Abstract
The Candida albicans Als adhesin Als5p has an amyloid-forming sequence that is required for aggregation and formation of model biofilms on polystyrene. Because amyloid formation can be triggered by force, we investigated whether laminar flow could activate amyloid formation and increase binding to surfaces. Shearing Saccharomyces cerevisiae cells expressing Als5p or C. albicans at 0.8 dyne/cm2 increased the quantity and strength of cell-to-surface and cell-to-cell binding compared to that at 0.02 dyne/cm2. Thioflavin T fluorescence showed that the laminar flow also induced adhesin aggregation into surface amyloid nanodomains in Als5p-expressing cells. Inhibitory concentrations of the amyloid dyes thioflavin S and Congo red or a sequence-specific anti-amyloid peptide decreased binding and biofilm formation under flow. Shear-induced binding also led to formation of robust biofilms. There was less shear-activated increase in adhesion, thioflavin fluorescence, and biofilm formation in cells expressing the amyloid-impaired V326N-substituted Als5p. Similarly, S. cerevisiae cells expressing Flo1p or Flo11p flocculins also showed shear-dependent binding, amyloid formation, biofilm formation, and inhibition by anti-amyloid compounds. Together, these results show that laminar flow activated amyloid formation and led to enhanced adhesion of yeast cells to surfaces and to biofilm formation.
INTRODUCTION
Biofilms are communities of microorganisms that form on surfaces. They are ubiquitous and exist in locations as diverse as the mouth, on indwelling catheters, and in fast-moving streams (1–3). Biofilms influence the spread of infections and can clog medical tubing. In the formation of biofilms, adherence of microbes to a surface is followed by cell division and/or capture of free-flowing microbes into the growing biofilm and production of an extracellular matrix of macromolecules. There are functional amyloids present in biofilms made by bacteria and yeast (4–7). These functional amyloids play roles in cell adhesion and in biofilm matrices (4, 5, 8).
In yeasts, adherence to substrate and cell-to-cell aggregation is mediated by cell surface glycoprotein adhesins. The Candida albicans Als adhesins and the Saccharomyces cerevisiae Flo flocculins are examples of adhesins that have little or no homology, but they have similar architecture. Each has an N-terminal secretion signal sequence, a globular ligand-binding region, a midregion containing threonine-rich tandem repeats (which are not homologous between the proteins), a long Ser/Thr-rich glycosylated stalk, and a C-terminal glycosylphosphatidylinositol (GPI) anchor (9). During cell wall biogenesis, the GPI anchor is cleaved in the glycan, and the remnant covalently attaches to cell wall polysaccharide (10). Within the midregions of Als5p, Flo1p, and Flo11p are 6- to 7-amino-acid sequences predicted by TANGO to form amyloids (http://tango.crg.es/) (11, 12). The amyloid sequence in Als5p is important for cell-to-cell aggregation and cell-to-substrate adhesion (7). A single-site amino acid substitution in the amyloid region of Als5pV326N decreases in vitro cell-to-cell aggregation, cell-to-substrate adhesion, and fluorescence of the amyloid-reporting dye thioflavin T (ThT) (7). Results with cells expressing the S. cerevisiae flocculins Flo1p and Flo11p are consistent with this model: anti-amyloid dyes Congo red (CR) and thioflavin S (ThS) decrease the rate and extent of flocculation of cells expressing either flocculin (12).
Tensile forces present in the environment often increase the strength of bonds formed between microbes, between microbes and surfaces, and between leukocytes and endothelia. These strengthened bonds, called “catch bonds,” result in enhanced interactions (13–15). Cell adhesion proteins, such as mammalian selectins and Escherichia coli FimH, form catch bonds. Leukocytes sheared at 0.3 dyne/cm2 or higher switch from a freely moving state to an immobilized state (16), and E. coli organisms sheared at 20 dyne/cm2 or higher switch from rolling adhesion to stationary adhesion (17). To our knowledge, catch bonding has not been reported in fungi, so we looked for similar behavior. We have carried out parallel experiments to determine the roles of Als5p, Flo1p, and Flo11p, three unrelated adhesins, in fungal biofilm formation. We report here that yeast cell adhesion shows similar behavior, and that such whole-cell catch bonding is dependent on force-sensitive amyloids.
MATERIALS AND METHODS
Strains and media.
S. cerevisiae strain W303-1B MATα leu2 ura3 ade2 trp1 (Rodney Rothstein, Columbia University), harboring the empty vector (pJL1-EV) or expressing Als5pWT or Als5pV326N, was grown in complete synthetic medium (CSM) lacking tryptophan with galactose as the carbon source (7). S. cerevisiae variant diastaticus MATa ura3 leu2-3,112 his4, expressing Flo11p, and the Δflo11 deletion strain MATa ura3 leu2-3,112 his4 flo11::URA3 were kindly gifted by Anne Dranginis (St. John's University). S. cerevisiae strain BX24-2B FLO1 MATα FLO1 gal1 was purchased from the ATCC (Manassas, VA). These cells and C. albicans SC5314 were grown in yeast extract-peptone-dextrose (YPD) at 30°C at 170 rpm for 24 h. Overnight cultures of C. albicans SC5314 were diluted 10-fold into fresh YPD and incubated for 45 min at 30°C and 170 rpm to induce increased expression of Als1p (18, 19). Yeast were washed 3 times with TE (10 mM Tris with 1 mM EDTA), pH 7.0, and then resuspended in TE at 1.4 × 106 cells/ml before binding to channels in the laminar-flow device.
Shear flow experiments.
