Abstract
Background
Hyperuricemia and markers of inflammation are correlated with the risk for hypertension. Whether hyperuricemia has any impact on the association between C‐reactive protein (CRP) and hypertension is not known.
Methods and Results
We analyzed cross‐sectional data from the National Health and Nutrition Examination Survey, 2009–2010, using ordinary least squares and logistic regression models. Those who met the criteria for metabolic syndrome, had self‐reported gout, or were <20 years old were excluded. For each 1‐SD increase in serum urate, the serum CRP concentration was 20% higher in unadjusted linear regression models and 13% higher in multivariable linear regression models, after accounting for the effects of age, sex, race, socioeconomic and educational strata, renal function, lipids, smoking, and body mass index. In multivariable models adjusting for the same covariates, hyperuricemia was associated with hypertension with an odds ratio of 2.21 (1.71 to 2.85). When analyzed separately, this was observed in men and women. In multivariable analyses of the overall sample, elevated CRP levels were not associated with hypertension.
Conclusions
Among adults free of metabolic syndrome, elevated uric acid, but not elevated CRP, is independently associated with prevalent hypertension.
Keywords: effect modification, hypertension, hyperuricemia, inflammation, National Health and Nutrition Examination Survey (NHANES), uric acid
Introduction
Uric acid is a byproduct of normal purine catabolism that is excreted mostly in urine but also through the gastrointestinal tract. Numerous studies have identified serum urate concentrations >7 mg/dL as an independent, major risk factor for hypertension; lowering serum urate is associated with reduction in blood pressure.(2005)–(2012) The mechanism that links hyperuricemia and these adverse clinical outcomes has not been elucidated. Both pro‐oxidant and antioxidant properties have been attributed to uric acid, depending on the context.(2010)–(2000) One pathophysiological model proposes that the oxidative stress associated with hyperuricemia leads to lipid oxidation that in turn becomes antigenic, triggering an immune response and systemic vascular inflammation.(2010),(2005)–(2005)
C‐reactive protein (CRP) is one biomarker of systemic inflammation that has been linked to cardiovascular disease and mortality.(2009),(2010) Ruggerio et al found that hyperuricemia was associated with elevated CRP and other inflammatory markers in a cohort of elders.(2006) Other studies have examined the urate–CRP link in populations with high cardiovascular risk due to factors such as metabolic syndrome,(2009) renal disease, hypertension,(2011) or diabetes.(2011) In the context of the general population free of metabolic syndrome, it is uncertain whether hyperuricemia is associated with elevated markers of systemic inflammation, whether hyperuricemia and CRP are associated with a higher prevalence of hypertension, and whether the presence of one of these modifies the association of the other with hypertension.
If the hyperuricemia–oxidative stress–inflammation model is correct, it follows that hyperuricemia will be associated with higher serum levels of markers of inflammatory response. The first objective of this study was to assess the relationship between hyperuricemia and CRP among those without gout or metabolic syndrome in a general population setting. The second objective of this study was to assess the statistical association of urate concentration on the previously reported CRP–hypertension link.(2003)–(2001) Furthermore, we studied the effect of the concurrent presence of hyperuricemia and CRP on the prevalence of hypertension.
Methods
Data Source
For this analysis, we used data from the National Health and Nutrition Examination Survey (NHANES) 2009–2010 cycle, a cross‐sectional, nationally representative sample of the noninstitutionalized adult US population. An exhaustive description of the survey design, data collection strategies, and instruments is available online (http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/nhanes09_10.htm). Briefly, this survey is a complex multistage sample of the US population where the basic geographic unit is the county. The survey deliberately oversamples difficult‐to‐enroll patient subgroups. The survey has 3 major data collection components: a telephone interview; an in‐person visit with additional questionnaires and anthropometry, other biometric measurements, and blood pressure measurement; and a laboratory test including a fasting phlebotomy. All participants provided informed consent for the data to be disseminated in a deidentified format, the format obtained for this study. Deidentified data are freely available in the public domain, and an institutional review board approval was not required. Dr Krishnan possesses the source data and computer code for data analyses and serves as the guarantor of this report.
Inclusion and Exclusion Criteria
Overall, there were 10 537 observations in the NHANES 2009–2010 datasets, from which we first excluded participants <20 years of age (n=4319), those who were missing values for serum urate (n=508), or those with gout (n=277) and examined the bivariate relationship between metabolic syndrome and CRP concentrations. Subsequently, those with metabolic syndrome (n=1194) were excluded, leaving a final analysis dataset with 4368 observations.
Blood Pressure Measurement
Standardized blood pressure measurement was performed by trained personnel and/or physicians using a mercury sphygmomanometer. Details of blood pressure measurement, calibration protocol and quality control measures are available (http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/BP.pdf). All measurements were performed in the mobile examination center. After 5 minutes of resting in sitting position, 3 arm measurements were performed using a cuff size appropriate for the individual. If needed, a fourth measurement was performed. For our analyses, we calculated the mean of these measures for each participant.
Laboratory Testing
Fasting serum specimens were processed, stored, and shipped to Collaborative Laboratory Services for analysis. Serum creatinine was assayed using the Jaffe rate method, and urate was assayed by using the uricase method. CRP was assayed using a Behring Nephelometer. The lower limit of detection of the CRP assay was 0.2 ng/dL. As per NHANES protocol, CRP measured below the threshold of detection (0.02 ng/dL) was divided by the square root of 2. Exhaustive technical details of these assays including calibration and standardization protocols are available in the NHANES Laboratory/Medical Technologists Procedures Manual (http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/labdoc_f.htm;accessed).
