Abstract
Among pathogenic environmental fungi, spores are thought to be infectious particles that germinate in the host to cause disease. The meningoencephalitis-causing yeast Cryptococcus neoformans is found ubiquitously in the environment and sporulates in response to nutrient limitation. While the yeast form has been studied extensively, relatively little is known about spore biogenesis, and spore germination has never been evaluated at the molecular level. Using genome transcript analysis of spores and molecular genetic approaches, we discovered that trehalose homeostasis plays a key role in regulating sporulation of C. neoformans, is required for full spore viability, and influences virulence. Specifically, we found that genes involved in trehalose metabolism, including a previously uncharacterized secreted trehalase (NTH2), are highly overrepresented in dormant spores. Deletion of the two predicted trehalases in the C. neoformans genome, NTH1 and NTH2, resulted in severe defects in spore production, a decrease in spore germination, and an increase in the production of alternative developmental structures. This shift in cell types suggests that trehalose levels modulate cell fate decisions during sexual development. We also discovered that deletion of the NTH2 trehalase results in hypervirulence in a murine model of infection. Taken together, these data show that the metabolic adaptations that allow this fungus to proliferate ubiquitously in the environment play unexpected roles in virulence in the mammalian host and highlight the complex interplay among the processes of metabolism, development, and pathogenesis.
INTRODUCTION
A fundamental requirement for organisms to survive is the ability to adapt to changes in the availability of metabolic fuels. Metabolic acclimation enhances survival in a given environment or provides the capacity to exploit a new one. In response to nutrient limitation, organisms generally decrease their growth rates; however, when nutrients become severely limiting, many microbes undergo a transition from active growth to dormancy (1). In some bacteria and fungi, this transition results in the development of a new cell type, the spore, and is coupled with structural and metabolic adaptations that allow spores to remain dormant and withstand severe environmental stresses (2–4). Once spores encounter more favorable growth conditions, they undergo another transition (germination) to resume vegetative growth (5).
For environmental human fungal pathogens, spores likely are infectious particles (6–9). The properties of spores that allow them to withstand harsh conditions and disperse throughout the environment (e.g., small size, tolerance for high temperatures, resistance to oxidative stress, etc.) also allow them to encounter hosts and initiate disease-causing infections. This is true of Cryptococcus neoformans, a human fungal pathogen for which both yeast and spore forms are plausible infectious particles (10). C. neoformans spores are produced on aerial fruiting bodies (basidia), allowing for dispersal which, along with their resistance to external stresses and ability to cause disease in a murine model of infection, has led to the hypothesis that C. neoformans spores are natural infectious particles (11, 12). C. neoformans infects people from environmental sources through a respiratory route in which the organism is inhaled and proliferates within the lung. In immunocompromised individuals, such as people with AIDS, this infection can disseminate to the central nervous system and cause meningoencephalitis (13). C. neoformans spores, also known as basidiospores, are produced during sexual development that occurs in response to nutritional signals and involves the formation of several new cell types that reside in multicellular structures. While the morphological changes that occur during sexual development have been described, relatively little is known about the metabolic signals or molecular pathways that influence cell fate decisions and ultimately lead to the production of spores and their ability to germinate in new environments.
To begin to understand spore biogenesis and germination, we carried out an analysis of spore transcripts in both dormant spores and a germinating population of spores. Transcripts of genes important for trehalose metabolism were highly overrepresented in dormant spores, suggesting a role for the sugar trehalose in C. neoformans spore biology. Trehalose is a disaccharide of two α-linked glucose molecules (α-1,1-glucose) that has been broadly linked to stress resistance and development in many organisms (14–18). In several fungi, trehalose biosynthesis has been intimately linked to virulence, morphogenesis, and the production of viable spores. In the human pathogen Candida albicans, trehalose biosynthesis is required for resistance to oxidative stress, and trehalose metabolism has been linked to both stress resistance and the yeast-to-hypha transition, both of which are important for virulence. Trehalose synthesis mutants of C. albicans also show decreased virulence (19–22). Similarly, trehalose has well-established roles in both virulence and spore production in the filamentous fungal pathogen Aspergillus fumigatus, in which trehalose synthesis is important for general stress response and the production of robust, stress-resistant spores (23). In contrast to other fungal pathogens, deletion of the trehalose biosynthesis genes in A. fumigatus, TPSA and TPSB, resulted in increased virulence in a murine model of invasive aspergillosis, likely caused by alterations in the cell wall, making mutant hyphae resistant to phagocytosis by macrophages (23). Taken together, these findings reveal a complex and diverse role for trehalose metabolism in regulating the physiology of fungal pathogens.
In C. neoformans, disruption of either of the genes involved in trehalose synthesis (TPS1 or TPS2) results in severe defects in both sexual development and virulence, but the mechanisms by which trehalose controls these functions are not known (24–26). Previously, a single trehalose-degrading enzyme, NTH1 (neutral trehalase 1), was studied, and surprisingly, deletion of this gene caused no observable phenotypes (25). Through our gene expression analysis of spores, we discovered a second predicted trehalose-degrading enzyme that we have designated NTH2 (neutral trehalase 2) that was overrepresented within dormant spores.
The discovery of this trehalase allowed us to assemble a more complete picture of trehalose metabolism and the relationships between trehalose synthesis and breakdown during spore formation, dormancy, and germination. We found that trehalase mutants harbor defects in sexual development, including aberrant fruiting body (basidium) formation, a decrease in spore formation, and decreased spore viability. We also observed that aberrant basidium formation was accompanied by an increase in the production of apparent chlamydospores, a previously described alternate developmental cell type (26), implicating trehalose as a signaling molecule in C. neoformans cell fate determination. Finally, we determined that the absence of NTH2 led to a significant increase in virulence in a murine model of infection, suggesting that the trehalose catabolic pathway is active in the mammalian host. Overall, our results indicate that trehalose metabolism is a core component of both sexual development and pathogenesis and underscore the importance of trehalose homeostasis in these diverse processes in C. neoformans.
MATERIALS AND METHODS
Strains and media.
All strains used were of the serotype D background (Cryptococcus neoformans var. neoformans strains JEC20 [a] and JEC21 [α]) (27) (Table 1). All were handled using standard techniques and media as described previously (28). Sexual development assays were conducted on V8 agar medium, pH 7.0, at 22°C in the dark for 2 to 7 days.
TABLE 1.