Flow experiments were carried out in a Bioflux 200 laminar-flow device, and cells were visualized on an Olympus inverted phase microscope. Zero- to 20-dyne/cm2 48-well plates were used at room temperature. In some experiments, channels were precoated with heat-denatured bovine serum albumin (BSA), 1 mg/ml for 1 h, and then washed in TE before introduction of cells. Cell counts were done with ImageJ software using the cell counter analysis tool. Counts were done for images after 2 h of shearing and after 10 min of shearing at 20 dyne/cm2. Each cell was counted whether it was in a cluster or not. Cells with small buds were counted as one cell. Clusters were binned as 2, 3 to 9, or >10 cells. Error bars represent standard deviations (SD) from triplicate determinations.
Two-hour assays with cells in coated channels.
Volumes of 850 μl of each strain of cells at 1.4 × 106/ml were pumped from the inlet well of the plate to the outlet well for 2 h at each respective shear flow into the coated channel surface. These cells were not allowed to settle under stationary conditions. After 2 h, the flow rate was increased to shear at 20 dyne/cm2 for 10 min.
ThT staining of cells in channels.
Cells were seeded onto the uncoated channel surface from the outlet well. They were allowed to adhere without flow for 15 min. TE buffer with 1 μM ThT was added to the inlet well and flowed over the cells at specified rates. Images were taken with a 405/450-nm-wavelength filter set (one per min for 1 h). The images shown are negatives.
Biofilm growth.
Cells suspended at 6 × 106/ml were allowed to adhere to noncoated channel surfaces for 15 min without flow. Growth medium then was pumped through the channels for 48 h at the shear stress specified. To assay the effects of anti-amyloid dyes on biofilm growth, 0.2 mM ThS or 10 μM CR was added to the medium during growth.
Peptides were purchased from the Rockefeller University Proteomics facility and were previously described (7, 12).
RESULTS
Effect of shear on cell-to-substrate binding of Candida albicans.
The Als adhesins of C. albicans mediate both cell-to-substrate and cell-to-cell binding (20, 21). When an atomic force microscope (AFM) is used to apply an extending force to cell surface-attached Als5p, the adhesins cluster on the cell surface through amyloid-like interactions (7, 22). Therefore, we determined whether there was activation under laminar-flow conditions similar to those the fungi encounter in vivo. C. albicans strain SC5314 cells were flowed over the surface of a channel coated with heat-denatured BSA in a laminar-flow device. The flow of buffer with the same number of cells (1.4 × 106/ml) continued for 2 h at rates yielding shear stresses of 0.02 to 2.6 dyne/cm2. At the lowest applied force, about 30 cells remained bound within the observed field (Fig. 1; also see Video S1 in the supplemental material). When the cells were subjected to higher shear, 0.2 to 1.6 dyne/cm2, there was an increasing number of cells bound to the surface, up to 18-fold greater (Fig. 1; also see Video S2). At the highest stress tested, 2.6 dyne/cm2, few cells remained bound. To ensure that flow rate did not affect the number of cells bound to the surface, the samples sheared at 0.02 dyne/cm2 were monitored for an additional 4 h. There was no increase in the binding of the cells to the channel. Similarly, when the cell density was increased 4-fold to 5.6 × 106/ml, there was no significant increase in the number of cells bound at low shear stress (data not shown). Time-lapse analyses showed that cells moved along the substrate under low shear but not under high shear (see Fig. S1 and Videos S1 and S2). These results demonstrated enhanced cell-to-substrate binding under moderate flow.
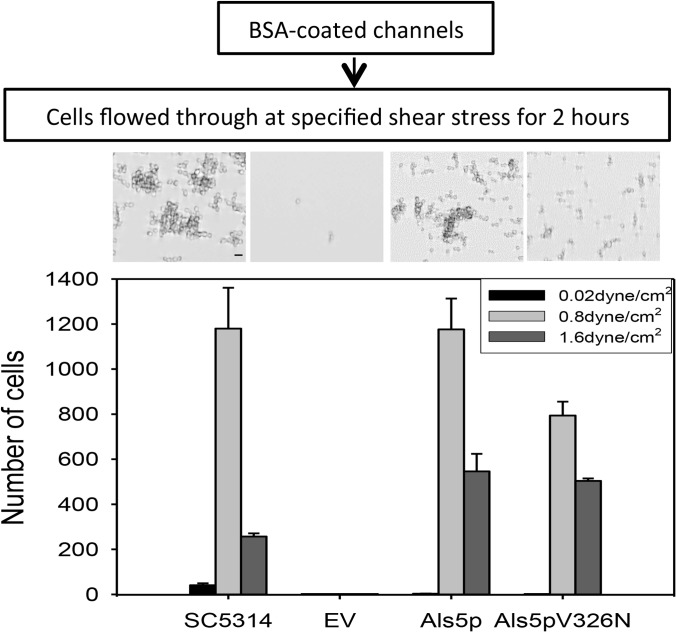
FIG 1.
Effect of shear stress on adhesion. C. albicans SC5314 or S. cerevisiae cells were allowed to adhere to surfaces under flow. (Top) Micrographs of representative field enlargement of the channel surface at 0.8 dyne/cm2 for each strain. Scale bar, 5 μm. (Bottom) Adherent cells were photographed after 2 h and quantified with Image J. EV, empty vector.
Shear stress also increased the formation and retention of clusters of cells. Cells subjected to a force of 0.2 to 1.6 dyne/cm2 formed clusters, with clusters of maximal size formed at 0.8 dyne/cm2 (see Fig. S2 in the supplemental material). At 0.8 dyne/cm2 there was a slight decrease in the number of cells bound compared to the level at 0.2 dyne/cm2, but the clusters were larger. Therefore, increased force mediated both cell-to-substrate and cell-to-cell binding. This increased binding under stronger shear is characteristic of catch bonding.
Als5p mediates catch bonding.
