Abstract
SUMMARY
In global health, critical challenges have arisen from infectious diseases, including the emergence and reemergence of old and new infectious diseases. Emergence and reemergence are accelerated by rapid human development, including numerous changes in demographics, populations, and the environment. This has also led to zoonoses in the changing human-animal ecosystem, which are impacted by a growing globalized society where pathogens do not recognize geopolitical borders. Within this context, neglected tropical infectious diseases have historically lacked adequate attention in international public health efforts, leading to insufficient prevention and treatment options. This subset of 17 infectious tropical diseases disproportionately impacts the world's poorest, represents a significant and underappreciated global disease burden, and is a major barrier to development efforts to alleviate poverty and improve human health. Neglected tropical diseases that are also categorized as emerging or reemerging infectious diseases are an even more serious threat and have not been adequately examined or discussed in terms of their unique risk characteristics. This review sets out to identify emerging and reemerging neglected tropical diseases and explore the policy and innovation environment that could hamper or enable control efforts. Through this examination, we hope to raise awareness and guide potential approaches to addressing this global health concern.
INTRODUCTION
The 21st century has ushered in an era when globalization of infectious diseases is occurring frequently and at an unprecedented speed (1). In this “globalized” environment of interdependent trade, travel, migration, and international economic markets, many factors now play an important role in the rise, emergence, and reemergence of infectious disease, which necessitates a coordinated, global response (1, 2). Of note, zoonotic diseases (i.e., those infectious diseases that can be transmitted from an animal to humans) account for the majority of emerging and reemerging infectious diseases occurring due to increased contact between humans and animals as a by-product of development, industrialization, and encroachment on wildlife habitats, resulting in a dynamic upward trajectory of these diseases (3–6). Yet many of these emerging and reemerging infectious diseases are also “neglected,” meaning they impact the world's poorest and lack adequate funding and innovation for prevention and treatment, with some not adequately identified or studied (7, 8).
Emerging infectious diseases (EIDs) and reemerging infectious diseases (ReIDs) can arise due to a multitude of factors and influences and must be addressed dynamically by diverse sectors of society; these include public health, medicine, environmental science, animal health, food safety, economics, and public policy stakeholders. A host of human-sourced and environmental factors complicate these actions, such as societal influences, human susceptibility to infection, demographics, availability of health care, food production, human behavior, trade and travel, environmental and ecological changes, economic development, war and famine, adequacy of public health infrastructures, man-made events with intent to harm, and pathogen adaptation or evolution (3, 9).
Striking examples of these EID events in play can be seen throughout history, with the majority originating from zoonotic pathogens from wildlife (3). These include the black plague of the 14th century, caused by Yersinia pestis. That plague event was largely attributable to regional trade and societal influences, with overcrowding, poor hygiene, and destruction of the predator of the animal reservoir being leading causes for the rapid transmission of the illness (10). Human behavior and mobility can further be implicated in sexually transmitted diseases such as HIV disease (also originally caused by cross-species transmission), hepatitis, gonorrhea, syphilis, and others, including in the context of rural and low-income settings (11–14). Immunosuppression due to HIV/AIDS coincided with the rise of opportunistic EIDs and ReIDs in the 1980s (3).
The invention of new drugs to fight cancer or autoimmune disease have also led to immunosuppression. This, along with the development of antimicrobial resistance, has resulted in the emergence of diseases that were otherwise rare (15, 16). Most notably, the development of antimicrobial resistance with new pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli, multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis, and a multitude of other microbial pathogens that were once easily treated is now leading to new infectious disease threats (17–20).
Beyond human action, extremes of weather and natural disasters have also influenced vector-borne infectious disease spread, suggesting a role for climate change in these events (3, 21–23). Environmental changes such as the introduction of a new insect or plant vector into a region or population have also led to rapid transmission of diseases that were not previously prevalent, such as in the case of Rift Valley fever, dengue, and malaria (3, 24). Food-borne illnesses are another area of emergence, with outbreaks of Salmonella, E. coli, and bovine spongiform encephalitis all occurring due to poor food processing practices (25). Hence, understanding the cause of a disease's emergence can be critical to its prevention and treatment (26).
Setting the stage for more recent attention to EIDs and ReIDs is globalization, which is fueled by growing worldwide travel and interdependent trade. Increasing globalization is linked to recent seminal infectious disease events as well as future concerns for global health emanating from large-scale population movement and migration. For example, the 2002 severe acute respiratory syndrome (SARS) outbreak and the 2009 H1N1 influenza pandemic were considered to be directly related to globalization and international travel (1, 27, 28). Large-scale international gatherings such as the Hajj in Saudi Arabia may also represent newly recognized social mechanisms for rapid spread of infectious diseases, such as the emergence of Middle Eastern respiratory syndrome (MERS), caused by the second novel coronavirus that has been identified in just the past decade (29, 30). The emergence of new influenza virus strains is also a growing concern. These include highly pathogenic avian influenza (HPAI) virus, with concerns over its high virulence and case fatality rate having possible disastrous public health effects should it mutate to maintain human-to-human transmission (31). Further, worrisome disease outbreak events continue to occur worldwide. This includes the first reported cases of infection with the highly contagious Ebola virus (with its estimated mortality rate as high as 90%) being reported in Guinea in March 2014 (32, 33). The Ebola outbreak has now spread to other countries in West Africa, with close to 2,000 cases and approximately 1,000 deaths as of August 2014, and has been declared a “public health emergency of international concern” by the World Health Organization (WHO) (55).
Within the current discourse on emerging and reemerging infectious diseases, there has also been criticism that global attention has been unjustifiably focused on novel or newly recognized pathogens at the expense of other, “older” diseases with higher global disease burdens (27). This criticism can also be translated to lack of global priority setting and attention to a group of historically neglected tropical diseases that currently infect more than 1 billion people and that have a high combined global burden of disease, estimated at 56.6 disability-adjusted life years (DALYs) (7, 34, 35). Despite their deleterious social, economic, and health impact, these “neglected” diseases continue to be an impediment to human development and progress, though some international efforts to address them are under way (36). The majority of these neglected diseases are zoonotic and impacted by factors similar to those associated with other emerging and reemerging infectious diseases. However, there has never been a detailed identification and examination of the subset of neglected tropical diseases (NTDs) that are also classified as emerging and reemerging, despite their unique threats to global public health.
This article presents an overview of emerging and reemerging infectious diseases within the context of neglected tropical disease concepts. It also identifies, characterizes, and assesses the common risk factors of a newly identified group of emerging and reemerging neglected tropical diseases (EReNTDs) that combine these infectious disease categories. This article expands on previous work that first identified and defined the category of EReNTDs by reviewing the medical literature for key topics regarding EReNTD-related risk factors, treatment options, public health responses, recent developments in diagnosis and treatment, organizations and initiatives addressing EReNTDs and NTDs as a broader category, and existing and proposed innovation mechanisms.
We conducted our review by searching the PubMed/MEDLINE and Google Scholar databases to review the scientific literature for discussion of these EReNTD subject areas. We also conducted general Google search engine inquiries using key words associated with EReNTD subject areas and supplemented the peer-reviewed literature with information from data sources including news reports, press releases, organization websites, and program and intervention descriptions.
Though this is not a systematic review, through this detailed examination we aim to raise awareness of the unique threats and challenges posed by EReNTDs, identify the current policy and innovation environment for EReNTDs, and inform global efforts moving forward.
EMERGING AND REEMERGING INFECTIOUS DISEASES
Definitions and Identification
National, regional, and international organizations, such as the U.S. Centers for Disease Control and Prevention (CDC) and WHO, have focused on EIDs and ReIDs due to the widespread and often disastrous consequences that an emerging or reemerging pathogen can inflict across a population. The importance of quarantine, border control, contact tracing, and disease surveillance for EIDs/ReIDs has long been recognized, as well as the need for proper assessment and identification of the changing nature of these diseases.
The CDC defines “emerging infectious diseases” as those infections that are increasing over time or threaten to increase (37). It further defines emerging infectious diseases as new infections resulting from new unknown pathogens, known infections which are increasing over new geographic areas, and known infections that are reemerging as a result of both resistance to antimicrobial therapies and the failure of public health measures (37). The CDC currently recognizes over 50 emerging or reemerging diseases (Table 1) and also publishes research on EIDs and ReIDs in its journal Emerging Infectious Diseases (38). In addition, in the mid-1990s, the CDC implemented the Emerging Infections Program, which was begun as a response to the 1994 CDC strategy titled “Addressing Infectious Disease Threats: a Prevention Strategy for the United States,” which was also later expanded to international collaborations through its International Emerging Infections Program as part of the CDC's Global Disease Detection Program (39, 40).
TABLE 1.
CDC list of emerging and reemerging pathogens or diseases
| Pathogen or disease |
|---|
| Bovine spongiform encephalopathy |
| Campylobacteriosis |
| Chagas disease |
| Cholera |
| Cryptococcus |
| Cryptosporidiosis |
| Cyclosporiasis |
| Cysticercosis |
| Dengue fever |
| Diphtheria |
| Drug-resistant infections (antimicrobial resistance) |
| Ebola hemorrhagic fever |
| Escherichia coli infection |
| Group B streptococcus |
| Hantavirus pulmonary syndrome |
| Hendra virus |
| Hepatitis C |
| Histoplasmosis |
| HIV/AIDS |
| Influenza |
| Lassa fever |
| Legionnaires' disease |
| Leptospirosis |
| Listeriosis |
| Lyme disease |
| Malaria |
| Marburg hemorrhagic fever |
| Measles |
| Monkeypox |
| MRSA |
| Nipah virus |
| Norovirus |
| Pertussis |
| Plague |
| Poliomyelitis |
| Rabies |
| Rift Valley fever |
| Rotavirus |
| Salmonellosis |
| Severe acute respiratory syndrome |
| Shigellosis |
| Sleeping sickness (trypanosomiasis) |
| Smallpox |
| Tuberculosis |
| Tularemia |
| Valley fever (coccidioidomycosis) |
| Vancomycin-intermediate or -resistant Staphylococcus aureus |
| West Nile virus |
| Yellow fever |
The U.S. National Institutes of Health (NIH) also recognizes emerging and reemerging diseases as a distinct category. The NIH defines emerging and reemerging disease by dividing them into three groups (Tables 2 and 3) (41). Group 1 diseases include those newly recognized in the last 20 years, group 2 diseases include reemerging diseases, and group 3 diseases include those caused by agents with potential for bioterrorism threat (Table 3) (41). Agencies in other countries, such as the United Kingdom's Public Health England, the executive agency of the Department of Health, also maintain their own lists of emerging infections and agents, though the CDC and NIH lists are the most utilized (42).
TABLE 2.
