Abstract
Background
The association between high‐density lipoprotein cholesterol (HDL‐C) and coronary heart disease (CHD) events is not well described in individuals with very high levels of HDL‐C (>80 mg/dL).
Methods and Results
Using pooled data from 6 community‐based cohorts we examined CHD and total mortality risks across a broad range of HDL‐C, including values in excess of 80 mg/dL. We used Cox proportional hazards models with penalized splines to assess multivariable, adjusted, sex‐stratified associations of HDL‐C with the hazard for CHD events and total mortality, using HDL‐C 45 mg/dL and 55 mg/dL as the referent in men and women, respectively. Analyses included 11 515 men and 12 925 women yielding 307 245 person‐years of follow‐up. In men, the association between HDL‐C and CHD events was inverse and linear across most HDL‐C values; however at HDL‐C values >90 mg/dL there was a plateau effect in the pattern of association. In women, the association between HDL‐C and CHD events was inverse and linear across lower values of HDL‐C, however at HDL‐C values >75 mg/dL there were no further reductions in the hazard ratio point estimates for CHD. In unadjusted models there were increased total mortality risks in men with very high HDL‐C, however mortality risks observed in participants with very high HDL‐C were attenuated after adjustment for traditional risk factors.
Conclusions
We did not observe further reductions in CHD risk with HDL‐C values higher than 90 mg/dL in men and 75 mg/dL in women.
Keywords: CHD events, total mortality, very‐high HDL‐C
Introduction
The association between high‐density lipoprotein cholesterol (HDL‐C) and coronary heart disease (CHD) events is inverse and linear across a large range of HDL‐C (30 to 60 mg/dL) values.1–2 Over the last several decades limited observational cohort data have suggested a possible threshold effect or increased risks for CHD and total mortality events in participants with HDL‐C >80 mg/dL.3–4 However, the sample size of individuals with very high HDL‐C was very small in most studies, limiting the ability to draw conclusions about the risks for CHD and total mortality associated with extremely high levels of HDL‐C. Since the guideline‐based use of lipid‐lowering pharmacotherapy is based on risk assessment, patients with very high levels of HDL‐C present a clinical dilemma, as the CHD risk associated with HDL‐C >80 mg/dL is not well described.
The Lifetime Risk Pooling Project is a very large dataset with extensive phenotyping that provides a unique opportunity to study CHD risks across the full range of HDL‐C values, including those in individuals with extreme elevations in HDL‐C.5 In addition, the detailed covariate data from these cohorts allow for adjustment for many potential confounding variables. In this study, we aimed to quantify, in unadjusted and adjusted models, the hazards for CHD events and total mortality in individuals with very high HDL‐C. We hypothesized that we would see independent increased hazards for CHD events in individuals with very high levels of HDL‐C when compared with a 45 mg/dL and 55 mg/dL referent in men and women, respectively.
Methods
Participants
The cohorts included in the analysis met the following a priori criteria: (1) community‐ or population‐based sampling or large volunteer cohort; (2) availability of at least one baseline examination at which participants provided demographic, personal, and medical history information and underwent direct measurement of physiologic and/or anthropometric variables (eg, blood pressure, weight), lipid assessment (including HDL‐C), and alcohol consumption; (3) availability of total mortality and cardiovascular mortality data also with ascertainment of nonfatal myocardial infarction events. We selected the following 6 cohorts: Framingham Heart Study6 (FHS), Framingham Offspring Study (FOS),7 Cardiovascular Health Study (CHS),8 Atherosclerosis Risk in Communities Study (ARIC),9 Honolulu Heart Study10–11 (HHP), and the Women's Health Initiative12 (WHI) study. Participants were excluded from the analysis if they had pre‐existing CHD (previous diagnosis of myocardial infarction, known angina pectoris, a history of hospitalization for unstable angina, or previous coronary revascularization). The Institutional Review Board at Northwestern University approved this project.
