Abstract
The incidence of neonatal abstinence syndrome (NAS) has increased dramatically during the past 15 years, likely due to an increase in antepartum maternal opiate use. Optimal care of these patients is still controversial because of the available published literature lacking sufficient sample size, placebo control, and comparative pharmacologic trials. Primary treatment for NAS consists of opioid replacement therapy with either morphine or methadone. Paregoric and tincture of opium have been abandoned because of relative safety concerns. Buprenorphine is emerging as a treatment option with promising initial experience. Adjunctive agents should be considered for infants failing treatment with opioid monotherapy. Traditionally, phenobarbital has been used as adjunctive therapy; however, results of clonidine as adjunctive therapy for NAS appear to be beneficial. Future directions for research in NAS should include validating a simplified scoring tool, conducting comparative studies, exploring home management options, and optimizing management through pharmacogenomics.
INDEX TERMS: abstinence syndrome, buprenorphine, clonidine, methadone, morphine
INTRODUCTION
Neonatal abstinence syndrome (NAS) is withdrawal symptoms that develop in infants born to mothers with antepartum use of certain drugs, such as opioids; the symptoms develop following the postpartum cessation of in utero exposure.1 The 2012 National Survey on Drug Use and Health stated that 5% of pregnant women used illicit drugs, including primarily opioids, central nervous system (CNS) depressants and stimulants, and hallucinogens, in the past 30 days, with the highest rate among girls ages 15 to 17 years at 20.9%.2 Alcohol use in the past 30 days, binge drinking, and heavy drinking were reported in 9.4%, 2.6%, and 0.4% of pregnant women, respectively. Cigarette smoking in the past month remained similar to previous reports at 17.6%. Beyond signs and symptoms of withdrawal and toxicity in the neonate, antepartum use of these substances may result in increased risk of fetal growth restriction, preterm birth, fetal death, congenital anomalies, and impaired neurodevelopment.1,3 Although this survey reflects the multitude of substances women may use during pregnancy, intrauterine opioid exposure is most commonly the cause of clinically significant NAS and will be the focus of this review.1
A recent study reported antepartum maternal opiate use in the United States increased almost 5-fold, from 1.19 to 5.63 per 1000 births per year in 2000 to 2009, respectively.4 During this same time period, the incidence of NAS increased nearly 3-fold, from 1.2 to 3.39 per 1000 births per year. These results indicate that not all newborns born to mothers dependent on or using opiates at the time of delivery will develop signs of NAS. The impact of NAS on health care expenditures also increased significantly, from $39,400 to $53,400 for mean hospital charges (converted to 2009 US dollars), reflecting the clinical and financial burden of this condition.4
In utero exposure to heroin and methadone has been associated with a 60% to 80% incidence of NAS.4 Buprenorphine has been suggested to be associated with a lower risk of NAS; however, studies have had inconsistent results.5 The Maternal Opioid Treatment: Human Experimental Research (MOTHER) project compared neonatal outcomes in infants exposed to buprenorphine versus methadone. Although methadone-exposed infants required an increased amount of morphine for NAS and longer hospital stay compared with buprenorphine-exposed infants, there was no difference in need for treatment of NAS, NAS peak score, or infant’s head circumference. More women discontinued treatment in the buprenorphine group (33%) versus the methadone group (18%), which warrants careful interpretation of these results. The onset and duration of symptoms vary by the drug, amount of maternal usage, duration and timing of intrauterine exposure, and maternal and newborn metabolism and elimination of the drug. Withdrawal from heroin typically occurs within the first 24 hours of birth, whereas symptoms from methadone generally manifest within 48 to 72 hours, or may even be delayed up to 4 weeks.1 Less information is available for buprenorphine-exposed infants; however, symptoms have been shown to appear within 12 to 48 hours and peak at 72 to 96 hours.3
CLINICAL MANIFESTATION
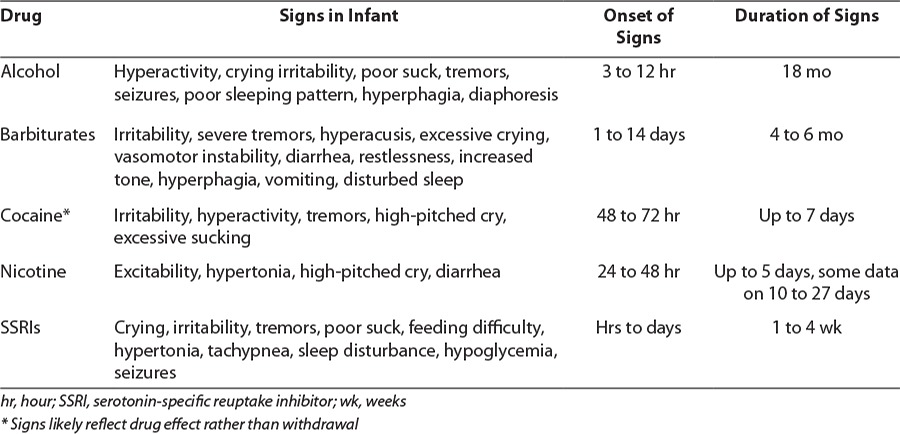
Signs and symptoms of NAS may be classified by affected systems, including neurologic, gastrointestinal, and autonomic. Common clinical manifestations of opioid withdrawal are listed in Table 1. As mentioned previously, there are many other substances that the mother may have used during pregnancy that exhibit overlapping signs and symptoms of withdrawal or toxicity, complicating the evaluation and treatment of these infants. Table 2 summarizes effects on the newborn of maternal use of common non-opioids, as well as typical onset and duration of signs.