National Institutes of Health emerging pathogens or diseases, groups 1 and 2
| Pathogen or diseasea |
|---|
| Group 1 |
| Acanthamebiasis |
| Australian bat lyssavirus |
| Babesia, atypical |
| Bartonella henselae |
| Ehrlichiosis |
| Encephalitozoon cuniculi |
| Encephalitozoon hellem |
| Enterocytozoon bieneusi |
| Hendra or equine morbillivirus |
| Human herpesvirus 8 |
| Human herpesvirus 6 |
| Lyme borreliosis |
| Parvovirus B19 |
| Group 2 |
| Enterovirus 71 |
| Clostridium difficile |
| Mumps virus |
| Streptococcus, group A |
| Staphylococcus aureus |
Group 1, pathogens or diseases newly recognized in the past two decades; group 2, reemerging pathogens.
TABLE 3.
NIH emerging pathogens or diseases, group 3: potential bioterrorism threats
| Pathogen and/or diseasea |
|---|
| Category A |
| Bacillus anthracis (anthrax) |
| Clostridium botulinum toxin (botulism) |
| Yersinia pestis (plague) |
| Variola major virus (smallpox) and other related poxviruses |
| Francisella tularensis (tularemia) |
| Viral hemorrhagic fevers |
| Arenaviruses (lymphocytic choriomeningitis virus, Junin virus, Machupo virus, Guanarito virus; Lassa fever) |
| Bunyaviruses (hantaviruses; Rift Valley fever) |
| Flaviviruses (dengue virus) |
| Filoviruses (Ebola virus, Marburg virus) |
| Category B |
| Burkholderia pseudomallei |
| Coxiella burnetii (Q fever) |
| Brucella species (brucellosis) |
| Burkholderia mallei (glanders) |
| Chlamydia psittaci (Psittacosis) |
| Ricin toxin (from Ricinus communis) |
| Epsilon toxin of Clostridium perfringens |
| Staphylococcus enterotoxin B |
| Rickettsia prowazekii (typhus fever) |
| Food- and waterborne pathogens (bacteria, diarrheagenic E. coli, pathogenic vibrios, Shigella species, Salmonella, Listeria monocytogenes, Campylobacter jejuni, Yersinia enterocolitica; viruses, caliciviruses, hepatitis A virus; protozoa, Cryptosporidium parvum, Cyclospora cayatanensis, Giardia lamblia, Entamoeba histolytica, Toxoplasma; fungi, microsporidia; additional viral encephalitides, West Nile virus, La Crosse virus, California encephalitis virus, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, Western equine encephalitis virus, Japanese encephalitis virus, Kyasanur Forest virus) |
| Category C |
| Emerging infectious disease threats such as Nipah virus and additional hantaviruses |
| Tick-borne hemorrhagic fever viruses (Crimean-Congo hemorrhagic fever virus) |
| Tick-borne encephalitis viruses |
| Yellow fever |
| Tuberculosis, including drug-resistant tuberculosis |
| Influenza |
| Other rickettsias |
| Rabies |
| Prions |
| Chikungunya virus |
| Severe acute respiratory syndrome associated coronavirus |
| Antimicrobial resistance, excluding research on sexually transmitted organisms*: Research on mechanisms of antimicrobial resistance, Studies of the emergence and/or spread of antimicrobial resistance genes within pathogen populations, Studies of the emergence and/or spread of antimicrobial-resistant pathogens in human populations, Research on therapeutic approaches that target resistance mechanisms, Modification of existing antimicrobials to overcome emergent resistance |
| Antimicrobial research, as related to engineered threats and naturally occurring drug-resistant pathogens, focused on development of broad-spectrum antimicrobials |
| Coccidioides immitis (added February 2008) |
| Coccidioides posadasii (added February 2008) |
| NIAID category C antimicrobial resistance-sexually transmitted excluded organisms (bacterial vaginosis, Chlamydia trachomatis, cytomegalovirus, Granuloma inguinale, Haemophilus ducreyi, hepatitis B virus, hepatitis C virus, herpes simplex virus, human immunodeficiency virus, human papillomavirus, Neisseria gonorrhea, Treponema pallidum, Trichomonas vaginalis |
Category A priority, pose highest risk to national security and public health, easily disseminated/high mortality; category B priority, second-highest priority, moderately easy to disseminate/low mortality; category C priority, third-highest priority, includes emerging pathogens that could be mass produced and easily disseminated.
Though arguably the NIH provides a more comprehensive listing of potential EIDs/ReIDs than the CDC, it is important to note that the lists do not completely overlap, with some infectious diseases on one list and not on the other. In addition, the majority of diseases on both the NIH and CDC lists are categorized in NIH list group 3, emphasizing a focus and political prioritization on funding for public health issues categorized as having a bioterrorism threat potential. We specifically provide these lists that identify EIDs and ReIDs for later discussion and identification of NTDs that overlap this category (i.e., EReNTDs).
NEGLECTED TROPICAL DISEASES
Background
Neglected tropical diseases (NTDs) are historically overlooked diseases that have been neglected at the community, national, and international levels and are endemic in many resource-poor populations and developing countries (7, 43, 44). The majority of individuals and communities in these regions have far less access to the resources necessary to address the social determinants of NTDs and may live in poor sanitary conditions, have inadequate nutrition, and lack access to necessary public health and health care systems for treatment, despite many of these diseases being preventable and/or treatable through specific low-cost interventions (44–47). Efforts to protect the health of these populations have been insufficient, with the global focus to identify and prioritize NTDs by the international community only “reemerging” in the last decade following efforts by leading NTD researchers and advocates such as the current president of the Sabin Vaccine Institute, Peter Hotez (43).
WHO has specifically identified 17 core NTDs: dengue, rabies, trachoma, buruli ulcer, endemic treponematoses, leprosy, Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, taeniasis/cysticercosis, dracunculiasis, echinococcosis, food-borne trematodiases, lymphatic filariasis, onchocerciasis, schistosomiasis, and soil-transmitted helminthiases (Fig. 1 shows the taxonomy of these diseases) (36, 45). NTDs are comprised primarily of viral, protozoan, helminthial, and bacterial infections.
FIG 1.
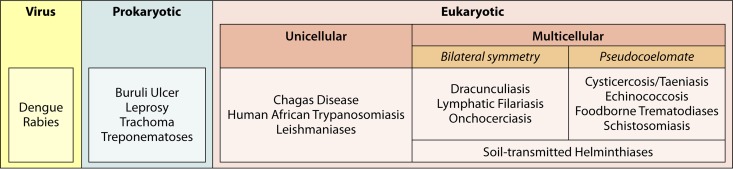
Neglected tropical disease agent taxonomy.
Many NTDs are also zoonotic and/or vector borne. Control of human exposure to vectors and animal reservoirs can protect susceptible populations from NTDs and is a critical component of prevention in the absence of effective therapeutics and vaccines (7, 45). These efforts can begin with surveillance of vector populations for signs of infection (34, 48). When it is impossible to accurately monitor the distribution of vectors and intermediate hosts, as it is for several insect vectors, educational campaigns to promote avoidance may be an effective means to reduce the incidence of associated NTDs (49, 50). Additionally, other methods of the overall integrated strategy in controlling NTDs include the control of intermediate hosts and vectors by use of environmentally safe insecticides, the use of insecticide-treated nets and other personal protective measures, alteration of the environment through clearance of vegetation, improved sanitation to disrupt breeding sites, and biological control though introduction of competitor species to a vector (7, 51–53).
In support of raising awareness for vector-borne NTDs, WHO highlighted that more than half of the world's population is at risk from vector-borne diseases (such as malaria and dengue) as its main topic for World Health Day 2014 and provided the public with information on how to prevent disease transmission (54).
Socioeconomics and NTDs
NTDs are well categorized from a socioeconomic perspective. NTDs, according to WHO, impact more than one billion people (including an estimated 500 million children) from almost 150 countries and territories where they are endemic (7). In addition, NTDs can also have a “hidden burden,” as they may also be prevalent in poorer populations living in wealthy countries, such as the United States (36).
Exacerbating the impact of NTDs is inadequate or absent health care capacity, especially since many NTDs are associated with chronic conditions and are also made worse by ineffective case detection/management, poor environmental conditions, rapid urbanization, public health deficiencies, and poverty (7). Poverty in particular is a key social determinant of uncontrolled NTD spread and can lead to reduced economic productivity due to long-term disability and morbidity, maternal-fetal and maternal-child health issues, and other health-related challenges that lead to infected individuals and their communities being caught in a health-related “poverty trap” (7, 34, 43, 47, 56). The impact of NTDs is hence disastrous in resource-poor settings and has been associated with broader societal disruptions, including political instability, civil strife, stigmatization, and destabilization of local communities (56, 57).
Consequently, given that developed countries have relatively low NTD transmission, this results in NTDs disproportionately impacting the poorest and most vulnerable, and unlike certain EIDs and ReIDs, they have also been historically neglected in drug development efforts (43, 44, 58–60). This, coupled with overall mortality rates that are lower (though possibly underestimated) then those for other infectious diseases such as HIV/AIDS or malaria, relegates NTDs to being “hidden” diseases with limited diagnostic, treatment, and public health interventions despite their substantial global disease burden, which combined is estimated to have higher disability-adjusted life years (DALYs) than malaria and tuberculosis (TB) (7, 34, 35, 43, 44, 56, 61–64).
NTD Support, Funding, and Ongoing Challenges
Despite challenges associated with NTDs, some progress has been made toward treating, controlling, eliminating, and possibly eradicating certain NTDs. This includes the elimination of leprosy in 116 of 122 countries where it is endemic, a massive reduction in the number of people infected with and ongoing global efforts to completely eradicate dracunculiasis, worldwide declines in incidence of onchocerciasis, and reductions in the transmission and prevalence of a number of other NTDs in certain regions of endemicity (65–67). This progress has been made possible in part due to medicine donations (primarily in the form of preventive chemotherapy) from a number of pharmaceutical manufactures, including Bayer, Eisai, Gilead Sciences, GlaxoSmithKline, Johnson & Johnson, Merck & Co., Inc., Novartis, Pfizer, and Sanofi. These donations are often in the form of commitments for large or unlimited quantities of medicines, leading to increased treatment coverage that has been enabled by campaigning and fund raising by the WHO and Carter Center (45). NTD policies have also benefited from recent increased political attention and prioritization, new partnerships, and funding commitments specifically devoted to combating NTDs, including some US$1.2 billion in grants from the Bill and Melinda Gates Foundation (BMGF) aimed to fill gaps in, rather than duplicate, disease research funding streams (35, 44, 45, 68, 69).