C. albicans expresses many adhesins, so it is difficult to attribute a specific behavior to any specific cell surface component (21, 23, 24). Therefore, we studied C. albicans Als5p in an S. cerevisiae surface display model. Als5p-expressing S. cerevisiae cells were allowed to settle on a surface coated with heat-denatured BSA and sheared at 0.02, 0.8, or 1.6 dyne/cm2 for 2 h. Fewer than five Als5p-expressing cells in the whole image field remained on the surface at 0.02 dyne/cm2 after 2 h, but at 0.8 dyne/cm2 more than 103 cells bound (Fig. 1; also see Videos S3 and S4 in the supplemental material). At 1.6 dyne/cm2, about one-third as many cells bound. The bound cells also formed clusters, as did the C. albicans cells (see Fig. S3). There were more large clusters formed at 0.8 dyne/cm2 than at 0.02 dyne/cm2 and 1.6 dyne/cm2. Cells transformed with an empty vector did not bind well at any shear stress (Fig. 1).
After 2 h, we assessed the resistance to flow by increasing shear stress to 20 dyne/cm2 for 10 min (see Fig. S4 in the supplemental material). No cells were retained that had been exposed to low shear (0.02 dyne/cm2). About 20% of the cells that adhered at 0.8 dyne/cm2 remained bound after this high-shear washing. Of the cells that were bound after washing at 1.6 dyne/cm2, about 50% remained after washing at 20 dyne/cm2. Therefore, expression of Als5p on the surface of S. cerevisiae led to stronger binding after the cells had been sheared at moderate stress, i.e., catch-bonding behavior similar to that of C. albicans.
Catch bonding increases biofilm formation.
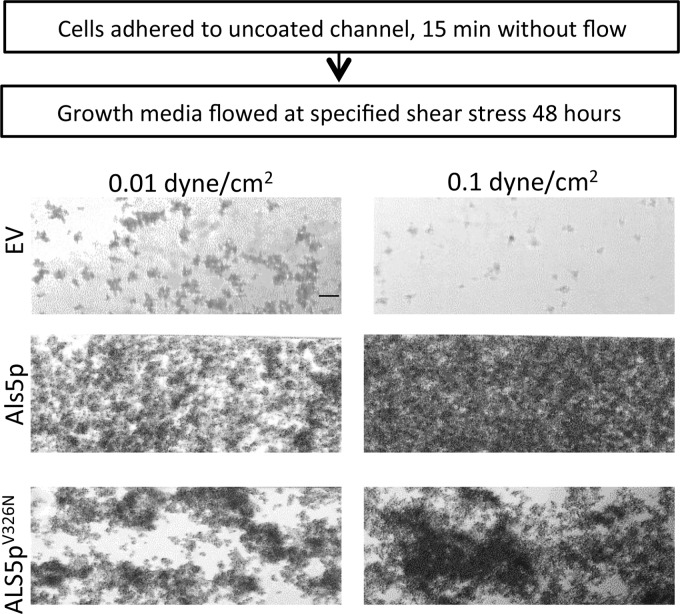
To assess effects of shear on biofilm growth and attachment, Als5p-expressing S. cerevisiae cells were allowed to adhere without flow on an uncoated surface, and then nonadherent cells were removed and the adherent cells sheared at 0.01 dyne/cm2 or 0.1 dyne/cm2 for 48 h in growth medium. Als5p-expressing cells remained bound to the channel and proliferated into a biofilm. The biofilm was denser and more extensive at higher shear than at lower shear (Fig. 2). The cells transformed with empty vector did not bind well to the channel and did not form a biofilm. Results were similar for C. albicans: the biofilm was thicker if the cells were sheared in medium at 0.1 dyne/cm2 than when sheared at 0.01 dyne/cm2 (data not shown).
FIG 2.
Biofilm growth for S. cerevisiae strains at two shear stresses. Biofilms of S. cerevisiae expressing Als5p or Als5pV326N or harboring an empty vector. Adherent cells were grown under laminar flow for 48 h at the designated shear stress. Scale bar, 25 μm.
Amyloid dependence of catch bonding and biofilm formation.
This force-dependent activation prompted us to determine whether shear stress induced increased binding through formation of amyloid nanodomains under laminar-flow conditions. Cells expressing the nonamyloid mutant Als5pV326N have impaired formation of surface amyloid nanodomains after stimulation by AFM and are poorly activated in aggregation assays (7, 22, 23). Such cells did not bind as well to the channels, the clusters that formed were smaller than those of the Als5p-expressing cells, and the amyloid-impaired mutants were more easily removed by washing at 20 dyne/cm2 (Fig. 1 and 3; also see Fig. S3 and S4 in the supplemental material). Similarly, cells expressing Als5pV326N formed biofilms that were sparser and less flow activated than cells expressing wild-type Als5p (Fig. 2).
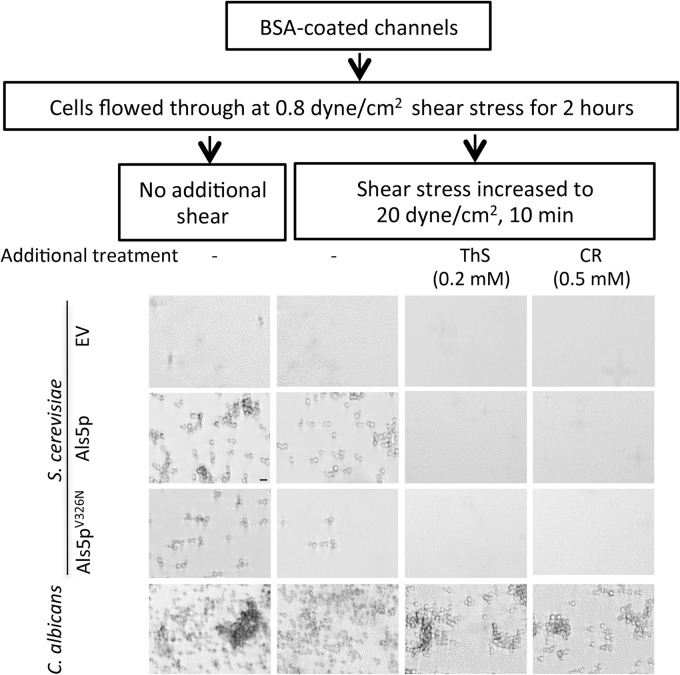
FIG 3.