Case Definitions
We used the standard NHANES case definition for gout that was dependent on self‐reported health care provider diagnosis. Hyperuricemia was defined as a serum urate of >7.0 mg/dL, similar to the definition used in other studies.(2007) Elevated CRP was defined as a concentration of ≥75th percentile (≥0.38 mg/dL). Hypertension was defined as a mean blood pressure of ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg. Current use of antihypertensive drugs categorized the individual as hypertensive regardless of the actual blood pressure measurement. Diabetes was defined as a fasting glucose concentration of ≥126 mg/dL. Oral glucose test results were not available. Metabolic syndrome was defined per the Adult Treatment Panel guidelines described by Grundy et al. In patients in whom waist circumference was not available (n=456), we considered a body mass index of ≥30 kg/m2 as equivalent to meeting the waist circumference criterion for metabolic syndrome. Estimated glomerular filtration rate was calculated per the CKD‐EPI creatinine equation.(2009) Income was measured using the poverty income ratio, the ratio of a family's income to the US Census Bureau's poverty threshold, which varies with the number and ages of family members and is revised yearly.()
Statistical Analyses
Unless specified otherwise, all analyses were performed using the survey suite of commands in STATA 11 (StataCorp). These analyses incorporated the study visit weights, primary sampling unit, and stratification design of the study. All SE values and 95% CIs were computed using the Taylor‐linearized variance estimation. Because the results from this study were from weighted analyses, all descriptive measures are presented with 95% CIs as opposed to SDs. A P value of <0.05 was deemed to indicate statistical significance.
We dichotomized serum urate and CRP measures for our primary analyses but also present analyses with these as continuous measures wherever needed. The choice of cutoffs for defining elevated CRP was based on published literature; the performance of this cutoff was assessed in our data using receiver operator characteristic (ROC) curves. The distribution of CRP measures was skewed and, therefore, we log‐transformed CRP measurements for the purpose of fitting regressions where it was modeled as a continuous measure.
Analysis of Hyperuricemia and Elevated CRP
In these models, the key independent variable was serum urate. We used ordinary least squares regressions where the key dependent variable was log‐transformed serum CRP. We estimated percent difference in the CRP concentration for each 1‐SD increase in serum urate (1.4 mg/dL) after adjustment for age, estimated glomerular filtration rate per CKD‐EPI creatinine equation, total cholesterol, poverty ratio, HDL cholesterol, and body mass index as continuous variables and sex, ethnicity, education level (less than high school, high school, greater than high school), and ever smoking as categorical variables. The regression coefficient associated with serum urate was assessed as the percent change in CRP per finite change in serum urate.
CRP and the Hyperuricemia–Hypertension Link
We addressed the statistical association between CRP concentration, hyperuricemia, and hypertension using ordinary least square (OLS) and logistic regression models.
OLS Models
We used multivariable OLS models where systolic and diastolic blood pressures were modeled separately as dependent variables. In these models, the covariates adjusted for included all those described in the previous section. We entered serum urate and log‐transformed values of CRP separately and then together along with other covariates. Subsequently, we calculated the magnitude and significance of linear combination of the respective β coefficients.
Logistic Regression Models
Here, too, we assessed the multivariable adjusted odds ratios of hyperuricemia and elevated CRP on the prevalence of hypertension. The covariates adjusted were the same as the OLS models. We calculated odds ratios in unadjusted and in age‐, sex‐, and ethnicity‐adjusted models. In final models, age, estimated glomerular filtration rate per CKD‐EPI creatinine equation, total cholesterol, poverty ratio, HDL cholesterol, and body mass index were included as continuous variables and sex, ethnicity, education level (less than high school, high school, greater than high school), and ever smoking were included as categorical variables. To study the statistical impact of the presence or absence of hyperuricemia on the CRP–hypertension association, we combined hyperuricemia and CRP into a single variable with 4 strata: low urate/low CPR, low urate/high CRP, high urate/low CRP, and high urate/high CRP concentrations. Odds ratios for these strata were examined for potential effect modifications.
Results
Participants Included in the Analyses
Before exclusions, we examined the prevalence of metabolic syndrome among adults overall. The overall prevalence of metabolic syndrome was 16.8% (95% CI 15.4% to 18.4%), the prevalence of hyperuricemia was 12.7% (95% CI 11.6% to 13.9%), and the prevalence of elevated CRP was 4.7% (95% CI 3.8% to 5.7%). The mean CRP concentration was lower among those without metabolic syndrome than those with the syndrome across the range of serum urate (Figure 1).
Figure 1.
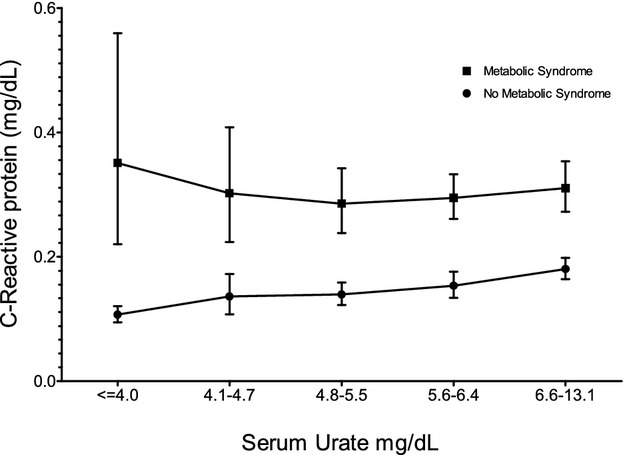
Weighted analysis of association between serum urate and CRP among those with and without metabolic syndrome in NHANES 2009–2010. Means were calculated using log‐transformed values of CRP, which was then back transformed. In weighted, bivariate ordinary linear regressions where both log‐transformed CRP and serum urate were analyzed as continuous variables, there was no significant trend among those with metabolic syndrome but the trend among those without was statistically significant at <0.0001. CRP indicates C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
After exclusions, the analysis dataset consisted of data from 4368 participants. Table 1 provides the characteristics of participants separated by hyperuricemia level and inflammation status. In this population, the prevalence of hypertension was 24%; among the nonhypertensive group, 31% had no other components of metabolic syndrome, 39% had 1 component, and 30% had 2 other components.