Collectively these efforts aid in attaining global goals outlined in the WHO “Roadmap for Implementation” published in 2010, which sets targets for the eradication (i.e., permanent reduction to zero of the worldwide incidence of infection) of dracunculiasis and yaws by 2015 and 2020, respectively, and elimination (i.e., reduction to zero of the incidence of disease or infection in a defined geographical area) of five other NTDs by 2015 and of 10 others by 2020 (45, 70). However, even though there have been recent declarations increasing financial commitments to NTDs, overall funding is lower than and insufficient compared with that for other global health issues (71). This situation continues despite findings that investment in NTD control can generate high rates of economic return and social benefit (66, 72).
As an example, the incidences of lymphatic filariasis, onchocerciasis, schistosomiasis, and soil-transmitted helminthiases would likely dramatically decrease if NTD-preventive chemotherapy (also known as mass drug administration) were more broadly implemented and scaled-up in countries where these diseases are endemic (7, 73–76). Several regions would also clearly benefit from the delivery of rapid-impact packages. These packages are disseminated quickly by community-based organizations and generally contain four of the six following drugs: albendazole, mebendazole, diethylcarbamazine, ivermectin, praziquantel, and azithromycin (8). Providing Africa with packages of albendazole, ivermectin, azithromycin, and praziquantel has been evaluated as having a yearly cost of $200,000,000 for the preventive chemotherapy of 500,000,000 individuals (with any of 7 NTDs), resulting in significant treatment cost-effectiveness of only $0.40 per person per year (77). Including indirect costs to the broader economy suggests even greater cost-efficacy, as there is considerable savings in averting decreased labor productivity from NTD infection (78).
Specific to NTD funding, the relatively small total share of global public health financing allocated for NTDs acts as critical factor hampering effective scale-up of NTD control and treatment and meeting WHO's goals of NTD elimination and eradication, many of which are less than a decade away (35, 45, 57, 69). At a mere average of 0.6% of total official development assistance for health as tracked by the Institute for Health Metrics and Evaluation, NTD-dedicated funding remains dwarfed by investments in diseases such as HIV/AIDS (36.3%), malaria (3.6%), and TB (2.2%) (69, 72).
Research and development (R&D) funding for NTDs, reported at some $3 billion in 2011, has also experienced recent nominal declines following the global financial crisis, after steady gains up until 2009 (71). Relatively unchanging levels of resources committed to NTD R&D funding have also seen shifts in donor sources, with public and philanthropic funding decreasing and industry-based funding increasing (71). Overall, funding inadequacies continue despite the high global disease burden of NTDs and availability of cost-effective interventions that can decrease morbidity and mortality from them. Decreased morbidity and mortality can subsequently lead to economic development that can improve the underlining social determinants that worsen NTD susceptibility, exposure, and transmission (47, 57, 69).
Key Characteristics and Factors Associated with NTDs
Provided adequate funding and international commitment to NTDs is achieved, global elimination and eradication goals will also need to take into account the unique characteristics and challenges associated with NTD control, treatment and prevention in order to be successful. Below we highlight in brief some of the key characteristics and factors associated with NTDs.
Neglected Zoonotic Diseases
Neglected zoonotic diseases (NZDs) are a subset of NTDs prioritized by the WHO that are transmitted between humans and other vertebrate animals (51). The transmission of these NZDs may be direct or indirect through vectors such as mosquitos, ticks, animal reservoirs, or other zoonotic agents found in water, food, and soil (51). The existence of these diseases raises the importance of broad ecologies that influence the protection of human populations, the need for more robust surveillance in animal vectors, and the reality that they are especially difficult to control or eliminate due to their nonhuman reservoirs (79). Integrated control and management of NZDs is important given their disproportionate impact on the poor, possible underreporting in incidence, availability of simple and relatively low-cost tools and strategies for control, and that they present a dual burden of disease on both humans and animals (80). Seven of the 17 NTDs are identified as targeted NZDs by WHO: rabies, human African trypanosomiasis, leishmaniasis, taeniasis/cysticercosis, echinococcosis, food-borne trematodiases, and schistosomiasis (81).
Geographic, Environmental, and Social Determinants
Several geographic, environmental, and sociocultural qualities may predispose individuals to contracting tropical diseases (82–88). The climate between the Tropic of Cancer and the Tropic of Capricorn (i.e., the “tropics”) is especially conducive to NTD vectors. Given that over 40% of the human population (about 3 billion people) currently live in the tropics, there is a high potential for NTD risk in the overall global population and for an increase of NTD prevalence, especially absent enhanced public health efforts (89, 90). However, several factors have also permitted these diseases to affect populations outside these tropical regions that are typically areas of endemicity (91, 92). These include ecology and broader environmental factors such as climate change and its association with changes in disease vectors, as well as a lack of adequate public health efforts specifically targeted at NTDs in these regions (45, 93).
As an example, individuals living near bodies of water are more susceptible to NTDs whose vectors (such as mosquitos) rely on aquatic ecosystems (94). Several insect vectors are likely to better reproduce and transmit diseases within certain ranges of temperature and humidity that may be geographically specific (95, 96). Therefore, global trends in changing climate may facilitate the migration of NTD vectors into new regions where they previously were not endemic, such as in the case of association between changes in weather and increased dengue incidence (93, 97, 98).
Elimination of an NTD can also be complicated by environmental issues, such as in the case of regions that present beneficial environments for NTD vectors, requiring more aggressive and targeted vector control efforts than in regions and environments less conducive to the NTD's vector (95, 99). Short epidemics of visceral leishmaniasis (also known as kala-azar) were observed in the northeast South American cities of São Luis and Teresina during major droughts caused by El Niño in 1983 to 1985 and 1992 to 1994 (100, 101). Such events are examples of temporary occasions where it is more advantageous for the sand fly vector to exist in a novel environment, possibly due to brief, dramatic decreases in humidity or food supply in their usual environment (102–105). Hence, more intense public health efforts would be required to facilitate the end of leishmaniasis endemicity in perpetually conducive regions than in the natural ending of El Niño in these northeast South American cities (99).
The distribution of social roles may also result in differential risks for NTD transmission and infection (106). As an example, women responsible for collecting water and children who play in it or in mud may expose themselves to NTD vectors that thrive near aquatic environments. This represents a social dynamic in disease transmission that requires targeted intervention such as providing better access to clean water, education, and improved sanitation (97, 107).
Other social determinants of health, including labor and workforce issues, can also have an impact (108–110). Families and communities that more often rely on outdoor activity for labor and income, such as agricultural or sustenance farming, are more likely to encounter NTDs than those who are engaged in other professions (108). This also ties into risks associated with individuals who lack access to education, who may consequently be less able to avoid riskier or more infectious disease-related occupational exposures than those that have received higher levels of education (47, 111–113). Likewise, interventions that utilize local school systems to provide health education on vector avoidance, though representing a cost-effective health promotion intervention to reduce the NTD disease burden among children, require those at the highest risk to have adequate access to primary education (114).
As discussed above, poverty also has strong links to NTDs, specifically by its social expression as substandard housing conditions, lack of access to safe water, and poor environmental sanitation (115–118). A major determinant of risk for NTD transmission is poor housing (e.g., cracked mud walls, thatched roofs, damp earthen floors, or lack of indoor plumbing), which can obstruct and complicate vector control efforts, leading to increased exposure to diseases such as dengue, leishmaniasis, lymphatic filariasis, and Chagas disease. This occurs in resource-poor and even developed country settings in low-income communities (115–118). Poor sanitation due to inadequate garbage disposal/collection can also result in breeding sites for many NTD arthropod vectors (such as the sandfly) and subsequently increased risk (115, 116). Vector control efforts aimed at controlling infestation through spraying of insecticides is often relied upon but may have limited effectiveness (119). Other strategies aimed at community-based housing improvement in rural settings, including the use of low-cost techniques for housing construction, selection of housing sites away from vector habitats, improving water storage, building latrines for improved sanitation, and minimizing clustering or crowding, may be more effective long-term strategies for reducing transmission (51, 120, 121).
Ecology and Economic Development
With the exceptions of Singapore, Hong Kong (Special Administrative Region of People's Republic of China), and Equatorial Guinea, no tropical countries/territories are classified as high income using data and definitions from the World Bank. Lower relative wealth in equatorial regions can be considered both an associated risk and an enabling factor for the spread and incidence of NTDs and other tropical diseases (34).
Ecology and economic development also play important contributing roles in this risk dynamic (88, 122). Regions around the equator receive more direct sunlight than less-equatorial regions, thereby increasing the ability of plants to survive (123). This proliferation in primary energy production through plant life results in higher levels of biodiversity throughout ecological food webs in tropical regions (124). Specifically, this can result in an environment with a higher presence of parasites (organisms associated with many NTDs) and their vectors that ultimately infect human hosts (3). Favorable weather conditions with high levels of heat and humidity in these regions provide an environment conducive to existing NTD parasitism, as they are similar to the environments in which the coevolutionary relationships between parasites and their environment were originally formed (125, 126). Therefore, humans living in tropical regions are subject to a greater exposure to parasitic diseases, which has historically inhibited human development and also leads to greater levels of poverty (127).
Negative impacts on human and economic development activity are also exacerbated by NTD-associated disabilities, which are often severe (88, 128). Disability and morbidity arise from a host of NTD-related symptoms, which may include excessive bleeding, paralysis, hallucinations, delirium, blindness, seizures, elephantiasis, and extreme pain (7, 45). In this sense, factors related to the ecology, weather, and environment in regions where NTDs are endemic all intersect and contribute to negatively impact economic and human development, which leads to increased risks and health consequences of NTDs (129).
Maternal and Child Health Impact
Over one-third of pregnant women in sub-Saharan countries are estimated to be infected with an NTD agent (130, 131). These diseases can have a significant negative impact on maternal and child health if not detected and treated appropriately (132, 133). This includes exacerbating blood loss during pregnancy (a leading cause of maternal death), mother-to-child transmission (e.g., of Chagas disease), and gender-specific consequences of NTDs such as female urogenital schistosomiasis (36, 134).
NTDs may also prevent adequate nutrient delivery for proper fetal and childhood development (135, 136). This can lead to low birth weight, which is associated with impaired cognitive and physical development (137). For example, intestinal worms arising from organisms such as soil-transmitted helminths may inhibit proper nutrient absorption, thereby hindering proper physical growth, and can also lead to impairments related to chronic anemia (138, 139). This can result in long-term health, physical, and cognitive impairments in children who contract an NTD, a result that can have a significant impact on the global burden of disease and economic development through increased disability and morbidity impacting future productivity and income generation (7, 133, 140).
