Summary
Background:
to evaluate the short and long term effectiveness of ultrasonography (US)-guided percutaneous needle lavage in calcific tendinopathy of the rotator cuff. To study the evolution of the size of calcifications and pain in the two years after treatment.
Methods:
study design: A 2 year longitudinal prospective study is carried out after applying the UGPL technique on a number of patients diagnosed with calcific tendinitis of the rotator cuff. Clinical, ultrasound and radiology follow-up controls were performed, 3 months, 6 months, one year and two years after the treatment. The Visual Analog Scale (VAS) was used to assess the pain. The degree and point of pain is selected on a 10 cm line, arranged horizontally or vertically. The “0” represents no pain and “10” represents worst pain. The population studied was made up of 121 patients that required our service as a result of suffering from a painful shoulder.
Results:
the pain (VAS) and the size of the calcification significantly decreased with the application of the technique (p< 0,001 in both cases) and regardless of the sex (p: 0.384 for pain and p: 0.578 for the size of the calcification). This occurred from the first check-up (3 months) and was maintained for two year.
Conclusions:
we consider this technique to be a valid alternative as a first-choice treatment of calcific tendinitis of the shoulder. The intervention is simple, cost-effective, does not require hospitalization, involves no complications, rehabilitation treatment is not required and it shows very few side effects without sequelae, significantly reducing the size of the calcification and pain in the majority of patients.
Keywords: calcified tendinopathy, ultrasound, percutaneous lavage, puncture
Introduction
Rotator cuff tendinopathy is the most common cause of shoulder pain, with the prevalence in the general population found to range between 5 and 39%1,2. The frequency of calcific tendinopathy ranges, according to the authors, between 7.5 and 22% of all cases of shoulder tendinopathy3–5 and the prevalence of the condition is highest among the third and fifth decade of life6. The etiopathogeny of calcific tendinopathy is still not clear, although its origin may be multifactorial, the result of a combination of extrinsic factors (anatomical or biomechanical) and intrinsic factors (changes related to age, vascularity, overloading, genetic, hormonal factors, etc.)2,4,7–10.
There is currently no universally accepted treatment or international protocols agreed upon by consensus to manage the disease, with many being the techniques described for this pathology, the results of which vary from one author to another1,4,11,12. Among the described treatments that have been most effective are shock waves13–16, ultrasound-guided percutaneous lavage17–23 and surgical treatment24,25.
Work is currently being carried out on the application of safer, non-invasive techniques with fewer side effects; in this regard, ultrasound-guided percutaneous lavage (UGPL) may be the first-choice, therapeutic alternative in the treatment of calcific tendinopathy of the shoulder. Initial percutaneous punctures were performed in 1978 with fluoroscopic control26 but the technique was dropped as a result of the radiation on the patient during such treatment. It wasn’t until the 90s, with the use of ultrasound scans, that this treatment was used again, when excellent results were obtained in identifying and locating calcifications in the rotator cuff and guiding the needle to perform the lavage of the calcific deposits17.
Since then, a number of documented studies have been produced using UGPL with various technical modifications19–21,27,28. However, to date, very little literature has been published on the study of the medium and long-term results of applying UGPL with a wide variety of case studies.
The main objective of our study is to assess the results after applying the UGPL technique for the treatment of calcific tendinopathy of the rotator cuff with a 2 year follow-up, assessing both the evolution of pain as well as the size of the calcification over time and the relationship between both parameters.
Material and methods
Study design: a 2 year longitudinal prospective study is carried out after applying the UGPL technique on a number of patients diagnosed with calcific tendinitis of the rotator cuff. Clinical, ultrasound and radiology follow-up controls were performed, 3 months, 6 months, one year and two years after the treatment. The Visual Analogy Scale (VAS) was used to assess the pain. The degree and point of pain is selected on a 10 cm line, arranged horizontally or vertically. The “0” represents no pain and “10” represents worst pain29.