Effects of high shear and amyloid dyes on binding of yeast to BSA-coated surfaces. Designated strains were allowed to adhere to BSA-treated channels for 2 h under laminar flow at 0.8 dyne/cm2 before imaging (left column). In the second column, adherent cells were washed at high shear (20 dyne/cm2). In the third and fourth columns, anti-amyloid compounds were added to the flow buffer during adherence. Micrographs are representative field enlargements of the channel surface. Scale bar, 5 μm.
If fungal catch-bonding behavior is dependent on formation of surface amyloids, then treatments that disrupt amyloids should inhibit the shear-dependent strengthening of adhesive bonds. The amyloid-perturbing dye thioflavin S (ThS; 0.2 mM) or Congo red (CR; 0.5 mM) was added to the flowing buffer. ThS and CR each inhibited binding of Als5p-expressing cells to BSA-coated surfaces, with ThS being more effective (Fig. 3). Similarly, CR and ThS each inhibited binding of C. albicans cells (Fig. 3). ThS and CR did not affect binding of cells harboring the empty vector. With cells expressing the amyloid-reduced mutant Als5pV326N, CR and ThS also inhibited the binding to the channel surface. Therefore, amyloid-perturbing dyes inhibited the shear-activated adhesion of the cells.
To assay for the effects of amyloid-perturbing dyes on biofilm formation, Als5p-expressing S. cerevisiae or C. albicans SC5314 was seeded onto channel surfaces and then subjected to flow at 0.1 dyne/cm2 for 24 h in growth medium with or without 0.2 mM ThS or 10 μM CR. Biofilms developed in the control channels but not in ThS-treated channels. In channels treated with CR, there was a significant decrease in the size of the biofilm (see Fig. S5 in the supplemental material).
Anti-amyloid peptide decreased cell adhesion.
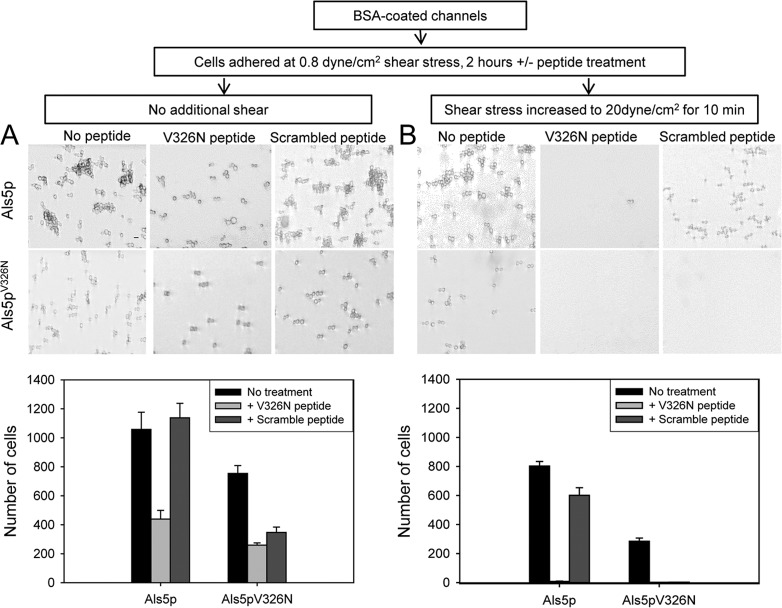
An anti-amyloid peptide (SNGINIVATTRTV) specifically inhibits nanodomain formation and activation of adhesion in Als5p-expressing cells (7, 23). We tested whether this peptide would inhibit shear-activated binding of cells to the substrate in BSA-coated channels. Als5p-expressing cells were flowed through channels at 0.8 dyne/cm2 in the absence or presence of the peptide (200 μg/ml). After 2 h, the cells were sheared at high force (20 dyne/cm2). The anti-amyloid peptide reduced both the number of cells bound and the number retained after high shear (Fig. 4). A peptide with identical composition but random sequence (VITGVTNIRTSVA) did not prevent attachment or retention of the Als5p cells. For cells expressing amyloid-reduced Als5pV326N, there was reduced attachment to the channel and much less flow resistance, and the two peptides were similar in facilitating removal of the attached cells. Therefore, the anti-amyloid peptide caused sequence-specific inhibition of binding and retention of Als5p-expressing cells.
FIG 4.
Effects of peptides on binding of yeast to BSA-coated surfaces. Micrographs and cell counts of channel surfaces with cells expressing Als5p or Als5pV326N sheared at 0.8 dynes/cm2 for 2 h. Micrographs are representative field enlargements of the channel surface. Scale bar, 5 μm. (A) Cells were sheared in the absence or presence of anti-amyloid V326N peptide or a scrambled peptide of the same composition (200 μg/ml). (B) Channels after an additional 10 min of high shear at 20 dyne/cm2.
Effect of shear on surface amyloids.