In addition, the potential for pain and discomfort from immunological reactions, including fever and inflammation, may further diminish pediatric health. A child's abilities to play, learn, and intellectually develop are seriously impaired by NTDs (141–144). Accordingly, children with NTDs have higher rates of absenteeism than their healthier counterparts, which decreases their capacity to benefit from education, can lead to stigmatization, and can reduce their future earning potential and economic output (45, 142). Administering antihelminthic drugs to schoolchildren as part of a deworming program can be an effective way to decrease the burden of parasitic infection on children and has been associated with increased school performance (145–147).
EMERGING AND REEMERGING NEGLECTED TROPICAL DISASES
Background
Emerging and reemerging neglected tropical diseases (EReNTDs) (sometimes denoted emerging and reemerging infectious neglected tropical diseases) are an aggregation of disease states that are significant due to challenges associated with their prevention and treatment, their geographical expansion, and their negative impact on economic and social progress (8). Specifically, they are a subset of the 17 NTDs identified by WHO that are also classified by the CDC as emerging and reemerging infectious diseases as first identified and defined by Mackey and Liang (8). Five specific EReNTDs are identified: dengue, Chagas disease, cysticercosis, human African trypanosomiasis, and rabies. Human African trypanosomiasis has been included as an EReNTD in this work, as it fits the criteria outlined and was previously not well recognized for its emergence/reemergence.
The global importance and challenges of NTDs are magnified for EReNTDs, which present their own unique global public health challenges that have yet to be adequately identified or addressed. EReNTDs are defined as NTDs with a human incidence that has rapidly increased in the last 2 decades and/or “threatens to increase in the near future” (8). Compared with the broader category of NTDs, EReNTDs present the additional risks associated with being both EIDs and ReIDs: they pose a potential “dual” threat given that they are spreading and emerging in areas where they were previously not endemic and are also “neglected.”
Consequently, EReNTDs impact the world's poorest populations who have few treatment options, are often not a priority in global and national disease prevention and control programs, and hence are absent for the most part in private sector pharmaceutical pipelines (7, 9). In fact, the “neglected” aspect of many diseases may be a contributing factor leading to their emergence or reemergence as infectious diseases. Additionally, since EReNTDs lack adequate prioritization in global health interventions and policy and innovation efforts, they have the potential to continue to spread and impact millions who are least able to combat them.
At present, although deemed a subset of NTDs, EReNTDs are important to address independently. Not only do EReNTDs have devastating impacts on affected countries and regions, their potential to spread to other areas provides a crucial case study in the globalization of diseases of poverty that do not respect geopolitical borders (8). Sharing common characteristics of EIDs and ReIDs, the spread of EReNTDs is accelerated by ever increasing globalization, travel, and trade, as well as environmental factors, climate change, population growth, migration, urbanization and new unregulated medical practices such as transplant tourism (7, 148–150). Table 4 provides a list and summary of some of the key characteristics of the identified EReNTDs.
TABLE 4.
Key Characteristics of EReNTDs
| EReNTD | List (CDC, NIH, or both | Potential for bioterrorism (NIH) | WHO priority neglected zoonotic disease | Disease agent class | Etiological agent | Animal vectors/reservoirs |
|---|---|---|---|---|---|---|
| Chagas disease | CDC | Not listed | No | Parasitic (protozoon) | Trypanosoma cruzi | Triatomine bug (genus Triatoma) |
| Cysticercosis | CDC | Not listed | Yes | Parasitic (helminthiases) | Taenia solium | Porcine (family Suidae) |
| Dengue | Both | Category A | No | Viral | Flaviviruses | Mosquito (Aedes aegypti) |
| Human African trypanosomiasis | CDC | Not listed | Yes | Parasitic (protozoon) | Trypanosoma gambiense; Trypanosoma rhodesiense | Tsetse fly (genus Glossina) |
| Rabies | Botha | Category C | Yes | Viral | Rabies virus | Multiple animal vectors (e.g., genus Canis and family Suidae) |
Rabies as a broad category is listed both on the CDC list and as an NIH list group 3, category C, infectious disease. Australian bat lyssavirus, which causes a form of rabies is also listed as an NIH list group 1 infectious disease newly recognized in the past 2 decades.
Three of the five EReNTDs are also categorized as targeted WHO NZDs (Table 4), emphasizing the importance of assessing these diseases from a multidisciplinary approach that considers the interaction between human and animal health ecosystems (8, 151–153). All EReNTDs are caused by either parasitic or viral disease agents, some with single/primary animal vectors/reservoirs and others (e.g., rabies) with multiple animal reservoirs. Two of the EReNTDs are also listed on the NIH group 3 list (dengue [category A] and rabies [category C]), indicating a possible need for assessment of their potential use as bioterrorism agents. Below, the characteristics of each of the identified EReNTDs, their unique risk factors, prevention and treatment options, and recent developments are reviewed.
Identified EReNTDs
Dengue.
Dengue, also known as “breakbone fever,” is an acute febrile disease caused by one of five serotypes of arthropod-borne dengue viruses and is characterized as an “old” disease that has reemerged in the last half of the 20th century (154, 155). Its arthropod vector is the Aedes aegypti mosquito, with transmission resulting in symptoms that become more pathognomonic as the disease progresses (156).
Infection can lead to dengue hemorrhagic fever (DHF) and in some severe cases, such as in those who suffer from dengue shock syndrome (DSS), can lead to death (7, 156). Common symptoms are high fever, headache, abdominal pain, myalgia, arthralgia, and rash; in severe cases of DHF and DSS, symptoms are accompanied by thrombocytopenia, vascular leakage, and hypotension (154).
Dengue and its clinical and social manifestations are a tremendous public health concern, and due to its undifferentiated presentation at early onset in roughly half of cases, it may be difficult to diagnose (156). Dengue virus infects between 50 million and 100 million people globally, has a geographic distribution in more than 125 countries, has increased in incidence 30-fold in the past 50 years, represents a leading cause of childhood hospitalization and mortality, and is endemic in all WHO regions except Europe (7, 45, 157). Hence, it is one of the most widespread flaviviruses globally (7, 9, 158, 159).
Dengue flourishes in tropical and subtropical regions, and it is estimated that 40% of the world's population inhabit areas where transmission occurs (160). It is especially prevalent in environments that have limited or no public health water management systems, leading to uncontrolled mosquito breeding of the arthropod vector (7, 155).
According to WHO, dengue outbreaks are increasing in frequency and expanding geographically, even given underreporting, which would tend to significantly underestimate the actual severity and impact; WHO has hence identified dengue as an international public health priority (7, 161). In fact, recent disease surveillance modeling has estimated that the annual global incidence could be closer to 390 million, approximately three times higher than current WHO estimates (155). Adjusting incidence reports may bring this number even higher, while mechanistic pathological characterization remains a challenge to identification, surveillance, and diagnostic and vaccine development (154, 162–165). Further complicating these challenges, a fifth new serotype of dengue virus was reported in late 2013, confounding efforts to develop a potential vaccine that can effectively protect against all types of the disease (155).
As it is an EReNTD, dengue resurgence or emergence is occurring in many poorer regions with no previous experience in preventing, combatting, or controlling it or in regions that have not had a reported case in greater than 20 years (159, 166). As an example, Latin America has experienced a constant increase in dengue and DHF cases since 2003 (160). Specific factors that lead to increased local outbreaks echo some of the challenges of other NTDs as well as its own contextual vector and transmission mechanisms, and these include rapid urbanization, global warming/climate change, lack of vector control, fundamental public health and social infrastructure failures (e.g., in waste management and disposal), and poor hygienic household water storage (167–169). These factors and individual country resource challenges have created social vulnerabilities for those regions with limited clinical or disease surveillance capacity. The fact that dengue is often left underprioritized in comparison to the push for economic development may also create enabling conditions (8).
Yet despite its characterization as an EReNTD and its severe impact on developing regions, dengue is not confined solely to resource-poor settings of endemicity. The disease spread has been increasingly associated with global travel to tropical and subtropical regions that are popular tourist destinations for travelers from high-income countries. It has also been identified as a risk for military populations who operate in areas of endemicity (161, 170, 171). Outbreaks and related seasonality-based transmission have led to dengue's spread internationally, particularly to vector-friendly habitats such as heavily urbanized regions (167). Indeed, outbreaks in the U.S. states of Texas, Hawaii, and Florida and other areas where it is not endemic highlight the growing global health risk of disease transmission of this EReNTD (9, 172). It has been estimated that dengue accounts for 2% of all travel-related illness of those returning from regions of endemicity, especially Southeast Asia (161). Risk factors include length of stay, season of travel, and prevalence of dengue at the destination country. Early diagnosis and appropriate clinical management/treatment are viewed as crucial prevention and treatment responses to limit international spread (161, 170).
Generally, public health interventions for dengue focus on environmental and vector management. These include employing integrated multiagent insecticides as a vector control methodology and an emphasis on early case detection, despite its challenges (7, 156, 173). Although there may be a positive impact from these environmentally driven interventions, integrated vector control has been criticized for being largely ineffective and costly and for its negative impact on the environment due to insecticide resistance and toxicity (160). Unfortunately, the lack of any prophylaxis for cost-effective and direct treatment means that vector control is currently the only feasible response for this widespread EReNTD despite criticism of these measures (154). This makes clear the need for renewed research efforts into preventive health strategies to address dengue (160).
Research efforts to develop a dengue vaccine have been ongoing for more than 70 years; however, these are still in experimental stages, and no licensed vaccines, or, indeed, antiviral agents, are available to prevent or treat the disease (171, 174). Vaccine development for dengue is inherently complex and rife with challenges, such as its unique pathogen-host interaction, absence of a viable animal model for vaccine development, issues of possible vaccine immunogenicity, and need for a vaccine that responds to all dengue virus serotypes administered in a single formulation (which is necessary because vaccination against a single serotype can lead to DHS when infection occurs from another serotype due to antibody-dependent enhancement) (154, 171, 175, 176). Reflecting these challenges, in late 2012, Sanofi Pasteur announced that its much anticipated development of a live-attenuated, tetravalent chimeric dengue-yellow fever vaccine showed limited protective immunity against the first four dengue virus serotypes (177).
Dengue is an important EReNTD focus beyond its direct global health impact on patient populations. It is also of concern because of an increase in mortality due to a increasing incidence of DHF and DSS as well as its complex immunopathophysiology (154). This may reflect viral evolution toward greater virulence, potentially from patient reinfection by one of the now five different dengue virus serotypes, which results in only partial immunity (9, 159, 168). Such shifts emphasize the need to control the spread of disease through development of effective prevention and surveillance controls, particularly in environments with accelerating resurgence (169, 178–180).
Hence, dengue specifically raises concerns regarding the increasing incidence and clinical severity of this EReNTD. It is a growing concern due to dengue's spread through global travel to and from tropical destinations, its intensification, and the lack of a primary vaccine for prevention or drug treatment. The increasing incidence and disease severity of dengue are occurring due to globalization, yet combating the disease has not sufficiently been addressed in drug innovation and discovery efforts, illustrating how an EReNTD can quickly develop from a regional issue to a global health concern.