Table 1.
Characteristics of Study Population Segregated by Serum Urate and Elevated CRP Levels
| Characteristics | Serum Urate ≤7.0 mg/dL | Serum Urate >7.0 mg/dL | Overall | |||
|---|---|---|---|---|---|---|
| Nonelevated CRP | Elevated CRP | Nonelevated CRP | Elevated CRP | Nonelevated CRP | Elevated CRP | |
| Unweighted number of observations in dataset | 2922 | 967 | 328 | 151 | 3250 | 1118 |
| Population size | 114 million | 33 million | 13 million | 5 million | 127 million | 38 million |
| Mean serum urate, mg/dL | 4.96 (4.91 to 5.01) | 5.08 (4.96 to 5.20) | 7.82 (7.71 to 7.93) | 7.94 (7.75 to 8.12) | 5.25 (5.19 to 5.31) | 5.44 (5.29 to 5.59) |
| % Women | 52.3 (50.6 to 54.0) | 68.6 (65.6 to 71.3) | 11.9 (8.6 to 16.2) | 29.9 (21.9 to 39.3) | 48.2 (46.5 to 49.9) | 63.7 (60.9 to 66.4) |
| Mean CRP, mg/dL | 0.12 (0.11 to 0.13) | 1.02 (0.96 to 1.08) | 0.14 (0.13 to 0.16) | 1.03 (0.86 to 1.20) | 0.12 (0.12 to 0.13) | 1.02 (0.96 to 1.08) |
| Age, y | 45 (44 to 46) | 45 (43 to 46) | 45 (43 to 48) | 52 (49 to 50) | 45 (44 to 46) | 46 (44 to 47) |
| Poverty ratio (0 to 5) | 3.12 (3.05 to 3.20) | 2.75 (2.54 to 2.95) | 3.05 (2.83 to 3.27) | 2.91 (2.60 to 3.22) | 3.12 (3.04 to 3.19) | 2.77 (2.57 to 2.96) |
| Ethnicity, % | ||||||
| Hispanic/Mexican | 13.3 (9.0 to 19.4) | 15.8 (9.1 to 25.9) | 11.9 (5.9 to 22.5) | 5.8 (2.7 to 11.9) | 13.2 (8.7 to 19.6) | 14.5 (8.5 to 23.8) |
| Non‐Hispanic White | 68.8 (61.8 to 75.1) | 65.2 (55.9 to 73.5) | 69.8 (58.3 to 79.3) | 66.3 (58.9 to 73.1) | 69.0 (61.7 to 75.4) | 65.4 (57.1 to 72.8) |
| African American | 9.5 (8.1 to 11.2) | 13.9 (11.1 to 17.3) | 10.2 (7.9 to 13.1) | 23.8 (16.3 to 33.3) | 9.6 (8.2 to 11.2) | 15.2 (12.2 to 18.7) |
| Other or multiracial | 8.3 (5.7 to 11.9) | 5.1 (4.1 to 6.4) | 8.0 (3.8 to 15.9) | 4.1 (1.2 to 12.7) | 8.2 (5.7 to 11.8) | 5.0 (3.9 to 6.4) |
| Lifestyle factors | ||||||
| Ever smoked, % | 42.6 (38.2 to 47.1) | 47.4 (41.6 to 53.4) | 46.0 (40.7 to 51.4) | 50.8 (40.1 to 61.5) | 43.0 (38.7 to 47.4) | 47.9 (42.2 to 53.6) |
| 57.4 (52.9 to 61.8) | 52.6 (46.6 to 58.4) | 54.0 (48.6 to 59.3) | 49.2 (38.5 to 59.9) | 57.0 (52.6 to 61.3) | 52.1 (46.4 to 57.8) | |
| Medications | ||||||
| Cholesterol medications, % | 88.2 (86.1 to 89.9) | 88.6 (85.5 to 91.9) | 86.5 (81.6 to 90.2) | 87.0 (80.6 to 91.5) | 88.0 (86.0 to 89.7) | 88.4 (85.6 to 90.7) |
| 11.8 (10.1 to 13.9) | 11.4 (8.9 to 14.5) | 13.5 (9.8 to 18.4) | 13.0 (8.5 to 19.4) | 12.0 (10.3 to 14.0) | 11.6 (9.3 to 14.4) | |
| Diabetes medications, % | 98.9 (98.3 to 99.4) | 98.4 (96.9 to 99.2) | 99.1 (97.9 to 99.6) | 98.2 (94.5 to 99.4) | 99.0 (98.4 to 99.3) | 98.4 (97.1 to 99.1) |
| 1.1 (0.6 to 1.7) | 1.6 (0.8 to 3.1) | 0.9 (0.4 to 2.1) | 1.8 (0.6 to 5.5) | 1.0 (0.7 to 1.6) | 1.6 (0.9 to 2.9) | |
| Blood pressure medications, % | 86.1 (83.3 to 88.5) | 78.8 (75.4 to 81.8) | 73.6 (64.7 to 80.9) | 51.5 (40.5 to 62.4) | 84.8 (81.7 to 87.5) | 75.3 (72.6 to 77.9) |
| 13.9 (11.5 to 16.7) | 21.2 (18.2 to 24.6) | 26.4 (19.1 to 35.3) | 48.5 (37.6 to 59.5) | 15.2 (12.5 to 18.3) | 24.7 (22.1 to 27.4) | |
| Diagnoses | ||||||
| Diabetes, %* | 4.6 (3.7 to 5.6) | 5.1 (3.5 to 7.3) | 3.7 (2.1 to 6.3) | 4.1 (2.4 to 6.7) | 4.5 (3.7 to 5.4) | 5.0 (3.5 to 7.0) |
| Hypertension, %* | 20 (17 to 23) | 27 (24 to 31) | 38 (31 to 40) | 55 (44 to 65) | 22 (19 to 25) | 31 (28 to 34) |
| Chronic kidney disease, %* | 3.2 (2.5 to 3.8) | 4.6 (2.2 to 7.0) | 13.1 (9.7 to 16.3) | 19.5 (14.0 to 25.0) | 4.2 (3.6 to 4.9) | 6.5 (4.4 to 8.6) |
| Physical examination data | ||||||
| Waist circumference, cm | 91.50 (90.48 to 92.52) | 103.04 (101.41 to 104.66) | 101.21 (99.09 to 103.32) | 111.95 (108.68 to 115.21) | 92.48 (91.43 to 93.54) | 104.09 (102.33 to 105.85) |
| Body mass index, kg/m2 | 26.02 (25.68 to 26.37) | 31.49 (30.79 to 32.19) | 29.41 (28.51 to 30.32) | 34.63 (32.11 to 37.14) | 26.37 (26.02 to 26.73) | 31.88 (31.06 to 32.71) |
| Systolic blood pressure, mm Hg | 117.34 (116.41 to 118.27) | 118.03 (116.37 to 119.68) | 122.47 (120.56 to 124.39) | 124.14 (121.87 to 126.40) | 117.86 (116.92 to 118.79) | 118.78 (117.17 to 120.38) |