Strains and oligonucleotides used in this study
| Strain | Genotype | Reference/source or descriptiona |
|---|---|---|
| C. neoformans var. neoformans serotype D | ||
| JEC20 | MATa | 27 |
| JEC21 | MATα | 27 |
| CHY2083-2085 | MATa nth2::NEOR | This study |
| CHY2089-2091 | MATα nth2::NEOR | This study |
| CHY1705-1707 | MATa nth1::NATR | This study |
| CHY1711-1713 | MATα nth1::NATR | This study |
| CHY2182-2183, 2188 | MATa nth1::NATR nth2::NEOR | This study |
| CHY2180-2181, 2184 | MATα nth1::NATR nth2::NEOR | This study |
| Oligonucleotides used in cloning and PCR | ||
| CHO1565 | TAATCAGTGGGTGAGGTGTG | NTH1 5′ Flank FWD |
| CHO1566 | GCTCACATCCTCGCAGCCTCGAAGTTGGAGTGAAGGAGA | NTH1 5′ Flank REV |
| CHO1567 | CTAGTTTCTACATCTCTTCCCTGGAAACATTCTTGATGG | NTH1 3′ Flank FWD |
| CHO1568 | TCTCGCCCCTCCTAAACTAT | NTH1 3′ Flank REV |
| CHO1569 | TCTCCTTCACTCCAACTTCGAGGCTGCGAGGATGTGAGC | NATR cassette FWD |
| CHO1570 | CCATCAAGAATGTTTCCAGGGAAGAGATGTAGAAACTAG | NATR cassette REV |
| CHO2636 | GTGTATCCAATCCAAGCACT | NTH2 5′ Flank FWD |
| CHO2637 | GTCATAGCTGTTTCCTGGAGCTTTCGTTCATCAAGTC | NTH2 5′ Flank REV |
| CHO2764 | CATCCTCGCAGCAAGGGCGTTTTGAGGTTCGAGGGTGAG | NTH2 3′ Flank FWD |
| CHO2765 | CCCATACTACCGCAGCAATC | NTH2 3′ Flank REV |
| CHO2640 | GACTTGATGAACGAAAGCTCCAGGAAACAGCTATGAC | NEOR cassette FWD |
| CHO2641 | GGCTATGTTCAATGGGTATCCGCCCTTGCTGCGAGGATG | NEOR cassette REV |
| CHO2979 | GCCTTCTCGAGGGGCTGGGA | NTH1 northern probe FWD |
| CHO2980 | CGTGGGCTTCGCCAGTCGTT | NTH1 northern probe REV |
| CHO2938 | GCCGCCCCATGCGTGTAAGT | NTH2 northern probe FWD |
| CHO2939 | CTCACCACCTCCTCCAGCGG | NTH2 northern probe REV |
FWD, forward; REV, reverse.
Microscopy.
All micrographs were created using an Axio Scope.A1 with long-working-distance objectives and an AxioVision MRM camera running AxioVision software.
RNA isolation and amplification.
Spores were isolated by scraping sexually developing populations of cells from V8 agar and suspending them in 65% Percoll made isotonic with phosphate-buffered saline (PBS). Suspensions were subjected to centrifugation (10,000 × g, 30 min, 4°C), and spores were collected from the bottom of the gradient and counted. An aliquot of 4 × 108 spores was frozen in liquid N2 to serve as the zero-time-point sample. The remaining spores (1.6 × 109) were resuspended in 6 ml of liquid yeast extract-peptone-dextrose (YPD) and incubated at 30°C with shaking at 200 rpm. Samples were collected every 2 h for 10 h and frozen in liquid nitrogen. RNA was extracted from frozen spores using hot acid-phenol as described previously and was further purified using the RNeasy minikit (Qiagen, Valencia CA) (29). RNA amplification was carried out using the MessageAmp II amplification kit (Ambion) according to manufacturer specifications. Five hundred ng of total RNA from each germinating time point was used as the starting material. After production of antisense RNAs containing aminoallyl-UTPs (aRNAs), each sample was assessed for quantity and average length of aRNA using an RNA Pico chip and an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Before hybridization, equal amounts of aminoallyl-UTP-incorporated aRNA were conjugated to oyster dyes (Boca Scientific) according to the manufacturer's instructions.
Microarray hybridization and analysis.
Fluorescently labeled aRNAs were hybridized to C. neoformans whole-genome spotted microarrays of 70-mer oligonucleotides containing 7,765 open reading frame (ORF) probes. The data presented for each experiment represent 8-fold coverage of ORFs: quadruplicate experiments (including dye swap), with each slide containing ORF probes spotted in duplicate. Slides were incubated as reported previously (30). Extracted data were analyzed using the GeneSpring 10.0 software package (Agilent Technologies, Santa Clara, CA). Data were normalized using the LOWESS (locally weighted scatter plot-smoothing) algorithm and assessed for statistical significance utilizing analysis of variance (ANOVA) (31). A total of 2,512 genes meeting the criterion of P < 0.05 were considered statistically significant and used in further analyses (32). Significant genes meeting a 2-fold-change threshold between any two time points (1,499) were clustered using Cluster 3.0. Clusters were assessed for KEGG database pathway and Gene Ontology (GO) term enrichment using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (33–35).
Generation of deletion strains.
Gene deletion constructs were created using fusion PCR (36) (oligo sequences are listed in Table 1). The NTH1 5′ region was amplified with primers CHO1565 and CHO1566, and the 3′ region was amplified with CHO1567 and CHO1568. The NATR cassette was amplified with CHO1569 and CHO1570. PCR fusion using CHO1565 and CHO1568 was used to create the final nth1::NATR deletion cassette. The NTH2 5′ region was amplified with primers CHO2636 and CHO2637, and the 3′ region was amplified with CHO2638 and CHO2639. The NEOR cassette was amplified with CHO2640 and CHO2641. PCR fusion using CHO2636 and CHO2639 was used to create the final nth2::NEOR deletion cassette. The nth1::NATR and nth2::NEOR deletion cassettes were transformed into JEC20 and JEC21 by biolistic transformation, grown on medium containing 1 M sorbitol, and selected on medium containing 200 μg/ml G418 or 200 μg/ml nourseothricin (37). Resulting colonies were screened using PCR for correct insertion of the knockout construct and assessed via Southern blotting for single integration of the construct to identify multiple independent knockouts (29). Each mutant then was crossed with wild-type yeast to obtain knockout progeny in both mating types. The resulting F1 progeny then were rescreened by PCR for the absence of the deleted gene to confirm the strain genotype. nth1Δ nth2Δ double mutants were constructed by backcrossing a nth2Δ (CHY2082) with α nth1Δ (CHY1710), isolating and germinating spore progeny from this cross, and then replica plating these progeny onto solid medium containing both nourseothricin and G418. Double mutants then were rescreened by PCR to confirm the absence of NTH1 and NTH2 open reading frames (CHY2184).
NTH1 and NTH2 transcript analysis.
RNA was prepared from C. neoformans yeast or crosses as described previously (30). Northern blots were conducted according to standard protocols using 10 μg total RNA per sample. Probes were generated by PCR amplification utilizing the Ex Taq PCR system (TaKaRa Bio Inc.) and oligonucleotides CHO2979 and CHO2980 (NTH1), CHO2938 and CHO2939 (NTH2), and CHO1696 and CHO1697 (GPD1). Probes were radiolabeled using Ready-To-Go DNA labeling beads (GE Healthcare Life Sciences, Piscataway, NJ) according to the manufacturer's instructions and hybridized to blots at 65°C as described previously (38). Blots were exposed to a phosphor screen and imaged with a Molecular Dynamics Storm 860 phosphorimager (GE, Amersham).
Quantification of trehalose.
Trehalose was quantified in C. neoformans crosses using a colorimetric enzyme-linked assay previously used in S. cerevisiae (39). Crosses were spotted on V8 for 5 days, harvested into 100 mM sodium citrate, pH 5.7, and boiled for 10 min. The resulting suspensions were lysed using sonication on a 30% duty cycle at level 2 for 5 min, and lysis was confirmed microscopically. Insoluble cell debris was pelleted and discarded. Lysates (100 μl) were treated with 1 U of porcine kidney trehalase (Sigma-Aldrich) for 4 h at 37°C. Glucose produced was quantified using a glucose assay kit (Sigma-Aldrich) according to the manufacturer's instructions. Values of absorbance at 540 nm for lysates were compared to a standard curve of glucose and normalized to the starting mass of the cell pellet. Calculated trehalose levels were subjected to one-way ANOVA comparing each sample to an a × α cross.
