Abstract
OBJECTIVES: With an increase in vancomycin resistance and the prevalence of obesity in children, alterations of vancomycin dosing regimens may be necessary to achieve target serum concentrations. The primary objective of this study was to describe initial vancomycin dosing with resulting serum concentrations in healthy-weight and overweight/obese children. Secondary objectives include comparing vancomycin dosing regimens of healthy-weight and overweight/obese patients that produced target trough serum concentrations and evaluating the likelihood of attaining target concentrations by patient characteristics.
METHODS: This retrospective review evaluated healthy-weight and overweight/obese patients, aged 2 to 18 years, who had vancomycin trough serum concentrations obtained between 2005 and 2010. Vancomycin dosing, initial trough serum concentrations, pharmacokinetic parameters, and patient demographics were collected for analysis. Target trough serum concentrations were defined as 10 to 20 mg/L.
RESULTS: The study included 98 patients (48 healthy weight, 50 overweight/obese) of which only 14 patients (14.2%, 6 healthy weight, 8 obese) reached a target trough serum concentration with empiric dosing. No difference was found between the mean daily dosing of vancomycin that produced target trough serum concentrations in healthy-weight or overweight/obese patients (53.63 mg/kg/day vs 51.6 mg/kg/day, respectively). Demographic or clinical characteristics were not found to be associated with the likelihood of target trough serum concentration attainment.
CONCLUSIONS: Vancomycin dosing in healthy-weight and overweight/obese pediatric patients did not reach target trough serum concentrations most of the time. In obtaining initial target serum concentrations, no dosing difference was identified for overweight/obese patients compared with healthy-weight patients. Alternate dosing strategies, therapeutic monitoring, and clinical outcomes should continue to be evaluated in this population.
INDEX TERMS: children, dosing, obese, pharmacokinetics, vancomycin
INTRODUCTION
It is well recognized that the pharmacokinetic and pharmacodynamic characteristics of vancomycin differ in the pediatric population as compared to adults.1 The effect of obesity on vancomycin pharmacokinetic parameters has been previously studied in adults, while such effects are beginning to be studied in pediatric patients.2–5 It has been recommended that vancomycin be dosed on total body weight for obese adult patients with subsequent doses changed per therapeutic drug monitoring, since total body weight has improved correlation with volume of distribution and clearance.6 As the pediatric population continues to show an increase in rates of children who are overweight or obese, appropriate dosing of vancomycin in this population warrants investigation.
In 2009, vancomycin guidelines were developed by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists for the adult population, which recommend increased target trough serum concentrations to combat microbiologic resistance.7 Serum concentrations of 10 to 15 mg/L were recommended for most infections with a higher concentration of 15 to 20 mg/L for the following complicated infections: bacteremia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA). These ranges were based on modeling data to provide a target area under the curve to minimum inhibitory concentration ratio (AUC:MIC) of 400 in adults. Although these trough serum concentration monitoring recommendations were not specific for pediatrics, many practitioners began to extrapolate the data to children. More recently, Le and colleagues8 determined that an AUC:MIC ≥ 400 was a “more realistic target in children” of exposure to vancomycin, compared to trough serum concentrations.
In 2011, the IDSA released a guideline for the treatment of MRSA infections and specifically recommended dosing vancomycin at 15 mg/ kg/dose every 6 hours for invasive diseases in pediatric patients.9 It also recommended vancomycin target trough serum concentrations of 15 to 20 mg/L in children with complicated infections (bacteremia, endocarditis, osteomyelitis, meningitis, pneumonia, and severe skin and skin structure infections) but acknowledged that data were limited in pediatrics and additional studies were needed. These recommendations were based on moderate evidence from clinical experience, opinions, descriptive studies, or reports of expert committees. Trough serum concentrations were recommended to be monitored in patients with serious infections, renal dysfunction (including those undergoing dialysis), fluctuating volumes of distribution, and the morbidly obese patient population. Although the AUC:MIC ratio may be the best pharmacodynamic predictor of efficacy of vancomycin, obtaining trough serum concentrations is the most practical way of monitoring at this time.9
With an increase in vancomycin resistance as well as an increase in the prevalence of obesity in pediatric patients, consideration of alterations in dosing regimens in this patient subset may be necessary to achieve target trough serum concentrations. The primary objective of this study was to describe vancomycin dosing with resulting serum concentrations in healthy-weight and overweight/obese children. Secondary objectives include comparing vancomycin dosing regimens of healthy-weight and overweight/ obese patients that produced target trough serum concentrations based upon disease indication and evaluating the likelihood of attaining target concentrations by patient characteristics.