Chagas disease.
Chagas disease, also known as American trypanosomiasis, is an EReNTD caused by the protozoan parasite Trypanosoma cruzi. It is a common chronic, systemic infection, impacting approximately 7 to 8 million people worldwide, and has an annual mortality rate of approximately 10,000 (7, 45, 181, 182). New cases arise primarily in the poor within areas of Latin America where it is endemic, although like other EReNTDs, it is spreading outside regions of endemicity (45, 183). Chagas disease can also lead to significant economic losses due to reduced productivity from early-age mortality and disability (47).
The parasite can infect a number of wildlife and domestic animal species reservoirs and is spread to humans generally through contact with T. cruzi-containing fecal matter deposited by the triatomine insect near the site of its bite or mucous membranes. This insect vector often infests poorly constructed or substandard housing (183, 184). However, Chagas disease can also be transmitted in the blood through congenital exposure, transfusion, organ transplantation, and reactivation due to immunosuppression and through oral transmission after ingesting contaminated food or liquids (7, 182, 185). Chronic infection is seen in fewer than 10% of infections but can result in severe organ damage leading to malnutrition or sudden cardiac death, with approximately 30 to 40% of cases developing into digestive megasyndromes, cardiomyopathy, or both (7, 182).
Chagas disease, like dengue, is being transmitted beyond its endemic presence in Latin America (7, 186, 187). Usually through vector migration occurring through population movement, travel, and trade, Chagas disease has spread to areas where it is not endemic, including high-income countries such as Australia, Canada, Europe, Japan, and the United States (7, 182, 187). Transmission is also facilitated by socioeconomic factors, including immigration, urbanization, factors leading to poor prenatal care and vertical transmission from mother to child, and potentially through tainted blood and/or organ tissue use (7, 188). Illustrating the potential risk for disease migration across borders, approximately 14 million persons have migrated from countries where Chagas disease is endemic to areas where it is not (188).
Efforts to combat the disease focus upon large-scale vector control programs, blood donor screenings, and surveillance (182, 189). However, although Chagas disease can be controlled when appropriate public health vector control systems are implemented, like for dengue, the development of a vaccine or new, cost-effective antiprotozoal drugs is critical for management of the disease (34). Chagas disease treatment primarily involves the use of the antiparasitic drug benznidazole or nifurtimox, which requires a long-term treatment course and careful monitoring and carries a substantial risk of adverse effects; supportive therapy may also be required for the management of Chagas cardiomyopathy or digestive disease (183, 190). Indeed, many other NTD-preventative chemotherapy treatments suffer from similar challenges of being expensive, highly toxic, long term (presenting compliance issues), often difficult to administer, and rife with follow-up failures, lack pediatric formulations, are prone to drug resistance, and/or are experimental (7, 56, 64, 158, 191).
In addition, comorbid infectious diseases such as HIV disease can lead to immunosuppression and reactivation of T. cruzi infection and other latent diseases, significantly complicating appropriate clinical management. Other comorbid conditions such as Chagas cardiomyopathy may require implantable cardiac defibrillator devices and expensive medications for those suffering from cardiac failure (185, 192–194). At present, there is no vaccine to prevent Chagas disease; however, experimental efforts for a postexposure vaccine for cardioprotection are in testing, particularly for use in disproportionately impacted areas such as Latin America (7, 64, 183, 195–199).
Rabies.
A well-known zoonotic EReNTD is rabies, which is an acute, progressive encephalitis caused by a group of RNA viruses that has close to a 100% mortality rate if left untreated (200). Rabies is caused by members of the genus Lyssavirus in the family Rhabdoviridae and is generally spread via contact with infected animal saliva (7). A wide array of animal species have been identified as reservoirs for potential rabies transmission. This is generally a function of regional and geographic variation worldwide for these animal vectors (7). Human acquisition is predominantly via dog bites, and although greater than 15 million people each year are given specific postexposure treatment, there are still an estimated 50,000 annual rabies deaths worldwide, mainly in Africa and Asia (7, 201). Fortunately, rabies infection is treatable, whereby the development of disease (manifest rabies) can be prevented, generally with postexposure prophylaxis (PEP) involving adequate wound care and administration of rabies immunoglobulin and vaccine (200, 202, 203). However, in resource-challenged environments with poor case detection or lack of affordable access to postexposure prophylaxis, untreated rabies results in eventual paralysis, coma, end-organ damage, and death in nearly all cases (7, 204, 205).
Challenges to the treatment of rabies even where prompt PEP is available include the need for multiple vaccine doses and multiple clinic visits to complete a rabies vaccination regime, which may be cost prohibitive, inconvenient, or inaccessible for resource-poor populations (206). Specifically, the cost and supply of different forms of PEP (including expensive but effective purified rabies immunoglobulin) can be prohibitive or inaccessible in low-income settings due to differences in regime, clinic throughput, cost of vaccine materials and vials, method of delivery, and possible shortages in supply (207, 208). Compounding these treatment access challenges is limited awareness regarding public health prevention and individual preventive measures (including failure of affected individuals to seek care), both of which represent significant challenges in attempting to effectively control rabies (7). Despite these challenges, treatment is effective, with an estimated 270,000 lives saved due to effective postexposure prophylaxis among worldwide rabies cases (7).
The highest risk for rabies incidence occurs in the poor in regions such as Africa, Asia, and Latin America, although rabies is distributed globally in high-income and resource-poor countries alike and is endemic on all continents except Antarctica (7, 209–212). In addition, international travelers are the largest population group to receive preexposure prophylaxis, though it has also been recommended for use in vulnerable populations such as children who live in countries where rabies is endemic and are at increased risk for exposure (205, 213). Large social events, such as the FIFA World Cup hosted in South Africa in 2010, also raised concerns about rabies due to large population movements and tourism and led to increased education and travel advisories on how to prevent exposure (211). Negative economic consequences can also occur due to loss of livestock from canine rabies exposure, especially in Asia (214).
Rabies prevention efforts have focused upon canines, as they are the primary animal reservoirs for disease transmission in both humans and livestock, although often canine vaccination coverage information for countries with the highest rabies disease burden is unavailable (212, 214, 215). Effective strategies have included the use of canine mass immunization and sterilization, as well as preexposure immunization for persons in high-risk occupations in certain settings (7). Mass or routine vaccination of animal reservoirs has been utilized to decease transmission of rabies, with oral rabies vaccination leading to rabies-free status in Switzerland and France in 1998 and 2000, respectively (216). Additionally, neutering of stray dogs may help to inhibit overpopulation, thereby facilitating a decrease in the incidence of rabies (217). However, it should also be noted that reducing the population density of canines through culling has not been found to be an adequate control measure against rabies, emphasizing the need for evaluation of evidence-based approaches to prevention strategies (218).
Rabies also illustrates additional global health thematic challenges associated with EReNTDs beyond limited prevention and challenges in delivering treatment. Primarily, the disease has seen recent emergence or reemergence in diverse country settings, including South Korea, Indonesia, Israel, Bhutan, and South Africa, a worrying trend in itself (7, 219–222). In addition, it appears that transmission is expanding to a larger set of animal species, which raises concerns regarding optimal global disease prevention strategies focused on vector control via targeted immunizations of specific animal reservoirs (7, 219–221). Indeed, new virus serotypes of rabies infection are being detected in previously uncommon reservoirs. For example, enzootic bat lyssaviruses are being detected across Europe, Africa, Asia, and Australia, bats are transmitting rabies from themselves to humans in Latin America, monkeys serve as potential sources of rabies transmission to travelers in Bali, fox-to-dog transmission of rabies has occurred following widespread vaccination efforts, and international animal trade poses a largely unchecked and significant potential for rabies zoonotic disease spread (7, 219, 220, 223–227).
All of these factors emphasize the need for coordinated strategies engaging both medical and animal science as well as across developing and developed countries to effectively monitor and control the incidence and transmission of the rabies EReNTD (220, 223, 224, 228–230). This is particularly important as travel and globalization may increase the spread of this disease, creating additional need for convenient, effective, and cost-sensitive vaccines (such as additional development of intradermally administered vaccines) for more advanced cases as well as potential passive immunity protections (231). In addition, potential development of more rapid and cost-effective rabies diagnostic tests may promote expanded and earlier detection, which is critical to treatment and reduction of rabies-related mortality (232).
Taeniasis/cysticercosis.
Human cysticercosis is an infectious disease caused by the ingestion of eggs of the pork tapeworm, Taenia solium, through fecal-oral transmission (233, 234). This typically occurs through a 2-host life cycle where the intermediate host (pigs) ingests food or water contaminated by eggs that are excreted in the feces of humans infected with the adult tapeworm, which then disseminate and mature into the larval stage in the tissue of pigs and develop into cysts (234). The cycle is completed with the definitive host (humans) ingesting eggs (typically transmitted from contaminated hands, food, or water) or possibly through autoinfection (234, 235). The completion of this life cycle leads to human cysticercus tissue infections and neurocysticercosis when the larvae invade the central nervous system (7).
The estimated prevalence of cysticercosis is very large, with estimates generally greater than 50 million people possibly infected worldwide and 50,000 deaths annually (7, 233, 236). These figures establish cysticercosis as one of the most common causes of acquired epilepsy in developing countries (234, 236). Indeed, clinical infection with cysticerci can lead to cysts in neural tissue, resulting in untreated outcomes including epileptic seizures, convulsions, learning difficulties, and possible death (7). Even with appropriate diagnosis of cysticercosis, treatment regimes can be complex, including possible drug intervention, surgery, or simply observation, depending on the location, size, stage, and number of parasites/cysts as well as the clinical symptoms of each case (234). The disease is particularly a problem in areas of endemicity, including many countries in Latin America and Southeast Asia, India, Haiti, and parts of China (7, 237). However, it is most recognized as a public health crisis in sub-Saharan Africa (7). As well, poor strategies for interrupting transmission are the rule rather than the exception, with the primary enabling factor associated with human activities of food preparation and animal husbandry practices (237).
Socioeconomic considerations are also significant for this EReNTD, as it has been associated with poverty in populations within high-income countries and poorer populations in areas of endemicity that may lack access to important diagnostic tests needed to refer patients for appropriate treatment (47, 233, 238). In addition, subsistence farmers in developing countries are tremendously affected by the disease due to loss of livestock. Cysticercosis creates economic chaos in agricultural systems dependent on pig/porcine production if and when infected pig carcasses are condemned for public health purposes (7). Further, due to the increasing popularity of pork consumption and flow of international workers from large cross-border migration, it may be imported into regions where it is not endemic and then spread locally (233). Reflecting this risk, cysticercosis transmission has been detected in high-income countries such as the United States and Canada through migration of agricultural workers and travel of these workers to and from regions of endemicity (117, 233, 237, 239).