Phenotype testing.
A battery of common phenotypes was assessed, including melanin production, capsule production, growth at 37°C, and cell wall integrity. In each case, wild-type, nth1Δ, nth2Δ, and nth1Δ nth2Δ strains were evaluated. For melanin production, we grew cells on l-3,4-dihydroxyphenylalanine (l-DOPA) agar at 37°C for 3 days and visually assessed the production of pigment. For capsule production, we cultured strains in Dulbecco's modified eagle medium (DMEM) with 5% fetal calf serum at 37°C in 5% CO2 (40) for 2 days and then stained them with India ink and assessed them microscopically (41). For growth at 37°C, we spotted serial dilutions of cells onto YPD agar, incubated them at 37°C for 2 days, and assessed them visually (25). For cell wall integrity, we spotted serial dilutions of cells onto medium containing calcofluor white MR2, sodium dodecyl sulfate, or caffeine and grew them for 2 days at 37°C (42). For resistance to heat shock, we grew cells on V8 agar, pH 7.0, at 25°C for 2 days; suspended them in PBS; incubated them at 42°C and 50°C for 5-, 10-, 15-, and 30-min intervals; diluted them; and plated them onto YPD for 3 days at 30°C (3). For resistance to oxidative stress, we spread 1 × 106 yeast onto a 100-mm-diameter V8 agar plate with a 6-mm disc of Whatman paper containing 10 μl 10% H2O2 in the center and incubated them at 30°C for 3 days (43). Germination frequencies were determined by carrying out crosses on V8 for 5 days and isolating spores. Spores were counted, plated onto rich medium, and allowed to germinate and grow at 30°C for 3 days. Germination frequencies were determined by dividing the number of colonies formed by the number of spores plated. Three replicates were performed for each strain, and the mean results for each strain were compared to those of the wild type using a Student t test.
Virulence analysis.
Strain virulence was assayed using a murine intravenous model of infection (44). Wild-type α, α nth1Δ, α nth2Δ, and α nth1Δ nth2Δ yeast were grown to mid-log phase and washed three times in PBS. Five female BALB/c mice were inoculated via tail vein with 1 × 106 yeast of each strain. Only the yeast cell type was used to infect the mice, because we could not recover sufficient numbers of mutant spores to carry out spore-mediated infections. The mice were observed daily for rapid weight loss or signs of pain, at which point moribund mice were euthanized as described previously (12). The resulting survival times were plotted on a Kaplan-Meier plot, and median survival times of mice were analyzed using a Wilcoxon rank-sum test. This experiment was carried out twice with independently derived mutant strains, and the results were identical between experiments. The animal studies were reviewed and protocol approved by the University of Wisconsin–Madison Institutional Animal Care and Use Committee (protocol A01506). The University of Wisconsin-Madison is AAALAC accredited, and the facilities meet and adhere to the standards in the Guide for the Care and Use of Laboratory Animals (59).
Microarray data accession number.
The full data set determined in the course of this work has been deposited in the NCBI Gene Expression Omnibus database (GEO) and can be accessed through GEO accession number GSE53912 (45).
RESULTS
Trehalose metabolism genes are overrepresented in dormant spores.
To determine changes in gene expression that occur during spore germination into yeast, total RNA was isolated from spores undergoing synchronous germination for whole-genome transcript analysis. Transcripts were competitively hybridized to a whole-genome microarray, and the resulting changes in transcript levels were assessed. Coexpressed groups of genes were submitted to DAVID to assess functional groups and to the KEGG database to determine placement within pathways.
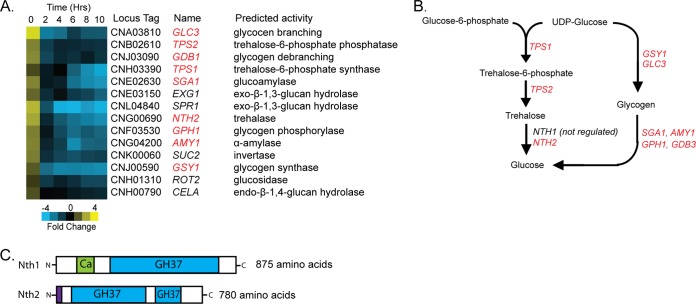
One of the most enriched pathways within dormant spores was the KEGG annotated pathway for starch and sucrose metabolism. Transcripts of 14 genes in this pathway showed a pattern of regulation in which their transcripts were overrepresented in dormant spores and became rapidly underrepresented during the first 2 h of germination (Fig. 1A). Some spore-enriched genes that fall into this metabolic pathway have been implicated previously in spore- or germination-specific functions in other systems: SGA1, which is highly similar to a spore-specific glucoamylase in S. cerevisiae, is responsible for glycogen degradation during sporulation; SPR1 is a sporulation-specific exo-β-1,3-glucan hydrolase in S. cerevisiae; and CELA is a germination-specific spore coat-modifying enzyme from the spore-forming amoeba Dictyostelium discoideum (46–48). Detection of these transcripts suggests that their roles are conserved across phyla. In addition, nine of the spore-enriched transcripts in this pathway are involved directly in the biosynthesis and breakdown of trehalose or glycogen (Fig. 1A and B).
FIG 1.
Trehalose metabolism gene transcripts are overrepresented in spores. (A) Heat map of genes in the KEGG database starch and sucrose metabolism pathway that were spore enriched. Relative fold change is represented from −4 (blue) to +4 (yellow). Each column represents a time point of germination in hours. Dormant spore transcripts are represented in 0 h. Yeast transcripts are represented in 10 h. Each row represents a gene. (B) A schematic of genes predicted to act in the trehalose and glycogen metabolic pathway, with those found to be spore enriched in red. Only one gene, NTH1, was found not to be spore enriched. (C) A schematic of predicted C. neoformans trehalases, Nth1 and Nth2, and their functional domain content, including the family 37 glycosyl hydrolase α,α-trehalase domain (GH37; blue), calcium binding regulatory domain (Ca; green), and predicted signal peptide (purple).
Among these genes was CNG00690, predicted to encode a trehalose-degrading enzyme. The presence of CNG00690 was unexpected, because previous analyses of trehalose synthesis and degradation in C. neoformans had not identified this gene (24, 25). Trehalose biosynthesis genes had been shown to be critical for both the initiation of sexual development (TPS1) and virulence in animal models (TPS1 and TPS2), but deletion of the only known neutral trehalase, NTH1, yielded only a modest decrease in growth on trehalose and no discernible phenotypes in sexual development or virulence (24). This finding was somewhat surprising, because many fungi harbor more than one trehalase gene, and their products often play key roles in fungal metabolism (49–51). Our discovery of the enrichment of the CNG00690 transcript in dormant spores led us to hypothesize that this gene (which we have named NTH2, for neutral trehalase 2) could encode an uncharacterized trehalase with specialized functions in sexual development and/or spore biology.