| Diastolic blood pressure, mm Hg | 68.77 (67.33 to 70.22) | 68.05 (66.22 to 69.88) | 70.27 (67.45 to 73.10) | 69.73 (66.90 to 72.57) | 68.93 (67.38 to 70.47) | 68.26 (66.44 to 70.08) |
| Pulse pressure, mm Hg | 48.56 (46.98 to 50.15) | 49.97 (47.90 to 52.04) | 52.20 (49.41 to 54.99) | 54.40 (51.07 to 57.74) | 48.93 (47.27 to 50.59) | 50.52 (48.53 to 52.50) |
| Laboratory data | ||||||
| Total cholesterol, mg/dL | 194.54 (192.67 to 196.41) | 194.59 (189.45 to 199.72) | 202.00 (192.61 to 211.40) | 196.27 (186.29 to 206.25) | 195.30 (193.02 to 197.58) | 194.80 (190.25 to 199.35) |
| LDL cholesterol, mg/dL | 114.17 (112.57 to 115.76) | 114.31 (108.47 to 120.16) | 120.75 (108.97 to 132.53) | 125.51 (112.02 to 138.99) | 114.75 (112.68 to 116.81) | 115.76 (110.08 to 121.44) |
| HDL cholesterol, mg/dL | 57.65 (56.52 to 58.78) | 53.31 (51.87 to 54.75) | 49.51 (47.67 to 51.36) | 47.30 (44.25 to 50.36) | 56.82 (55.82 to 57.81) | 52.56 (51.06 to 54.05) |
| Triglycerides, mg/dL | 101.49 (96.95 to 106.04) | 106.48 (101.04 to 111.93) | 117.07 (105.96 to 128.19) | 112.76 (100.04 to 125.49) | 102.86 (98.98 to 106.73) | 107.29 (102.06 to 112.52) |
| Serum glucose, mg/dL | 91.74 (90.67 to 92.80) | 94.00 (92.15 to 95.85) | 94.34 (92.53 to 96.14) | 95.82 (92.36 to 99.28) | 92.01 (91.03 to 92.98) | 94.23 (92.62 to 95.84) |
| Creatinine clearance per CKD‐ EPI method, mL/min per 1.73 m2* | 97.31 (96.09 to 98.54) | 99.83 (97.83 to 101.83) | 87.43 (83.66 to 91.20) | 83.18 (77.37 to 88.99) | 96.30 (95.02 to 97.58) | 97.73 (95.74 to 99.72) |
Weighted means, proportions, and 95% CIs are provided unless otherwise specified. Elevated CRP status was determined by a serum CRP concentration ≥0.38 mg/dL that corresponded to the 75th percentile of distribution. CRP indicates C‐reactive protein.
Diabetes was defined as the use of diabetes medications and/or fasting serum glucose >126 mg/dL.
Hypertension was defined by ≥1 of the following criteria: systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 or use of medications to treat hypertension.
Chronic renal disease was defined as an estimated glomerular filtration rate <60 mL/min per 1.73 m2.
CKD‐EPI indicates the method described by Levey et al.(2009)
Choice of Cutoffs for Hyperuricemia and Elevated CRP
In ROC analyses within the analysis dataset, the definition of hyperuricemia in the present study (serum urate >7.0) correctly classified hypertension in 71% of observations with a positive likelihood ratio of 2.6 and a negative likelihood ratio of 0.9. The area under the ROC curve was 0.56. The definition that we used for elevated CRP classified hypertension correctly in 64% of the observations with positive and negative likelihood ratios of 1.3 and 0.89, respectively. The area under the ROC curve was 0.54.
When hyperuricemia was redefined as serum urate >7.0 mg/dL for men and >6.0 mg/dL for women, the ROC characteristics did not significantly improve. The sex‐specific definition correctly classified hypertension in 73% of observations, with positive and negative likelihood ratios of 2.89 and 0.80, respectively. The area under the curve was 0.59 (95% CI 0.58 to 0.61), which was less than that under the study definition that we used. Similarly, a single cutoff value of 3.0 mg/dL in our dataset was not superior to the distribution‐based measure that we used. The former correctly classified hypertension in 73% of the observations with positive and negative likelihoods of 2.89 and 0.80, respectively, and an area under the curve of 0.50 (95% CI 0.49 to 0.51).
Analysis of Association of Serum Urate and CRP
Table 2 shows the results of the linear regression analyses that suggest a statistically significant relationship between urate and CRP levels overall and in all subgroups except Hispanics, the “other ethnicities” category (that included Americans of Asian, Pacific Island, and Native American heritage) and among the lowest age tertile.
Table 2.