This EReNTD is even more challenging as a public health concern because little reliable epidemiological information is available, while it also appears to evolve in concert with events associated with its hosts (7, 237). Cysticercosis treatment and prevention methods center around chemotherapy strategies, generally focused upon helminthic infection treatments and control of animal reservoirs through mass vaccinations of pigs (7, 240, 241). Unfortunately, the latter strategy—to inoculate pigs to reduce disease incidence and spread—creates and represents high costs and challenging environments to reach the most at-risk areas; consequently, these vaccination efforts have been of limited success (240). Efforts are under way to improve animal reservoir vaccination, with two recombinant antigens for Taenia solium shown to be 98.6% and 99.9% effective at protecting pigs from infection after oral administration of T. solium eggs, thereby drastically reducing the likelihood of human neurocysticercosis infection after consumption of the intermediate porcine host (242). These results were replicated in several different field trials in rural Mexico, an area of endemicity, which found statistically significant decreases in porcine infection after vaccination with antigenic extracts or a potentially more cost-effective synthetic peptide (243–246).
Strategies to increase awareness of the need to improve animal husbandry practices may also aid in reducing transmission. For example, providing pig handlers with oxfendazole, an anthelmintic benzimidazole that prevents the parasitic worm's glucose uptake by binding to tubulin proteins in microtubules, can effectively treat porcine Taenia solium infections and likely lead to decreased incidences of taeniasis and cysticercosis in both pig and human populations (247).
Currently no vaccine is available for prevention of the disease in humans, although promising entities are being tested (234, 248, 249). However, access to these potential treatments as well as more sophisticated molecular diagnostic techniques may be limited, exacerbating the incidence of untreated disease (238).
HAT.
Human African trypanosomiasis (HAT), also known as the “sleeping sickness,” is a complex vector-borne parasitic infection caused by the Trypanosoma brucei protozoan transmitted to humans via bites from blood-feeding tsetse flies (genus Glossina) that have previously acquired an earlier stage of the infection from a human. There are two related geographically distinct Trypanosoma brucei subspecies: T. b. gambiense and T. b. rhodesiense. (7, 250, 251). T. b. gambiense is responsible for the majority of cases and occurs in West and Central Africa, with the transmission cycle involving humans as the reservoir for the parasite and the tsetse fly acting as both a disease host and a vector for human-human transmission, though direct zoonotic transmission can occasionally occur (252–254). Specifically, domestic and wild animals can become infected with both parasitic subspecies and act as carriers or reservoirs for the tsetse fly vector (252, 255). T. b. rhodesiense infection involves a number of wildlife and domestic animal species (such as livestock) as reservoirs in eastern and southern Africa and involves zoonotic transmission and outbreaks often involving cattle (252, 254, 255). Vertical transmission from mother to child can also occur for HAT (255).
The disease is restricted to sub-Saharan Africa, where the tsetse vector resides, and is especially prevalent in remote rural sub-Saharan African regions which lack health system capacity. It is endemic in many of these countries and has a wide range of infection areas, from as small as the village level to as large as an entire district (7, 254). The vast majority (approximately 90%) of cases occur in Africa, with the remaining reported cases occurring in the eastern Mediterranean region (7). Reported cases of HAT have experienced global declines of an estimated 76% from 1999 to 2012, and since 2009 fewer than 10,000 new cases have been reported per year (256–258). However, it has also been estimated that 70 million people may continue to be at risk for contracting HAT, reflecting its potential for reemergence, similar to its large increase in incidence in the 1980s and 1990s (256, 257).
Clinical symptoms of HAT can be complex and difficult to diagnose, as it initially presents with mild or nonspecific symptoms, and the onset of major symptoms can be significantly delayed by months or even years depending on the subspecies of Trypanosoma brucei involved (T. b. gambiense leads to chronic infection, and T. b. rhodesiense leads to acute infection) (7).
Common symptoms after transmission include fever, headache, pain and weakness in joints, and, as the parasite migrates to the central nervous system, severe neurological and psychiatric disorders that if left untreated can result in death (7, 250). The disease can also cause amenorrhea, sterility, and abortion and can be contracted congenitally, all of which impact maternal-child health outcomes in resource-poor populations (7, 259). Early screening, case detection, diagnosis, and treatment are critical strategies in the clinical management of this debilitating and potentially fatal disease. Advanced stages can require complex treatment that lowers the chance of a successful intervention, although the strain of the agent can also influence virulence and outcomes (7, 260–263).
Treatment of HAT depends on the subspecies and stage of the disease but is largely characterized by the use of older drugs that carry concerns of toxicity, poor efficacy, possible drug resistance, and inconvenient route of administration (e.g., Suramin, Melarsoprol, Pentamidine, and Eflornithine) (251, 264–267). Second-line treatments are associated with potential fatal adverse patient safety events and complex and lengthy treatment regimes and administration, and they can come at a prohibitively high cost (7, 190, 250, 251). Fortunately, access to therapy has been aided by international coordination and medicine donations by pharmaceutical firms (268, 269).
Despite safety risks of existing treatment, drug development for new treatments has been largely ineffective, though partnership and initiatives aimed at development of new drugs and diagnostic tools are increasing (262, 265, 266, 270). Given the limitations of available treatments, control of the disease requires active surveillance, vector control, and strengthening of health system capacity, particularly through specialized training, establishment of fixed health facilities, and possible use of mobile teams of health care workers to screen and diagnose the disease in rural areas of endemicity (7, 271).
The negative socioeconomic impact of HAT is particularly severe due to the devastating physical and mental disabilities associated with disease progression, particularly in the late stage, that can also lead to stigmatization of affected individuals (7). This can result in a significant direct and indirect economic burden to households and communities when an infected person becomes incapacitated and can no longer work, especially given that rural populations engaged in agriculture, fishing, animal husbandry and hunting activities for livelihood are at particular risk of exposure (7, 113). Children engaged in activities that lead to increased exposure to the disease vector can also experience problems in growth and intellectual development that can lead to learning retardation and loss of future labor resources (7). Additionally, livestock and agricultural production in rural communities are at risk of exposure to “nagana,” the animal form of HAT (7, 272, 273). This and other factors have contributed to stagnation in economic development in Africa, with an estimated US$1.5 billion in agricultural income loses annually as a result of the disease reported by the Food and Agriculture Organization (FAO) (7).
Similar to the case for other vector-borne EReNTDs, HAT spread is also impacted by environmental and climate changes that can alter possible human contact with the tsetse fly vector, including declines in rainfall and loss of vector habitat through increased agricultural production (7, 274). The disease and its prevalence are also linked to social determinants similar to those for other EIDs/ReIDs, such as population growth, economic development, war, poverty, and the displacement of populations, that can lead to increased transmission in affected areas of endemicity (7, 130, 274).
Though classified as an EID/ReID by the CDC, HAT appears to largely be limited in distribution to the continent of Africa. Despite this geographical containment, the disease's long incubation period in the T. b. gambiense subspecies can lead to importation into regions where it is not endemic and to misdiagnosis if clinicians are unaware of the disease (275). Hence, HAT exhibits many of the risk factors associated with other EReNTDs, and although it does not currently maintain a wider global distribution, it has been reported as increasing in a number of cases in regions where it is not endemic, often connected to migration, international travel, and tourism (276, 277).
Risk Factors and Challenges of EReNTDs
Common risk factors.
The clinical, socioeconomic, and environmental factors exhibited by the EReNTDs show they share many important characteristics that warrant more detailed discussion. These common risk factors include vector-related risk factors, disease-related risks, drug treatment and development challenges, social determinants of health-related risk factors, environmental/climate change risk factors, and disease control-related challenges (Table 5). Primarily, the diseases within the EReNTD category are largely widespread, they impact millions worldwide, they disproportionately impact impoverished, resource-poor communities, and they recently have spread to higher-income, developed country settings through globalization, primarily via migration and importation through international trade and travel (8, 278).
TABLE 5.
EReNTD common risk factors
| Risk factor or challenge |
|---|
| Vector-related risk factors |
| Complex stages of disease exposure and transmission through animal vectors that act as intermediary hosts |
| Present in a variety of different animal vectors (e.g., arthropod, porcine. mammalian) |
| Possible spread of transmission to broader group of animal species vectors (rabies) |
| Disease-related risks |
| Increasing in geographic spread and distribution, including to certain high-income settings |
| Possibility of mortality if EReNTD cases are not treated appropriately |
| Associated with chronic disease and long-term disability |
| Sometimes undifferentiated disease symptoms at early stage of infection |
| Complex cases and costly interventions arising from disease progression (e.g., DHF/DSS, cardiomyopathy, neurocysticercosis) |
| Difficulty of diagnosis or lack of available diagnostic tools |
| Possible vertical transmission from mother to child for certain EReNTDs |
| Actual prevalence may be underreported |
| Drug treatment and development challenges |
| Often lack of safe drug treatments |
| Prolonged and inconvenient treatment regimes and routes of administration |
| Reliance on “older” treatments and failure of license approval for new drug treatments |
| Multiple serotypes/strains may make vaccine development difficult |
| Social determinants of health-related risk factors |
| Poverty |
| Globalization |
| Rapid urbanization |
| Lack of adequate vector control |
| Poor hygiene and sanitation |
| Migration and mass movement of populations |
| Travel and tourism |
| Loss of agriculture/livestock |
| Large social gatherings/events |
| Environmental risk factors |
| May be affected by climate change and its impact on disease vectors |
| Disease control-related challenges |
| High cost and potential environmental impact of vector control measures |
| Possible insecticide resistance |
| Lack of adequate public health and health system infrastructure |
| Lack of awareness and education for EReNTDs |
Importantly, EReNTDs are also characterized by a broad array of risk factors also associated with EIDs/ReIDs, including societal and human behavioral influences, changes in population demographics, food production, spread through international travel, and environmental and ecological changes, and they have had their control limited due to inadequacies of public health and health care system capacity. This includes similar concerns for developing antimicrobial resistance, such as developing resistance to insecticides from vector control measures and drug resistance associated with mass drug administration (190, 279, 280).
It should also be noted that the use of zoonotic agents for bioterrorism purposes by deliberately introducing an infectious agent into wildlife has been raised as a concern. Such a possibility has been highlighted by unconfirmed reports of the use of mosquito vectors and dengue as a potential weapon for bioterrorism and points to the need for dynamic assessment for this particular risk characteristic from a global health security standpoint in concert with global public health approaches (281–285).
Improving EReNTD prevention and treatment.