Determination of Lipid Phenotype
Blood from each study was collected and stored at −70°C after venipuncture until the time of analysis. To determine HDL‐C, all cohorts (except WHI) utilized centrifugation followed by removal of all apolipoprotein B containing lipid particles with a magnesium/dextran precipitation assay.13 HDL‐C was then quantified by standardized enzymatic techniques.7–9 Total cholesterol (TC) and triglycerides were determined by direct enzymatic techniques. LDL‐C was calculated via the Friedewald equation.14 The WHI cohort utilized the Hitachi 917 analyzer (Roche Diagnostics) for determination of HDL‐C, LDL‐C, Triglycerides, and TC. This system uses a direct enzymatic colorimetric assay for determination of all lipid subfraction concentrations.12 Non‐HDL cholesterol was calculated as the difference between TC and HDL‐C.
Determination of Covariates
Details of demographic and traditional risk factor assessment are presented elsewhere.6–7,6–12,6–9 Briefly, age, sex, race, smoking status, alcohol consumption, diabetes status, anti‐hypertension medication use, and lipid medication use were determined by self‐report. In ARIC, medication use was confirmed by bottle inspection. Diabetes was defined as self‐reported physician diagnosis, fasting blood sugar ≥126 mg/dL, or diabetes medication use. A drink was defined as one serving of alcohol (12 oz beer, 6 oz glass of wine, or 1.5 oz of hard liquor) or as 0.6 fluid oz, 14 g, or 18 mL or ethanol depending on the cohort. Self‐reported weekly and monthly alcohol consumption was converted to daily consumption and defined as the equivalent of >1 drink a day for women and >2 drinks a day for men. Systolic blood pressure was an average of 2 to 3 measurements taken while seated (supine for HHP). A mercury sphygmomanometer was used for all blood pressure measurements. BMI was calculated as the weight in kilograms divided by the square of the height in meters.
Determination of Outcome
CHD events (nonfatal myocardial infarction [MI] and CHD death) and total mortality events were ascertained using cohort‐specific criteria, as described elsewhere.6–7,6–12,6–9 Briefly, the Framingham Heart Study adjudicated CHD events via medical history, physical examinations, and electrocardiograms. All suspected CHD events were reviewed by a panel of 3 physicians, who applied established criteria for such events.7 CHS, ARIC, HHP, and WHI generally utilized similar criteria: myocardial infarction was defined as an evolving Q‐wave MI or cardiac pain associated with abnormal cardiac enzymes and either an evolving ST‐ or T‐wave pattern or new left bundle branch block on electrocardiogram. A panel of study physicians within each study adjudicated CHD death. Follow‐up for vital status and date of death is nearly 100% complete in each of the cohorts.
Statistical Analyses
To examine the potential nonlinear association between continuous HDL‐C and the risks of events, we used penalized cubic splines in Cox proportional hazards models, which can provide nonparametric estimates for the hazard ratio of HDL‐C.15–16 The proportional hazards assumption was tested using Schoenfeld residuals and was found to be appropriate.17–18 The hazard ratios were scaled such that the hazard ratio was 1 for the HDL‐C level of 45 mg/dL and 55 mg/dL in men and women, respectively. The follow‐up time was truncated at 20 years, as there were few person‐years beyond that point for most of the cohorts. The survival models were initially adjusted for cohort; then adjusted for age, race, systolic blood pressure, body mass index, current smoking status, diabetes, non‐HDL‐C, antihypertensive therapy, antihyperlipidemia therapy, and alcohol drink status (>1 drink/day for women and >2 drinks/day for men).
Due to minor differences in mean HDL‐C across cohorts and slight differences in the measurement of HDL‐C across cohorts, we conducted a sensitivity analysis. For the sensitivity analysis, data were pooled by cohort‐ and sex‐specific Z‐scores of HDL‐C. The hazard ratios were scaled such that the hazard ratio was 1 for the HDL‐C Z‐Score of 0. We then re‐ran the analysis using penalized cubic splines as described above.
