Abstract
Background
Verapamil is traditionally applied prophylactically in transradial procedures to prevent radial artery spasm. However, verapamil may have side effects and is contraindicated in some clinical settings.
Methods and Results
During an investigator‐initiated, randomized, double‐blind trial, we evaluated the need for preventive verapamil administration. After vascular access was established, patients received either 5 mg verapamil (n=297) or placebo (n=294). We compared the rate of access site conversions as primary end point using a superiority margin of 5%. Occurrence of code breaks (composite of conversions and unplanned use of verapamil), overall verapamil use, procedural and fluoroscopic times, contrast volume, and subjective pain were investigated as secondary end points. The rate of access site conversions was not different in the 2 arms (placebo 1.7% versus verapamil 0.7%, P=0.28, difference 1.0%, 95% CI for the difference −1.1% to 3.3%). Proportion of code breaks was similar in the 2 groups (3.4% versus 1.3%, P=0.11), whereas overall verapamil use was markedly lower in the placebo arm (2.0% versus 100%, P<0.0001). Procedural time (median [IQR] 16.0 minutes [9.0 to 30.0 minutes] versus 17.0 minutes [10.0 to 31.0 minutes], P=0.37), fluoroscopic time (4.4 minutes [2.1 to 9.6 minutes] versus 4.8 minutes [2.4 to 10.7 minutes], P=0.28), contrast volume (72.5 mL [48.0 to 146.0 mL] versus 75.5 mL [47.0 to 156.5 mL], P=0.74), and pain score (P for trend=0.12) were comparable in the 2 groups.
Conclusions
The preventive use of verapamil may be unnecessary for transradial procedures. The omission of prophylactic verapamil may not only reduce the rate of potential complications related to the drug but also allow the safe extension of the transradial method to those with contraindications to verapamil.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01402427.
Keywords: coronary angiography, drug policy, percutaneous coronary intervention, transradial, vasodilator, verapamil
Introduction
Radial access has been proved to be a safe and effective technique for both diagnostic and therapeutic procedures. Advantages of the transradial approach (TRA) include less bleeding and vascular complications,1 increased patient comfort,2 and cost savings3 compared with the traditional transfemoral route. Moreover, transradial access seems to reduce the mortality of patients with ST‐segment elevation myocardial infarction who are undergoing primary percutaneous coronary intervention.4–6 Nevertheless, the TRA is sometimes technically challenging and requires special skill by the operator. This is largely due to smaller vessel diameter, anatomical variations (eg, tortuosity of radial, brachial, and subclavian arteries, loops, variations of the brachiocephalic trunk/ascending aorta), and spasm of the radial artery (RAS). According to the literature, the incidence of RAS varies between 2% and 34%, frequently resulting in transfemoral conversion.7–10 Therefore, intra‐arterial vasodilators, most frequently verapamil, are routinely used prophylactically,11 because it is generally believed that ad hoc administration is less effective.7 Indeed, most experts agree that the administration of prophylactic intra‐arterial vasodilators is mandatory.12–14 Yet, with the technological advances in recent years (thinner devices, hydrophilic coating) and increasing operator expertise, RAS is likely to be of less importance as vasodilator use shows wide geographic variations, suggesting that it may not be essential.11 On the other hand, verapamil is contraindicated in some clinical settings, and even in patients without known contraindications, adverse reactions may occur.15–17
Methods
Study Design, Population, and Outcome Measures
Our goal was to evaluate the need for prophylactic application of verapamil in transradial coronary procedures. Therefore, we conducted an investigator‐initiated, randomized, double‐blind, parallel‐group, placebo‐controlled trial with an all‐comers design. Eligible were all patients aged ≥18 years with clinical indication for coronary angiography or percutaneous coronary intervention, in whom the right or left radial artery could be successfully cannulated (multiple puncture attempts were allowed). Only patients with known contraindications to verapamil were excluded (significant aortic stenosis, heart rate <50/min, high‐grade atrioventricular block, myocardial infarction complicated with cardiogenic shock, or left ventricular ejection fraction <35%).
Figure 1 shows an outline of the trial design. After insertion of the arterial sheath, patients received either 5 mg verapamil hydrochloride diluted with 0.9% w/v saline to 10 mL (n=297) or placebo (10 mL 0.9% w/v saline, the diluent of verapamil in the vials, alone, n=294) intra‐arterially. In the case of procedural failure, operators determined the underlying cause (spasm or anatomical variation), then they were unblinded and, at their discretion, continued either “unplanned” intra‐arterial administration of 5 mg verapamil (first 5‐mg dose in the placebo arm [“ad hoc”] or a second 5‐mg dose in the verapamil arm [“extra”]) or with primary access site conversion (ie, without prior unplanned use of verapamil). If verapamil was ineffective, the access site was changed (secondary conversion). Arterial spasm was defined by the operator as severe difficulty advancing the guidewire or catheter, with or without (1 case) angiographic evidence, while anatomical variations were all confirmed with angiography. Nonprotocol medications (eg, other vasodilators, analgesics, sedatives) were not administrated during the procedures.
Figure 1.
