risk based on any lipid marker in individuals treated with statins.
The differences we have demonstrated are based on differences in the mass of cholesterol present in apoB particles, differences in the response of LDL‐C, non‐HDL‐C, and apoB to statin therapy and differences in the rate at which different LDL particles are cleared by the LDL pathway. The primary effect of statins is to reduce hepatic cholesterol synthesis, which in turn, will increase uptake of LDL particles by the liver. Because statins reduce LDL‐C substantially more than VLDL triglycerides, cholesterol ester transfer protein mediated exchange of cholesterol ester and triglyceride between LDL particles and VLDL particles will increase with the net result that LDL particles become triglyceride‐enriched and cholesterol‐ester depleted. The triglyceride then tends to be hydrolyzed making the LDL particles smaller and denser. Smaller and denser LDL particles bind less avidly to the LDL receptor and therefore tend to be removed less effectively from plasma. The overall result is that statins reduce LDL‐C substantially, non‐HDL‐C less, and apoB even less.24
As we have illustrated, apoB may identify a subgroup of subjects with cholesterol‐depleted apoB particles that point to an incomplete response to statin therapy. Thus, particularly in subjects with on‐treatment levels of apoB above the mean of the clinical trials we have analyzed, a level that corresponds to approximately the 40th percentile of the American population, significant additional benefit appeared to be possible. Given these results and given that the response to statin therapy is variable,26 we believe the suggestion by AHA/ACC that the response to statin therapy be assessed is reasonable. On the other hand, we acknowledge that until further research is completed, the definition of an incomplete response within an individual subject requires clinical judgment, which as we have argued elsewhere,27 is core to clinical care and well within the options outlined by AHA/ACC guidelines.
This analysis has several limitations that deserve mention. We included only statin trials since our comparison of the markers is to benefit (ie, risk reduction) and only this agent has been consistently associated with benefit. We did not include the statin trials that compared different regimens (eg, high vs low potency statins) since the results of these studies are less clear with respect to benefit attributed to each lipid marker than placebo‐controlled trials. We also related overall statin benefit to changes in cholesterol or apoB only over the first year of treatment when the largest change in cholesterol and apoB are expected to occur. Our meta‐analyses were also limited to published reports and we did not have access to individual‐level data. However, our finding of no significant heterogeneity, our use of various prior distributions based on independent estimates of the correlations and the agreement between our frequentist and Bayesian analyses suggest that our findings are highly consistent and robust. We also used the correlations between lipid markers rather than the correlation between change in markers for our analyses to calculate the standard errors, which may have somewhat slightly overestimated these correlations but would be expected to have only trivial differences in the overall results. Finally, the several included RCTs excluded severe hypertriglyceridemia and therefore limited entry to participants with less extreme elevations in apoB, which may have attenuated the association between changes in apoB and benefit.
Summary
In summary, using both frequentist and Bayesian approaches we demonstrate that reduction in apoB is more closely associated with the risk reduction produced by statin therapy and that apoB‐targeted statin therapy may produce significantly greater benefit than therapy targeted to either LDL‐C or non‐HDL‐C.
Supplementary Material
Appendix A Detailed Frequentist Meta-Analysis Results
Appendix B Bayesian Analysis for the difference of Log HR/SD between apoB and non-HDL-C
Disclosures
Allan Sniderman has received speaker's fees from Genzyme and Merck. George Thanassoulis has received speaker fees from Servier Canada. The other authors have no relevant disclosures.
Acknowledgments
Dr Thanassoulis is supported by a Clinician‐Scientist Award by the Fonds de Recherche en Santé du Québec and by operating grants from the Canadian Institutes of Health Research (MOP‐119380 and MOP‐126033). Dr Sniderman is supported by unrestricted grants from Jean deGranpre and the Louis and Sylvia Vogel Foundation.