In an effort to further examine the effect of alcohol consumption and smoking on elevated HDL‐C and total mortality and CHD events, we also conducted separate stratified (by alcohol and smoking) penalized cubic spine models on the pooled cohort. Baseline hormone replacement therapy (HRT) status and lipid concentrations were available in FH, FOS, and ARIC cohorts only. To assess possible confounding or effect modification due to HRT in women, we ran cohort‐specific, HRT‐stratified Cox proportional hazard models. We also assessed the significance of a HDL‐C (continuous) by HRT interaction term. We used separate sex‐stratified univariable Cox proportional hazard models to determine the traditional risk factor characteristics that were associated with CHD events in individuals with HDL‐C >75 mg/dL.
A P value <0.05 was considered statistically significant, and all statistical tests for significance were 2‐tailed. Statistical analyses were performed using SAS statistical software v. 9.1. The penalized cubic spline model was implemented using coxph and pspline functions in R version 2.14.1.
Results
Study Sample
Baseline characteristics for the 11 515 men included in the analysis are presented in Table 1. The mean ages across all strata of HDL‐C varied from 54 to 60 years. The median HDL‐C in the highest stratum (>90 mg/dL) was 100 mg/dL. The mean non‐HDL‐C, triglyceride levels, and prevalence of cholesterol medication use were lowest in the very high HDL‐C strata. Men within the 2 highest HDL‐C strata had higher mean systolic blood pressures. When compared with the lower strata of HDL‐C (≤50 mg/dL) men in the higher strata had lower BMI. Reported alcohol consumption and smoking were highest in the highest strata of HDL‐C at 40% and 52%, respectively.
Table 1.
Baseline Characteristics by HDL‐C Strata: Men*
| HDL‐C (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≤30 | 31 to 40 | 41 to 50 | 51 to 60 | 61 to 70 | 71 to 80 | 81 to 90 | >90 | |
| N | 1063 | 3520 | 3497 | 2058 | 796 | 335 | 129 | 117 |
| Age, y | 54.4 (9.6) | 56.0 (11.1) | 57.5 (12.1) | 58.1 (12.2) | 59.9 (12.3) | 59.8 (12.7) | 60.1 (11.7) | 59.2 (9.7) |
| SBP, mm Hg | 126.7 (20.0) | 127.4 (19.7) | 128.8 (21.0) | 129.8 (21.1) | 131.8 (22.5) | 131.8 (20.8) | 136.1 (24.9) | 141.4 (26.0) |
| DBP, mm Hg | 77.2 (11.6) | 77.1 (11.6) | 77.1 (11.6) | 78.0 (11.6) | 77.8 (12.2) | 77.5 (12.0) | 80.7 (12.7) | 83.2 (15.0) |
| BMI, m/kg2 | 28.5 (4.0) | 27.7 (4.0) | 26.8 (3.7) | 26.1 (3.9) | 25.1 (3.7) | 24.6 (4.0) | 23.8 (4.4) | 23.4 (3.8) |
| TC, mg/dL | 203.1 (43.4) | 209.6 (40.3) | 210.4 (39.1) | 212.1 (40.9) | 210.4 (38.6) | 215.0 (41.5) | 216.3 (44.0) | 220.5 (39.8) |
| HDL‐C, mg/dL | 26.4 (3.7) | 35.8 (2.7) | 45.0 (2.8) | 54.5 (3.0) | 64.5 (2.8) | 75.0 (2.9) | 84.5 (2.8) | 104.0 (13.6) |
| Non‐HDL‐C, mg/dL | 176.7 (43.6) | 173.8 (40.5) | 165.4 (39.2) | 157.5 (40.9) | 145.9 (38.6) | 140.1 (41.4) | 131.8 (44.0) | 116.5 (36.5) |
| LDL‐C, mg/dL | 132.0 (40.8) | 140.8 (36.6) | 139.9 (35.4) | 136.1 (38.3) | 128.9 (35.0) | 124.3 (37.5) | 117.2 (39.3) | 102.0 (34.7) |
| Triglycerides, mg/dL | 273.9 (182.0) | 219.8 (155.7) | 169.9 (123.7) | 147.1 (119.5) | 128.5 (113.5) | 114.8 (88.0) | 116.0 (110.5) | 76.0 (44.0) |
| Cholest Rx, % | 5 | 4 | 3 | 2 | 2 | 1 | 2 | 1 |
| HTN Rx, % | 22 | 22 | 20 | 19 | 17 | 22 | 15 | 18 |
| Hx DM, % | 14 | 10 | 7 | 6 | 5 | 5 | 9 | 10 |
| Smoker*, % | 43 | 35 | 31 | 31 | 29 | 33 | 37 | 52 |
| Alcohol, % | 18 | 16 | 20 | 25 | 31 | 34 | 35 | 40 |
BMI indicates body mass index; Cholest Rx, cholesterol medication use; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HTN Rx, blood pressure‐lowering medication use; Hx DM, history of diabetes mellitus; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol;.