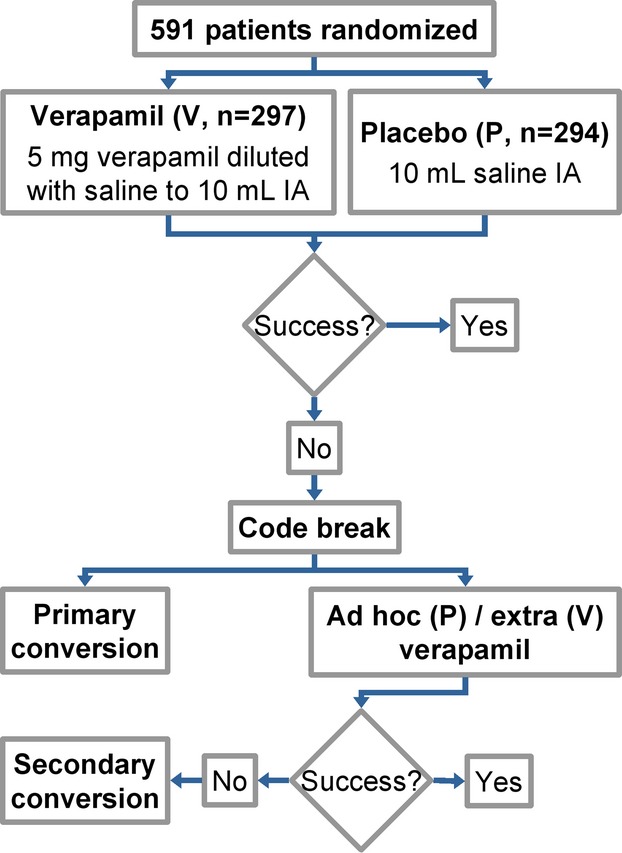
Study algorithm. A total of 591 patients were randomized to receive either 5 mg verapamil diluted with 0.9% w/v saline or placebo (0.9% w/v saline alone) intra‐arterially. For details, see text. IA indicates intra‐arterial; P, placebo arm; V, verapamil arm.
We compared the occurrence of overall access site conversions (primary and secondary, transfemoral or contralateral transradial) as primary end point, whereas rates of RAS defined by the operator, code breaking (composite of access site crossovers and unplanned use of verapamil), overall verapamil use, procedural and fluoroscopic times, radiocontrast volume, and subjective pain of the patients were investigated as secondary end points. Pain sensation was assessed using a questionnaire with a semiquantitative scale (1=no pain to 6=unbearable pain) in the catheterization laboratory, immediately after the procedure. In addition to comparison of the pain score distributions using the Cochran–Armitage test, we analyzed the rates of “significant pain” in the 2 groups by using Fisher's exact test. Significant pain was defined as pain score ≥4 on the 1‐to‐6 scale. Furthermore, we analyzed the underlying cause of code breaks (RAS or anatomical variation) and evaluated the efficacy of prophylactic verapamil on the incidence of RAS. We also studied the efficacy of ad hoc administered verapamil on manifest RAS and analyzed the rates of access site crossovers due to RAS. The study protocol was approved by the Institutional Ethics Committee on Human Research and was conducted according to the principles of the Declaration of Helsinki. The study is registered at ClinicalTrials.gov, number NCT01402427.
Transradial Procedure
The skin was infiltrated using 1% procaine hydrochloride (Teva). After insertion of the introducer with hydrophilic coating (Radifocus Introducer II Transradial Kit; needle 20 gauge, sheath 4 to 7 French, 10 cm; Terumo Europe), heparin sodium (70 IU/kg bolus for diagnostics and percutaneous coronary intervention [PCI] with planned use of a glycoprotein IIb/IIIa receptor inhibitor or 100 IU/kg bolus for elective PCI; Merckle), and the study medication (5 mg verapamil hydrochloride; Sanofi‐Aventis Chinoin, or 10 mL 0.9% w/v sodium chloride; Teva) were given intra‐arterially. Transradial coronary angiography and PCI were then performed according to the study protocol using standard techniques. The arterial sheath was removed immediately after the procedure and bleeding was stopped using a compression device (TR Band; Terumo Europe) for 6 to 8 hours.
Statistical Analysis
To our knowledge, there are no published data about the treatment effect of verapamil on access site conversions. In the Survenue Per Angiographie d'un Spasme Majeur (SPASM) study, Varenne et al detected a 14.3–percentage point absolute risk reduction (ARR, 22.2% versus 7.9%) in the occurrence of RAS between the placebo and 5‐mg verapamil groups.18 In a trial comparing a cocktail (5 mg verapamil and 200 μg nitroglycerin) versus placebo, Kiemeneij et al found a 20–percentage point difference (14% versus 34%) in incidence of severe pain during sheath removal and a 14–percentage point change (8% versus 22%) in the rate of maximal pullback force indicating significant RAS.9 Based on these data, we considered a 5–percentage point treatment effect as evidence of clinical superiority. Assuming a conversion rate of 2.0% in the verapamil group based on our registry data, the enrollment of 572 patients (286 in each arm) would have provided the study with a statistical power of 80.1% to detect a 5.0% difference in the rate of access site conversions at a 2‐sided α level of 0.05 (computed with Fisher's exact test). The final sample size was 591 patients: 294 in the placebo arm and 297 in the verapamil group. For the primary end point, the groups were compared using the 2‐sided CI approach. Categorical variables in 2×2 contingency tables were analyzed using Fisher's exact test, while the Cochran–Armitage test was used to detect trend in data from 2×k contingency tables. After examination of the distribution of continuous variables for normality with use of the D'Agostino–Pearson test, normally distributed variables were compared with use of the Student t‐test for independent samples, whereas not normally distributed parameters were assessed using the Mann–Whitney test. A 2‐tailed P value <0.05 was considered statistically significant. Because of the design and short time‐frame of the study, no patient was lost for follow up, the intention‐to‐treat and per‐protocol populations were identical, and both analyses yielded the same results. Sample size and statistical power calculations were performed using IBM SPSS SamplePower 3.0.1 software (IBM Corporation); all other analyses were carried out with MedCalc 12.7.7.0 (MedCalc Software).