The presence of NTH2 is consistent with the fact that fungi often harbor both cytosolic and secreted trehalases with characteristic features: cytosolic trehalases usually contain a calcium binding domain and a single trehalase domain, while secreted trehalases harbor a secretion signal and two trehalase domains (52, 53). Through their predicted functional domains and features, NTH1 and NTH2 appear to be the only two genes encoding trehalase activity in the C. neoformans genome; NTH1 is a predicted cytosolic trehalase, and NTH2 is a predicted secreted trehalase (Fig. 1C). The presence of NTH2 may account for the lack of phenotypes in NTH1 deletion strains in previous studies and opens the possibility that the degradation of trehalose plays key roles in C. neoformans biology.
NTH1 and NTH2 are differentially regulated under sexual development conditions.
To determine the expression profiles of NTH1 and NTH2 under different conditions, we carried out RNA blot hybridization using RNA from wild-type strains grown in rich medium (YPD broth, mid-log phase), from a cross (a × α on V8, pH 7.0, for 72 h), and from purified spores. As suspected based on previous reports, NTH1 transcript was abundant in cells grown in rich medium, cross conditions, and in spores, suggesting that this trehalase is expressed constitutively (25). In contrast, expression of NTH2 was differentially regulated. While NTH2 transcript was abundant in both crosses and purified spores, none was detected from cells grown in rich medium (Fig. 2A).
FIG 2.
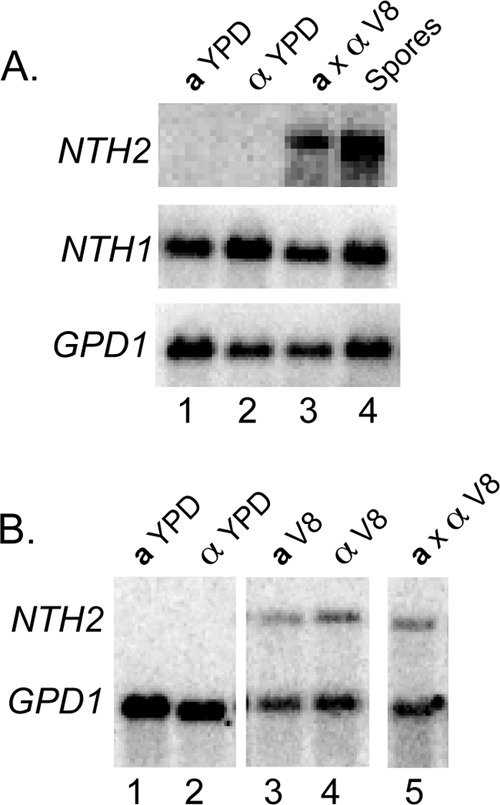
NTH1 and NTH2 are differentially expressed. (A) NTH2 is induced during sexual development. Lanes: 1 and 2, a and α yeast grown in rich medium (YPD liquid); 3, a and α yeast mixed under cross conditions (V8 agar, pH 7, at 22°C for 72 h); 4, spores from a cross after 72 h. Top, middle, and bottom images reflect probes to the transcripts of interest: NTH2, NTH1, and GPD1, respectively. (B) NTH2 is induced on V8 agar. Lanes: 1 and 2, a and α yeast grown on rich medium (YPD agar); 3 and 4, a and α yeast grown individually on V8 agar for 48 h; 5, a and α yeast grown in mixed culture on V8 agar for 48 h. Probes used were to NTH2 and GPD1. GPD1 was used as a loading and hybridization control.
To determine the extent to which NTH2 expression was dependent on signals from V8 agar medium versus the process of sexual development, we analyzed RNA from yeast propagated on V8 agar in the absence or presence of a mating partner for 48 h. NTH2 transcript levels increased on V8 medium both in the absence and presence of a mating partner. This finding indicates that NTH2 is induced largely by V8 medium alone (the same condition that promotes sexual development) (Fig. 2B). In contrast to the constitutive expression of NTH1, NTH2 expression is responsive to changes in metabolic conditions.
NTH1 and NTH2 are required for trehalose metabolism.
To determine the functions of NTH1 and NTH2, we created single and double deletion strains and evaluated them for discernible phenotypes, including the ability to use trehalose as a sole carbon source. Wild-type and mutant strains (nth1Δ, nth2Δ, and nth1Δ nth2Δ) were grown on solid synthetic medium containing 2% glucose or 2% trehalose as the sole carbon source (Fig. 3A). The wild-type strain and all of the trehalase deletion strains (nth1Δ, nth2Δ, and nth1Δ nth2Δ) grew similarly on medium containing glucose. However, on medium containing trehalose as the sole carbon source, nth1Δ strains grew more slowly and to a lower density than wild-type strains, nth2Δ strains showed no difference in growth compared to the wild type, and nth1Δ nth2Δ double mutants showed severe growth defects with little or no growth on trehalose-containing medium. Together with the observation that NTH1 and NTH2 contain the only identifiable trehalase domains in the C. neoformans genome, these results indicate that both NTH1 and NTH2 encode functional trehalases and provide compelling evidence that these are the only trehalases in C. neoformans. Furthermore, given that NTH2 can partially compensate for the loss of NTH1, these trehalases harbor overlapping (redundant) functions in trehalose catabolism in vegetatively growing cells.
FIG 3.
Deletion of NTH1 and NTH2 causes a severe synthetic defect in trehalose utilization. (A) Wild-type a, wild-type α, a nth1Δ, α nth1Δ, a nth2Δ, α nth2Δ, a nth1Δnth2Δ, and α nth1Δnth2Δ strains (left) were struck onto synthetic defined media containing either 2% glucose (middle) or 2% trehalose (right) as a sole carbon source and grown for 2 days at 30°C. (B) Trehalose was quantified in wild-type and mutant crosses after 5 days on V8, pH 7.0. Mean trehalose values from three biological replicates were compared using one-way ANOVA statistics, and those crosses that differed significantly (P < 0.05) from the wild type (a × α) are marked with an asterisk.
Given this relationship during vegetative growth, we hypothesized that the contributions of NTH1 and NTH2 during sexual development also would be at least partially redundant. In the absence of NTH1 and NTH2, we predicted that trehalose would accumulate and that this accumulation would be additive in the double mutant. To test this hypothesis, trehalose levels were quantified from wild-type and mutant crosses after 5 days on V8 medium. In contrast to our expectations, we observed that deletion of NTH1 and NTH2 had opposing effects on trehalose accumulation: nth1Δ crosses accumulated significantly more trehalose than wild-type crosses, whereas nth2Δ crosses harbored significantly less trehalose than the wild type (P < 0.05 in both cases). The double mutant crosses (nth1Δ nth2Δ) produced levels of trehalose similar to those of the wild type (Fig. 3B). These data indicate a complex relationship between NTH1 and NTH2 in trehalose homeostasis during development that may be due (at least in part) to the predicted differences in localization between Nth1 and Nth2 (Nth1 cytoplasmic and Nth2 secreted). The levels of intracellular trehalose and glucose likely are influenced by both external and internal sources, and the activities of Nth1 and Nth2 on different pools of trehalose could have significant effects on the outcome of overall trehalose levels. It also is possible that differences in the localization of trehalase activity among specific cell types (e.g., yeast versus basidia) that control local trehalose levels were not detected using this assay due to homogenization of trehalose levels across the entire population of developing cells (yeast, filaments, basidia, and spores).