Data are expressed as mean (SD) unless otherwise indicated.
Smokers current.
Baseline characteristics for the 12 925 women included in the analysis are presented in Table 2. The mean ages across all strata of HDL‐C ranged from 55 to 58 years. The median HDL‐C in the highest stratum was 99 mg/dL. Similar to men, non‐HDL‐C, triglyceride levels, and BMI were lowest in the highest stratum. Systolic blood pressure was slightly lower across higher HDL‐C strata. The prevalence of drinking >1 drink a day was greatest in the 2 highest strata of HDL‐C. Smoking prevalence was generally lower across higher HDL‐C strata.
Table 2.
Baseline Characteristics by HDL‐C Strata: Women*
| HDL‐C (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≤30 | 31 to 40 | 41 to 50 | 51 to 60 | 61 to 70 | 71 to 80 | 81 to 90 | >90 | |
| N | 282 | 1299 | 2986 | 3330 | 2325 | 1441 | 728 | 534 |
| Age, y | 55.2 (10.35) | 56.8 (10.9) | 57.2 (11.4) | 57.1 (11.6) | 57.4 (11.8) | 57.8 (11.2) | 57.9 (10.9) | 58.4 (9.9) |
| SBP, mm Hg | 128.5 (20.2) | 128.9 (22.3) | 126.9 (21.7) | 125.8 (21.3) | 125.0 (21.3) | 124.7 (21.3) | 125.0 (21.4) | 123.2 (20.1) |
| DBP, mm Hg | 75.0 (10.5) | 75.1 (12.3) | 74.0 (11.6) | 73.7 (11.2) | 73.2 (11.3) | 73.2 (11.1) | 73.2 (11.2) | 72.2 (11.3) |
| BMI, m/kg2 | 28.5 (5.6) | 28.7 (5.8) | 27.8 (5..6) | 26.7 (5.3) | 25.6 (4.9) | 24.9 (4.7) | 24.4 (4.3) | 24.0 (4.4) |
| TC, mg/dL | 221.4 (56.6) | 217.7 (46.6) | 221.2 (45.0) | 218.3 (41.0) | 220.3 (41.5) | 220.3 (41.5) | 222.3 (36.4) | 227.9 (37.0) |
| HDL‐C, mg/dL | 26.6 (3.6) | 36.5 (2.7) | 45.7 (2.8) | 55.2 (2.9) | 65.1 (2.8) | 74.8 (2.9) | 84.7 (2.7) | 101.8 (10.8) |
| Non‐HDL‐C, mg/dL | 194.8 (57.0) | 181.3 (46.8) | 175.5 (44.4) | 165.9 (45.0) | 153.1 (41.1) | 145.5 (41.6) | 137.6 (37.5) | 126.1 (37.8) |
| LDL‐C, mg/dL | 147.6 (50.4) | 143.8 (42.4) | 144.9 (39.2) | 140.0 (40.8) | 130.7 (37.3) | 125.0 (38.5) | 118.8 (34.4) | 107.4 (35.0) |
| Triglycerides, mg/dL | 263.3 (180.6) | 206.5 (135.2) | 168.0 (113.5) | 147.0 (101.2) | 125.9 (91.5) | 115.2 (82.8) | 113.2 (81.2) | 95.3 (66.6) |
| Cholest Rx, % | 5 | 3 | 3 | 2 | 2 | 2 | 2 | 2 |
| HTN Rx, % | 30 | 29 | 26 | 23 | 21 | 19 | 18 | 17 |
| Hx DM, % | 18 | 12 | 8 | 5 | 3 | 3 | 2 | 2 |
| Smoker*, % | 45 | 39 | 33 | 28 | 25 | 24 | 24 | 21 |
| Alcohol, % | 17 | 10 | 10 | 13 | 16 | 19 | 23 | 26 |
BMI indicates body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
Data are expressed as mean (SD) unless otherwise indicated.