Population: the population studied was made up of 121 patients that required our service as a result of suffering from a painful shoulder. Between 30–39 years: 16 patients; 40–49 years: 60 patients; 50–65 years: 45 patients, made up of 81 women (66.9%) and 40 men (33.1%). 78 patients (64.46%) had jobs that required low levels of physical strain (administrative, teachers, nurses…etc.) and 43 patients (35.53%) had jobs that required moderate to high levels of physical strain (operators, firemen, policemen…etc.).
All the patients included in the survey were clinically diagnosed and underwent radiology and ultrasound scans to diagnose the calcific tendinopathy of the rotator cuff. 104 patients only show calcifications in the tendon of the supraspinatus (SP) muscle, 8 patients in the SP and other tendons of the rotator cuff and 8 patients in the subscapularis (SC). Inclusion criteria were established: minimum size of calcification 5 mm, minimum value of 6 on the visual analogy scale (VAS), no known allergies to the drugs used. All the individuals were informed of the nature and characteristics of the study beforehand and they signed the informed consent, pursuant to the principles of the Declaration of Helsinki concerning medical research involving human subjects30 and study meets the ethical standards of the Muscles, Ligaments and Tendons Journal31.
Material: TOSHIBA Xario SSA-660A ultrasound, with multi-frequency probe (8–12 MHz), for the diagnosis and measurement of the size of the calcification. I-Scan 4400 (FM.Control ®) portable fluoroscopic imaging system.10 ml syringes and 18G, 20G and 21G needles for the calcification lavage.
Description of the procedure: for the intervention, patients do not have to fast or perform any preliminary actions other than taking an anxiolytic pill orally 30 minutes before the procedure (Bromazepam 1,5 mgr), in order to reduce the possibility of vagal syndrome.
During the preliminary ultrasound scan, we establish the position of the shoulder that will make the route to the calcific deposits most accessible, through the skin. The technique is performed with the patient sitting in a chair with armrests in front of the doctor and if possible with an internal rotation of the shoulder positioning the forearm behind the patient’s back. This position increases the pressure inside the tendon and enables the ejection of the calcium into the syringe. This area of skin is marked and the doctor explains to the patient that this position must be held throughout the procedure, approximately 30 minutes. The area shall be prepared with an aseptic technique using a povidone-iodine solution. The following shall be prepared using a sterile drape on the laboratory table, applying the aseptic technique: A 10 cc syringe containing 2% mepivacaine, various syringes containing a sterile physiological saline solution and a 5 cc syringe containing Triamcinolone and 18G and 20G needles.
First the needle is inserted with the syringe containing mepivacaine and the ultrasound probe is placed on the path to be followed. We always start the procedure with the 20G needle. The anaesthesia is then administered to the area from the point of entry on the skin into the subacromial bursa. The needle is placed below the calcification and the needling and lavage commences with the rest of the anaesthetic through the actual pressure placed on the embolus of the syringe from the outside with a pulsating or pumping mechanism (Fig.1). These impulses continue until the calcic material is extracted. When the syringe is full of calcium deposits, the syringe is changed for another containing a physiological saline solution, without removing the needle from its position. The pumping, pressure and lavage continues, successively changing the syringes until no further calcium deposits are extracted or until the patient shows signs of discomfort. Once the lavage is completed, the syringe containing the physiological saline solution is changed for the one containing Triamcinolone. The needle is gradually extracted until the bursa, where 2 cc of Triamcinolone shall be introduced. Finally the area is covered with a sterile gauze. If the calcification is extremely hard or the exit is obstructed by very thick material, an 18G needle should be used.
Figure 1.
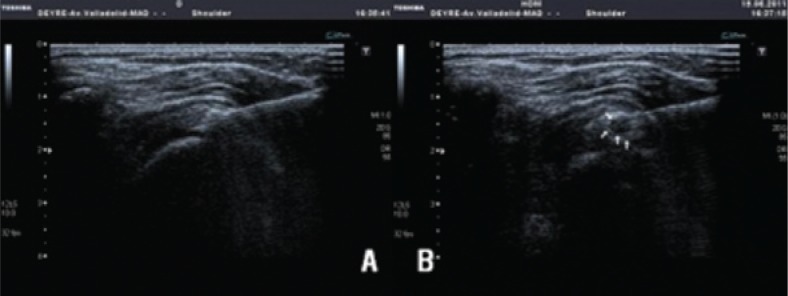
A–B. (A) Needle point entering below the calcification. (B) The introduction of liquid produces a cavity through the pumping effect enabling the lavage.