Table 1.
Clinical Manifestations of Opioid Withdrawal1

Table 2.
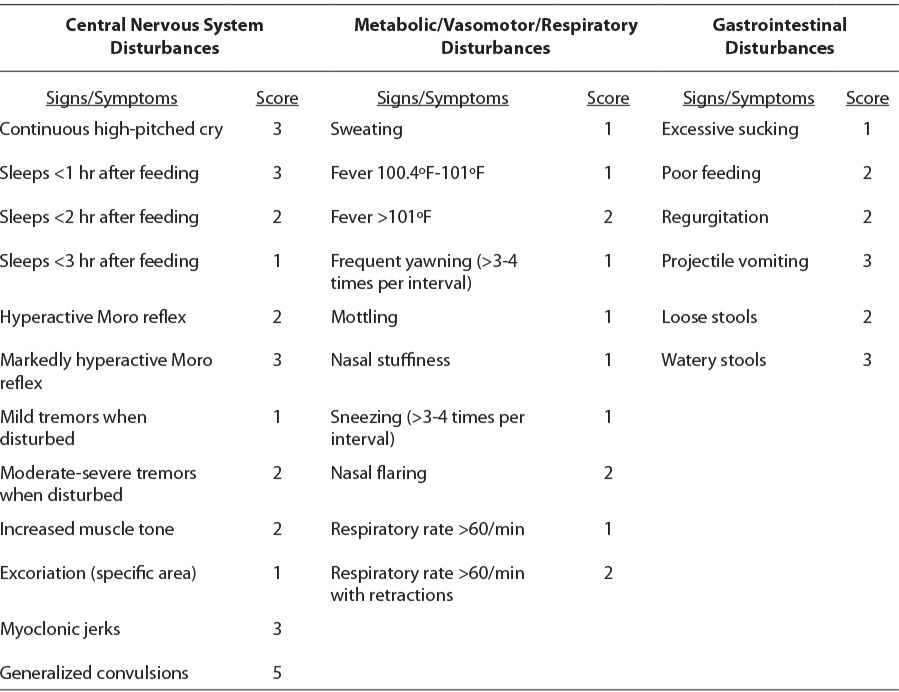
Opioid withdrawal is rarely life-threatening, but it may be associated with weight loss, fever, or seizures. Using a validated scoring system, such as the Modified Finnegan (Table 3) or Lipsitz tool, clinicians may qualify the severity of symptoms, but definitions of severity are not standardized. The American Academy of Pediatrics (AAP) highlighted the Lipsitz tool in their 1998 guidelines because of its simplicity; however, by the time of the 2012 guidelines, the AAP recognized that the Modified Finnegan was most commonly used in practice.1,8 Common thresholds for starting drug therapy are persistent scores 8 or above on the Finnegan scale and 4 and above on the Lipsitz scale; however, this varies per institution protocols. Even within institutions, standardization of definitions for each item should be educated to avoid interrater variability.
Table 3.