Smokers current.
In men, 1825 CHD events and 3311 deaths from any cause occurred during 139 624 person‐years of follow‐up. In women, 977 CHD events and 2435 deaths from any cause occurred during 167 622 person‐years of follow‐up. In men, unadjusted total mortality rates were highest in the 4 highest strata of HDL‐C; in contrast, unadjusted CHD event rates were highest in the 2 lowest strata of HDL‐C (Table 3). In women, unadjusted total mortality events were similar across all HDL‐C strata >60 mg/dL, but death rates were higher for individuals in lower strata (HDL‐C <40 mg/dL). The unadjusted CHD death and nonfatal MI rates were lowest in the higher (>60 mg/dL) strata of HDL‐C (Table 3).
Table 3.
Sample Size and Unadjusted Event Rates* by HDL‐C Strata
| HDL‐C (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≤30 | 31 to 40 | 41 to 50 | 51 to 60 | 61 to 70 | 71 to 80 | 81 to 90 | >90 | |
| Men | ||||||||
| N | 1063 | 3520 | 3497 | 2058 | 796 | 335 | 129 | 117 |
| Mortality event rate | 21.8 | 22.2 | 22.9 | 24.1 | 29.8 | 31.9 | 32.7 | 43.4 |
| CHD event rate | 18.8 | 15.7 | 13.0 | 10.7 | 12.0 | 9.7 | 6.4 | 10.0 |
| Women | ||||||||
| N | 282 | 1299 | 2986 | 3330 | 2325 | 1441 | 728 | 534 |
| Mortality event rate | 21.0 | 19.2 | 15.2 | 14.0 | 12.1 | 13.2 | 14.0 | 13.5 |
| CHD event rate | 8.9 | 9.2 | 7.0 | 6.2 | 4.2 | 3.9 | 4.1 | 3.2 |
CHD indicates coronary heart disease; HDL‐C, high‐density lipoprotein cholesterol.
Events per 1000 person‐years.
CHD Events
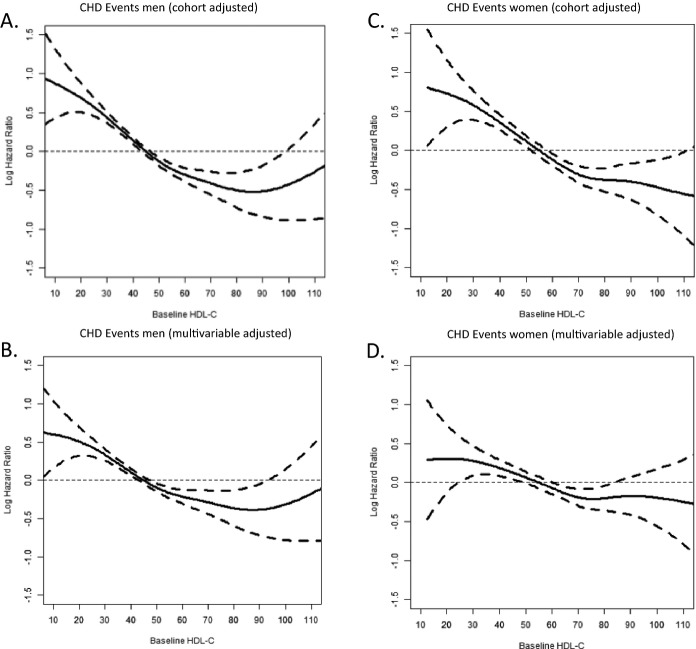
The unadjusted and multivariable‐adjusted cubic spline of the log‐transformed hazard ratios for CHD death and nonfatal MI for men and women are presented in Figure. Men with HDL‐C values less than the referent had higher hazards for CHD events (Figure – Panel A). This relationship is inverse and linear up to an HDL‐C value of ≈90 mg/dL. At HDL‐C values above 90 mg/dL no further reduction in CHD risk was observed. In traditional risk factor and alcohol‐adjusted models, the point estimate was no longer significantly different from the referent when the HDL‐C >95 mg/dL (Figure – Panel B). However, the sample size of this group is substantially less (causing the very wide 95% CI) than the size of the referent category, which likely accounts for the lack of significance. In cohort‐adjusted models, women with HDL‐C <55 mg/dL had higher hazards for CHD events; hazards for CHD events were significantly <1 across all HDL‐C values >55 mg/dL (Figure – Panel C). In multivariable adjusted models, the pattern of association is inverse and linear up to HDL‐C of 75 mg/dL. At HDL‐C strata >75 mg/dL there was no further reduction in the point estimate of CHD risk (Figure – Panel D). Above HDL‐C values of 85 mg/dL hazard ratios were not significantly different from the referent in multivariable adjusted models, though the sample size is modest at such high levels of HDL‐C, which likely accounts for the lack of significance. In sensitivity analysis, the patterns of association between HDL‐C and CHD events were similar in alcohol‐ and tobacco‐ and HRT‐stratified models. There was no significant HRT by HDL‐C interaction term. Likewise, pooling by cohort‐specific HDL‐C Z‐score did not alter the pattern of association.