MATERIALS AND METHODS
Research was conducted in a 231-bed, community, women and children’s hospital. Institutional review board approval was granted for this project from both the hospital and the academic institution. An automatic pharmacy pharmacokinetic consult is generated per the hospital Pharmacy and Therapeutics Committee protocol when vancomycin is prescribed. The pharmacy pharmacokinetic consult allows pharmacists to initiate individualized patient doses, adjust physician-ordered doses, order serum concentrations and corresponding laboratory values associated with vancomycin dosing and monitoring. A standard pharmacokinetic data form is used for each patient for monitoring purposes and stored in the pharmacy. Patient demographics, dosing regimens, laboratory and microbiology culture data, and monitoring information were collected on the form.
Pediatric patients, aged 2 to 18 years, prescribed vancomycin from January 2005 to May 2010 were evaluated in this retrospective chart review. Only the initial vancomycin dose and resulting trough serum concentration were included in this analysis. Serum trough concentrations were obtained 30 minutes before dispensing a dose following 3 or more consecutive doses. From these data set, patients were further classified by body mass index (BMI) percentile by using the Centers for Disease Control and Prevention and American Association of Pediatrics recommendations for defining healthy-weight, overweight, and obesity in the pediatric population.10,11 Healthy weight is defined as a BMI that is 5th to <85th percentile, while overweight is defined as a BMI of 85th to 94th percentile and obesity, as a BMI of ≥ 95th percentile. Overweight and obese patients were combined into a single group. Vancomycin dosing (based on total body weight), serum concentration, indication of use, as well as patient demographics, were analyzed. Creatinine clearance for patients was estimated by the Schwartz equation.12 Target trough serum concentrations were defined as 10 to 14.9 mg/L in patients without complicated infections and 15 to 20 mg/L in patients with complicated infections. A complicated infection in this study was defined by using the IDSA 2011 guideline’s definition. Children who were underweight (BMI of <5th percentile) were excluded from the analysis. Patients younger than 2 years were also excluded owing to the differences in recommendations for determination of obesity in this respective age group as compared to pediatric patients between 2 and 18 years of age.
The analysis of variance (ANOVA) procedure and chi-square test were used to compare the demographic characteristics of the patients. Descriptive statistics were used to obtain frequencies of vancomycin dosing (of intervals at every 6, 8, and 12 hours) with resulting trough serum concentrations and to summarize characteristics of patients that attained target trough concentrations. Logistic regression was used to determine the likelihood of attaining target concentrations by patient characteristics and vancomycin regimen characteristics. Statistical differences were determined at p=0.05 and conducted by using SAS 9.2 statistical software (Cary, NC).
RESULTS
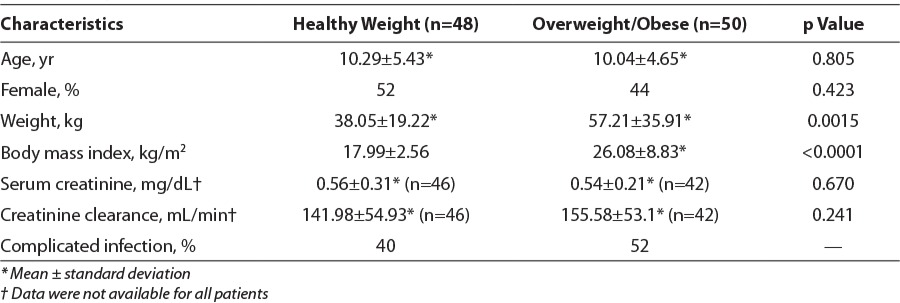
A total of 98 patients met inclusion criteria during the study period. Most (49%, n=48) patients were aged 12 to 18 years with 23% (n=23) between 6 to 11 years of age and 28% (n=27) aged 2 to 5 years. Fifty patients (51%) were classified by BMI percentile as overweight or obese. Specifically, 18 patients (18%) were overweight and 32 patients (33%) were obese. Table 1 provides patient characteristics for the study sample. No significant difference was found for age, sex, serum creatinine, or renal function between groups for baseline characteristics. Sixty-six percent of the healthy-weight and 50% overweight/obese children who reached goal trough were diagnosed with a complicated infection. Most patients received vancomycin owing to a skin or soft tissue infection (33%, n=32) or a respiratory tract infection (22%, n=22). Moreover, 9 and 6 patients had an indication of osteomyelitis or meningitis, respectively. Overall, groups were similar for indications except that 6 patients with healthy weights received vancomycin for neutropenic fever, whereas there were no overweight/obese patients with this indication. Most patients were on the general pediatric ward (n=62, 63%) versus the pediatric intensive care unit.