Results
Patient Characteristics
A total of 591 patients were randomized; all of them completed the protocol and were included in the analyses. Baseline characteristics of the populations and procedures and operator's annual volumes are summarized in Tables 1 and 2. The demographic, clinical, and procedural parameters of the patients were similar.
Table 1.
Baseline Demographic and Clinical Characteristics
| Variable | Placebo (n=294) | Verapamil (n=297) |
|---|---|---|
| Age, mean±SD y | 62.5±10.8 | 61.8±10.5 |
| Female, % | 35.4 | 37.4 |
| BMI, median (IQR) kg/m2 | 27.9 (25.2 to 31.3) | 28.7 (25.5 to 32.1) |
| Hypertension, % | 84.0 | 79.5 |
| Diabetes mellitus, % | 25.9 | 31.6 |
| Verified dyslipidemia, % | 38.4 | 38.4 |
| Current smoker, % | 32.6 | 31.2 |
| PAD, % | 5.4 | 8.8 |
| CVD, % | 13.6 | 11.8 |
| CHF, % | 8.5 | 5.1 |
| CRF, % | 2.0 | 2.7 |
| Previous MI, % | 22.4 | 29.3 |
| Previous PCI, % | 25.2 | 25.6 |
| Previous CABG, % | 4.4 | 6.1 |
| Concomitant CCB use, % | 19.4 | 26.3 |
| Concomitant BB use, % | 68.4 | 71.7 |
| Concomitant CCB+BB use, % | 14.3 | 19.9 |
| Concomitant NG use, % | 32.0 | 32.0 |
| Concomitant ACEI use, % | 54.1 | 52.2 |
| Concomitant ARB use, % | 21.8 | 23.6 |
BMI indicates body mass index; PAD, peripheral artery disease; CVD, cerebrovascular disease; CHF, congestive heart failure; CRF, chronic renal failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; CCB, calcium channel blocker; BB, β‐blocker; NG, nitroglycerin; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 2.
Procedural Characteristics and Operators' Annual Volume
| Variable | Placebo: Total (n=294) Dx (n=187) PCI (n=107) | Verapamil: Total (n=297) Dx (n=191) PCI (n=106) |
|---|---|---|
| Right radial artery, % | 93.5 | 95.3 |
| ACS rate, % | 31.6 | 32.7 |
| PCI rate, % | 36.4 | 35.7 |
| Primary PCI rate, % | 8.5 | 9.1 |
| FFR rate, % | 3.1 | 4.7 |
| No. of arterial sheaths, % | ||
| 1 | 99.7 | 99.0 |
| 2 | 0.3 | 1.0 |
| Arterial sheath size, % | ||
| 5 French | 0.3 | 1.0 |
| 6 French | 99.7 | 98.7 |
| 7 French | 0.0 | 0.3 |
| Sheath upgrade for PCI, % | 0.7 | 0.7 |
| 6 French to 7 French, % | 0.0 | 0.3 |
| 6 French to 7.5 French sheathless, % | 0.7 | 0.3 |
| Total number of catheters (Dx and PCI)/No. of catheter exchanges | ||
| 1 (%)/0 (%) | 24.2 | 23.0 |
| 2 (%)/1 (%) | 49.8 | 42.9 |
| 3 (%)/2 (%) | 18.0 | 25.3 |
| ≥4 (%)/≥3 (%) | 8.0 | 8.8 |
| No. of catheters during Dx, % | ||
| 1 | 22.7 | 24.1 |
| 2 | 67.0 | 58.1 |
| 3 | 7.6 | 13.1 |
| ≥4 | 2.7 | 4.7 |
| No. of catheters during PCI, % | ||
| 1 | 26.9 | 21.0 |
| 2 | 19.2 | 15.2 |
| 3 | 36.5 | 47.6 |
| ≥4 | 17.3 | 16.2 |
| No. of diseased vessels (%, PCI) | ||
| 1 | 43.0 | 46.2 |
| 2 | 32.7 | 33.0 |
| 3 and/or LM | 24.3 | 20.8 |
| No. of dilated vessels (%, PCI) | ||
| 1 | 82.2 | 85.8 |
| 2 | 14.0 | 11.3 |
| 3 and/or LM | 3.7 | 2.8 |
| Vessel dilated, % | ||
| LAD | 42.3 | 48.4 |
| LCX | 26.2 | 19.4 |
| RCA | 27.7 | 26.6 |
| D, IM | 2.3 | 3.2 |
| LM | 1.5 | 1.6 |
| Bypass graft | 0.0 | 0.8 |
| Lesion characteristics (%, PCI) | ||
| A | 6.2 | 3.2 |
| B | 38.5 | 40.3 |
| C | 55.4 | 56.5 |
| Thrombus aspiration (%, PCI) | 19.6 | 11.3 |
| Other adjunctive devices during PCI | Not applicable | Not applicable |
| Operator's annual volume | ||
| Total number of procedures, median (IQR) | 467 (467 to 633) | 467 (467 to 572) |
| Percent radial procedure, median (IQR) | 92.9 (84.2 to 96.6) | 92.9 (84.2 to 96.6) |
| PCI per year, median (IQR) | 210 (210 to 262) | 210 (210 to 262) |
| Percent radial PCI, median (IQR) | 94.9 (79.8 to 95.2) | 94.9 (80.8 to 95.2) |
Dx indicates diagnostic procedures (including fractional flow reserve [FFR] estimation without percutaneous coronary intervention [PCI]); ACS, acute coronary syndrome; LM, left main artery; LAD, left anterior descending artery artery; LCX, left circumflex artery; RCA, right coronary artery; D, diagonal branch; IM, intermediate artery.