After the intervention, we recommend that the patient should continue with normal life and should avoid overloading that shoulder during the week after the treatment.
Statistical analysis: the statistical methods used were as follows: descriptive statistics of the quantitative variables (Descriptive procedure) and qualitative (Frequencies procedure). Kolmogorov-Smirnov test (NPAR Tests procedure) for normality study. Contingency tables and chi-squared tests (c2) (CROSS-TABS procedure). ANOVA (one-way procedure), with Bonferroni test. ANOVA repeated measures (GLM procedure). Pearson correlation analysis (CORR procedure). The alpha level of significance in all the cases was 0.05 (95%). The statistical analysis of the information was carried out with the programme, Statistical Package for the Social Sciences (SPSS) 19.0 for Windows32.
Results
The average size of the calcific deposits before the application of the treatment was 12.07 +/− 4.8, while the VAS was 7.46 +/− 0.92, without any significant differences between men and women in either of the two parameters (p=0.451 and p= 0.680 respectively). In our series we found a greater percentage of calcific tendinopathy in the dominant side (54.5%) but without significant differences (p: 0.09).
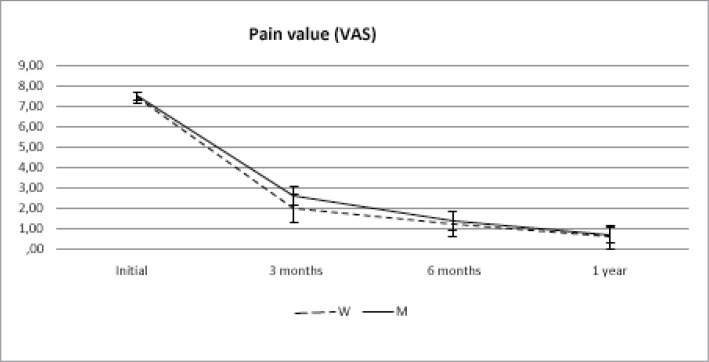
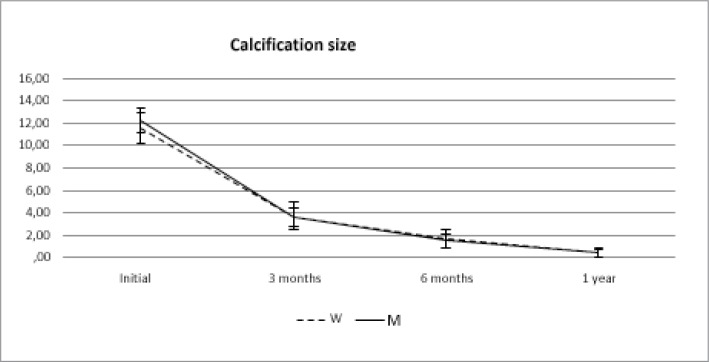
The pain (VAS) and the size of the calcific deposits significantly decreased with the application of the technique (p< 0,001 in both cases) and regardless of the sex (p: 0.384 for pain and p: 0.578 for the size of the calcification). This occurred from the first checkup (3 months) and was maintained for two year. (Figs. 2, 3).
Figure 2.
VAS behaviour over time between women (W) and Men (M).
Figure 3.
Behaviour of the size of the calcification over time between women (W) and Men (M).
The size of the initial calcification showed a wide spectrum, which is why we established four groups. Less than 6 mm, from 7–12 mm, from 13–18 mm and over 19 mm. 77.68% showed calcifications ranging between 7–18 mm. We have correlated the VAS value with the size of the initial calcific deposits and we have confirmed that the pain does not depend on the size thereof; however, we did find a positive correlation between the pain and the size of the calcific deposits after the percutaneous lavage. In other words, the pain decreases as the residual calcific deposits decreases after the treatment (p< 0,001 a year in all cases).