Deletion of NTH1 and NTH2 leads to a defect in sporulation.
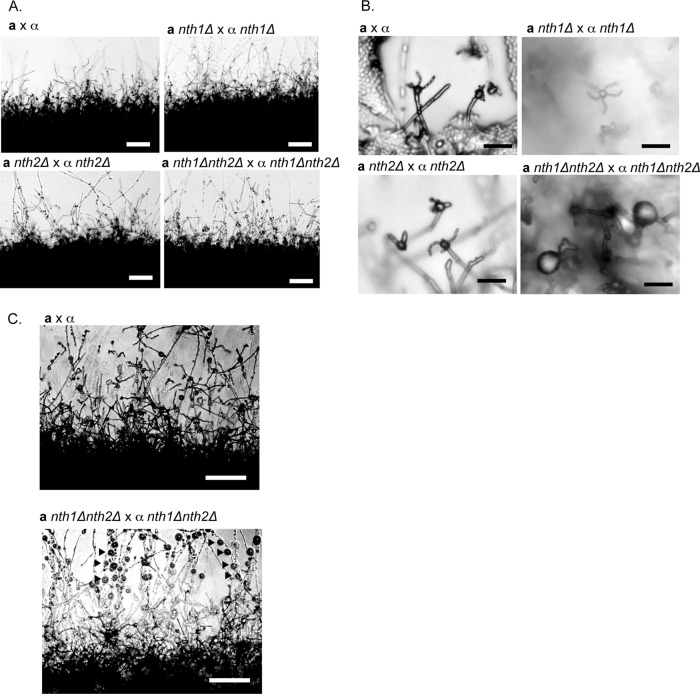
To investigate the roles of trehalases in sexual development, nth1Δ, nth2Δ, and nth1Δ nth2Δ mutant strains were crossed with wild-type strains and with each other under sexual development conditions. Crosses between wild-type strains and all of the mutants (nth1Δ, nth2Δ, and nth1Δ nth2Δ) were indistinguishable from wild-type a × α crosses, as were nth1Δ × nth1Δ and nth2Δ × nth2Δ crosses (Fig. 4). In stark contrast, however, crosses between nth1Δ nth2Δ mutants (nth1Δ nth2Δ × nth1Δ nth2Δ) led to severe defects in spore formation, indicating that both genes have redundant functions in this process.
FIG 4.
Deletion of NTH1 and NTH2 leads to aberrant sexual development. (A) The wild-type, nth1Δ, nth2Δ, and nth1Δ nth2Δ crosses after 5 days all show normal filament formation. Magnification, ×100; bar, 100 μm. (B) nth1Δ nth2Δ crosses form large, ball-like structures in place of basidia and do not produce spore chains. Magnification, ×400; bar, 10 μm. (C) After 7 days of development, nth1Δ nth2Δ crosses produced numerous large, swollen nodules on filaments, giving them a beads-on-a-string appearance (black arrows), consistent with previous descriptions of chlamydospores. Magnification, ×200; bar, 50 μm.
At low microscopic resolution, nth1Δ nth2Δ × nth1Δ nth2Δ crosses were observed to be similar to wild-type crosses, with abundant filaments radiating from the periphery of cells undergoing sexual development (Fig. 4A). However, under careful examination at higher magnification, it was clear that the majority of basidia in nth1Δ nth2Δ crosses did not develop spore chains, and strikingly, these sporeless basidia appeared extremely enlarged compared to wild-type basidia (Fig. 4B). In addition, nth1Δ nth2Δ crosses exhibited the production of an unusually high number of large, ball-like structures, giving the developing filaments a beads-on-a-string appearance (Fig. 4C). Structures like these have been described previously and referred to as chlamydospores (26). The purpose of this cell type is poorly understood; however, they have been observed to be metabolically active, to harbor glycogen, and to be capable of budding off new yeast. It has been hypothesized that C. neoformans chlamydospores offer an alternative fate for sexually developing cells and a means of producing new satellite colonies independent of sporulation (26). The structures produced in abundance by nth1Δ nth2Δ crosses also were capable of budding off yeast, were found to harbor glycogen via iodine staining (data not shown), and were morphologically indistinguishable from the previous description of chlamydospores. These features led us to conclude that the nth1Δ nth2Δ structures very likely were the same type of cells designated previously as chlamydospores in C. neoformans (26). Given the phenotypes of nth1Δ nth2Δ mutant strains, we posit that the ratio of trehalose to glucose in basidia is a determinant of cell fate. The cleavage of trehalose by NTH1 and NTH2 drives spore formation, whereas the accumulation of trehalose in the nth1Δ nth2Δ strain shifts the developmental path, resulting in a marked increase in alternative cell type (chlamydospore) production.
Spores harboring trehalase deletions show defects in germination.
Given the enrichment of NTH2 transcripts in spores and the importance of trehalase function in sporulation, we hypothesized that trehalases also are important for germination. Despite the observation that nth1Δ nth2Δ × nth1Δ nth2Δ crosses show a marked decrease in spore numbers, these crosses still produced a limited number of spores that were morphologically indistinguishable from wild-type spores by light microscopy (data not shown). To test whether spores from trehalase-deficient crosses were viable, spores were isolated from wild-type, nth1Δ, nth2Δ, and nth1Δ nth2Δ crosses, counted, and grown on rich medium. As expected, nearly 100% of wild-type spores germinated and grew into colonies (Fig. 5). Similarly, spores from nth1Δ and nth2Δ crosses also germinated at wild-type frequencies. In contrast, however, spores from nth1Δ nth2Δ crosses showed significant decreases in germination frequency, with only 33% of spores forming colonies (Fig. 5). These data indicate that nth1Δ nth2Δ crosses not only lead to aberrant basidium formation and a decrease in spore formation but also result in substantial numbers of spores that are unable to grow in response to germination conditions. These findings again indicate overlapping functions of NTH1 and NTH2.
FIG 5.
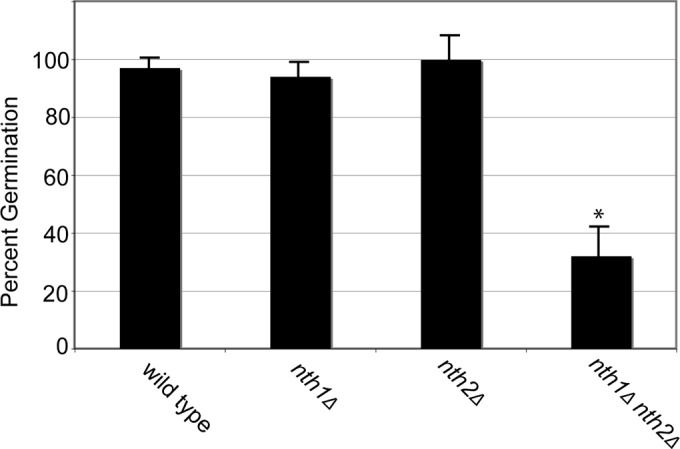
Trehalase-deficient spores are defective for growth. Spores from the wild type and nth1Δ, nth2Δ, and nth1Δ nth2Δ crosses were isolated, counted, and cultured on rich medium for 3 days at 30°C. The x axis represents the genotype of spores. The y axis represents the percentage of spores plated that produced a colony. Resulting mean germination frequencies from three independent biological replicates were compared using ANOVA, with only nth1Δ nth2Δ spores having a significant reduction (P < 0.05) in germination.