Primary End Point
There was no significant difference in the crossover rate between the 2 groups (Fisher's exact test, placebo 5/294=1.7% versus verapamil 2/297=0.7%, P=0.28). Because the 2‐sided 95% CI for the difference in proportion of conversions overlaps zero and does not cross the prespecified superiority margin (Figure 2), the strategy of preventive verapamil use may not be considered to be superior to the policy of ad hoc administration. The fact that the observed conversion rate in the verapamil group was lower than expected, along with the final sample size, allowed the study to detect a 5.0–percentage point difference in the primary end point with 96.3% statistical power at a 2‐sided α level of 0.05. Conversely, the trial had 80.0% power to verify superiority of even a 3.4–percentage point change at a 2‐sided α=0.05 (computed using Fisher's exact test).
Figure 2.
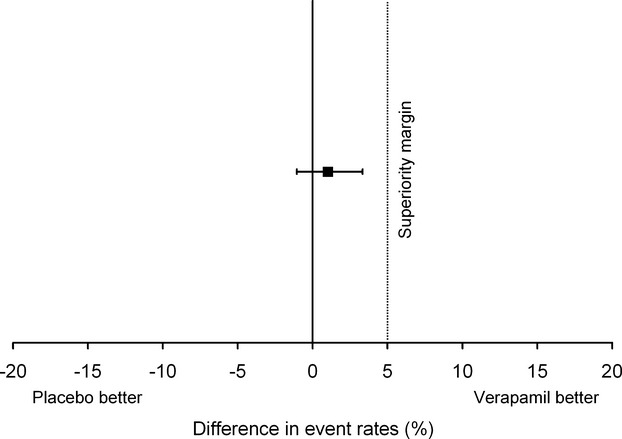
Primary study end point. There was no significant increase in the conversion rate of the placebo arm (Fisher's exact test, placebo 5/294=1.7% vs verapamil 2/297=0.7%, P=0.28). Superiority margin for the primary end point was set as low as 5.0 percentage points. Since the 95% CI for the difference in effect of the 2 regimens overlaps zero not crossing the superiority margin, the strategy of preventive verapamil use may not be considered superior to the policy of ad hoc administration (difference in conversion rates 1.0%, 95% CI −1.1% to 3.3%).
Secondary End Points
The results are summarized in Table 3.
Table 3.
Secondary End Points
| Placebo: Total (n=294) Dx (n=187) PCI (n=107) | Verapamil: Total (n=297) Dx (n=191) PCI (n=106) | P Value | |
|---|---|---|---|
| RAS, % | 1.7 | 1.0 | 0.50 |
| Crossover due to RAS, % | 0.0 | 0.3 | 1.00 |
| Rate of code breaks, % | 3.4 | 1.3 | 0.11 |
| Overall verapamil use, % | 2.0 | 100 | <0.0001 |
| Procedural time (min), median (IQR) | 16.0 (9.0 to 30.0) | 17.0 (10.0 to 31.0) | 0.37 |
| Dx | 10.0 (8.0 to 15.8) | 11.0 (8.0 to 17.0) | 0.16 |
| PCI | 34.0 (25.0 to 50.8) | 36.0 (24.0 to 51.0) | 0.67 |
| Fluoroscopic time (min), median (IQR) | 4.4 (2.1 to 9.6) | 4.8 (2.4 to 10.7) | 0.28 |
| Dx | 2.5 (1.7 to 4.6) | 3.1 (1.8 to 4.8) | 0.20 |
| PCI | 11.4 (7.3 to 17.9) | 13.1 (7.4 to 18.4) | 0.48 |
| Contrast volume (mL), median (IQR) | 72.5 (48.0 to 146.0) | 75.0 (47.0 to 156.5) | 0.74 |
| Dx | 53.0 (41.0 to 70.8) | 53.0 (41.0 to 73.8) | 0.89 |
| PCI | 182.0 (117.0 to 252.3) | 179.5 (133.0 to 265.0) | 0.58 |
| Subjective pain (arbitrary, 1 to 6), % | 0.12 | ||
| 1 | 12.6 | 14.8 | |
| 2 | 54.8 | 56.2 | |
| 3 | 23.8 | 21.9 | |
| 4 | 5.1 | 5.7 | |
| 5 | 2.7 | 1.3 | |
| 6 | 1.0 | 0.0 | |
| Dx | 0.19 | ||
| 1 | 12.8 | 15.7 | |
| 2 | 55.1 | 53.4 | |
| 3 | 23.5 | 24.6 | |
| 4 | 3.2 | 5.2 | |
| 5 | 3.7 | 1.0 | |
| 6 | 1.6 | 0.0 | |
| PCI | 0.42 | ||
| 1 | 12.1 | 13.2 | |
| 2 | 54.2 | 61.3 | |
| 3 | 24.3 | 17.0 | |
| 4 | 8.4 | 6.6 | |
| 5 | 0.9 | 1.9 | |
| 6 | 0.0 | 0.0 | |
| Significant pain (arbitrary, ≥4), % | 8.8 | 7.1 | 0.45 |
| Dx | 8.6 | 6.3 | 0.44 |
| PCI | 9.3 | 8.5 | 1.00 |
Dx indicates diagnostic procedures (including fractional flow reserve [FFR] estimation without percutaneous coronary intervention [PCI]); RAS, radial artery spasm.