Estimated Change in Serum CRP With Each 1‐SD Increase in Serum Urate Among Those Without Metabolic Syndrome in NHANES 2009–2010
| Model | No. of Observations in the Regression Model | Proportion of Variance in Log‐Transformed CRP Levels in the Population Explained by the Model, %* | Estimated Change (%) in CRP Concentrations per Each 1‐SD (1.44 mg/dL) Increase in Serum Urate* |
|---|---|---|---|
| Unadjusted | 4372 | 1.6 | 19.4 (14.1 to 25.1) |
| Adjusted for age, race, and sex | 4372 | 8.5 | 39.6 (20.0 to 26.1) |
| Final adjusted model, overall (*) | 3947 | 29.0 | 13.4 (7.6 to 19.6) |
| Final adjusted model, subgroups (*) | |||
| Men | 1915 | 23.7 | 14.6 (7.6 to 22.0) |
| Women | 2032 | 32.9 | 17.0 (7.0 to 28.1) |
| Hispanics and other Latinos | 1041 | 28.1 | 5.3 (−1.7 to 8.4) |
| Whites | 1990 | 27.5 | 12.1 (3.6 to 21.3) |
| African Americans | 686 | 35.3 | 25.8 (15.5 to 37.0) |
| Other ethnicities | 230 | 31.4 | 10.3 (10.6 to 36.1) |
| Age, y | |||
| <36 | 1249 | 34.4 | 7.2 (−2.2 to 17.6) |
| 37 to 54 | 1365 | 32.4 | 17.4 (1.6 to 35.6) |
| >55 | 1333 | 19.8 | 15.2 (4.3 to 27.1) |
| Body mass index, kg/m2 | |||
| <25 | 1364 | 13.9 | 16.9 (−0.8 to 37.8) |
| 25 to 30 | 1396 | 14.8 | 14.9 (6.4 to 24.0) |
| >30 | 20.5 | 20.5 | 10.4 (0.9 to 20.7) |
CRP indicates C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
Model fit assessed by R2 statistic.
Assessed by survey weighted linear regression models where CRP values were log transformed first. Regression coefficients were then transformed back and interpreted accordingly.
Final model included serum urate, age, estimated glomerular filtration rate per CKD‐EPI creatinine equation, total cholesterol, poverty ratio, HDL cholesterol, and body mass index as continuous variables and sex, ethnicity, education level (less than high school, high school, greater than high school), and ever smoking as categorical variables.
Full models were fitted within each subgroup of interest. The stratum variable of interest was not included in such models.
Prevalence of Hypertension
Overall, the prevalence of hypertension in the group of participants without metabolic syndrome was 23.9% (95% CI 21.2% to 26.7%). The prevalence was similar between sexes (men: 24.6%, 95% CI 21.2% to 28.4%; women: 23.2%, 95% CI 20.6% to 26.0%). There were no significant ethnic differences in the prevalence between African Americans and whites (31.3%, 95% CI 25.9% to 37.4%, versus 25.2%, 95% CI 21.8% to 28.9%). Hispanics had the lowest prevalence at 12.7% (95% CI 10.4% to 15.4%).
Hyperuricemia and Hypertension
Hyperuricemia was a significant correlate of hypertension with an unadjusted odds ratio of 2.72 (95% CI 2.20 to 3.38) and an age‐, sex‐, and race‐adjusted odds ratio of 2.94 (95% CI 2.27 to 3.81). In the final model, the odds ratio was 2.21 (95% CI 1.71 to 2.85). Figure 2 shows the odds ratios for participants grouped by age, sex, and ethnicity.
Figure 2.
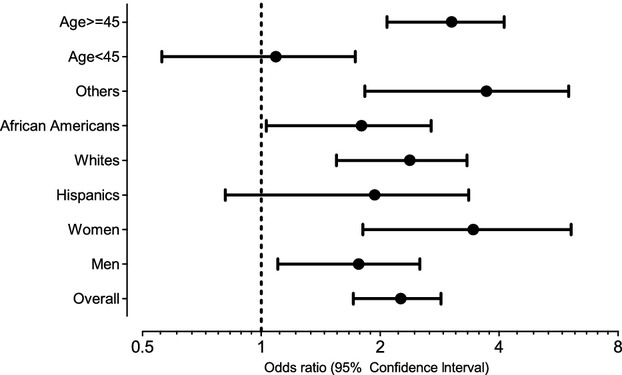
Hyperuricemia and the odds ratios for hypertension. Multivariable logistic regression models adjusting for age, estimated glomerular filtration rate per CKD‐EPI equation, total cholesterol, poverty ratio, HDL cholesterol and body mass index as continuous variables and sex, ethnicity, education level (less than high school, high school, greater than high school), and ever smoking as categorical variables.
Elevated CRP and Hypertension
Elevated CRP was associated with an unadjusted odds ratio of 1.74 (95% CI 1.29 to 2.40) and an age‐, sex‐, and ethnicity‐adjusted odds ratio of 1.60 (95% CI 1.05 to 2.44). In the final model, elevated CRP was not associated with prevalence of hypertension (odds ratio 0.99, 95% CI 0.34 to 1.54). When the multivariable analyses were repeated in separate OLS models where systolic and diastolic blood pressures were the dependent variables and log‐transformed CRP was the independent variable, no statistically significant associations were observed.
Hyperuricemia, Elevated CRP, and the Prevalence of Hypertension
OLS Models
We first examined OLS models where systolic, diastolic, and pulse pressures were dependent variables; serum urate and log‐transformed CRP as continuous variables were the independent variables of interest. Figure 3 shows the mean systolic and diastolic blood pressure measurements stratified by quintiles of serum urate and quartiles of log‐transformed CRP measures. In unadjusted OLS models where these were entered as continuous measures, both serum urate and log‐transformed CRP variables were statistically significant correlates of systolic blood pressure. Of the 2, only serum urate was correlated with diastolic pressure. In multivariable (final) models where urate and log‐transformed CRP were entered, systolic blood pressure was associated with serum urate levels, and diastolic pressure was not correlated with urate or CRP levels. The combined β coefficient of CRP and serum urate was 0.90 (95% CI 0.32 to 1.48) compared with the individual effect of CRP alone at 0.28 (95% CI −0.26 to 0.82). Such differences were not observed for diastolic blood pressure.