Components of the Modified Finnegan1
TREATMENT
Treatment goals in the management of NAS include preventing complications associated with NAS and restoring normal newborn activities, such as sleep, nutrition intake, weight gain, and adaptation to the social environment. As with all drug therapies, the potential benefits and risks of treatment must be considered. Relief of symptoms and prevention of potential complications are benefits of treatment, whereas prolonged drug exposure and hospitalization are disadvantages. In addition, maternal-infant bonding may be affected.
Non-pharmacologic measures should be used in all infants with NAS and may be sufficient to provide comfort. Adequate sleep and nutrition are essential to provide consistent weight gain and socialization. Supportive options include swaddling, rocking, minimizing sensory or environmental stimulation, maintaining temperature stability, increasing the frequency of feeds, early response to infant’s signals, and achieving a caloric intake of 150 to 250 kcal/kg/day.9 Breast-feeding is also strongly encouraged, even with mothers receiving methadone at treatment programs.10 If clinical manifestations persist despite optimized supportive or nonpharmacologic measures, then drug therapy should be considered.
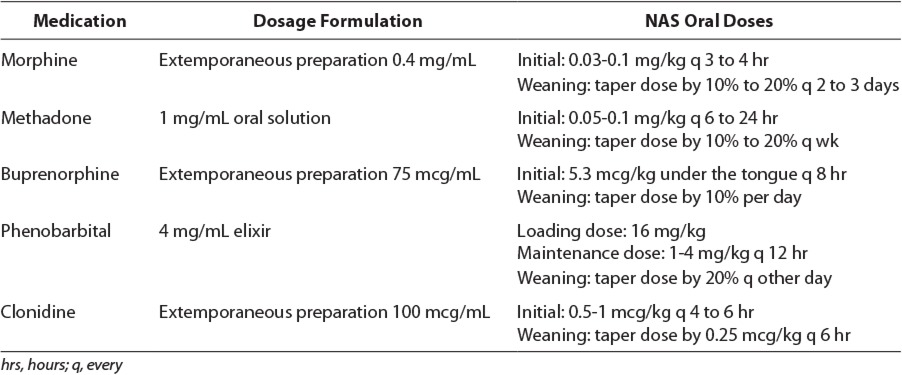
Pharmacotherapy remains the mainstay of treatment for managing NAS. Based on the current recommendation from the AAP on Neonatal Drug Withdrawal, initiation of pharmacotherapy is indicated in newborns with moderate to severe signs of withdrawal to prevent further complications, such as seizures, fever, and weight loss or dehydration due to vomiting, diarrhea, or poor feeding.1 The incidence of newborns requiring pharmacologic therapy for the treatment of NAS ranges from 60% to 80%.9 Pharmacologic treatments studied for NAS have included a variety of agents, such as opioids (paregoric, tincture of opium, morphine, methadone, and buprenorphine), barbiturates (phenobarbital), benzodiazepines (diazepam and lorazepam), clonidine, and phenothiazines (chlorpromazine). Table 4 lists the medications used for NAS that are reviewed in this article.
Table 4.
Medications and Dosage Formulations Used for the Treatment of Neonatal Abstinence Syndrome11–13
OPIOIDS
Surveys completed in the United States and United Kingdom reported that most clinicians (83% and 94%, respectively) choose to select morphine or methadone as the first-line agent in the management of NAS.13,14 Opioids interact with opiate receptors, which modulate neurotransmitter release of norepinephrine, serotonin, acetylcholine, dopamine, and substance P. The primary therapeutic outcome of opioids is analgesic effects activated via the mu receptors. Other opiate receptors include delta, kappa, and sigma. Opioids are small, lipophilic molecular-weight compounds that readily cross the blood-brain barrier and placenta. Adverse effects associated with opioids include sedation, respiratory depression, constipation, urinary retention, twitching, and hypotension.
Paregoric and Tincture of Opium
The earliest opioid used to control NAS was paregoric, an anhydrous morphine available as 0.4 mg/mL. Similar to other opioids, paregoric decreases neuronal activity, preventing withdrawal symptoms associated with NAS. Although paregoric affects motility, peristalsis, and digestive secretions, and reduces the frequency of diarrhea, its use is no longer recommended for the treatment of NAS. Paregoric contains various potentially toxic ingredients, such as noscapine and papaverine, camphor, ethanol 44%, anise oil, benzoic acid, and glycerin.8 Although tincture of opium contains fewer toxic additives than paregoric, the solution still contains ethanol 19% and multiple narcotic alkaloids (i.e., codeine). Tincture of opium contains opium 10 mg/mL and requires a 25-fold dilution to produce 0.4 mg/mL morphine equivalent. A report from the Institute for Safe Medication Practices (ISMP) addresses the dangers of mistaking tincture of opium for paregoric.16 Failure to dilute and prepare tincture of opium in a 25-fold dilution to a final concentration of 0.4 mg/mL can potentially lead to dangerous medication errors. As a result, many pharmacies choose not to prepare diluted tincture of opium for NAS.