Rate of RAS
Prophylactically used verapamil did not prevent the occurrence of RAS (placebo 5/294=1.7% versus verapamil 3/297=1.0%, P=0.50, Fisher's exact test), and the rate of spasm was low in both arms.
Rate of Code Breaks
The composite end point of conversions and unplanned use of verapamil did not differ significantly in the 2 groups (placebo 10/294=3.4% versus verapamil 4/297=1.3%, P=0.11, Fisher's exact test).
Overall Verapamil Use
The overall rate of verapamil use was markedly lower in the placebo group (6/294=2.0% versus 297/297=100%, P<0.0001, Fisher's exact test), showing that the observed low conversion rate was not at the cost of frequent ad hoc use of verapamil.
Safety End Points
There was no difference regarding procedural time between the placebo and verapamil groups (Mann–Whitney test, median [IQR]: placebo 16.0 minutes [9.0 to 30.0 minutes] versus verapamil 17.0 minutes [10.0 to 31.0 minutes], P=0.37). Fluoroscopic times did not differ considerably in the 2 arms (Mann–Whitney test, median [IQR]: placebo 4.4 minutes [2.1 to 9.6 minutes] versus verapamil 4.8 minutes [2.4 to 10.7 minutes], P=0.28). The volume of injected contrast media was similar in both groups (Mann–Whitney test, median [IQR]: placebo 72.5 mL [48.0 to 146.0 mL] versus verapamil 75.0 mL [47.0 to 156.5 mL], P=0.74). Subjective pain of the patients was assessed using a questionnaire with an arbitrary scale (1=no pain to 6=unbearable pain) in the catheterization laboratory, immediately after the procedure. Pain score was similarly distributed in the 2 arms (Cochran–Armitage test, P for trend=0.12, Figure 3). Likewise, there was no considerable difference in the rates of “significant pain” defined as pain score ≥4 on the 1‐to‐6 scale (Fisher's exact test, placebo 8.8% versus verapamil 7.1%, P=0.45, Table 3). Analysis of patients undergoing diagnostics or PCI showed no significant differences between the placebo and verapamil groups regarding any of the studied safety end points (Table 3).
Figure 3.
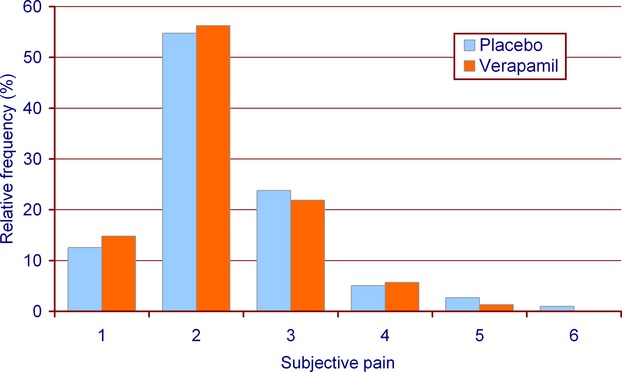
Subjective pain. Pain score measured on a semiquantitative scale was equally distributed in the 2 arms (Cochran‐Armitage test, P for trend=0.12). The majority of the patients had no or minimal pain in both groups.
Analysis of Code Breaks, Efficacy of Ad Hoc Administered Verapamil, and Access Site Crossovers Due to RAS
Half of the 10 events that comprised the composite end point of conversions and unplanned verapamil use in the placebo group were caused by RAS and the other half by anatomical variations. In the verapamil arm, RAS accounted for 3 composite outcomes and anatomical variations accounted for 1 (Figure 4). Although code breaks were infrequent in both arms, there was no statistical difference between the 2 groups regarding the underlying cause (P=0.58, Fisher's exact test).
Figure 4.
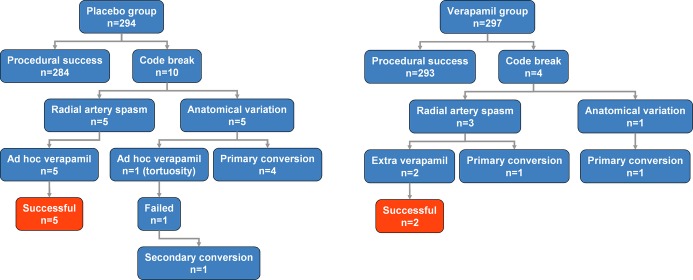
Analysis of code breaks and efficacy of ad hoc used verapamil. Ad hoc used verapamil was effective in all cases of radial artery spasm, the conversions were due to anatomical variations.