EReNTDs are also notable for their significant negative economic impacts on afflicted, poorer rural communities, tremendous morbidity and mortality exacerbated by poverty and social determinants of health, and fatality in many cases if left untreated (47).
The prevention of spread of EReNTDs, as primarily zoonotic diseases, is heavily dependent upon strategic integrated vector control strategies. This dependence on vector control is due to the frequent lack of access to safe or effective treatment. However, even when treatment exists and is available, it can be costly and complex, especially in later stages of disease progression. Communities impacted by EReNTDs often lack the necessary health care delivery infrastructure to support integrated prevention and treatment approaches that include screening, diagnosis, treatment, and case management, the absence of which can translate into a significant global burden of disease (8, 56). Hence, encouraging and funding health care institutions, clinics, and community health facilities to engage in surveillance, prevention, and treatment programs may help to reduce the risk and aid in decreasing the incidence of EReNTDs (34, 48).
Specifically, ensuring that clinics in areas of endemicity are adequately stocked with medications that are low-cost and accessible may encourage infected individuals to seek care (60). Misdiagnosis of infectious diseases may be prevented if programs are initiated to ensure that rural providers (including traditional healers and those who practice ethnomedicine) are capable of identifying early symptoms of infection and have proper training to either treat or refer cases (286, 287). This can be enhanced if efforts are made to increase accessibility of basic literature and education that aid in making an accurate EReNTD diagnosis (288). Providing periodic forums for feedback from community health workers may prioritize more cost-efficient primary prevention strategies over strategies related to the clinical management of symptoms (289, 290). However, managers of rural clinics should also be encouraged to facilitate and reward workers for suggesting innovative ideas about how to provide better care for patients infected with EReNTDs (291).
Vaccine availability.
Further complicating NTD control and treatment, no EReNTDs have an approved vaccine, with the exception of targeted preexposure rabies immunization in both animals and humans (8). Providing protection against EReNTDs through the use of vaccination in human populations in areas where the diseases are endemic and that have a high number of cases is an approach that is likely to drastically decrease target disease incidence should vaccines be developed and made accessible (292).
Vaccine development for other infectious diseases may have potential, including the bacillus Calmette-Guérin vaccine for tuberculosis, which has been found to offer limited, temporary protection from the bacterium that causes leprosy and the bacteria that cause Buruli ulcer (293). Though these NTDs are not classified as EReNTDs, the successful utilization of existing vaccines used for other infectious diseases may provide another method for encouraging EReNTD research and innovation. Vaccine development for dengue, Chagas disease, and other EReNTDs is also ongoing, although multiple technical challenges remain prior to their full development and administration (176, 177, 196, 293–300).
Need for continued investment and innovation.
A critical area of need for many EReNTDs is continued investment, innovation, and development to ensure equitable access to safe and effective diagnostic tools and drug treatments. The development of rapid, high-quality, low-cost diagnostic tools for EReNTDs is crucial, as many of these diseases present with nonspecific symptoms or currently require laboratory capacity for accurate diagnosis (301, 302), and current diagnostic testing can be highly variable and of heterogeneous sensitivity and specificity, limiting its effectiveness (34, 303). A particular concern is that common EReNTD treatment regimes, through preventive chemotherapy and rapid-impact packages containing a combination of drugs delivered acutely to interrupt transmission of different EReNTD-related parasites, often are dangerous, are difficult to administer, lack pediatric formulations, and do not necessarily address the causative agents or downstream public health impact (34, 190, 303).
In addition, the quality of NTD medicines has come into question as part of larger global concerns regarding the ongoing public health problem of counterfeit, falsified, and substandard medicines in low-resource settings (304–307). Concerns regarding possible substandard NTD medicines have arisen from the detection of locally manufactured miltefosine (used to treat visceral leishmaniasis) that contained no active ingredient in Bangladesh, which was discovered only due to abnormally high numbers of treatment failures (304, 308). This important concern regarding the quality and efficacy of EReNTD treatments has not been adequately researched or addressed.
Importance of environmental issues.
Strategies to improve environmental sanitation and provide adequate human hygiene are also critical components of addressing several risk factors associated with the transmission of EReNTDs. Greater access to clean water may also lead to improved hygiene in populations susceptible to EReNTDs, including increased handwashing, a practice which is likely to prevent cases of certain NTDs that are spread through human-to-human transmission (309).
On a broader environmental scale, reducing factors contributing to climate change that can lead to environmental impacts as noted above may decrease the potential spread of EReNTD-carrying vectors, although the conceivable positive effects of such efforts will likely be far in the future (98, 99, 310). While high-income countries historically have emitted a much larger amount of greenhouse gases than lower-income countries, it is the residents of lower-income countries whose health is often most directly impacted by the NTD health-related consequences of climate change (311). Broad private sector adoption of voluntary standards, including the Greenhouse Gas Protocol and the Carbon Disclosure Project, may promote increased environmental stewardship that protects global social and economic well-being and can have a downstream positive impact on NTD control and prevention (312, 313).
Overview.
Consequently, although there is some variation by specific EReNTD, there are at present significantly underdeveloped public health strategic interventions, health promotion and education efforts, and available diagnostic tools and treatment options for effectively fighting the increasing EReNTD spread across developing and developed countries. The global distribution of endemic EReNTDs is shown in Fig. 2.
FIG 2.
Global intensity map of EReNTD regions of endemicity. Data are from the World Health Organization (2013). We used the following definitions when coding the data for regions of endemicity for each EReNTD according to WHO data: dengue, most of the country is at risk for dengue; rabies, most of the country is at high risk for rabies; Chagas disease, the disease is present in the country; cysticercosis, the disease is reported as endemic in the country; human African trypanosomiasis, the disease is present in the country.
However, the categories and spread have the potential to expand. Specifically, the list of EIDs and ReIDs compiled by the CDC and NIH may grow as new diseases are detected and emerge/reemerge and the debate over the definition of NTDs and whether the 17 WHO-identified diseases are adequately representative of the term continues (43). Although not “officially” classified by CDC as emerging or reemerging, other NTDs may also be subject to epidemiological or geographical shifts, transformations through genetic mutation, or changes in host/vector distribution that require further research, including mapping, monitoring, and surveillance, to assess their risk (46). These developments may portend a greater potential for regional spread in areas where the diseases are not endemic, which needs to be captured in global policy efforts to prioritize the surveillance, control, and development of cost-effective interventions for current and future EReNTDs (56).
EMERGING GLOBAL HEALTH POLICY ENVIRONMENT FOR EReNTDs
Background
NTDs have been a topic of interest for the WHO since as early as 1948, shortly after the organization was constituted, when the World Health Assembly (WHA) (the decision-making body of WHO) first issued a resolution recognizing the need for international action to address vector-borne diseases (45). Since then, numerous WHA resolutions have been adopted, addressing prevention, surveillance, control, elimination, and eradication of specific NTDs, supply and use of insecticides, intensification and coordination of research on NTDs and vector control, prevention and control of NTDs associated with food-borne illnesses, water supply and sanitation issues, and environmental issues, including organic pollutants (Table 6) (45).
TABLE 6.
Summary of EReNTD policy environmenta
| EReNTD | Disease-specific WHA resolution(s) (yr) | WHO targets and milestones for 2015 and 2020 | Examples of EReNTD-specific initiatives |
|---|---|---|---|
| Chagas disease | WHA51.14 (1998), “Elimination of transmission of Chagas disease”; WHA63.20 (2010), “Chagas disease: control and elimination” | 2015, regional transmission through blood transfusion interrupted; 2020, Regional intradomiciliary transmission interrupted in the region of the Americas | Global partnerships, WHO- and PAHO-led initiatives (e.g., WHO Global Network to combat Chagas disease) and Global Chagas Disease Coalition (DNDi and ISGlobal); medicine donations and other support, Bayer and Sanofi; drug development, DNDi |
| Cysticercosis | No specific WHA | 2015, validated strategy for control and elimination; 2020, interventions scaled up in selected countries for control and elimination | Global partnerships, Cysticercosis Working Group in Europe, Cysticercosis Working Group in Eastern and Southern Africa; drug development, Global Alliance for Livestock Veterinary Medicines-Indian Immunological Limited-University of Melbourne, International Livestock Research Institute |
| Dengue | WHA46.31 (1993), “Dengue prevention and control”; WHA55.17 (2002), “Prevention and control of dengue fever and dengue haemorrhagic fever” | 2015, sustainable vector control interventions established in 10 priority countries of endemicity; 2020, control and surveillance system established in all regions, no. of cases reduced by more than 25%, no. of deaths reduced by 50% | Global partnerships, WHO- and PAHO-led initiatives, Dengue Vaccine Initiative (DVI), Asia-Pacific Dengue Prevention Partnership, DengueTools Project; drug development, DVI, Pediatric Dengue Vaccine Initiative, Sanofi Pasteur |
| Human African trypanosomiasis | WHA36.31 (1983), “African human trypanosomiasis”; WHA50.36 (1997), “African trypanosomiasis”; WHA56.7 (2003), “Pan African tsetse and trypanosomiasis eradiation campaign”; WHA57.2 (2004), “Control of human African trypanosomiasis” | 2015, country elimination in 80% of foci | Global partnerships, WHO Collaboration with Programme Against African Trypanosomiasis (PAAT); medicine donations and other support, Bayer and Sanofi; drug development, DNDi and Sanofi |
| Rabies | WHA3.20 (1950), “Rabies” | 2015, regional elimination in Latin America; 2020, regional elimination in Southeast Asia and Western Pacific regions | Global partnership, Partnership for Rabies Prevention (Global Alliance for Rabies Control initiative) |
Sources: WHA resolutions.
The NTD movement has also capitalized on momentum gained from international adoption of the 2000 Millennium Development Goals (MDGs), which commit United Nations (UN) member states to global targets to reduce extreme poverty by 2015. International commitment to combating NTDs can lead to progress in achieving all the MDGs, a concept established by WHA and other UN resolutions (7).
The MDG targets are as follows: eradicating extreme poverty (MDG1); achieving universal primary education (MDG2); promoting gender equality and empowering women (MDG3); reducing child mortality (MDG4); improving maternal child health (MDG5); combating HIV/AIDS, malaria, and other diseases (MDG6); ensuring environmental sustainability (MDG7); and developing a global partnership for development (MDG8) (7). EReNTDs exemplify MDG priorities as they are diseases of poverty and have negative socioeconomic impact (MDG1, targets 1A and B), can lead to child mortality and poor maternal child health outcomes (MDGs 4 and 5, targets 4A and 5A), are impacted by environmental changes, drinking water, and sanitation (MDG7, target 7.C), and clearly fall in the category of “other” diseases that impede human development (MDG6, target 6.C) (7, 36, 314).