Figure 3.
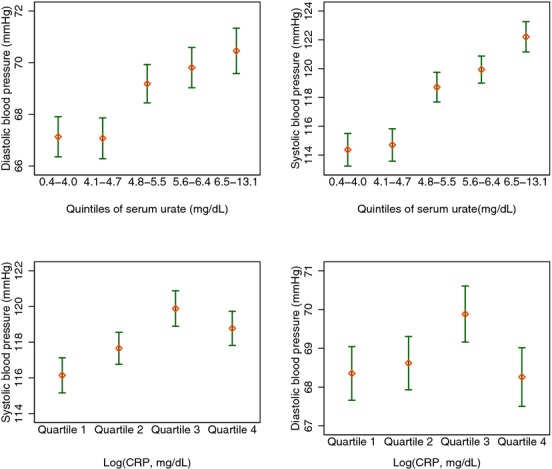
Bivariate associations between log‐CRP (quartiles), serum urate (quintiles) and blood pressure. Mean and 95% CIs were calculated using survey weights. CRP indicates C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
Logistic Regression Models
In separate multivariable logistic regression models, hyperuricemia was associated with hypertension with an odds ratio of 2.21 (95% CI 1.71 to 2.85) but elevated CRP was not (odds ratio 0.99, 95% CI 0.64 to 1.54). Results of logistic regression models where the statistical impacts of concurrent hyperuricemia and elevated CRP were assessed are given in Table 3. Notably, elevated CRP was associated with significantly higher prevalence of hypertension among those with hyperuricemia only.
Table 3.
Results of Logistic Regression Analyses for the Odds Ratios of Elevated Urate and CRP Concentrations on Hypertension
| No Hyperuricemia/Low CRP | No Hyperuricemia/High CRP | Hyperuricemia/Low CRP | Hyperuricemia/High CRP | |
|---|---|---|---|---|
| Odds Ratio | Odds Ratio (CI) | Odds Ratio (CI) | Odds Ratio (CI) | |
| Unadjusted | 1.00 (reference) | 1.48 (1.23 to 1.77) | 2.49 (1.93 to 3.22) | 4.79 (3.05 to 7.52) |
| Age, sex, and race adjusted | 1.00 (reference) | 1.63 (1.32 to 2.01) | 3.11 (2.31 to 4.19) | 3.71 (1.87 to 7.38) |
| Final model, overall* | 1.00 (reference) | 1.11 (0.83 to 1.50) | 2.33 (1.63 to 3.34) | 2.12 (1.05 to 4.25) |
| Final model, subgroups | ||||
| Sex and race | ||||
| Men | 1.00 (reference) | 0.86 (0.59 to 1.26) | 1.72 (1.03 to 2.87) | 1.37 (0.74 to 2.53) |
| Women | 1.00 (reference) | 1.53 (1.01 to 2.34) | 7.36 (1.64 to 33.03) | 7.98 (1.36 to 46.82) |
| Hispanics | 1.00 (reference) | 1.23 (0.87 to 1.74) | 1.96 (0.97 to 3.98) | 0.90 (0.30 to 2.70) |
| Non‐Hispanic white | 1.00 (reference) | 1.07 (0.72 to 1.59) | 2.40 (1.51 to 3.82) | 1.98 (0.77 to 5.12) |
| African Americans | 1.00 (reference) | 1.21 (0.80 to 1.83) | 1.62 (0.69 to 3.82) | 2.01 (1.00 to 4.06) |
| Others | 1.00 (reference) | 1.09 (0.23 to 5.22) | 3.59 (2.17 to 5.95) | 6.43 (1.16 to 35.54) |
| Tertiles of age, y | ||||
| <36 | 1.00 (reference) | 1.59 (0.69 to 3.69) | 0.47 (0.18 to 1.22) | N/A |
| 37 to 54 | 1.00 (reference) | 1.23 (0.77 to 1.99) | 2.84 (1.89 to 4.27) | 2.11 (0.51 to 8.81) |
| >55 | 1.00 (reference) | 1.03 (0.58 to 1.81) | 2.37 (1.16 to 4.83) | 2.67 (1.22 to 5.84) |
| Body mass index, kg/m2 | ||||
| <25 | 1.00 (reference) | 0.93 (0.17 to 5.00) | 3.12 (1.48 to 6.56) | 1.83 (0.85 to 3.93) |
| 25 to 30 | 1.00 (reference) | 0.90 (0.37 to 2.17) | 3.29 (1.16 to 6.73) | 24.0 (4.08 to 150.50)* |
| ≥30 | 1.00 (reference) | 0.95 (0.55 to 1.65) | 1.32 (0.66 to 2.65) | 4.62 (0.96 to 22.28) |
Elevated CRP status was determined by a serum CRP concentration ≥0.38 mg/dL that corresponded to the 75th percentile of distribution. N/A, odds ratios could not be computed as the observations in these categories predicted hypertension perfectly and were dropped by the regression. CRP indicates C‐reactive protein.
Final model included serum urate, age, estimated glomerular filtration rate per CKD‐EPI creatinine equation, total cholesterol, poverty ratio, HDL cholesterol, and body mass index as continuous variables and sex, ethnicity, education level (less than high school, high school, greater than high school), and ever smoking as categorical variables.
Imprecise estimates owing to large SE value and should be interpreted with caution.