Morphine
Morphine is one of many natural opioids extracted from the opium poppy plant and is the most frequently used agent for the treatment of NAS. Morphine is commercially available in several oral solution concentrations: 2, 4, and 20 mg/mL. Currently, two recommendations for the preparation of oral morphine for NAS exist. Morphine oral solution requires further dilution from its original dosage form to a final concentration of 0.4 mg/mL, similar to paregoric and diluted tincture of opium.12 The ISMP also recommends aqueous oral solution of morphine prepared from the morphine injection dosage formulation.16
Methadone
Methadone is a full mu opioid agonist, similar to heroin, and is currently approved by the Food and Drug Administration for withdrawal maintenance programs in adults. Methadone acts on the N-methyl-d-aspartate receptor antagonist blocking glutamate, the primary excitatory neurotransmitter. Limited research is available for the use of methadone for NAS; however, studies report that methadone suppresses abstinence symptoms and prevents fetal distress.17,18 Newborns born to mothers receiving methadone generally exhibit signs and symptoms of withdrawal at 48 to 72 hours after birth. Because of its longer half-life—26 hours versus 8 hours with morphine—methadone requires less frequent dosing and may potentially lead to drug accumulation.11 Unlike the other opioid preparations discussed earlier, methadone 1 mg/mL oral solution does not require further dilution. Other methadone oral solution concentrations are also available in 2 and 10 mg/mL. Although no reports have been documented in pediatric patients, cases of QT prolongation from the use of methadone have been described in adults.19
Buprenorphine
More recently investigated for the treatment of NAS is buprenorphine. Buprenorphine is indicated for the management of opioid withdrawal in adults.20 As a partial agonist, buprenorphine selectively acts at the mu opioid receptor and blocks the binding of other mu agonists, but it has limited activity at this site, resulting in a mild analgesic effect. Buprenorphine is metabolized by the cytochrome P450 enzyme 3A4 to the active metabolite, norbuprenorphine. Commercially, buprenorphine is only available as an injection of 300 mcg/mL and as a sublingual tablet requiring extemporaneous compounding to a final concentration of 75 mcg/mL.13
Comparison of Different Opioids
Five comparative clinical trials evaluating opioid agents in neonates treated for withdrawal have been published since the AAP 1998 update.13,17,21–23 In a prospective, randomized, double-blind, controlled clinical trial, 33 neonates gestational ages 32.9 to 41 weeks (mean 38.6 weeks) received tincture of opium or oral morphine for the treatment of NAS.21 Both tincture of opium and morphine were diluted to a concentration of 0.4 mg/mL, and regimens started with initial dosages at 2 drops/kg orally every 4 hours. Initiation of therapy was assessed using the Finnegan scores and outcome measures focused on duration of treatment and total opioid dose required. There was no statistically significant difference in the duration of hospital (32.4 versus 37.4 days) and treatment (26.9 versus 29.8 days) days in neonates receiving tincture of opium or morphine, respectively. Additionally, no differences were found between the two groups on weight gain and Finnegan scores.
Another trial also evaluated the use of tincture of opium and oral morphine for the treatment of NAS.22 This retrospective chart review was conducted to determine the time to medical clearance for discharge in neonates receiving tincture of opium from 2007 to 2009 versus morphine in 2009 to 2011. No statistically significant difference was found in terms of primary outcome measures. Mean duration of days until medical clearance was found to be 32.7 days in the tincture of opium group versus 39 days in the morphine group. No differences were observed in the use of adjunctive therapy with phenobarbital, nor were variations in Finnegan scores found.
Lainwala et al17 compared the length of hospital stay in neonates receiving oral morphine versus methadone treatment for NAS. A total of 46 neonates, gestational age ≥36 weeks, were evaluated, and results revealed no significant difference in median length of stay between both groups, 36 days in the morphine group versus 40 days in the methadone group. Higher maternal methadone dose and increased birth weight were associated with longer length of stay.