Ad hoc administration of verapamil was successful in 5 of 5 cases of RAS in the placebo group; all 5 crossovers were due to anatomical variations (Figure 4). Although prophylactic application did not prevent the occurrence of spasm (Table 3) in the verapamil arm, a repeated 5‐mg dose was effective in 2 of the 3 patients with manifest spasm despite preventive verapamil use. In 1 case, however, the operator did not believe a repeated 5‐mg dose would be clinically appropriate and decided primary conversion was preferable. Thus, rates of access site crossovers due to RAS were similar (placebo 0/294=0.0% versus verapamil 1/297=0.3%, P=1.00, Fisher's exact test). Figure 5 shows the treatment effect of verapamil for 3 of the studied end points: access site conversion, RAS, and access site conversion due to RAS. All 2‐sided 95% CIs for the differences overlap zero and do not cross the prespecified superiority margin, suggesting that the policy of prophylactic verapamil use may not be superior to the strategy of ad hoc administration.
Figure 5.
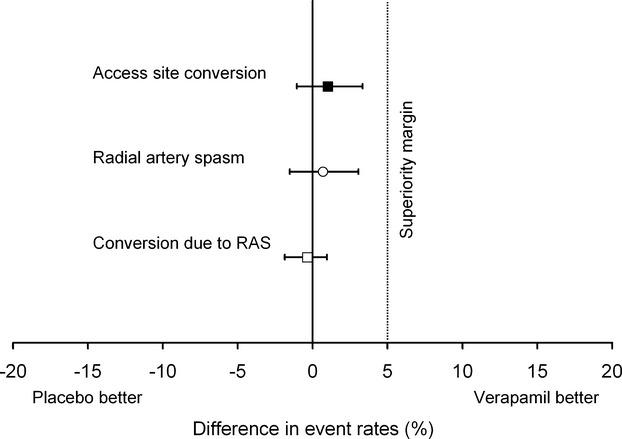
Treatment effect of verapamil for access site conversion, radial artery spasm, and access site conversion due to radial artery spasm. All 2‐sided 95% CIs for the differences in event rates overlap zero and do not cross the prespecified superiority margin of 5%, suggesting that the policy of prophylactic verapamil application may not be superior to the strategy of ad hoc administration (difference in access site conversion rates: 1.0%, 95% CI −1.1% to 3.3%; difference in rate of radial artery spasm [RAS]: 0.7%, 95% CI −1.5% to 3.1%; difference in occurrence of access site crossover due to RAS: −0.3%, 95% CI −1.9% to 1.0%).
Discussion
Although with the development of thinner catheters, use of hydrophilic coating,19–20 and increasing operator expertise the importance of RAS is likely to be reduced, most experts agree that prophylactic administration of intra‐arterial vasodilators is still mandatory.12–14 Indeed, according to a recent survey across 75 countries, the majority (85.9%) of operators use vasodilators prophylactically, most frequently verapamil (75.3%), alone or in combination with other agents.11 On the other hand, 72.2% of the Japanese operators do not use any medication for spasm prophylaxis. This geographic disparity suggests that the preventive use of vasodilators may not be crucial. Lacking a uniform definition, incidence of RAS varies considerably in the literature. Although the angiographic evidence used by Fukuda et al was objective, a number of patients diagnosed with RAS were clinically asymptomatic.21 Kiemeneij et al used an automatic pullback device for removal of sheaths and to establish a parameter (maximal pullback force) to quantify RAS.22 Although the authors found a good correlation with clinical symptoms, RAS could be detected only postprocedurally, at the time of sheath removal. Clinically relevant RAS is characterized by subjective pain of the patient and severe difficulty/inability of guidewire/catheter manipulation, frequently resulting in access site conversion.18,23–24 Even if there are data demonstrating that verapamil is effective in reducing the incidence of RAS9,18 and increases the volume of the radial artery detected with intravascular ultrasound,25 to our knowledge, there are no reports about its efficacy on the clinically more important (and objective) end point: the rate of access site conversions. On the other hand, the safety of intra‐arterial use of verapamil is not fully known. Although data from the SPASM trial18 show that intra‐arterial application of vasodilators is safe, recent data from the active‐controlled SPasmolytic Agents to avoid SpasM during transradial percutaneous coronary interventions (SPASM3) trial17 suggest that the administration of any of the tested vasodilators may be accompanied by adverse events, even if, lacking a placebo arm, a causal inference may not be justified. In view of potential side effects, the Transradial Committee of the Society for Cardiovascular Angiography and Interventions suggests that “femoral access may need to be favored in hemodynamically unstable patients, who may not tolerate the spasmolytic cocktail used in TRA.”26 Patients with these conditions are excluded from the benefits of the TRA.
Because we aimed to evaluate the need for the prophylactic application of verapamil, the most commonly used vasodilator, we chose a relatively high (ie, likely effective) dose of 5 mg in order to avoid type II error due to inadequate dose selection. In our study, we not merely assessed the occurrence of RAS but also investigated clinically more important variables related to RAS: the objective rate of access site crossovers as primary end point and subjective pain of the patients along with procedural parameters. We found that the strategy of preventive high‐dose verapamil use is not superior to the policy of provisional application of verapamil (Figure 2). Moreover, the need for ad hoc verapamil in the placebo arm was as negligible as 2.0% (Table 3). Also, there were no differences observed between the groups regarding any of the investigated safety end points (Table 3).