References
- 1.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013; 29:151-167 [DOI] [PubMed] [Google Scholar]
- 2.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad FEuropean Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012; 33:1635-1701 [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd‐Jones DM, Blum CB, McBride P, Eckel RH, Schwartz JS, Goldberg AC, Shero ST, Gordon D, Smith SC, Levy D, Watson K, Wilson PW. 2013. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol‐‐10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM, Jr, Ridker PM, Kastelein JJ. Association of LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins. J Am Med Assoc. 2012; 307:1302-1309 [DOI] [PubMed] [Google Scholar]
- 5.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins RHeart Protection Study Collaborative Group. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012; 125:2469-2478 [DOI] [PubMed] [Google Scholar]
- 6.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002; 360:7-2212114036 [Google Scholar]
- 7.Cholesterol Treatment Trialists' (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010; 376:1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sniderman A, Thanassoulis G, Couture P, Williams K, Alam A, Furberg CD. Is lower and lower better and better? A re‐evaluation of the evidence from the CTT meta‐analysis for LDL lowering. J Clin Lipidol. 2012; 6:427-433 [DOI] [PubMed] [Google Scholar]
- 9.Robinson JG, Wang S, Jacobson TA. Meta‐analysis of comparison of effectiveness of lowering apolipoprotein B versus low‐density lipoprotein cholesterol and non‐high‐density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012; 110:1468-1476 [DOI] [PubMed] [Google Scholar]
- 10.Pedersen TR, Olsson AG, Faergeman O, Kjekshus J, Wedel H, Berg K, Wilhelmsen L, Haghfelt T, Thorgeirsson G, Pyörälä K, Miettinen T, Christophersen B, Tobert JA, Musliner TA, Cook TJ. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation. 1998; 97:1453-1460 [DOI] [PubMed] [Google Scholar]
- 11.Simes RJ, Marschner IC, Hunt D, Colquhoun D, Sullivan D, Stewart RAH. Relationship between lipid levels and clinical outcomes in the long‐term intervention with pravastatin in the ischemic disease (LIPID) trial. To what extent is the reduction in coronary events with pravastatin explained by on‐study lipid levels? Circulation. 2002; 105:1162-1169 [DOI] [PubMed] [Google Scholar]
- 12.Gotto AM, Whitney E, Stein EA, Shapiro DR, Clearfield M, Weis S. Relation between baseline and on‐treatment lipid parameters and first acute major coronary events in the Air force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation. 2000; 101:477-484 [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJJUPITER Trial Study Group. Reduction in C‐reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009; 373:1175-1182 [DOI] [PubMed] [Google Scholar]
- 14.Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JAStroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006; 355:549-559 [DOI] [PubMed] [Google Scholar]
- 15.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton‐Menys V, Fuller JHCARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004; 364:685-696 [DOI] [PubMed] [Google Scholar]
- 16.The Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low‐density lipoprotein cholesterol levels and low‐dose anticoagulation on obstructive changes in saphenous‐vein coronary‐artery bypass grafts. N Engl J Med. 1997; 336:153-162 [DOI] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. 2009Chichester, UK: Wiley [Google Scholar]
- 18.NHANES dataset. http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Accessed April 22, 2013.
- 19.Bruno G, Merletti F, Biggeri A, Bargero G, Prina‐Cerai S, Pagano G, Cavallo‐Perin P. Effect of age on the association of non‐high‐density‐lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia. 2006; 49:937-944 [DOI] [PubMed] [Google Scholar]
- 20.Chien KL, Hsu HC, Su TC, Chen MF, Lee YT, Hu FB. Apolipoprotein B and non‐high density lipoprotein cholesterol and the risk of coronary heart disease in Chinese. J Lipid Res. 2007; 48:2499-2505 [DOI] [PubMed] [Google Scholar]
- 21.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004; 110:2824-2830 [DOI] [PubMed] [Google Scholar]
- 22.Bachorik PS, Lovejoy KL, Carroll MD, Johnson CL. Apolipoprotein B and AI distributions in the United States, 1988‐1991: results of the National Health and Nutrition Examination Survey III (NHANES III). Clin Chem. 1997; 43:2364-2378 [PubMed] [Google Scholar]
- 23.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non‐high‐density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005; 112:3375-3383 [DOI] [PubMed] [Google Scholar]
- 24.Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL lowering therapy: Implications for clinical practice. J Clin Lipidol. 2008; 2:36-42 [DOI] [PubMed] [Google Scholar]
- 25.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta‐analysis of low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011; 4:337-345 [DOI] [PubMed] [Google Scholar]
- 26.Awan Z, Seidah NG, MacFadyen JG, Benjannet S, Chasman DI, Ridker PM, Genest J. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem. 2012; 58:183-189 [DOI] [PubMed] [Google Scholar]
- 27.Sniderman AD, LaChapelle KJ, Rachon NA, Furberg CD. The necessity for clinical reasoning in the era of evidence‐based medicine. Mayo Clin Proc. 2013; 88:1108-1114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A Detailed Frequentist Meta-Analysis Results
Appendix B Bayesian Analysis for the difference of Log HR/SD between apoB and non-HDL-C