Kraft et al13,23 conducted two randomized, open-label clinical trials on the use of buprenorphine for the treatment of NAS. One study compared buprenorphine with tincture of opium.23 The trial enrolled 12 neonates who received buprenorphine 13.2 mcg/kg/day sublingually in 3 divided doses, whereas 13 neonates were administered initial dosing of tincture of opium at 0.4 mg/kg/day orally in 6 divided doses as monotherapy. No statistical differences were detected for mean length of treatment days and duration of hospitalization days. The authors concluded that additional buprenorphine pharmacokinetic studies in neonates are necessary, especially because norbuprenorphine plasma concentrations were undetectable in these patients. As a result, Kraft et al13 revised the dosage of buprenorphine to 15.9 mcg/kg/day sublingual in 3 divided doses in their follow-up trial. Applying similar methods as the previous study, buprenorphine significantly reduced the length of treatment days compared with morphine, 23 versus 38 days, respectively. Furthermore, buprenorphine therapy also showed decreased duration of hospitalization, 32 days versus 42 days in the morphine group.
PHENOBARBITAL
Phenobarbital is the drug of choice for non– narcotic-related withdrawal or polydrug abuse and is an option for neonates requiring adjunct therapy with opioids. Phenobarbital is a long-acting barbiturate that enhances the inhibitory neurotransmitter, γ-aminobutyric acid, thus causing CNS depression. Additionally, phenobarbital promotes the disposition and excretion of the opioid. Although phenobarbital modifies hyperactive behavior related to narcotic withdrawal, several disadvantages for its use in NAS exist. For example, phenobarbital lacks relief of gastrointestinal signs, causes CNS depression, impairs suck reflex, delays bonding between mother and infant, produces rapid tolerance to sedative effect, and possesses pharmacokinetic/pharmaco-dynamic properties, such as being a cytochrome P450 inducer and having a prolonged half-life (45–100 hours).11,24 Other unknown considerations for phenobarbital include the necessity of a loading dose for the treatment of NAS, therapeutic drug monitoring, duration of therapy, and the neurodevelopmental effects on the brain.25 When monitored, phenobarbital therapeutic drug levels of 20 to 30 mg/dL have shown effective control of NAS symptoms.24
Comparison of Phenobarbital to Opioids
A randomized, double-blind, controlled trial enrolled 75 infants ages 32 to 42 weeks’ gestation (median 40 weeks) with NAS.26 Patients with two consecutive Lipsitz scores >4 received initial dosing of either phenobarbitone 2 mg/kg orally 4 times a day or morphine 0.05 mg/kg orally 4 times a day. A significant difference in median duration of treatment was observed when comparing phenobarbitone with morphine, 12 versus 8 days, respectively. Although not significant, 47% of neonates in the phenobarbitone group required chloral hydrate for second-line therapy, compared with 35% in the morphine group. Higher maternal methadone dose and use of other drug classes correlated with increased length of treatment duration and need for second-line therapy with chloral hydrate.
Another prospective trial enrolled 32 infants who received initial dosing of phenobarbital 5 to 10 mg/kg/day orally or morphine 0.05 to 0.1 mg/kg orally for up to 6 doses.27 In contrast to previous trials, Ebner et al27 measured the duration of NAS in neonates requiring treatment as a secondary outcome. Similar to the prior study results, neonates treated with morphine significantly reported fewer days of treatment than in the phenobarbital group, 9.9 versus 17.7 days, respectively. No significant difference was noted with the Finnegan scores in both groups.
CLONIDINE
Clonidine is a centrally acting α2 adrenergic receptor agonist used as adjunct therapy in opioid withdrawal in children and adolescents. Clonidine works by stimulating presynaptic adrenergic receptors, consequently inhibiting CNS sympathetic outflow and reducing norepinephrine. In relation to withdrawal, clonidine ameliorates autonomic overactivity, such as tachycardia, hypertension, diaphoresis, restlessness, and diarrhea. As a result, rebound autonomic activity may occur with the abrupt withdrawal of clonidine. Other adverse effects associated with clonidine include metabolic acidosis and hypotension. Similar to clonidine, dexmedetomidine exhibits selective α2 adrenoceptor agonist activity and may have a potential role in the future for the treatment of NAS; however, published experience with this agent is lacking.