The decrease in pain and the size of the calcification occurred in all the cases (100%). 29.75% (36 patients) did not show any calcific deposits with the ultrasound scan after three months, 61.16% (74 patients) after six months and 83.78% (105 patients) after one year. With regard to pain, 63.64% (77 patients) were asymptomatic after 3 months, while after one year this percentage increased to 89.26% (108 patients). No patients suffered a relapse after two years of the procedure.
During the first check-up after three months, 19 patients (15.70%) did not show any clinical or sonographic signs of improvement, with more than 5 mm calcification and VAS above 7. The intervention was repeated on these patients. Finally, a total of 13 patients (10.74 %) showed no definitive improvement with the treatment one year after its application. None of these patients improved two years after the procedure.
As indicated in the section on material and methods, during the application of the technique, calcium deposits can be seen to be extracted through the syringe. This occurred in 98 patients (80.99%). However, pain reduction over time was not related to whether or not calcium deposits were extracted (p:0.503). Other authors have also confirmed this fact20,21, although there is not unanimity on the issue19, however, the number of patients and the monitoring time is lower in all the cases in our survey.
Six patients (5%) had a vagal reaction during the application of the procedure or as soon as it was finished which were spontaneously reverted with physical measures and in no case did it need to be interrupted or have other measures applied or subsequent medical treatments. No patients had any other complications during the intervention or during the 2 years of monitoring (rupture of the tendon, etc.).
Discussion
In our survey including 121 patients, with the application of the UGLP, we found that pain disappeared in 89.26% and the calcification disappeared in 86.78% of the patients, with low levels of complications (5% vagal reaction). More than 50% of the patients were asymptomatic 3 months later, with the largest percentage reached one year after the application of the UGLP and with the results remaining after two years. Therefore this technique may be effective in the medium and long term. Similar results to those published after arthroscopic treatment33,34 and higher, medium and long-term, to other minimally invasive treatments such as infiltrations with corticoids35, shock-waves16 and iontophoresis using acetic acid36. The etiopathogeny of calcific tendinopathy of the rotator cuff is still unknown and may be caused by a number of factors. The epidemiological analysis of our series could advance some of these factors. In our series, as with other authors, the majority of the calcific deposits were in the supraspinatus tendon21,37, which could be related to the critical or ischemic area, located in the distal area of this tendon38,39. Also, in our survey, it mainly affects women in the fourth decade of life18,20,23,37, with no relation to prior illnesses or traumas, with a greater presence in professions or activities that require low levels of physical exertion and with no significant differences between the dominant and non-dominant side18,20,40, this information could suggest a greater influence of intrinsic factors (genetic or hormonal) rather than extrinsic factors (traumas).
Physiotherapy is classically recommended as a first option for treating calcific tendinitis and surgical treatment is considered after an average period of six months if clear clinical results are not obtained22,41. Surgical treatment has seen better results in the treatment of this pathology that the conservative treatment25,33. However, there is a number of reasons for not prescribing surgical treatment as a first option. On the one hand, although surgery would appear to reduce the percentage of tendon ruptures25, it is not riskless, given that if the calcification is bulky, the surgical resection of it could leave to a defect in the tendon and even rupture it, with it possibly having to be repaired42,43. On the other hand, after surgical treatment, rehabilitating treatment is normally required in the majority of cases44. Finally, it has the inconvenience of having to stay in hospital, entailing greater health costs18,45.