The nth2Δ deletion strain is hypervirulent in a mouse model of cryptococcosis.
To evaluate the roles of trehalase function in pathogenesis, we assessed the virulence of the trehalase mutant strains in a mouse tail vein model of infection. It had been shown previously that deletion of NTH1 alone had no effect on virulence (25). This result was initially surprising because of the predicted important roles for trehalose in stress tolerance and the apparent absence of other trehalases in the genome (17). However, our identification of NTH2, and the obviously redundant roles of these genes described above, raised the possibility that deletion of NTH1 resulted in no phenotype due to the presence of NTH2-derived trehalase activity (Fig. 3A).
Wild-type, nth1Δ, nth2Δ, or nth1Δ nth2Δ strains were inoculated into mice via tail vein at 1 × 106 yeast cells/mouse. As expected, the effects of wild-type and nth1Δ strains were indistinguishable from one another, causing mean times to death of 34 and 35 days, respectively (Fig. 6). In contrast, however, the nth2Δ and nth1Δ nth2Δ strains both caused morbidity in mice significantly faster (P < 0.05) (average time to death of 27 and 28 days, respectively) than wild-type and nth1Δ strains, indicating increased virulence in the absence of NTH2. This result is consistent with the previous findings that strains harboring deletions of the trehalose biosynthesis genes TPS1 and TPS2 are less virulent. It seems likely that deletion of the biosynthesis genes would decrease the abundance of trehalose, while deletion of the NTH2 trehalase would increase overall abundance. Together, these results support the hypothesis that the level of trehalose, which has been linked to environmental stress resistance in other organisms, is important for survival against stressors in the host as well.
FIG 6.

Strains harboring nth2Δ are hypervirulent in mice. The x axis shows time, in days, after tail vein infection with 1 × 106 α wild-type (diamonds), α nth1Δ (squares), α nth2Δ (triangles), or α nth1Δ nth2Δ (cross hatches) yeast. The y axis shows percent survival of five BALB/c mice per strain. The difference in average time to death between nth2Δ and nth1Δ nth2Δ strains versus wild-type and nth1Δ strains is 7 days and is both statistically significant (P < 0.05 by Wilcoxon rank-sum test) and reproducible between experiments.
To test this hypothesis, we evaluated the ability of the strains to resist heat shock (at temperatures of 37°C, 42°C, and 50°C) and survive exposure to reactive oxygen species (in an H2O2 disk diffusion assay). We also tested capsule-inducing conditions (RPMI with 5% CO2 at 37°C), melanizing medium (l-DOPA agar), and cell wall stressing agents (1.5 mg/ml calcofluor white and 0.5 mg/ml caffeine) (54). In no case did the nth1Δ, nth2Δ, or nth1Δ nth2Δ mutant strains respond differently than the wild-type strain (data not shown). The trehalase mutants were not intrinsically more resistant to global stressors than their wild-type counterparts, suggesting that changes in trehalose levels alone were not responsible for the hypervirulence of nth2Δ strains.
A surprising aspect of the virulence data was that the functions of NTH1 and NTH2 were not overlapping. In the absence of NTH2 alone, there was a hypervirulence phenotype, indicating that NTH1 was not compensating for the loss of NTH2 activity. Furthermore, there was no change in the phenotype in the absence of both NTH1 and NTH2. While the NTH1 and NTH2 activities were largely redundant outside the host, it appeared that their functions diverge within the host, implicating an NTH2-specific function or feature in modulating virulence.
DISCUSSION
Using transcriptional profiling, we discovered that spores of the human fungal pathogen C. neoformans contain high levels of transcripts associated with the synthesis and breakdown of the disaccharide trehalose, including a previously unrecognized trehalase, NTH2. Through molecular and genetic analyses, we revealed that NTH2 and a previously characterized trehalase, NTH1, are fully responsible for trehalose utilization in C. neoformans and have largely overlapping trehalase functions, even though Nth1 is predicted to be cytosolic and Nth2 secreted. Significantly, we show for the first time that trehalose degradation is a key function in C. neoformans that is important for fruiting body formation, spore biogenesis, and germination. Trehalases play a critical role in signaling cell fate decisions. In the absence of trehalases, very few spores are produced during sexual development; instead, development shifts to the formation of chlamydospores. The phenotypes of NTH1 and NTH2 diverge in a mouse model of infection where the deletion of NTH2 results in hypervirulence.
Trehalose homeostasis determines cell fate during sexual development.
Trehalose has been shown to play two primary roles within microbes. First, trehalose is a reserve carbohydrate that can be accumulated and cleaved into two glucose molecules when external carbon sources are scarce, and second, trehalose acts as an osmostabilizer and stress protectant (17). In C. neoformans, trehalose biosynthesis is required for initiating sexual development, but roles for trehalose degradation in sexual development have not been documented in any system (23, 55). Our data now bring the full trehalose pathway to light and show that trehalose degradation influences many aspects of C. neoformans biology and is critical for complete sexual development. Notably, in the absence of trehalase genes, development shifts from the formation of fruiting bodies and spores to the formation of what appears to be an alternative cell type known as chlamydospores (26). While little is known about the properties of chlamydospores in C. neoformans, they have been postulated to be structures from which yeast can bud off and establish new colonies (26). Lin and Heitman propose that nutrient sensing through the accumulation of carbohydrate storage molecules, particularly glycogen, drives cell fate decisions between chlamydospore formation and sporulation. However, glycogen biosynthesis genes, whose transcripts are also highly enriched in spores, are dispensable for both chlamydospore formation and sporulation (26), implicating signaling roles for other carbohydrates. Our findings indicate that trehalose functions as a metabolic signal for determining whether filamentous growth should be terminated with spore production or whether conditions are sufficiently favorable to continue proliferating (via chlamydospores) and return to vegetative growth.
Based on these findings, we propose a model in which trehalose levels are a readout for glucose availability (refer to Fig. 1B). In this model, as glucose becomes limiting, sexual development can initiate, and spore formation is favored; trehalose levels remain low because of limited substrates for its production, indicating the metabolic need to differentiate into spores. Spores are the terminal consequence of development under these conditions; thus, the current colony is abandoned by dispersed spores for growth in a more favorable environment elsewhere. However, if nutrient stores are replenished at any point during filamentation, trehalose levels rise (as glucose-derived substrates become plentiful), indicating a change in nutrient availability, and normal growth and colony expansion can be restored through chlamydospore production.
This model is consistent with the seemingly paradoxical result that deletion of trehalose synthase (TPS1) and deletion of the trehalases (NTH1 and NTH2) both result in severe defects in sporulation. In both cases the substrates derived from glucose to make trehalose (i.e., glucose-6-phosphate and UDP-glucose) are not used (as in tps1Δ) or needed (as in nth1Δ nth2Δ), resulting in a change in metabolic flux toward carbohydrate storage as glycogen. Given that chlamydospores are hypothesized carbohydrate storage structures, a shift toward glycogen storage could result in a shift in development. In this way, trehalose concentration acts as a sensor of metabolic conditions to toggle between two different developmental pathways, controlling a critical cell fate decision by playing a novel signaling role rather than simply functioning only as a fuel source or osmoprotectant. This is akin to the role of trehalose signaling in plants that serves to regulate development and plant physiology. However, plants do not accumulate trehalose to the high levels typical of fungi; thus, the contribution of trehalose metabolism to signaling versus stress responses and nutrition is not well understood in plants. The increased development of chlamydospores in trehalase-deficient C. neoformans may serve as a platform to dissect the different contributions of trehalose metabolism to the physiology of fungal pathogens.