Table 1.
Comparison of Patient Characteristics
The empiric mean vancomycin dose for all patients combined was 46.59 ± 12.63 mg/kg/ day. When evaluating the 2 groups, the mean dose for the healthy-weight group was 48.86 ± 10.50 mg/kg/day and the mean dose for the overweight/obese group was 44.40 ± 14.14 mg/ kg/day (p=0.08). To evaluate the relationship between observed vancomycin trough concentrations and vancomycin dosing in the healthy-weight and overweight/obese pediatric patients, the doses were stratified as <40, 40 to 59, 60 to 79, and ≥80 mg/kg/day and the corresponding trough serum concentrations were stratified as <5, 5 to 9, 10 to 14, 15 to 20, >20 mg/L. Table 2 delineates these dose relationships for the time period studied. Shorter dosing intervals (every 6 and 8 hours) were used more frequently after 2009 and in younger children. Intervals of every 6 hours (47%) were used more often than either every 8 hours (35%) or every 12 hours (18%). Of patients who received a dose every 6 hours, 50% were 6 years of age or younger and 23% were 7 to 12 years of age. All 10 patients who received vancomycin at an interval of every 12 hours were 12 years of age or older with 80% being 15 years of age or older.
Table 2.
Serum Trough Concentration Following Empiric Vancomycin Dosing Every 6, 8, or 12 hours
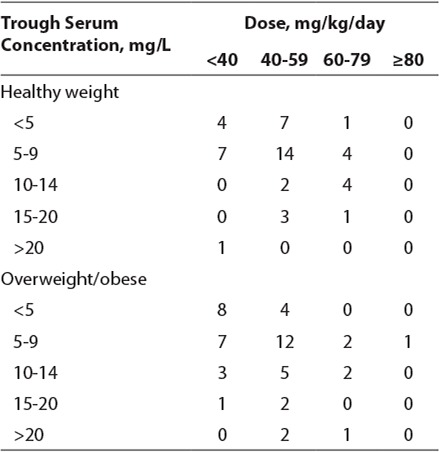
In specifically evaluating overweight/obese patients, 24% of all trough serum concentrations (n=12) observed during the period were <5 mg/L. Most serum concentrations (44%) were 5 to 9 mg/L. Three trough serum concentrations (6%) were >20 mg/L. Larger vancomycin doses did not necessarily result in a supratherapeutic (>20 mg/L) concentration as 2 serum concentrations greater than 20 mg/L were a result of 40 to 59 mg/kg/day dosing. Overall, 13 overweight/ obese patients (26%) reached a serum concentration of 10 to 20 mg/L, with only 8 patients (16%) achieving their target concentration based on indication.
Only 14 patients (healthy weight [n=6, 6%], overweight/obese [n=8, 8%]) in the total population reached a target serum trough concentration with their empiric vancomycin dose regimen. This mean dose was 53.6 ± 9.56 and 51.6 ±10.96 mg/kg/day for the healthy-weight and the over-weight/obese patients, respectively. The mean milligram dose per day was 2121.9 mg (healthy weight) and 2822.2 mg (overweight/obese) for those who reached target serum concentrations. The Figure depicts the number of patients who achieved a target serum trough concentration by dosing interval. The mean vancomycin dose was 54.13 ± 8.18, 53.11 ± 11.18, and 38 mg/kg/ day for those patients receiving a dose every 6, 8, and 12 hours, respectively. The logistic regression model was not significant to conclude association of demographic or clinical characteristics with target trough serum concentration attainment.
Figure.