As we described in the procedure, we used a single 20G needle, a smaller needle could cause an obstruction in the needle if calcium is extracted and a large one could damage the tendon. Various intervention techniques have been described, some authors use two needles18,22 and others puncture the calcification and aspirate the content19. No differences have been found between aspirating the calcium content21, therefore some authors do not recommend this aspiration technique as it could increase the risk of damaging the tendon20,21. In our study, the calcification was not punctured, a needle was introduced below it and we pressure-cleaned it with the saline solution, this is distributed throughout the calcification and the diluted calcium is retropulsed into the syringe, without using the aspiration technique and therefore reducing the risk of tendon damage and the possibility of the needle being obstructed by the calcific deposit. A significant reduction of the size of the calcification has been seen, regardless of whether calcium is extracted, therefore we do not believe that the aspiration technique is required, or the introduction of two needles as a single operator can perform the technique. With our procedure we have obtained similar results or even better results to those communicated by other authors using different techniques18,20,23. With a two-year follow-up in our series.
Calcification classification by Gärtner46 is based on morphology and density of these as they are shown in X-ray studies. Different phases of the illness are described based on the evolution of symptoms and images, and for the lack of response suggests a simple infiltration puncture. Classically, papers about calcifying tendinopathy mentioned Gartner`s Classification, but not data is found about long-term evolution of the Calcification size and its relation with symptoms release. That is the main reason why we improved our investigations in order to know how theses calcifications evolve post UGPL. Our study is mainly a Clinical trial, and we do not find other studies, which consider same stats such as size before and after treatment in such sample, comparing this evolution with clinical improving after treatment.
Limitations of our study are the lack of randomisation and control cohort. The sample is made up of all the individuals that attended our department diagnosed with calcific tendinitis of the shoulder. The positive results obtained in our experience with the application of the technique, has led us to use in this prospective study as a first-choice alternative. We measure and describe Calcification size, pain and relation between these two stats, considering two years free-of-pain period and regular work activity, as success in the treatment of these patients. Non measuring functional scales or quality life scores could be considered as a limitation of our study.
To conclude, we consider this technique to be a valid alternative as a first-choice treatment of calcific tendinitis of the shoulder. The intervention is simple, cost-effective, does not require hospitalization, involves no complications, rehabilitation treatment is not required and it shows very few side effects without sequel, significantly reducing the size of the calcification and pain in the majority of patients.
References
- 1.Maffulli N. Basic science and rotator cuff repair: where have we arrived? Med Sport Sci. 2012;57:VIII–X. [PubMed] [Google Scholar]
- 2.Longo UG, Berton A, Papapietro N, Maffulli N, Denaro V. Epidemiology, genetics and biological factors of rotator cuff tears. Med Sport Sci. 2012;57:1–9. doi: 10.1159/000328868. [DOI] [PubMed] [Google Scholar]
- 3.Welfling J, Kahn MF, Desroy M, Paolaggi JB, de Seze S. Calcifications of the shoulder. II. The disease of multiple tendinous calcifications. Rev Rhum Mal Osteoartic. 1965;32(6):325–334. [PubMed] [Google Scholar]
- 4.Oliva F, Via AG, Maffulli N. Calcific tendinopathy of the rotator cuff tendons. Sports Med Arthrosc. 2011;19(3):237–243. doi: 10.1097/JSA.0b013e318225bc5f. [DOI] [PubMed] [Google Scholar]
- 5.Friedman M. Calcified tendinitis of the shoulder. Am J Surg. 1957;94:56–61. doi: 10.1016/0002-9610(57)90618-9. [DOI] [PubMed] [Google Scholar]
- 6.Rupp S, Seil R, Kohn D. Tendinosis calcarea of the rotator cuff. Orthopade. 2000;10:852–867. [PubMed] [Google Scholar]