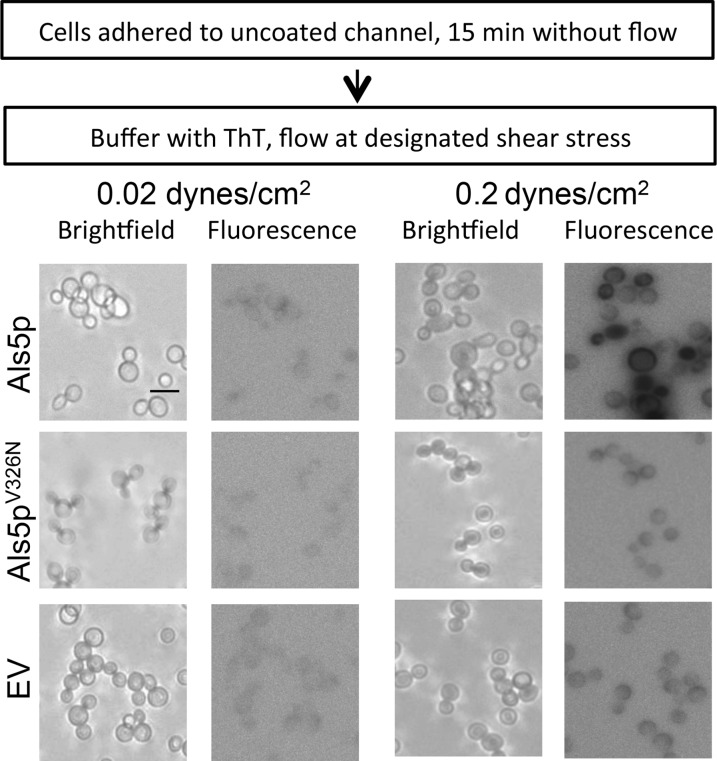
We assayed formation of surface nanodomains by real-time staining with the amyloid dye thioflavin T (ThT). Als5p-expressing S. cerevisiae cells were allowed to adhere to the surface in the laminar-flow device and then subjected to shear stress at 0.02 dyne/cm2 or 0.2 dyne/cm2 in TE containing 1 μM ThT, a concentration that does not affect cellular binding (2, 24). Als5p-expressing cells subjected to higher shear showed bright ThT fluorescence after 7 min relative to cells sheared at low force (Fig. 5). The nonamyloid mutant Als5pV326N and cells with the empty vector did not show increased fluorescence. This result showed that amyloid nanodomain formation accompanied force-dependent activation and catch bonding for Als5p.
FIG 5.
Effect of shear stress on thioflavin T fluorescence of Als5p-expressing S. cerevisiae cells. Bright-field and corresponding fluorescence micrographs of Als5p-expressing cells. Micrographs are representative field enlargements of the channel surface. Fluorescence micrographs are photographic negative representations. Scale bar, 5 μm.
Role of S. cerevisiae flocculins in amyloid-mediated catch bonding and biofilm formation.
The S. cerevisiae flocculin proteins Flo1p and Flo11p are not homologous to each other or to the Als adhesins. Nevertheless, they also contain TANGO-predicted amyloid sequences (12). Therefore, we tested whether high shear could activate the cell-to-surface binding. Flo1p- or Flo11p-expressing S. cerevisiae cells were allowed to adhere on the surface of the flow channel for 2 h in flowing buffer. More Flo11p-expressing cells remained attached to the channel surface after shear at 0.8 dyne/cm2 than at 0.02 dyne/cm2 (see Fig. S6 in the supplemental material). Similar results were seen with Flo1p-expressing cells: at 0.8 dyne/cm2, more cells remained bound than at 0.02 dyne/cm2. As with Als5p-mediated binding, cells washed at 0.8 dyne/cm2 were more resistant to dislodgement during washing at 20 dyne/cm2. When flocculin-expressing cells were sheared in the presence of 0.2 mM ThS or with 0.5 mM CR, ThS decreased the binding of cells expressing either flocculin. CR inhibited the binding of Flo11p-expressing cells but not Flo1p-expressing cells (see Fig. S6).
Flo11p-expressing cells also showed development of ThT surface fluorescence during shear at 0.5 dyne/cm2 after 35 min. Cells sheared at 0.01 dyne/cm2 did not show a fluorescence increase, nor did flo11-deleted cells (Fig. 6).
FIG 6.
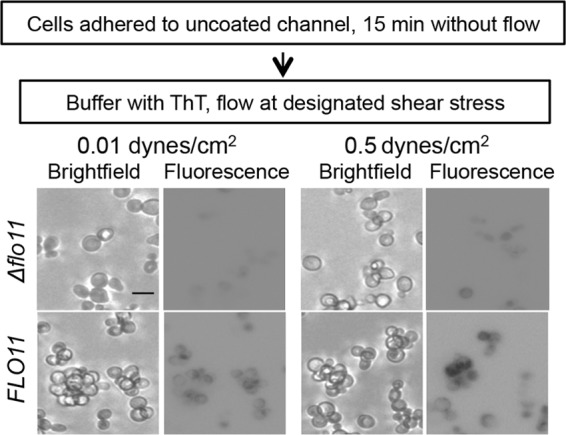
Effect of shear stress on thioflavin T fluorescence of S. cerevisiae var. diastaticus expressing Flo11p and a corresponding flo11 deletion strain. Cells were adhered to the surface without flow and then subjected to flow at the designated shear stress for 35 min in the presence of 1 mM ThT. Micrographs are representative field enlargements of the channel surface. Fluorescence micrographs are photographic negative representations. Scale bar, 5 μm.