Comparison of Clonidine in Conjunction With Opioids to Opioid Monotherapy
One smaller trial and one case report investigated the use of clonidine for the treatment of NAS.28,29 Leikin et al28 completed a retrospective chart review evaluating the use of clonidine alone for the prevention and management of NAS. Although only 3 of 14 patients were treated with clonidine 0.5 to 1 mcg/kg orally every 6 hours for NAS, results concluded that clonidine may be a reasonable alternative. The use of clonidine doses of up to 3 mcg/kg orally every 3 hours was reported in a 34 weeks’ gestational age infant born to a mother receiving tramadol 600 to 800 mg daily.29
A randomized, placebo-controlled trial to compare clonidine with diluted tincture of opium to standard care tincture of opium alone was evaluated.30 A total of 40 patients received initial dosing of clonidine at 1 mcg/kg orally every 4 hours plus diluted tincture of opium 0.08 mg morphine equivalent orally every 4 hours, whereas another 40 neonates received diluted tincture of opium and placebo. Median length of therapy was significantly less in patients who received combination therapy, 11 versus 15 days. Furthermore, the length of therapy in infants exposed to maternal methadone also was statistically significantly shorter in the clonidine plus diluted tincture of opium group than opioid alone, 12 versus 17 days. No treatment failure was reported with adjunctive therapy of clonidine. Seven infants in the clonidine and diluted tincture of opium group rebounded and one patient developed supraventricular tachycardia after therapy discontinuation. Overall, clonidine as adjunctive therapy for NAS was well tolerated.
Comparison of Clonidine With Phenobarbital
A recent prospective study compared the use of phenobarbital versus clonidine when used in conjunction with morphine for NAS.31 A total of 34 neonates in each group received either morphine plus phenobarbital or morphine plus clonidine. Standard dosing and weaning protocols for the two groups were used; with each morphine dose reduction, the respective clonidine or phenobarbital doses were escalated. In comparison with the clonidine, the phenobarbital group had fewer morphine treatment days, 18.2 versus 13.6 days, respectively. Although both groups received similar mean total morphine doses, the length of phenobarbital therapy continued for an average of 3.8 months, whereas all neonates in the clonidine group completed weaning prior to discharge.
SUMMARY
A standardized process should be developed for the identification, evaluation, treatment, and discharge management of infants with NAS. Primary treatment of NAS consists of opioid replacement therapy, such as morphine or methadone. Currently, there is no evidence to support that length of hospital stay and treatment duration differ between the various morphine preparations and methadone. Buprenorphine has demonstrated to shorten duration of treatment and hospital stay at higher dosages of 15.9 mcg/kg/day when compared with morphine preparation.13 Adjunctive agents, such as phenobarbital or clonidine, should be considered for infants with treatment failure. Although clonidine at 1 mcg/kg every 4 hours appears to be effective for the treatment of NAS, O’Mara et al29 determined that larger doses of 3 mcg/kg every 3 hours might be necessary in some neonates experiencing withdrawal.29 Large, well-designed clinical trials are needed to optimize evidence-based treatment for these neonates.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- CNS
central nervous system
- ISMP
Institute for Safe Medication Practices
- NAS
neonatal abstinence syndrome
Footnotes
Disclosure The authors declare no conflicts or financial interest in any products or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Hudak ML, Tan RC, The Committee On Drugs; The Committee On Fetus And Newborn; American Academy of Pediatrics Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–e560. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 2.Results From the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2013. HHS publication (SMA) 12–4713. [Google Scholar]
- 3.Committee on Health Care for Underserved Women, American Society of Addiction Medicine. ACOG Committee Opinion No. 524: opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 4.Patrick SW, Schumacher RE, Benneyworth BD et al. Neonatal abstinence syndrome and associated health care expenditures – United States, 2000–2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 5.Jones HE, Kaltenbach K, Heil SH et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godding V, Bonnier C, Fiasse L et al. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates. Pediatr Res. 2004;55(4):645–651. doi: 10.1203/01.PDR.0000112099.88740.4E. [DOI] [PubMed] [Google Scholar]
- 7.Stroud LR, Paster RL, Papandonatos GD et al. Maternal smoking during pregnancy and newborn neurobehavior: a pilot study of effects at 10–27 days. J Pediatr. 2009;154(1):10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics. 1998;101(6):1079–1088. [PubMed] [Google Scholar]
- 9.Wilson GS. Somatic growth effects of perinatal addiction. Addict Dis. 1975;2(1–2):333–345. [PubMed] [Google Scholar]
- 10.Jansson LM, Choo R, Velez ML et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121(1):106–114. doi: 10.1542/peds.2007-1182. [DOI] [PubMed] [Google Scholar]
- 11.Lexi-comp Online. Hudson (OH): Lexicomp, Inc; http://online.lexi.com/crlsql/servlet/crlonline. Accessed August 29, 2013. [Google Scholar]
- 12.NeoFax Online. Greenwood Village (CO): Truven Health Analytics Inc; http://neofax.micromedexsolutions.com/neofax/neofax.php. Accessed September 10, 2013. [Google Scholar]
- 13.Kraft WK, Dysart K, Greenspan JS et al. Revised dose schema of sublingual buprenorphine in the treatment of neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574–580. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 15.Green M, Suffet F. The Neonatal Narcotic Withdrawal Index: a device for the improvement of care in the abstinence syndrome. Am J Drug Alcohol Abuse. 1981;8(2):203–213. doi: 10.3109/00952998108999125. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Safe Medication Practices. Hazard alert!: recurring confusion between tincture of opium and paregoric. http://www.ismp.org/hazardalerts/recruting.asp. Accessed August 20, 2013.