- 7.Siegal DS, Wu JS, Newman JS, Del Cura JL, Hochman MG. Calcific tendinitis: a pictorial review. Can Assoc Radiol J. 2009;60(5):263–272. doi: 10.1016/j.carj.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Seitz AL, McClure PW, Finucane S, Boardman ND, 3rd, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon) 2011;26(1):1–12. doi: 10.1016/j.clinbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Oliva F, Giai Via A, Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Medicine. 2012;10(95) doi: 10.1186/1741-7015-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvie P, Pollard TC, Carr AJ. Calcific tendinitis: natural history and association with endocrine disorders. J Shoulder Elbow Surg. 2007;16(2):169–173. doi: 10.1016/j.jse.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database Syst Rev. 2003;(2):CD004258. doi: 10.1002/14651858.CD004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachewar SG, Kulkarni DS. Calcific tendinitis of the rotator cuff: a review. J Clin Diagn Res. 2013;7(7):1482–1485. doi: 10.7860/JCDR/2013/4473.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SY, Cheng B, Grimmer-Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. J Shoulder Elbow Surg. 2011;20(5):845–854. doi: 10.1016/j.jse.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Vavken P, Holinka J, Rompe JD, Dorotka R. Focused extra-corporeal shock wave therapy in calcifying tendinitis of the shoulder: a meta-analysis. Sports Health. 2009;1(2):137–144. doi: 10.1177/1941738108331197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioppolo F, Tattoli M, Di Sante L, et al. Clinical improvement and resorption of calcifications in calcific tendinitis of the shoulder after shock wave therapy at 6 months' follow-up: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94(9):1699–1706. doi: 10.1016/j.apmr.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Farin PU, Rasanen H, Jaroma H, Harju A. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 1996;25(6):551–554. doi: 10.1007/s002560050133. [DOI] [PubMed] [Google Scholar]
- 18.Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment--nonrandomized controlled trial. Radiology. 2009;252(1):157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 19.Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fine-needle technique. Radiology. 2001;221(2):455–461. doi: 10.1148/radiol.2212000830. [DOI] [PubMed] [Google Scholar]
- 20.del Cura J, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. Am J Roentgenol. 2007;189(3):128–134. doi: 10.2214/AJR.07.2254. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Jiang Y, Hu Y, Xing C, Hu B. Evaluating the long-term effect of ultrasound-guided needle puncture without aspiration on calcifying supraspinatus tendinitis. Adv Ther. 2008;25(11):1229–1234. doi: 10.1007/s12325-008-0115-x. [DOI] [PubMed] [Google Scholar]
- 22.Sconfienza LM, Viganò S, Martini C, et al. Double-needle ultrasound-guided percutaneous treatment of rotator cuff calcific tendinitis: tips & tricks. Skeletal Radiol. 2013;42(1):19–24. doi: 10.1007/s00256-012-1462-x. [DOI] [PubMed] [Google Scholar]
- 23.De Zordo T, Ahmad N, Odegaard F, et al. US-guided therapy of calcific tendinopathy: clinical and radiological outcome assessment in shoulder and non-shoulder tendons. Ultraschall Med. 2011;32(Suppl 1):S117–23. doi: 10.1055/s-0029-1245333. [DOI] [PubMed] [Google Scholar]
- 24.Balke M, Bielefeld R, Schmidt C, Dedy N, Liem D. Calcifying tendinitis of the shoulder: midterm results after arthroscopic treatment. Am J Sports Med. 2012;40(3):657–661. doi: 10.1177/0363546511430202. [DOI] [PubMed] [Google Scholar]
- 25.Wittenberg RH, Rubenthaler F, Wolk T, Ludwig J, Willburger RE, Steffen R. Surgical or conservative treatment for chronic rotator cuff calcifying tendinitis--a matched-pair analysis of 100 patients. Arch Orthop Trauma Surg. 2001;121(1–2):56–59. doi: 10.1007/s004020000195. [DOI] [PubMed] [Google Scholar]
- 26.Comfort TH, Arafiles RP. Barbotage of the shoulder with image-intensified fluoroscopic control of needle placement for calcific tendinitis. Clinical orthopaedics and related research. 1978;(135):171–178. [PubMed] [Google Scholar]
- 27.Cacchio A, Rompe JD, Serafini G, Sconfienza LM, Sardanelli F. US-guided percutaneous treatment of shoulder calcific tendonitis: some clarifications are needed. Radiology. 2010;254(3):990. doi: 10.1148/radiol.091542. ; author reply -1. [DOI] [PubMed] [Google Scholar]
- 28.Lee KS, Rosas HG. Musculoskeletal ultrasound: how to treat calcific tendinitis of the rotator cuff by ultrasound-guided single-needle lavage technique. AJR Am J Roentgenol. 2010;195(3):638. doi: 10.2214/AJR.10.4878. [DOI] [PubMed] [Google Scholar]
- 29.Huskisson EC. Measurement of pain. Lancet. 1974;2(7889):1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 30.2012. World, Medical, Association, editors. 64 WMA General Assembly. Declaration of Helsinki. Fortaleza (Brasil). http://www.wma.net/es/30publications/10policies/b3/.