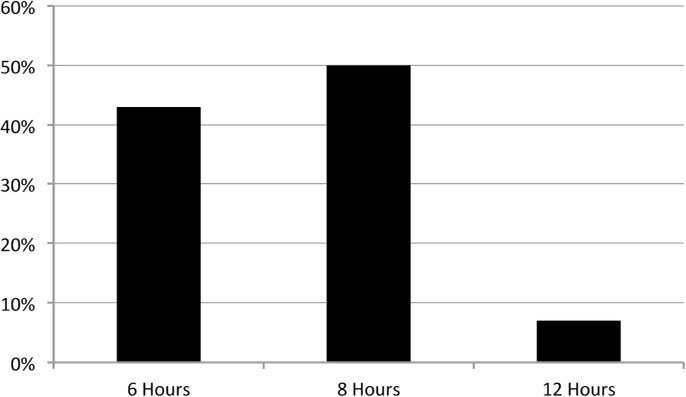
Patients who achieved target serum concentrations by dosing interval.
Four patients in the study had supratherapeutic trough serum concentrations of >20 mg/L. However, only 1 of these patients (a 3-year-old with obesity) had renal dysfunction. There were no apparent impacting factors in the other 3 patients.
DISCUSSION
Pediatric patients identified as overweight and obese have been examined for trends related to the dosing strategy of vancomycin to treat infections. Further comparisons have been made between this patient population and similar data collected from healthy-weight patients. Our study is one of the larger populations studied.
A dosing interval of every 6 hours was used more frequently, especially after 2009. Clinicians may have been aiming for higher trough serum concentrations, thus shortening the dosing interval. This was probably in response to the release of the adult vancomycin dosing and monitoring recommendations advocating higher trough concentrations. Although pediatric patients were not included in the adult guidelines, practitioners may have extrapolated the data. Younger children were given a dose more frequently than older children, which would be expected owing to higher vancomycin clearance rates.
A comparison of vancomycin dosing characteristics in healthy-weight patients and overweight/ obese patients did not reveal a statistically significant difference in dosing. Vancomycin dosing for the overweight/obese patients remained in the “traditional” recommendations for pediatric dosing of 40 to 59 mg/kg/day; yet, 42 overweight/obese patients (84%) did not reach target serum concentrations based on indication. Larger doses are needed to reach higher serum concentrations. However, it may be challenging to reach this target serum concentration in this population and balance adverse effects such as nephrotoxicity. The largest daily dose in our study, 4380 mg, was administered to a 146-kg, 14-year-old with sepsis and resulted in a trough of 19.7 mg/L. Four patients (ages 14, 16, 17, and 18 years) received daily doses of 4 g, which provided a trough serum concentration of 8.3, 20, 12.5, and 15.1 mg/L, respectively. Dosing intervals compared between the groups showed that every-6-hour and every-8-hour dosing had a stronger likelihood of achieving target trough serum concentrations than every-12-hour dosing, which is expected from pharmacokinetics principles. Intervals less frequent than every 8 hours may result in suboptimal trough serum concentrations, which correlate with increasing antimicrobial resistance.
Clinical improvement of the patient or AUC:MIC calculations may play a larger role in patient outcome than monitoring trough serum concentration. Before Le and colleagues8 published their recommendations, Gordon and colleagues13 reported that the AUC:MIC ratio may be a “more accurate and safe method” for monitoring vancomycin in children. This method could be the next step in evaluating vancomycin dosing for overweight and obese pediatric patients. More recently, Camaione and colleagues14 found that vancomycin dosing based on body surface area (BSA) provided isometric AUC24 outcomes in children and young adults. They determined that BSA was a better predictor of vancomycin clearance than weight, using maximum a posteriori probability Bayesian–derived system parameter estimates. Studies evaluating BSA dosing compared to weight-based dosing for vancomycin are warranted.
In our study, the average mg/kg/day dose for the overweight/obese patients to reach target trough serum concentrations was essentially the same as in healthy-weight patients. Miller and colleagues3 also did not find a difference in vancomycin dosing between healthy-weight, over-weight, and obese pediatric patients. Moffett and colleagues4 evaluated a vancomycin dose of 15 mg/kg/dose every 8 hours in matched obese and healthy-weight children, hypothesizing that the obese patients would have higher serum trough concentrations. They determined no difference in the serum concentrations between the 2 groups. Both studies advocate for empiric dosing to be calculated on actual body weight in overweight and obese patients, with which we concur. Nassar and colleagues5 also did not find a difference in dosing regimens between healthy-weight, obese, and underweight pediatric patients and stated that larger doses and more frequent dosing are needed to meet current guideline recommendations. Recently, one small retrospective study did find a difference in initial serum trough concentrations between overweight and obese patients compared to healthy-weight patients when an age-based dosing algorithm was used.15 They also found more trough concentrations > 20 mcg/mL in the overweight and obese group.