Discussion
Using data from the broad adult population without gout and metabolic syndrome, we were able to draw 3 conclusions. First, we observed that CRP concentrations were higher among those with greater serum urate concentrations, independent of age, sex, ethnicity, measures of obesity, or other potential confounders. Prior studies have documented an independent relationship between hyperuricemia and hypertension among middle‐aged men.(2007) In the present study, hyperuricemia was significantly associated with higher prevalence of hypertension among men and women without any other components of the metabolic syndrome. Last, our analysis showed that elevated CRP (>0.7 mg/dL) is not associated with higher prevalence of hypertension independent of presence of concurrent hyperuricemia or other risk factors.
The link between hyperuricemia and inflammation has been examined in vitro studies, animal models, and human studies.(2005),(2011)–(2008) Urate micro crystals are known to be released from dying cells, triggering a danger signal that results in activation of the inflammasome and the interleukin‐1 pathway.(1998)–(2009) It has been suggested that the ensuing activation of arterial wall immune cells drives atherosclerosis and arteriosclerosis and is responsible for the elevated inflammation markers among those with hyperuricemia.(2005),(2009) In epidemiological settings, elevated serum urate temporally precedes elevation of interleukin‐1 and CRP (with expression driven by interleukin‐6), suggesting that interleukin‐6/CRP may be a mediator in the hyperuricemia–cardiovascular link.(2007) Treatment with anti‐CRP antibody can reverse the stimulant effect of urate on vascular cell proliferation, migration of vascular smooth muscle cells, and nitric oxide release in human umbilical vein cells, suggesting that CRP expression may be responsible for urate‐induced vascular remodeling.(2005) A definitive interventional study involving >17 000 participants that examines the impact of interleukin‐1 inhibition on cardiovascular outcomes is under way.(2011)
A recent meta‐analysis of epidemiological literature on hyperuricemia and hypertension provided the striking observation that all except 3 of the 9 studies of men and all studies of women suggest that hyperuricemia and hypertension links are independent of other risk factors.(2011) Some have argued that conventional statistical analyses cannot avoid residual confounding from prevalent metabolic syndrome.(2005) To address this, we analyzed data from 3073 middle‐aged men at high risk for cardiovascular events but not metabolic syndrome and reported a 80% increase in the risk for those with serum urate >7.0 mg/dL.(2007) In the present study, we extended our observations to a sample of men and women from the general population in the United States who were free of metabolic syndrome.
The putative role of CRP in the etiology, diagnosis, prevention, and treatment of hypertension is under investigation. Elevated serum levels of CRP and interleukin‐6 have been associated with incident hypertension.(2003),(2007)–(2003) Elevated CRP levels are associated with greater vascular stiffness, an early indicator of hypertension.(2011) Studies using a Mendelian randomization approach suggest that the observed link between CRP and hypertension is unlikely to be causal.(2005) Thus, whether CRP is a marker, mediator, or causal factor remains controversial.
Despite evidence for a link between urate and inflammation, few studies have assessed whether the presence of hyperuricemia modifies the association between CRP and hypertension. The present study raises the interesting possibility that the high‐oxidative stress that occurs during hyperuricemia may be a necessary precondition for the links between systemic inflammatory state (represented by elevated CRP) and hypertension. The limitations of this study were the cross‐sectional design and the possibility of residual confounding by covariates that were not available for analysis such as medication data (eg, statins, diuretics). Although the study population was free of metabolic syndrome, it was by no means healthy; only 38% were free of all components of metabolic syndrome. Thus, although the risk profile of our analysis group is better than that of patients with metabolic syndrome, our results should not be construed as applicable to a healthy population.
Perspectives
Hyperuricemia is common and easily detected and bears consistent association with hypertension regardless of the study setting. Semelweis (1818–1865) observed that the mortality from puerperal fever was lower in women attending a clinic run by midwives than it was in those attending the clinic run by physicians. Without any understanding of the specific bacteriology, he was able to drastically reduce mortality by instituting better a hand‐washing regimen in the clinics. Similarly, despite ongoing research efforts, causal relation, if any, may never be proved or disproved beyond doubt in the near future. The early studies suggesting therapeutic benefits of urate reduction may or may not ultimately lead to preventative use of allopurinol, a urate‐lowering medication.(2011)–(2008)
Disclosures
Dr Krishnan has served as a consultant to Takeda Pharmaceuticals, Inc, URL Pharmaceuticals Inc, Metabolex, Inc, and UCB Pharmaceuticals, Inc, and has received grant support from URL, ARDEA Biosciences, and Takeda. However, these entities did not sponsor this study or have access to the contents prior to publication. Dr Krishnan was responsible for all aspects of this manuscript from concept to finalizing the manuscript. He serves as the guarantor for this article. There are no patents, products in development, or marketed products to declare.