- 31.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ. 2013;4:250–252. [PMC free article] [PubMed] [Google Scholar]
- 32. SPSS. SPSS Stadistics 19.0. Command Syntax Reference: SPSS Inc.; 2010. [Google Scholar]
- 33.Rebuzzi E, Coletti N, Schiavetti S, Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. J Orthop Traumatol. 2008;9(4):179–187. doi: 10.1007/s10195-008-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyer T. Arthroscopic treatment of calcifying tendinitis of the rotator cuff. Chir Main. 2006;25(Suppl 1):S29–35. [PubMed] [Google Scholar]
- 35.Thomas T, Beaudreuil J. Non-traumatic pathology of the shoulder: medical treatment. Rev Prat. 2006;56(14):1550–1555. [PubMed] [Google Scholar]
- 36.Leduc B, Caya J, Tremblays S, Bureau NJ, Dumont M. Treatment of calcific tendinitis of the shoulder by acetic acid iontophoresis: A double-blind randomized controlled trial. Arch Phys Med Rehabil. 2003;84(10):1523–1527. doi: 10.1016/s0003-9993(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 37.Seil R, Litzenburger H, Kohn D, Rupp S. Arthroscopic treatment of chronically painful calcifying tendinitis of the supraspinatus tendon. Arthroscopy. 2006;22(5):521–527. doi: 10.1016/j.arthro.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Burkhart S, Esch J, Jolson R. The rotator crescent and rotator cable: An anatomic description of the shoulder′s suspension bridge. Arthroscopy. 1993;9:611–616. doi: 10.1016/s0749-8063(05)80496-7. [DOI] [PubMed] [Google Scholar]
- 39.Ling SC, Chen CF, Wan RX. A study on the vascular supply of the supraspinatus tendon. Surg Radiol Anat. 1990;12(3):161–165. doi: 10.1007/BF01624517. [DOI] [PubMed] [Google Scholar]
- 40.Pfister J, Gerber H. Chronic calcyfying tendinitis of the shoulder-therapy by percutaneous and lavage: A prospective open study of 62 shoulders. Clin Rheumatol. 1997;16:269–274. doi: 10.1007/BF02238962. [DOI] [PubMed] [Google Scholar]
- 41.Ogon P, Suedkamp NP, Jaeger M, Izadpanah K, Koestler W, Maier D. Prognostic factors in nonoperative therapy for chronic symptomatic calcific tendinitis of the shoulder. Arthritis Rheum. 2009;60(10):2978–2984. doi: 10.1002/art.24845. [DOI] [PubMed] [Google Scholar]
- 42.Lubojacky J. Calcareous tendinitis of the shoulder. Treatment by needling. Acta Chir Orthop Traumatol Cech. 2009;76(3):225–231. [PubMed] [Google Scholar]
- 43.Weber SC, Abrams JS, Nottage WM. Complications associated with arthroscopic shoulder surgery. Arthroscopy. 2002;18(2 Suppl 1):88–95. doi: 10.1053/jars.2002.31801. [DOI] [PubMed] [Google Scholar]
- 44.Rompe J, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001;387:72–82. doi: 10.1097/00003086-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Haake M, Rautmann M, Wirth T. Assessment of the treatment costs of extracorporeal shock wave therapy versus surgical treatment for shoulder diseases. Int J Technol Assess Health Care. 2001;17(4):612–617. [PubMed] [Google Scholar]
- 46.Gärtner J, Heyer A. Calcific tendinitis of the shoulder. Orthopade. 1995;24(3):284–302. [PubMed] [Google Scholar]