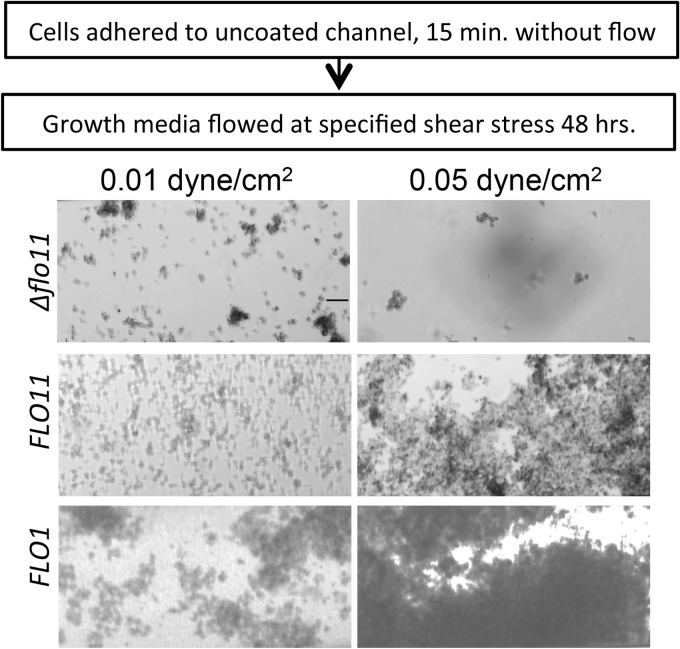
Flo11p- and Flo1p-expressing cells formed thicker biofilms after overnight shearing at 0.05 dyne/cm2 than cells that were sheared at 0.01 dyne/cm2 (Fig. 7). (Higher shear stress led to formation of biofilms that blocked the channels.) S. cerevisiae var. diastaticus cells with the genomic deletion for the flocculins did not form a thick biofilm. Therefore, Flo1p and Flo11p showed properties similar to those of Als5p under flow and formed similarly robust biofilms.
FIG 7.
Effect of shear stress on flocculin-mediated biofilm formation. Flo11p- or Flo1p-expressing S. cerevisiae cells were seeded on surfaces and then grown under flow at the designated shear stress for 24 h. Scale bar, 25 μm.
DISCUSSION
Our data support several findings on shear activation of yeast adhesins. First, laminar flow promoted increased cell binding to surfaces and increased cell aggregation. Second, this activation was dependent on shear-induced formation of functional surface adhesin amyloids. Third, this shear-dependent activation led to formation of robust biofilms. A model for the mechanism is shown in Fig. 8. Shear flow, like other extension forces, leads to the unfolding of T domains. This unfolding exposes the amyloid-forming sequences, which then associate strongly to form surface amyloid nanodomain patches that bind to surfaces and to other fungi with high avidity (7, 22, 23). We tested three unrelated yeast adhesins, Als5p, Flo1p, and Flo11p. Each adhesin was activated by forces greater than 0.02 dyne/cm2, and for each, activation was accompanied by formation of surface amyloids. Thus, formation of adhesin amyloid nanodomains led to increased binding under shear, behavior that characterizes catch bonding (13–17). To our knowledge, catch bonding has not been reported previously in yeast or other fungi.
FIG 8.
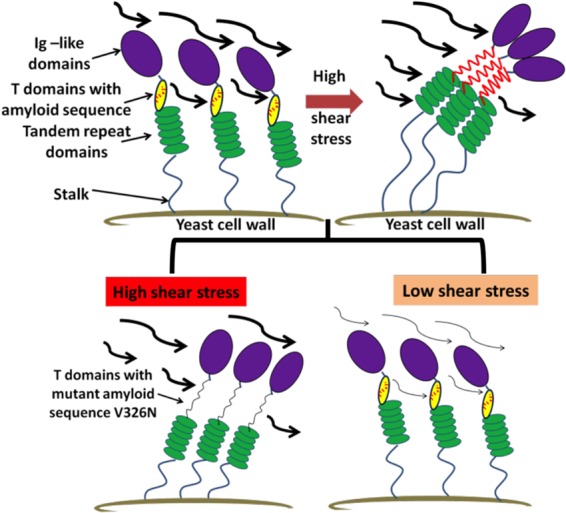
Model for shear stress activation of Als5p. (Top left) Model showing the domains of Als5p on the cell surface; the amyloid-forming sequences (red) are buried inside the folded T domains (yellow). (Top right) Under strong shear stress, the T domains unfold, the amyloid-forming sequences are exposed, and surface amyloid nanodomains form. (Bottom) If the amyloid-forming sequence is absent, as in Als5pV326N (left), or if the shear stress is weak, then the nanodomains cannot form.
Shear activation of yeast adhesins.
Single-molecule atomic force microscopy shows that pulling on individual Als5p molecules on the surface of a live cell leads to clustering of the adhesins into amyloid nanodomain patches (7, 22). As a result, the macroscopic dissociation rate is reduced as an exponential function of the number of arrayed adhesins, a phenomenon known as avidity. Thus, surface nanodomains mediate strong binding through increased avidity for ligand. The binding becomes extremely strong despite the relatively low affinity of an individual adhesin molecule for its ligand (23).
Our results here show similar behavior in cells subjected to laminar flow. Such flow activated C. albicans or S. cerevisiae cells expressing Als5p, Flo1p, or Flo11p to bind to coated and uncoated surfaces and to aggregate as well. Some of the cells bound at 0.8 dyne/cm2 were resistant to dislodgment from the channel surface at 20 dyne/cm2 shear stress. As a comparison, forces in the circulatory system can be in the range of 5 to 20 dyne/cm2 for large arteries and less in capillaries. In this range, bacteria change from rolling along the surface to stable binding as catch bonding is activated. The adhesin-expressing yeasts show similar behavior (see Videos S1 to S3 in the supplemental material). Thus, the fungal systems responded to forces of 0.1 to 1.6 dyne/cm2, similar to or slightly lower than those of other catch-bonding systems (22–27). Therefore, C. albicans and S. cerevisiae show catch-bonding behavior similar to that of bacteria and leukocytes.