Limitations to this study include the retrospective nature of data collection, the descriptive nature of the study, and the small number of patients in the subgroups. Owing to the small number of patients in the overweight group who met target serum concentrations, the patients were combined with the obese patients. The small sample size likely hindered finding a difference in dosing in the subgroups, if one exists. We extrapolated the adult target trough serum concentrations to children when evaluating the data, before the release of the 2011 IDSA guideline specifically including children. Some practitioners may have not done this, as the 2009 vancomycin recommendations were specific for adults. Clinical cure was not evaluated for these patients, thus we are not able to state that higher trough serum concentrations were required for efficacy. Data collection was dependent on review of paper pharmacokinetic forms maintained over time. It is possible that forms, and hence, some patient data, were inadvertently discarded during the study period. Although standardized forms are used, the documentation varied and a serum creatinine concentration was not available for each patient. Our study also only evaluated initial dosing for patients and did not look at multiple data points to identify if patients ever reached target serum concentrations.
CONCLUSION
Empiric vancomycin dosing in overweight/ obese pediatric patients did not reach initial target serum concentrations most of the time. From the results of this study, pediatric patients who are overweight and obese did not require empiric daily doses of vancomycin different from those of healthy-weight patients to reach target serum concentrations. At this time, initial vancomycin dosing for patients who are overweight or obese should not be different from dosing for healthy-weight patients. Actual weight should be used when dosing vancomycin in obese and overweight patients. Larger vancomycin doses for all patients are required to reach target trough serum concentrations. Dosing intervals that optimize the pharmacokinetic parameters of vancomycin in this population should be tailored to each patient (on an individualized basis), but more frequent dosing is recommended. Alternate dosing strategies, therapeutic monitoring, and clinical outcomes should continue to be evaluated in this population.
ABBREVIATIONS
- AUC:MIC
area under the curve to minimum inhibitory concentration
- BMI
body mass index
- BSA
body surface area
- IDSA
Infectious Diseases Society of America
- MRSA
methicillin-resistant Staphylococcus aureus
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Benner K, Worthington M, Kimberlin D et al. Correlation of vancomycin dosing to serum concentrations in pediatric patients: a retrospective database review. J Pediatr Pharmacol Ther. 2009;14(2):86–93. doi: 10.5863/1551-6776-14.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vance-Bryan K, Guay D, Gilliland S et al. Effect of obesity on vancomycin pharmaco-kinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37(3):436–440. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller M, Miller JL, Hagemann TM et al. Vancomycin dosage in overweight and obese children. Am J Health Syst Pharm. 2011;68(21):2062–2068. doi: 10.2146/ajhp110107. [DOI] [PubMed] [Google Scholar]
- 4.Moffett BS, Kim S, Edwards MS. Vancomycin dosing in obese pediatric patients. Clin Pediatr (Phila) 2011;50(5):442–446. doi: 10.1177/0009922810393500. [DOI] [PubMed] [Google Scholar]
- 5.Nassar L, Hadad S, Gefen A et al. Prospective evaluation of the dosing regimen of vancomycin in children of different weight categories. Curr Drug Saf. 2012;7(5):375–381. doi: 10.2174/157488612805076606. [DOI] [PubMed] [Google Scholar]
- 6.Pai MP, Bearden DT. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy. 2007;27(8):1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 7.Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 8.Le J, Bradley JS, Murray W et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(4):e155–e163. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):1–38. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Childhood overweight and obesity. http://www.cdc.gov/obesity/childhood/basics.html. Accessed May 12, 2014.
- 11.Barlow SE, Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rates in infants, children and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 13.Gordon CL, Thompson C, Carapetis JR et al. Trough concentrations of vancomycin: adult therapeutic targets are not appropriate for children. Pediatr Infect Dis J. 2012;31(12):1269–1271. doi: 10.1097/INF.0b013e31826a3eaf. [DOI] [PubMed] [Google Scholar]
- 14.Camaione L, Elliott K, Mitchell-Van Steele A et al. Vancomycin dosing in children and young adults: back to the drawing board. Pharmacotherapy. 2013;33(12):1278–1287. doi: 10.1002/phar.1345. [DOI] [PubMed] [Google Scholar]
- 15.Heble DE, McPherson C, Nelson MP et al. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy. 2013;33(12):1273–1277. doi: 10.1002/phar.1321. [DOI] [PubMed] [Google Scholar]