Metabolic adaptation, differentiation, and consequences for virulence.
In this work, we discovered that trehalose degradation affects spore germination, which is critical for the long-term survival of the organism in both the natural environment and a host lung. In the absence of NTH1 and NTH2, spore germination drops 3-fold, suggesting that trehalase function is important for stabilizing dormant spores, cleaving trehalose into glucose for fuel during germination, or both. The fact that both rich medium conditions and the mammalian lung are germination inducing (3, 11, 12) underscores a characteristic feature of C. neoformans, namely, the ability to survive and grow in rapidly changing environmental conditions. The tissues of a mammalian host are an unusual environment for C. neoformans to inhabit; thus, the ability to respond to these conditions likely is shaped by the selection pressures of its typical environmental niches (56, 57). The advantages potentially conferred by this relationship have been proposed to explain many of the survival behaviors exhibited by C. neoformans in the host. For example, Steenbergen et al. posit that the unusual ability of C. neoformans to survive and grow within macrophages results from adaptive strategies developed in the natural environment in response to soil amoeba (58).
In the same vein, it would seem likely that adaptations to efficiently access internal stores of energy (e.g., trehalase activity to cleave accumulated trehalose) and scavenge trehalose from the environment would enhance survival in the host. Surprisingly, however, we discovered that an nth2Δ strain is hypervirulent in a mouse model of infection, indicating a negative correlation between trehalase function and virulence in the mouse host. That is, the presence of an intact trehalose-degrading pathway confers a disadvantage in disease-causing ability. One possible explanation for this finding lies in the fact that Nth2 is predicted to be secreted. Perhaps Nth2 is an antigen that can be recognized by the adaptive immune system, allowing the host immune response more opportunity to inhibit C. neoformans growth.
Altering trehalose metabolism in other fungal pathogens has been shown to both positively and negatively affect virulence in murine models of infection. Broadly, trehalose accumulation has been associated with increased stress resistance that typically results in increased infectivity or virulence (17). However, changes in trehalose accumulation can result in changes in cell wall structure and morphogenesis that also may affect the ability of fungal pathogens to cause disease (20, 21, 23). There may be changes that occur in trehalase-deficient C. neoformans yeast inside a mammalian host, such as cell wall polysaccharide composition or titan cell formation (enlarged yeast cells associated with virulence), that can be elucidated in future studies of the interaction of trehalase-deficient C. neoformans and the host.
Intersections among sexual development, metabolism, and pathogenesis.
Spores, as infectious particles, form an obvious connection between the processes of sexual development and mammalian pathogenesis, but the full relationship is more complex. In this work, we reveal a link between the processes of spore production and virulence in a mammalian host through the actions of trehalose-degrading enzymes. This unexpected connection underscores the emerging relationships between sexual development and virulence among human fungal pathogens in general and the role of metabolic acclimation in C. neoformans in particular. Responses in C. neoformans to the extreme conditions between the natural environment and human host are of particular interest, because understanding metabolic adaptations promises to reveal pathways that influence pathogenesis.
The importance of these relationships is underscored by recent studies of C. neoformans morphology in which filamentous growth of C. neoformans results in a loss of virulence in mice, implying that yeast morphology plays a role in pathogenesis. A coincident possibility, however, is that morphology is secondary to changes in metabolic gene expression between yeast responding to environmental signals (such as those that initiate developmental changes) and yeast responding to host conditions. The resulting metabolic acclimation results in both positive and negative effects on pathogen survival in the host and could help to explain differences in virulence properties observed between clinical and environmental isolates. One possibility is that gene expression patterns that would normally be advantageous in an environmental niche but disadvantageous within a host are modified, making the host a selective environment for cells that have the capacity to rapidly modulate expression of metabolic or stress response pathways. Discovering these pathways provides another route to understanding how host resources are enhanced to prevent fungal infection and/or disease.
ACKNOWLEDGMENTS
We thank A. Adams, J. Ekena, S. Peters, and J. Kusiak for laboratory support and N. Walsh, M. Mead, and C. Fox for comments on the manuscript. We thank the University of Wisconsin Laboratory Animal Resource Staff for the care of animals used in this study.
This work was supported by NIH R01 AI064287, NIH R01 AI059370, and a Burroughs Wellcome Fund Career Award in Biomedical Sciences, all to C.M.H. M.R.B. was supported by the University of Wisconsin Molecular Biosciences Training Grant NIH T3GM07215.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Harder W, Dijkhuizen L. 1983. Physiological responses to nutrient limitation. Annu. Rev. Microbiol. 37:1–23. 10.1146/annurev.mi.37.100183.000245 [DOI] [PubMed] [Google Scholar]
- 2.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525. 10.1111/j.1365-2672.2005.02736.x [DOI] [PubMed] [Google Scholar]
- 3.Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. 2009. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot. Cell 8:595–605. 10.1128/EC.00352-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briza P, Breitenbach M, Ellinger A, Segall J. 1990. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 4:1775–1789. 10.1101/gad.4.10.1775 [DOI] [PubMed] [Google Scholar]
- 5.Haber JE, Halvorson HO. 1975. Methods in sporulation and germination of yeasts. Methods Cell Biol. 11:45–69. 10.1016/S0091-679X(08)60316-7 [DOI] [PubMed] [Google Scholar]
- 6.Converse JL, Reed RE. 1966. Experimental epidemiology of coccidioidomycosis. Bacteriol. Rev. 30:678–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein BS, Vergeront JM, Davis JP. 1986. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin. Respir. Infect. 1:29–39 [PubMed] [Google Scholar]
- 8.Latgé JP. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latgé J-P. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121. 10.1038/nature08264 [DOI] [PubMed] [Google Scholar]
- 10.Ellis DH, Pfeiffer TJ. 1990. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 336:923–925. 10.1016/0140-6736(90)92283-N [DOI] [PubMed] [Google Scholar]
- 11.Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 77:4345–4355. 10.1128/IAI.00542-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect. Immun. 77:3491–3500. 10.1128/IAI.00334-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell TG, Perfect JR. 1995. Cryptococcosis in the era of AIDS–100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie KF, Singh KK, Brown AD. 1988. Water stress plating hypersensitivity of yeasts: protective role of trehalose in Saccharomyces cerevisiae. J. Gen. Microbiol. 134:1661–1666 [DOI] [PubMed] [Google Scholar]
- 15.Mitsumasu K, Kanamori Y, Fujita M, Iwata K, Tanaka D, Kikuta S, Watanabe M, Cornette R, Okuda T, Kikawada T. 2010. Enzymatic control of anhydrobiosis-related accumulation of trehalose in the sleeping chironomid, Polypedilum vanderplanki. FEBS J. 277:4215–4228. 10.1111/j.1742-4658.2010.07811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nwaka S, Holzer H. 1998. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 58:197–237 [DOI] [PubMed] [Google Scholar]