Figure 1.
Log hazard ratios (95% CI) for CHD events by HDL‐C for men and women. CHD indicates coronary heart disease; HDL‐C, high‐density lipoprotein cholesterol.
We sought to describe the characteristics of men and women with very high HDL‐C who nonetheless had CHD events during follow up. There were 392 men with HDL‐C >75 mg/dL and 32 CHD events occurred during follow‐up. In men, systolic blood pressure (HR=1.01; 95% CI 1.0, 1.03), total cholesterol (HR=1.01; 95% CI 1.01, 1.02), and non‐HDL cholesterol (HR=1.01; 95% CI 1.00, 1.02) were significantly associated with an increased risk for CHD events. There were 1864 women with HDL‐C >75 mg/dL and 95 CHD events that occurred during follow‐up. In women, age (HR 1.1; 95% CI 1.06, 1.14), systolic blood pressure (HR 1.03; 95% CI 1.02, 1.04), diastolic blood pressure (HR=1.03; 95% CI 1.01, 1.05), and treatment for hypertension (HR=1.71; 95% CI 1.08, 2.71) were associated with increased risk for CHD events during follow‐up.
Total Mortality
In men, there was an unadjusted parabolic association between HDL‐C and total mortality with substantially elevated hazards for total mortality in the highest and lowest HDL‐C categories. However, after multivariable adjustment and further stratification by smoking status these risks were highly attenuated among individuals with very high HDL‐C. There were no significant associations between high HDL‐C and total mortality in women.
Discussion
In this pooled analysis from 6 community‐based cohorts we did not observe a monotonic inverse linear association between HDL‐C and CHD risk at very high levels of HDL‐C. Rather, we observed evidence of a plateau effect for CHD risk at high levels of HDL‐C in men and women.
Although CHD event rates were generally lower in cohort participants with very high HDL‐C, risk for CHD events persisted in this group as many participants with very high HDL‐C still experienced MI or CHD death during follow‐up. However, the significant associations observed between age, blood pressure, and non‐HDL‐C and CHD risk suggest that at‐risk individuals with very high HDL‐C are at least partially identifiable through assessment of traditional risk factors. We believe this observation suggests heterogeneity in the CHD risk of individuals with very high HDL‐C. Therefore, it is important for clinicians to continue to consider the entire spectrum of CHD risk factors, and not assume that a very high HDL‐C is automatically or fully protective against CHD.