Because our study was not primarily designed to evaluate the efficacy of ad hoc administered verapamil on RAS, there were only 5 such cases during the trial. Furthermore, the assessment might be influenced by the fact that the determination of the underlying cause of code breaks was done by the operator, not by an independent observer. However, it is not likely to cause any major bias in the rates of RAS and anatomical variations, because at the time of adjudication the investigator was blinded to the treatment. Keeping these limitations in mind, it is of interest that, in contrast to previous findings suggesting that prevention is likely to be more effective than treatment of a manifest spasm,7 ad hoc used verapamil was effective in 5 of the 5 cases of RAS, and the conversions were all due to anatomical causes (Figure 4).
The rate of RAS (and the more objective rate of access site conversions) was found to be low in both groups (Table 3). This may be partly explained by the fact that the operators were all experienced in radial procedures, beyond the learning curve (for details, see Table 2). In addition, we used exclusively arterial sheaths with hydrophilic coating, which could contribute to the observed low event rate.20 The similarly rare occurrence of RAS in the 2 groups also indicates that mechanisms such as heavy manipulation and multiple catheter exchanges, which more frequently occur during the learning curve, may play a role in the provocation of RAS. Therefore, despite our findings, the preventive use of spasmolytic drugs may be useful during the learning curve.
In our trial, we tested placebo against a relatively high dose of the vasodilator, verapamil, most frequently used worldwide. The second most frequently (63.7%) used substance, nitroglycerin,11 has a somewhat more favorable side effect profile but, like verapamil, is still contraindicated in patients with aortic stenosis or severe hypotension/cardiogenic shock. The choice of the vasodilator and its side effect profile might have minimal impact on our results, because we showed that, after the learning curve, not only is RAS a rare event but also there are no clinically relevant differences between the placebo and the likely effective high‐dose verapamil groups regarding any of the investigated end points. Nevertheless, we acknowledge that the application of substances with a more favorable side effect profile is preferable. Based on our results, we believe that the strategy of applying any vasodilator only to patients with manifest RAS (ie, 1.7% of the population, according to our data) in the lowest effective dose may not only contribute to the reduction of adverse reactions (regardless of their severity and incidence) but also allow the safe extension of the TRA to those with contraindications to vasodilators.
The “all‐comers” fashion of the trial is represented by the high proportion of patients with acute coronary syndromes and PCIs (Table 2). It is noteworthy that a high percentage of patients undergoing coronary angiography were receiving concomitant β‐blocker therapy (≈70% in both arms, see Table 1). While this may theoretically cause a proclivity toward RAS, it also means that this population may be more prone to develop bradycardia/hypotension when receiving intra‐arterial verapamil.
Limitations
The trial was conducted in a single, high‐volume institution by experienced operators (Table 2). Our results may not be applicable to lower‐volume centers or to operators with less experience. Multicenter studies are warranted to confirm the findings of the present study. We did not assess the possibility that omission of verapamil may have an effect on the rate of radial artery occlusion. Furthermore, being primarily an efficacy study, it did not address potential adverse effects related to intra‐arterial application of verapamil. Lacking previous data on the treatment effect of verapamil on access site conversion, the choice of the superiority margin was based on clinical judgment.
Conclusions
Our data indicate that beyond the learning curve preventive administration of intra‐arterial verapamil offers no advantage over ad hoc application in terms of access site conversion rates. Therefore, the prophylactic application of verapamil may not necessarily be required among high‐volume transradial operators. This finding may be clinically relevant, particularly considering the worldwide growing number of transradial procedures and increasing expertise, since the omission of prophylactic verapamil (or vasodilators in general) may not only contribute to the reduction of potential adverse reactions related to the drug but also allow the safe extension of the, in many respects favorable, TRA to those with contraindications to vasodilators.
Acknowledgment
The excellent technical assistance of Katalin Antal, Monika Basti, Melinda Fazakas, Tamasne Huszar, Olga Kiss, Attilane Rupp, and Olga Szaniszlo is gratefully acknowledged.
Disclosures
None.
References
- 1.Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997; 29:1269-1275 [DOI] [PubMed] [Google Scholar]
- 2.Cooper CJ, El‐Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999; 138:430-436 [DOI] [PubMed] [Google Scholar]
- 3.Roussanov O, Wilson SJ, Henley K, Estacio G, Hill J, Dogan B, Henley WF, Jarmukli N. Cost‐effectiveness of the radial versus femoral artery approach to diagnostic cardiac catheterization. J Invasive Cardiol. 2007; 19:349-353 [PubMed] [Google Scholar]
- 4.Hizoh I, Szabo G, Kecskes A, Kerecsen G, Kiss N, Korda A, Major L, Markus R, Kiss RG. Factors predicting outcome of primary percutaneous coronary intervention: role of transradial access. Eur Heart J. 2010; 31suppl 1:193-194 [Google Scholar]