- 17.Lainwala S, Brown ER, Weinschenk NP et al. A retrospective study of length of hospital stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care. 2005;5(5):265–272. doi: 10.1016/j.adnc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg S, Melia D, Khuri E et al. Adolescent and young adult heroin patients: drug use and success in methadone maintenance treatment. J Addict Dis. 2006;25(3):15–25. doi: 10.1300/J069v25n03_03. [DOI] [PubMed] [Google Scholar]
- 19.Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747–753. doi: 10.1002/pds.1112. [DOI] [PubMed] [Google Scholar]
- 20.Jones HE. Practical considerations for the clinical use of buprenorphine. Sci Pract Perspect. 2004;2(2):4–20. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langenfeld S, Birkenfeld L, Herkenrath P et al. Therapy of neonatal abstinence syndrome with tincture of opium or morphine drops. Drug Alcohol Depend. 2005;77(1):31–36. doi: 10.1016/j.drugalcdep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Kokotajlo S, Robinson CA, Presti A. Use of tincture of opium compared to oral morphine for the treatment of neonatal abstinence syndrome. J Opioid Manag. 2013;9(1):62–70. doi: 10.5055/jom.2013.0148. [DOI] [PubMed] [Google Scholar]
- 23.Kraft WK, Gibson E, Dysart K et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122(3):e601–e607. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coyle MG, Ferguson A, LaGasse L. Diluted tincture of opium (DTO) and phenobarbital vs. DTO alone for the treatment of neonatal opiate withdrawal in term infants. J Pediatr. 2002;140(5):561–564. doi: 10.1067/mpd.2002.123099. et al. [DOI] [PubMed] [Google Scholar]
- 25.Finnegan LP, Mitros TF, Hopkins LE. Management of neonatal narcotic abstinence utilizing a phenobarbital loading dose method. NIDA Res Monogr. 1979;27:247–253. [PubMed] [Google Scholar]
- 26.Jackson L, Ting A, McKay S et al. A randomized controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F300–F304. doi: 10.1136/adc.2003.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebner N, Rohrmeister K, Winklbaur B et al. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87(2–3):131–138. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Leikin JB, Mackendrick WP, Maloney GE et al. Use of clonidine in the prevention and management of neonatal abstinence syndrome. Clin Toxicol. 2009;47(6):551–555. doi: 10.1080/15563650902980019. [DOI] [PubMed] [Google Scholar]
- 29.O'Mara K, Gal P, DaVanzo C. Treatment of neonatal withdrawal with clonidine after long term, high-dose maternal use of tramadol. Ann Pharmacother. 2010;44(7–8):1342–1344. doi: 10.1345/aph.1M758. [DOI] [PubMed] [Google Scholar]
- 30.Agthe AG, Kim GR, Mathias KB et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5):e849–e856. doi: 10.1542/peds.2008-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surran B, Visintainer P, Chamberlain S et al. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome: a prospective randomized clinical trial. J Perinatol. 2013;33(12):954–959. doi: 10.1038/jp.2013.95. [DOI] [PubMed] [Google Scholar]