A recent nested case‐control analysis on participant samples from the Nurses' Health and Health Professionals Follow‐Up Studies suggests a structural variation in HDL particles that may mediate the atheroprotective properties of HDL. Specifically, Jensen et al found that people with HDL particles that contained Apolipoprotein CIII did not have the expected inverse association between HDL‐C and CHD risk.19 This finding raises the possibility that more prevalent dysfunctional HDL particle variants in people with very high HDL‐C could explain the plateau relationship between HDL and CHD risk observed in this study.
Interestingly, the triglyceride concentrations in Tables 1 and 2 are noticeably lower in individuals with very high HDL‐C. Higher triglyceride concentrations in individuals with very high HDL‐C suggest the possibility of decreased lipid exchange from HDL to VLDL particles that could be mediated by abnormalities in physiologic pathways for lipid exchange between lipoprotein particles, eg, cholesteryl ester transfer protein (CETP) activity.
Multiple epidemiologic studies have demonstrated a well‐known independent inverse linear association between HDL‐C and risk for CHD events across a broad range of HDL‐C concentrations <60 mg/dL.1–2 However, prior studies could not thoroughly assess the pattern of association in people with levels of HDL‐C >80 mg/dL, due to its uncommon occurrence. In many of these studies, when data were examined in ordinal bins of HDL‐C, the highest stratum of HDL‐C was >50 to 55 mg/dL. If a small number of cohort participants with HDL‐C >80 mg/dL were grouped with a much larger number of participants with HDL‐C values close to 50 mg/dL, the effect of the larger numbers of participants with HDL‐C close to 50 mg/dL would dominate and could mask a potential plateau pattern of association suggested in our larger sample. Furthermore, careful inspection of crude event rates presented from the Lipoprotein Phenotyping Study reveals a small increase in the event rate for CHD at HDL‐C values >75 mg/dL.1 More recent data from the Incremental Decrease in End Point Through Aggressive Lipid Lowering study (IDEAL) demonstrated a modest increase in the risk for major cardiac events in participants with HDL‐C values >80 mg/dL.4 The association became significant only after adjustment for traditional risk factors, Apolipoprotein‐A1 and Apolipoprotein‐B concentrations. Unlike our study, they were not able to adjust for alcohol consumption in the IDEAL study, which, given the confounding effects of ethanol abuse on HDL‐C and CHD events, was a major limitation of their analysis.
Our study benefited from a large aggregation of multiple community‐based samples and well‐phenotyped cohorts. As mentioned above, this sample allows for adjustment for many potential confounding variables—most notably alcohol. To the best of our knowledge, no other such analysis has been performed. With the pooling of multiple cohorts, it is possible that cohort‐specific or birth cohort effects are present. However, all individual‐level cohort data were reviewed and pooled only after similar patterns of association were seen between cohorts. Unfortunately we are unable to obtain sufficient data to adjust for the effects of dietary fat intake and frequency of aerobic exercise on HDL‐C values and CHD events.
In summary, we observed strong evidence of a plateau effect in terms of CHD risk reduction for men and women with very high levels of HDL‐C. Thus, CHD risk persists even among people with very high HDL‐C. These data suggest that clinicians should have continued vigilance about the detection, avoidance, and treatment of traditional risk factors in this patient population. We believe these data support the possibility of diversity and complexity in the function of HDL in constitutive human physiology. Further research is needed into whether specific biologic mechanisms related to health outcomes vary with HDL‐C level.