References
- Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005; 118:816-826 [DOI] [PubMed] [Google Scholar]
- Avram Z, Krishnan E. Hyperuricaemia—where nephrology meets rheumatology. Rheumatology (Oxford). 2008; 47:960-964 [DOI] [PubMed] [Google Scholar]
- Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken). 2010; 62:170-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta‐analysis. Arthritis Rheum. 2009; 61:885-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, Duranay M, Covic A, Johnson RJ. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011; 6:1887-1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008; 300:924-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentoukas E, Tsarouhas K, Tsitsimpikou C, Lazaros G, Deftereos S, Vavetsi S. The prognostic impact of allopurinol in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2010; 145:257-258 [DOI] [PubMed] [Google Scholar]
- Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009; 48:804-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassoulis G, Brophy JM, Richard H, Pilote L. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med. 2010; 170:1358-1364 [DOI] [PubMed] [Google Scholar]
- Krishnan E, Pandya BJ, Lingala B, Hariri A, Dabbous O. Hyperuricemia and untreated gout are poor prognostic markers among those with a recent acute myocardial infarction. Arthritis Res Ther. 2012; 14:R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford). 2010; 49:1229-1238 [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant‐ and radical‐caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981; 78:6858-6862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000; 148:131-139 [DOI] [PubMed] [Google Scholar]
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid‐induced C‐reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005; 16:3553-3562 [DOI] [PubMed] [Google Scholar]
- Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C‐reactive protein as a risk factor for coronary heart disease: a systematic review and meta‐analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009; 151:483-495 [DOI] [PubMed] [Google Scholar]
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005; 25:39-42 [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010; 375:132-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Lauretani F, Bandinelli S, Senin U, Ferrucci L. Uric acid and inflammatory markers. Eur Heart J. 2006; 27:1174-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah AR, Hasan HA, Raigangar VL. Analysis of the relationship of leptin, high‐sensitivity C‐reactive protein, adiponectin, insulin, and uric acid to metabolic syndrome in lean, overweight, and obese young females. Metab Syndr Relat Disord. 2009; 7:17-22 [DOI] [PubMed] [Google Scholar]
- Tsioufis C, Kyvelou S, Dimitriadis K, Syrseloudis D, Sideris S, Skiadas I, Katsi V, Stefanadi E, Lalos S, Mihas C, Poulakis M, Stefanadis C. The diverse associations of uric acid with low‐grade inflammation, adiponectin and arterial stiffness in never‐treated hypertensives. J Hum Hypertens. 2011; 25:554-559 [DOI] [PubMed] [Google Scholar]
- Li Q, Yang Z, Lu B, Wen J, Ye Z, Chen L, He M, Tao X, Zhang W, Huang Y, Zhang Z, Qu S, Hu R. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011; 10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C‐reactive protein and the risk of developing hypertension. JAMA. 2003; 290:2945-2951 [DOI] [PubMed] [Google Scholar]
- Bautista LE, Lopez‐Jaramillo P, Vera LM, Casas JP, Otero AP, Guaracao AI. Is C‐reactive protein an independent risk factor for essential hypertension? J Hypertens. 2001; 19:857-861 [DOI] [PubMed] [Google Scholar]
- Baker JF, Schumacher HR, Krishnan E. Serum uric acid level and risk for peripheral arterial disease: analysis of data from the multiple risk factor intervention trial. Angiology. 2007; 58:450-457 [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau: Social, Economic and Housing Statistics Division (September 13, 2011). “How the census bureau measures poverty” Retrieved January 25, 2012, from http://www.census.gov/hhes/www/poverty/about/overview/measure.html.
- Krishnan E, Kwoh C, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007; 49:298-303 [DOI] [PubMed] [Google Scholar]
- Meotti FC, Jameson GN, Turner R, Harwood DT, Stockwell S, Rees MD, Thomas SR, Kettle AJ. Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J Biol Chem. 2011; 286:12901-12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Caught red‐handed: uric acid is an agent of inflammation. J Clin Invest. 2010; 120:1809-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekic J, Jelic‐Ivanovic Z, Spasojevic‐Kalimanovska V, Memon L, Zeljkovic A, Bogavac‐Stanojevic N, Spasic S. High serum uric acid and low‐grade inflammation are associated with smaller LDL and HDL particles. Atherosclerosis. 2009; 203:236-242 [DOI] [PubMed] [Google Scholar]
- Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all‐cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008; 28:1186-1192 [DOI] [PubMed] [Google Scholar]
- Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole‐Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998; 19:1814-1822 [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003; 425:516-521 [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; 440:237-241 [DOI] [PubMed] [Google Scholar]
- Nozari Y, Geraiely B. Correlation between the serum levels of uric acid and HS‐CRP with the occurrence of early systolic failure of left ventricle following acute myocardial infarction. Acta Med Iran. 2011; 49:531-535 [PubMed] [Google Scholar]
- Tsai WC, Huang YY, Lin CC, Li WT, Lee CH, Chen JY, Chen JH. Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels. 2009; 24:371-375 [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Miller E, III, Maggio M, Najjar SS, Lauretani F, Bandinelli S, Senin U, Ferrucci L. Usefulness of uric acid to predict changes in C‐reactive protein and interleukin‐6 in 3‐year period in Italians aged 21 to 98 years. Am J Cardiol. 2007; 100:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin‐1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the canakinumab anti‐inflammatory thrombosis outcomes study (CANTOS). Am Heart J. 2011; 162:597-605 [DOI] [PubMed] [Google Scholar]
- Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken). 2011; 63:102-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG. Serum uric acid and risk of coronary heart disease. Curr Pharm Des. 2005; 11:4125-4132 [DOI] [PubMed] [Google Scholar]
- Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin‐6 and C‐reactive protein for the risk of developing hypertension in women. Hypertension. 2007; 49:304-310 [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C‐reactive protein, interleukin‐6, and TNF‐alpha) and essential hypertension. J Hum Hypertens. 2005; 19:149-154 [DOI] [PubMed] [Google Scholar]
- Boos CJ, Lip GY. Elevated high‐sensitive C‐reactive protein, large arterial stiffness and atherosclerosis: a relationship between inflammation and hypertension? J Hum Hypertens. 2005; 19:511-513 [DOI] [PubMed] [Google Scholar]
- Dauphinot V, Roche F, Kossovsky MP, Schott AM, Pichot V, Gaspoz JM, Gosse P, Barthelemy JC. C‐reactive protein implications in new‐onset hypertension in a healthy population initially aged 65 years: the Proof study. J Hypertens. 2009; 27:736-743 [DOI] [PubMed] [Google Scholar]
- Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C‐reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003; 16:429-433 [DOI] [PubMed] [Google Scholar]
- Kusche‐Vihrog K, Urbanova K, Blanque A, Wilhelmi M, Schillers H, Kliche K, Pavenstadt H, Brand E, Oberleithner H. C‐reactive protein makes human endothelium stiff and tight. Hypertension. 2011; 57:231-237 [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Lawlor DA, Harbord RM, Gaunt TR, Day IN, Palmer LJ, Hattersley AT, Ebrahim S, Lowe GD, Rumley A, Davey Smith G. C‐reactive protein and its role in metabolic syndrome: mendelian randomisation study. Lancet. 2005; 366:1954-1959 [DOI] [PubMed] [Google Scholar]