Amyloid formation in shear-activated yeast adhesins.
AFM-induced nanodomains are ThT fluorescent and birefringent, and their formation depends on the presence of amyloid-forming sequences in the adhesins (7, 12, 22, 28). Similarly, laminar-flow-induced activation had the characteristics of amyloid formation. Activation was accompanied by development of ThT surface fluorescence (Fig. 4 and 6). Anti-amyloid compounds, including ThS, CR, and an Als5p sequence-specific amyloid-blocking peptide, inhibited activation (Fig. 3 and 4; also see Fig. S5 and S6 in the supplemental material). There was also reduced activation of cells that expressed an amyloid-impaired Als5pV326N form of the adhesin. Together, these data imply that the mechanism of activation under flow is the same as that of AFM: formation of amyloid nanodomains with increased avidity.
The remaining activity of Als5pV326N reflected the normal functions of other domains of the adhesin. The peptide-binding Ig-like invasin domains and the tandem repeats, which mediate hydrophobic-effect interactions, remain intact and functional (7, 23, 29). Therefore, this amyloid-impaired form bound effectively to the surface, but the binding was weaker and was inhibited by both specific and nonspecific inhibitors. Such cells also were easily dislodged at high shear and formed reduced biofilms.
This shear-induced amyloid nanodomain activation appears to be general for yeast adhesins. The behavior of the S. cerevisiae Flo adhesins and C. albicans Als5p was similar, including flow-induced activation, sensitivity to CR and ThS, and development of surface fluorescence (Fig. 5 and 6; also see Fig. S8 and S9 in the supplemental material). C. albicans itself also showed similar behavior, probably reflecting amyloid-like properties of other Als and non-Als adhesins (Fig. 1; also see Fig. S6) (7, 12, 30). Additionally, C. albicans and other pathogenic fungi in abscesses in human tissue also display surface amyloids (31, 32, and T. Lundberg, M. C. Garcia-Sherman, R. E. Sobonya, P. N. Lipke, and S. A. Klotz, unpublished data). Thus, the activation by force appears to be a conserved mechanism for fungal adhesins (9, 12, 30).
Biofilm formation increased by shear stress.
Consequences of amyloid-dependent fungal catch bonding included larger aggregates and robust biofilms (Fig. 2 and 5; also see Fig. S2, S3, and S6 in the supplemental material). The amyloid-impaired Als5pV326N mutant did not form biofilms as thick or extensive as the wild type and showed reduced sensitivity to ThS inhibition. This result supports the idea that biofilm formation begins with the initial binding of cells to a surface and is dependent on amyloid-forming adhesion proteins on the cell surface. Bacterial biofilms show similar force dependence. The thickness of the Pseudomonas sp. strain CT07 gfp biofilms is reduced when flow is reduced from 0.95 dyne/cm2 to 0.09 dyne/cm2 (33). Seven Listeria monocytogenes strains had initial adhesion rates that were significantly greater for shear stress at 11 dyne/cm2 than at 1 dyne/cm2 on stainless steel (34). Biofilms of marine bacteria and water-supply bacteria show increased cohesion and durability as the shear stress is raised (35, 36). Thus, the behavior of the fungal biofilms under flow was similar to that of bacterial biofilms.
In summary, shear-flow-mediated activation of yeast adhesins illustrates several novel findings. First, the behavior is that of catch bonding, the strengthening of bonds under tension. Second, in yeast the catch-bonding behavior is explainable by the formation of amyloid-interacting arrays of cell adhesion molecules. To our knowledge, such a mechanism has not been reported previously. Third, amyloid nanodomain formation is a response to force in at least three different yeast adhesins in two species, and circumstantial evidence supports an even broader occurrence. Finally, this amyloid-dependent catch bonding is important in the formation of robust and flow-resistant biofilms.
Supplementary Material
ACKNOWLEDGMENTS
C.X.J.C. carried out the experiments. Both authors planned and analyzed experiments and wrote the paper.
We thank Alandra Mitchell, Yang Lin, Michael Cohen, Ivor Joseph, Melissa Garcia-Sherman, and Caleen Ramsook for assistance with some of the experiments and for helpful discussions.
This work was supported by NIH grants SC1 GM083756 and R01 GM098616.
Footnotes
Published ahead of print 28 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00068-14.