- 17.Tournu H, Fiori A, Van Dijck P. 2013. Relevance of trehalose in pathogenicity: some general rules, yet many exceptions. PLoS Pathog. 9:e1003447. 10.1371/journal.ppat.1003447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbein AD, Pan YT, Pastuszak I, Carroll D. 2003. New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R–27R. 10.1093/glycob/cwg047 [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Fresneda R, Guirao-Abad JP, Argüelles A, González-Párraga P, Valentín E, Argüelles J-C. 2013. Specific stress-induced storage of trehalose, glycerol and D-arabitol in response to oxidative and osmotic stress in Candida albicans. Biochem. Biophys. Res. Commun. 430:1334–1339. 10.1016/j.bbrc.2012.10.118 [DOI] [PubMed] [Google Scholar]
- 20.Pedreño Y, González-Párraga P, Martínez-Esparza M, Sentandreu R, Valentín E, Argüelles J-C. 2007. Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 153:1372–1381. 10.1099/mic.0.2006/003921-0 [DOI] [PubMed] [Google Scholar]
- 21.Serneels J, Tournu H, Van Dijck P. 2012. Tight control of trehalose content is required for efficient heat-induced cell elongation in Candida albicans. J. Biol. Chem. 287:36873–36882. 10.1074/jbc.M112.402651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaragoza O, Blazquez MA, Gancedo C. 1998. Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J. Bacteriol. 180:3809–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Bader N, Vanier G, Liu H, Gravelat FN, Urb M, Hoareau CM-Q, Campoli P, Chabot J, Filler SG, Sheppard DC. 2010. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect. Immun. 78:3007–3018. 10.1128/IAI.00813-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, Miller JL, Perfect JR. 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74:5877–5887. 10.1128/IAI.00624-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngamskulrungroj P, Himmelreich U, Breger JA, Wilson C, Chayakulkeeree M, Krockenberger MB, Malik R, Daniel H-M, Toffaletti D, Djordjevic JT, Mylonakis E, Meyer W, Perfect JR. 2009. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect. Immun. 77:4584–4596. 10.1128/IAI.00565-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin X, Heitman J. 2005. Chlamydospore formation during hyphal growth in Cryptococcus neoformans. Eukaryot. Cell 4:1746–1754. 10.1128/EC.4.10.1746-1754.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon-Chung KJ, Edman JC, Wickes BL. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman F. 1991. Getting started with yeast. Methods Enzymol. 194:3–21. 10.1016/0076-6879(91)94004-V [DOI] [PubMed] [Google Scholar]
- 29.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA. 1988. Current protocols in molecular biology. Wiley Interscience, Hoboken, NJ [Google Scholar]
- 30.Kruzel EK, Giles SS, Hull CM. 2012. Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone-response network by a change in transcription factor identity. Genetics 191:435–449. 10.1534/genetics.112.138958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr MK, Churchill GA. 2001. Statistical design and the analysis of gene expression microarray data. Genet. Res. 77:123–128. 10.1017/S0016672308009713 [DOI] [PubMed] [Google Scholar]
- 32.Quackenbush J. 2001. Computational analysis of microarray data. Nat. Rev. Genet. 2:418–427. 10.1038/35076576 [DOI] [PubMed] [Google Scholar]
- 33.Kanehisa M. 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28:27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25:25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang DW, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4:44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 36.Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, Wang P, Heitman J. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 37.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherman F, Fink GR, Hicks JB. 1986. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 39.Parrou JL, François J. 1997. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 248:186–188. 10.1006/abio.1997.2138 [DOI] [PubMed] [Google Scholar]
- 40.Zaragoza O, Fries BC, Casadevall A. 2003. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO2. Infect. Immun. 71:6155–6164. 10.1128/IAI.71.11.6155-6164.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFadden D, Zaragoza O, Casadevall A. 2006. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 14:497–505. 10.1016/j.tim.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 42.Hu G, Steen BR, Lian T, Sham AP, Tam N, Tangen KL, Kronstad JW. 2007. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 3:e42. 10.1371/journal.ppat.0030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giles SS, Stajich JE, Nichols C, Gerrald QD, Alspaugh JA, Dietrich F, Perfect JR. 2006. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot. Cell 5:1447–1459. 10.1128/EC.00098-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casadevall A, Perfect JR. 1998. Cryptococcus neoformans. ASM Press, Washington, DC [Google Scholar]
- 45.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207–210. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita I, Fukui S. 1985. Transcriptional control of the sporulation-specific glucoamylase gene in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 5:3069–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthukumar G, Suhng SH, Magee PT, Jewell RD, Primerano DA. 1993. The Saccharomyces cerevisiae SPR1 gene encodes a sporulation-specific exo-1,3-β-glucanase which contributes to ascospore thermoresistance. J. Bacteriol. 175:386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramalingam R, Blume JE, Ennis HL. 1992. The Dictyostelium discoideum spore germination-specific cellulase is organized into functional domains. J. Bacteriol. 174:7834–7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garre E, Matallana E. 2009. The three trehalases Nth1p, Nth2p and Ath1p participate in the mobilization of intracellular trehalose required for recovery from saline stress in Saccharomyces cerevisiae. Microbiology 155:3092–3099. 10.1099/mic.0.024992-0 [DOI] [PubMed] [Google Scholar]
- 50.Jules M, Beltran G, François J, Parrou JL. 2008. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization. Appl. Environ. Microbiol. 74:605–614. 10.1128/AEM.00557-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirimburegama K, Durnez P, Keleman J, Oris E, Vergauwen R, Mergelsberg H, Thevelein JM. 1992. Nutrient-induced activation of trehalase in nutrient-starved cells of the yeast Saccharomyces cerevisiae: cAMP is not involved as a second messenger. J. Gen. Microbiol. 138:2035–2043. 10.1099/00221287-138-10-2035 [DOI] [PubMed] [Google Scholar]
- 52.Parrou JL, Jules M, Beltran G, François J. 2005. Acid trehalase in yeasts and filamentous fungi: localization, regulation and physiological function. FEMS Yeast Res. 5:503–511. 10.1016/j.femsyr.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 53.He S, Bystricky K, Leon S, François JM, Parrou JL. 2009. The Saccharomyces cerevisiae vacuolar acid trehalase is targeted at the cell surface for its physiological function. FEBS J. 276:5432–5446. 10.1111/j.1742-4658.2009.07227.x [DOI] [PubMed] [Google Scholar]
- 54.Panepinto JC, Komperda KW, Hacham M, Shin S, Liu X, Williamson PR. 2007. Binding of serum mannan binding lectin to a cell integrity-defective Cryptococcus neoformans ccr4Δ mutant. Infect. Immun. 75:4769–4779. 10.1128/IAI.00536-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni M, Yu J-H. 2007. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2:e970. 10.1371/journal.pone.0000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, Geddes JMH, Griffiths EJ, Choi J, Cadieux B, Caza M, Attarian R. 2012. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot. Cell 11:109–118. 10.1128/EC.05273-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu G, Cheng P-Y, Sham A, Perfect JR, Kronstad JW. 2008. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol. Microbiol. 69:1456–1475. 10.1111/j.1365-2958.2008.06374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steenbergen JN, Shuman HA, Casadevall A. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. U. S. A. 98:15245–15250. 10.1073/pnas.261418798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, D.C [Google Scholar]