Sources of Funding
This work is supported in part by grant R21 HL085375 from the National Heart, Lung, and Blood Institute. The Atherosclerosis Risk in Communities Study, Framingham Heart Study, Framingham Offspring Study, and Honolulu Heart Program are conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the study investigators. This manuscript was prepared using limited access datasets obtained by the NHLBI from these studies, and does not necessarily reflect the opinions or views of the study investigators or the NHLBI. The research reported in this article was supported by contracts HHSN268201200036C, N01‐HC‐85239, N01‐HC‐85079 through N01‐HC‐85086, N01‐HC‐35129, N01 HC‐15103, N01 HC‐55222, N01‐HC‐75150, N01‐HC‐45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG‐023629, AG‐15928, AG‐20098, and AG‐027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosures
None.
Acknowledgments
We would like to thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf. In addition, we would like to thank the investigators, staff, and participants of the Framingham Heart Study, Framingham Offspring Study, Atherosclerosis Risk in Communities Study, Honolulu Heart Study, and Cardiovascular Health Study.
References
- 1.Castelli W, Doyle J, Gordon T, Hames C, Hjortland M, Hulley S, Kagan A, Zukel W. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977; 55:767-772 [DOI] [PubMed] [Google Scholar]
- 2.Gordon D, Probstfield J, Garrison R, Neaton J, Castelli W, Knoke J, Jacobs D, Jr, Bangdiwala S, Tyroler H. High‐density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989; 79:8-15 [DOI] [PubMed] [Google Scholar]
- 3.Stensvold I, Urdal P, Thurmer H, Tverdal A, Lund‐Larsen PG, Foss OP. High‐density lipoprotein cholesterol and coronary, cardiovascular and all cause mortality among middle‐aged Norwegian men and women. Eur Heart J. 1992; 13:1155-1163 [DOI] [PubMed] [Google Scholar]
- 4.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ESG, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw K‐T, Kastelein JJP. High‐density lipoprotein cholesterol, high‐density lipoprotein particle size, and apolipoprotein A‐I: significance for cardiovascular risk: the IDEAL and EPIC‐Norfolk studies. J Am Coll Cardiol. 2008; 51:634-642 [DOI] [PubMed] [Google Scholar]
- 5.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012; 366:321-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawber T, Kannel W, Lyell L. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963; 107:539-556 [DOI] [PubMed] [Google Scholar]
- 7.Feinleib M, Kannel W, McNamara P, Garrison R, Castelli W. The Framingham Offspring Study. Prev Med. 1975; 4:518-525 [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991; 1:263-276 [DOI] [PubMed] [Google Scholar]
- 9. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989; 129:687-702 [PubMed] [Google Scholar]
- 10.Kagan A, Harris B, Winkelstein W. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: demographic, physical, dietary and biochemical characteristics. J Chronic Dis. 1974; 27:345-364 [DOI] [PubMed] [Google Scholar]
- 11.Yano K, Reed D, McGee D. Ten‐year incidence of coronary heart disease in the Honolulu Heart Program: relationship to biologic and lifestyle characteristics. Am J Epidemiol. 1984; 119:653-666 [DOI] [PubMed] [Google Scholar]
- 12. Design of the women's health initiative clinical trial and observational study. Control Clin Trials. 1998; 19:61-109 [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR, Benderson J, Albers JJ. Dextran sulfate‐Mg2+ precipitation procedure for quantitation of high‐density‐lipoprotein cholesterol. Clin Chem. 1982; 28:1379-1388 [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502 [PubMed] [Google Scholar]
- 15.Eilers PH, Marx BD. Flexible smoothing with B‐splines and penalties. Stat Sci. 1996; 11:89-112 [Google Scholar]
- 16.Hurvich CM, Simonoff JS, Tsai C‐L. Smoothing parameter selection in nonparatemtric regression using an improved akaike information criterion. J R Stat Soc Series B. 1998; 60:271-293 [Google Scholar]
- 17.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994; 81:515-526 [Google Scholar]
- 18.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982; 69:239-241 [Google Scholar]
- 19.Jensen MK, Rimm EB, Furtado JD, Sacks FM. Apolipoprotein C‐III as a potential modulator of the association between HDL‐cholesterol and incident coronary heart disease. J Am Heart Assoc. 2012; 1‐10.1161/JAHA.111.000232 [DOI] [PMC free article] [PubMed] [Google Scholar]