REFERENCES
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108. 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6:199–210. 10.1038/nrmicro1838 [DOI] [PubMed] [Google Scholar]
- 3.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. 2013. Sticking together: building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 11:157–168. 10.1038/nrmicro2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR. 2012. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 20:66–73. 10.1016/j.tim.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otzen D, Nielsen PH. 2008. We find them here, we find them there: functional bacterial amyloid. Cell. Mol. Life Sci. 65:910–927. 10.1007/s00018-007-7404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, Nielsen PH. 2007. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 9:3077–3090. 10.1111/j.1462-2920.2007.01418.x [DOI] [PubMed] [Google Scholar]
- 7.Garcia MC, Lee JT, Ramsook CB, Alsteens D, Dufrene YF, Lipke PN. 2011. A role for amyloid in cell aggregation and biofilm formation. PLoS One 6:e17632. 10.1371/journal.pone.0017632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 107:2230–2234. 10.1073/pnas.0910560107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dranginis AM, Rauceo JM, Coronado JE, Lipke PN. 2007. A biochemical guide to yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 71:282–294. 10.1128/MMBR.00037-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez M, Goddard N, Hicks C, Ovalle R, Rauceo JM, Jue CK, Lipke PN. 2010. A screen for deficiencies in GPI-anchorage of wall glycoproteins in yeast. Yeast 27:583–596. 10.1002/yea.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otoo HN, Lee KG, Qiu W, Lipke PN. 2008. Candida albicans Als adhesins have conserved amyloid-forming sequences. Eukaryot. Cell 7:776–782. 10.1128/EC.00309-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O'Meally S, Otoo HN, Khalaf RA, Dranginis AM, Gaur NK, Klotz SA, Rauceo JM, Jue CK, Lipke PN. 2010. Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot. Cell 9:393–404. 10.1128/EC.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isberg RR, Barnes P. 2002. Dancing with the host; flow-dependent bacterial adhesion. Cell 110:1–4. 10.1016/S0092-8674(02)00821-8 [DOI] [PubMed] [Google Scholar]
- 14.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. 2003. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423:190–193. 10.1038/nature01605 [DOI] [PubMed] [Google Scholar]
- 15.Thomas W. 2008. Catch bonds in adhesion. Annu. Rev. Biomed. Eng. 10:39–57. 10.1146/annurev.bioeng.10.061807.160427 [DOI] [PubMed] [Google Scholar]
- 16.Alon R, Chen S, Fuhlbrigge R, Puri KD, Springer TA. 1998. The kinetics and shear threshold of transient and rolling interactions of L-selectin with its ligand on leukocytes. Proc. Natl. Acad. Sci. U. S. A. 95:11631–11636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas WE, Nilsson LM, Forero M, Sokurenko EV, Vogel V. 2004. Shear-dependent “stick-and-roll” adhesion of type 1 fimbriated Escherichia coli. Mol. Microbiol. 53:1545–1557. 10.1111/j.1365-2958.2004.04226.x [DOI] [PubMed] [Google Scholar]
- 18.Coleman DA, Oh SH, Zhao X, Hoyer LL. 2010. Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody. Microbiology 156:3645–3659. 10.1099/mic.0.043851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer LL, Scherer S, Shatzman AR, Livi GP. 1995. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol. Microbiol. 15:39–54 [DOI] [PubMed] [Google Scholar]
- 20.Hoyer LL, Green CB, Oh SH, Zhao X. 2008. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family–a sticky pursuit. Med. Mycol. 46:1–15. 10.1080/13693780701435317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur NK, Klotz SA. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alsteens D, Garcia MC, Lipke PN, Dufrene YF. 2010. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl. Acad. Sci. U. S. A. 107:20744–20749. 10.1073/pnas.1013893107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipke PN, Garcia MC, Alsteens D, Ramsook CB, Klotz SA, Dufrene YF. 2012. Strengthening relationships: amyloids create adhesion nanodomains in yeasts. Trends Microbiol. 20:59–65. 10.1016/j.tim.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279:30480–30489. 10.1074/jbc.M401929200 [DOI] [PubMed] [Google Scholar]
- 25.Wilson D, Hube B. 2010. Hgc1 mediates dynamic Candida albicans-endothelium adhesion events during circulation. Eukaryot. Cell 9:278–287. 10.1128/EC.00307-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walshe TE, Ferguson G, Connell P, O'Brien C, Cahill PA. 2005. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro coculture model of the retinal vasculature. Investig. Ophthalmol. Vis. Sci. 46:375–382. 10.1167/iovs.04-0806 [DOI] [PubMed] [Google Scholar]
- 27.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. 2003. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 81:177–199. 10.1016/S0079-6107(02)00052-4 [DOI] [PubMed] [Google Scholar]
- 28.Rauceo JM, Gaur NK, Lee KG, Edwards JE, Klotz SA, Lipke PN. 2004. Global cell surface conformational shift mediated by a Candida albicans adhesin. Infect. Immun. 72:4948–4955. 10.1128/IAI.72.9.4948-4955.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank AT, Ramsook CB, Otoo HN, Tan C, Soybelman G, Rauceo JM, Gaur NK, Klotz SA, Lipke PN. 2010. Structure and function of glycosylated tandem repeats from Candida albicans Als adhesins. Eukaryot. Cell 9:405–414. 10.1128/EC.00235-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N. 2013. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot. Cell 12:470–481. 10.1128/EC.00364-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilchrist KB, Garcia MC, Sobonya R, Lipke PN, Klotz SA. 2012. New features of invasive candidiasis in humans: amyloid formation by fungi and deposition of serum amyloid P component by the host. J. Infect. Dis. 206:1473–1478. 10.1093/infdis/jis464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Sherman MC, Lysak N, Filonenko A, Richards H, Sobonya RE, Klotz SA, Lipke PN. 2014. Peptide detection of fungal functional amyloids in infected tissue. PLoS One 9:e86067. 10.1371/journal.pone.0086067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bester E, Wolfaardt GM, Aznaveh NB, Greener J. 2013. Biofilms' role in planktonic cell proliferation. Int. J. Mol. Sci. 14:21965–21982. 10.3390/ijms141121965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skovager A, Whitehead K, Siegumfeldt H, Ingmer H, Verran J, Arneborg N. 2012. Influence of flow direction and flow rate on the initial adhesion of seven Listeria monocytogenes strains to fine polished stainless steel. Int. J. Food Microbiol. 157:174–181. 10.1016/j.ijfoodmicro.2012.04.028 [DOI] [PubMed] [Google Scholar]
- 35.Salta M, Capretto L, Carugo D, Wharton JA, Stokes KR. 2013. Life under flow: a novel microfluidic device for the assessment of anti-biofilm technologies. Biomicrofluidics 7:64118. 10.1063/1.4850796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paris T, Skali-Lami S, Block JC. 2007. Effect of wall shear rate on biofilm deposition and grazing in drinking water flow chambers. Biotechnol. Bioeng. 97:1550–1561. 10.1002/bit.21321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.