- 5.Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, Summaria F, Patrizi R, Borghi A, Di Russo C, Moretti C, Agostoni P, Loschiavo P, Lioy E, Sheiban I, Sangiorgi G. Radial versus femoral randomized investigation in ST‐segment elevation acute coronary syndrome. J Am Coll Cardiol. 2012; 60:2481-2489 [DOI] [PubMed] [Google Scholar]
- 6.Mehta SR, Jolly SS, Cairns J, Niemela K, Rao SV, Cheema AN, Steg PG, Cantor WJ, Dzavik V, Budaj A, Rokoss M, Valentin V, Gao P, Yusuf S, Investigators R. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST‐segment elevation. J Am Coll Cardiol. 2012; 60:2490-2499 [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SL, Renslo R, Sinow R, French WJ. Learning curve in the use of the radial artery as vascular access in the performance of percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn. 1998; 44:147-152 [DOI] [PubMed] [Google Scholar]
- 8.Hildick‐Smith DJ, Lowe MD, Walsh JT, Ludman PF, Stephens NG, Schofield PM, Stone DL, Shapiro LM, Petch MC. Coronary angiography from the radial artery—experience, complications and limitations. Int J Cardiol. 1998; 64:231-239 [DOI] [PubMed] [Google Scholar]
- 9.Kiemeneij F, Vajifdar BU, Eccleshall SC, Laarman G, Slagboom T, van der Wieken R. Evaluation of a spasmolytic cocktail to prevent radial artery spasm during coronary procedures. Catheter Cardiovasc Interv. 2003; 58:281-284 [DOI] [PubMed] [Google Scholar]
- 10.Spaulding C, Lefevre T, Funck F, Thebault B, Chauveau M, Ben Hamda K, Chalet Y, Monsegu J, Tsocanakis O, Py A, Guillard N, Weber S. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996; 39:365-370 [DOI] [PubMed] [Google Scholar]
- 11.Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodes‐Cabau J, Larose E, Costerousse O, Hamon M, Mann T. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010; 3:1022-1031 [DOI] [PubMed] [Google Scholar]
- 12.Kristic I, Lukenda J. Radial artery spasm during transradial coronary procedures. J Invasive Cardiol. 2011; 23:527-531 [PubMed] [Google Scholar]
- 13.Pyne C, Mann T. Overcoming anatomic challenges to transradial access. An overview for interventionists who are considering transradial access training. Cardiac Interventions Today. 2010:38-40http://bmctoday.net/citoday/2010/04/article.asp?f=overcoming-anatomic-challenges-to-transradial-access [Google Scholar]
- 14.Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, Sabate M, Mauri‐Ferre J, Huber K, Niemela K, Haude M, Wijns W, Dudek D, Fajadet J, Kiemeneij F. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013; 8:1242-1251 [DOI] [PubMed] [Google Scholar]
- 15. Verapamil hydrochloride for intravenous injection; 2011. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018925s008lbl.pdf Accessed August 13, 2013.
- 16.Lewis JG. Adverse reactions to calcium antagonists. Drugs. 1983; 25:196-222 [DOI] [PubMed] [Google Scholar]
- 17.Rosencher J, Chaib A, Barbou F, Arnould MA, Huber A, Salengro E, Jegou A, Allouch P, Zuily S, Mihoub F, Varenne O. How to limit radial artery spasm during percutaneous coronary interventions. The SPasmolytic Agents to avoid SpasM during transradial percutaneous coronary interventions (SPASM3) study. Catheter Cardiovasc Interv. 2013. 10.1002/ccd.25163 [DOI] [PubMed] [Google Scholar]
- 18.Varenne O, Jegou A, Cohen R, Empana JP, Salengro E, Ohanessian A, Gaultier C, Allouch P, Walspurger S, Margot O, El Hallack A, Jouven X, Weber S, Spaulding C. Prevention of arterial spasm during percutaneous coronary interventions through radial artery: the SPASM study. Catheter Cardiovasc Interv. 2006; 68:231-235 [DOI] [PubMed] [Google Scholar]
- 19.Kiemeneij F, Fraser D, Slagboom T, Laarman G, van der Wieken R. Hydrophilic coating aids radial sheath withdrawal and reduces patient discomfort following transradial coronary intervention: a randomized double‐blind comparison of coated and uncoated sheaths. Catheter Cardiovasc Interv. 2003; 59:161-164 [DOI] [PubMed] [Google Scholar]
- 20.Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND, Perry RA, Morris JL. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc Interv. 2010; 3:475-483 [DOI] [PubMed] [Google Scholar]
- 21.Fukuda N, Iwahara S, Harada A, Yokoyama S, Akutsu K, Takano M, Kobayashi A, Kurokawa S, Izumi T. Vasospasms of the radial artery after the transradial approach for coronary angiography and angioplasty. Jpn Heart J. 2004; 45:723-731 [DOI] [PubMed] [Google Scholar]
- 22.Kiemeneij F, Vajifdar BU, Eccleshall SC, Laarman G, Slagboom T, van der Wieken R. Measurement of radial artery spasm using an automatic pullback device. Catheter Cardiovasc Interv. 2001; 54:437-441 [DOI] [PubMed] [Google Scholar]
- 23.Chen CW, Lin CL, Lin TK, Lin CD. A simple and effective regimen for prevention of radial artery spasm during coronary catheterization. Cardiology. 2006; 105:43-47 [DOI] [PubMed] [Google Scholar]
- 24.Ruiz‐Salmeron RJ, Mora R, Masotti M, Betriu A. Assessment of the efficacy of phentolamine to prevent radial artery spasm during cardiac catheterization procedures: a randomized study comparing phentolamine vs. verapamil. Catheter Cardiovasc Interv. 2005; 66:192-198 [DOI] [PubMed] [Google Scholar]
- 25.Carrillo X, Fernandez‐Nofrerias E, Ciompi F, Rodriguez‐Leor O, Radeva P, Salvatella N, Pujol O, Mauri J, Bayes‐Genis A. Changes in radial artery volume assessed using intravascular ultrasound: a comparison of two vasodilator regimens in transradial coronary interventions. J Invasive Cardiol. 2011; 23:401-404 [PubMed] [Google Scholar]
- 26.Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011; 78:823-839 [DOI] [PubMed] [Google Scholar]