Yet, prioritization of the deleterious social, economic, and health consequences of NTDs has historically varied and has reemerged as a global health policy priority only in the past decade (43). The global adoption of the MDGs and various efforts by WHO calling for action on NTDs have acted as catalyst for this paradigm shift in advocacy, recognition, and action. Specifically, international interest in NTDs was formally reestablished in 2003 and 2005, when WHO held workshops that specifically called for international action to address the so-called “neglected” diseases (43). This subsequently led to eventual identification of the 17 NTDs, establishment of the WHO Department for Control of NTDs, and creation of the WHO's “Global Plan to Combat Neglected Tropical Disease 2008-2015,” which set forth a conceptual framework for a strategic integrated policy approach for combating NTDs, and of the aforementioned WHO “Roadmap for Implementation” setting NTD elimination and eradication targets (7, 315). Since then, growing international attention to and advocacy for NTDs have led to numerous technical reports, scientific research and journals, global meetings, collaborations, initiatives, organizations, and partnerships all focused on the prevention and control of NTDs (7, 36).
EReNTDs and Global Health Partnerships
The engagement of multistakeholder public-private partnerships (PPPs), which enable collaboration between national governments, the private sector, academia, private foundations, nongovernmental organizations (NGOs), and other nonprofit organizations, has been a growing trend in addressing critical global public health issues, and these include notable organizations such as the Global Fund to Fight AIDS, Tuberculosis, and Malaria (the Global Fund) and the GAVI Alliance (8, 316–318).
PPPs also include the subcategory of product development partnerships (PDP), which have a primary objective of developing a health product and have been widely adopted for NTDs (319). One example of an NTD PDP is the nonprofit Drugs for Neglected Disease initiative (“DNDi”) (64, 319, 320). The DNDi relies on financial contributions and cooperation from both public- and private-sector partners and focuses on drug development for six specific neglected diseases, taking a pragmatic and case-by-case approach to intellectual property (IP) (i.e., using intellectual property only to promote accessibility) (321). It has been successful in developing six new products, including a combination treatment for HAT and a pediatric formulation for Chagas disease, both EReNTDs (321). The collaboration with industry and management of IP by this groundbreaking initiative is an example of a new paradigm and precedent for future partnerships. Similarly, a new consortium called “WIPO Re:Search,” formed between the Biotechnology Industry Organization Ventures for Global Health and the World Intellectual Property Organization in 2011, aims to establish partnerships with industry and research institutions to facilitate sharing of IP for NTD innovation and development (383).
Other examples of PPPs devoted to NTDs have also taken shape (322). These include notable collaborations directly with or led by WHO, including WHO's Special Programme for Research and Training in Tropical Diseases (TDR) (cosponsored by UNICEF, the UN Development Programme, the World Bank, and WHO), in which GlaxoSmithKline, WHO, and Merck collaborate to provide medicines for lymphatic filariasis (34, 61, 158, 323). Additionally, private-sector collaborations involving WHO and other stakeholders have also been active, such as the partnership between GlaxoSmithKline and Merck & Co., Inc., to address lymphatic filariasis (384). NTD initiatives also include direct government-, industry-, or private foundation-led initiatives such as the U.S. Agency for International Development's NTD Program, Merck's partnership on onchocerciasis, Pfizer's partnership with the International Trachoma Initiative, the Global Network on Neglected Tropical Diseases (an initiative of the Sabin Vaccine Institute), and the BMGF-led Drugs for Neglected Diseases initiative (addressing African trypanosomiasis) and Grand Challenges in Global Health initiative (which provides funding for many NTD projects) (34, 43, 322–324). Further, PPPs supporting infectious disease funding and research and development (R&D) activity in “innovative developing countries,” such as Brazil, China, India, and Indonesia, where NTDs are also endemic will also be important models to consider moving forward (36, 61, 325, 326).
Other organizations have also attempted to tackle specific EReNTDs. These include the Partners for Rabies Prevention, an informal PPP that includes representative of WHO, the Pan American Health Organization, FAO, the World Organization for Animal Health (OIE), the European Commission, academic, NGOs, industry, and private global health institutions, with the goal of global elimination of canine rabies (212, 327).
The London Declaration
Though NTD-dedicated partnerships and investments are growing, NTDs nevertheless remain marginalized, and commitments have yet to translate into needed new drug treatments or necessary scale-up that could improve the lives of millions. However, the policy environment may rapidly be changing, as international prioritization and cooperation on combating NTDs have recently culminated in the January 2012 London Declaration on Neglected Tropical Diseases (the “London Declaration”).
The London Declaration brought together a consortium of stakeholders, including national governments (the United States, the United Kingdom, and the United Arab Emirates), UN agencies (WHO and World Bank), private foundations (BMGF), 13 pharmaceutical firms, and other organizations. Jointly, all endorse a renewed focused on the control or elimination of at least 10 NTDs by the end of the decade through international partnership and cooperation (36, 45).
This monumental declaration included commitments by stakeholders to expand drug access programs and other interventions; advance NTD treatment R&D through partnerships and funding, enhance collaboration and coordination at the public, private, national, and international levels, provide technical support to countries where NTDs are endemic, and provide adequate funding for implementation of NTD programs and health system strengthening (36, 45). This specifically included financial commitments from pharmaceutical firms for US$1.4 billion in NTD treatments, $363 million from the BMGF, and additional commitments from bilateral donors (328). This strong global commitment to combating NTDs has been hailed as a watershed event and may have the potential to build and add capacity to existing collaborations and partnerships, though other policy solutions should also continue to be explored and pursued.
Application of the One Health Initiative to EReNTDs
Building upon efforts of PPPs may be a viable component of addressing the current needs for innovation, financing, drug discovery, and medicine procurement and delivery. However, it is not enough alone to overcome the multitude of risk factors associated with EReNTDs that extend beyond therapeutic preventive or treatment interventions (8). Instead, a more macro approach may be necessary to promote greater effectiveness in addressing the underlining social, human health, zoonotic, and environmental challenges to prevent morbidity and mortality from these diseases.
One such approach that is growing in recognition is the “One Health” initiative, which engages interdisciplinary and multistakeholder participation locally, nationally, and globally in areas of human and animal health, agriculture, and the environment (8, 329–335). Specifically, the CDC, the American Society of Tropical Medicine and Hygiene, and a number of other professional organizations have recognized the importance of an integrated approach to addressing the prevention, detection, and control of EIDs/ReIDs (331). Additionally, some 90 One Health initiatives are currently ongoing in Europe and Asia and have been adopted by other networks and organizations (335).
One Health initiatives promoting cooperation and strategic planning between physicians, ecologists, and veterinarians with the aim of improving health for humans and animals and addressing the spread of diseases through zoonoses and environmental factors can serve as an important potential model for effective EReNTD interventions. They have earlier been proposed also as a model integrated approach to preventing pandemic-scale zoonotic infectious disease threats (329, 331, 336).
These One Health approaches should be sensitive to resource-poor settings and should leverage partners and broader global public health networks. If possible, they should also integrate potential technology-based solutions, such as the use of wireless and mobile technologies for health intervention/education delivery and utilization of household water treatment technologies (8, 337). One Health initiatives can also be conducive to broader engagement with organizations and individuals with knowledge, ability, and experience in core EReNTD programmatic areas of prevention, surveillance, vector control, and clinical case management, as well as those with training in economic development, trade and travel, food safety, genomics, geography, pharmacology, veterinarian sciences, wildlife management, farming and agriculture, climatology, molecular biology and microbiology, virology, parasitology, ecology, policy, and law (8, 332, 338). Hence, the interdisciplinary enabling environment of the One Health concept provides an important opportunity to combine field efforts addressing both NTDs and EIDs/ReIDs but requires sound global health governance to make it operational (330).
Supporting the compatibility of the One Health framework for addressing EReNTDs has been its direct application in infectious disease governance and integrated vector prevention and control strategies. This includes successful vector control interventions in Chad, where restricted application of insecticides to cattle leg extremities was used to address HAT, application of the One Health model for coordination and control efforts for rabies and other canine-related zoonoses, and the importance of One Health and the globalized food supply chain (201, 272, 339–341).
In order to address critical governance challenges in implementing a One Health framework for EReNTDs, United Nations specialized agencies and related programs and funds and intergovernmental organizations could take a leading role in their respective areas of focus; a potential framework is outlined in Fig. 3 (330). Specifically, potential UN agency cooperation under a One Health framework for EReNTDs could include the WHO Special Programme for Research and Training in Tropical Diseases (TDR) with its history of public-private engagement, the World Bank with its development projects and global health strengthening programs, the UN Environment Program (UNEP) and World Meteorological Organization (WMO), which can provide environmental assessments and interventions, the UN Development Programme (UNDP) and UN Population Fund (UNFPA) with their collective expertise in poverty alleviation and reproductive health, the World Organization for Animal Health (OIE) with its expertise in animal health and veterinarian sciences, the FAO with expertise in agriculture and food safety, and UNICEF with its specific maternal and child health intervention focus (8).
FIG 3.
Proposed United Nations “One Health” framework. FAO, Food and Agriculture Organization; OIE, World Organization for Animal Health; TDR, WHO Special Programme for Research and Training in Tropical Diseases; UNDP, United Nations Development Programme; UNEP, United Nations Environment Programme; UNFPA, United Nations Population Fund; UNICEP, United Nations Children's Fund; WHO, World Health Organization; WMO, World Meteorological Organization.
INNOVATION ENVIRONMENT FOR EReNTDs
Intellectual Property, Innovation, and NTD Policy Proposals
Within drug development, a number of intellectual property rights (IPRs) recognized under international agreements such as the World Trade Organization (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS agreement) are employed, including patents (typically granted for new chemical entities, molecules, or biological preparations), data exclusivity (surrounding data generated when potential new drugs undergo clinical trials), market exclusivity (when new drugs are granted exclusive marketing rights by a drug regulatory agency, generally developed for government-identified priority areas), trademarks (to give brand names to drugs), and secrecy (to protect prepatented data as well as manufacturing techniques) (342, 343). In addition, IPR management tools such as differential/tiered pricing, voluntary licensing, patent pools, and promotion of local manufacturing through technology transfer and licensing have also been utilized for developing markets to provide enhanced access to medicines (343–346). However, in the case of NTD innovation and R&D, incentives offered through IPRs may not be sufficient to provide access to life-saving medicines despite their necessity and demand, leading to market failure (347, 348).
Other Proposals
Beyond these traditional IPR-based approaches, a number of governance, financial, and policy mechanisms have been proposed to address the lack of NTD innovation and financing (343). Central to understanding these incentives is an understanding of how “push” (direct incentives that provide for funding and investment) and “pull” (incentives that offer rewards for the final outcome of R&D) mechanisms can facilitate innovation (343). These include proposals for an international treaty on global R&D, establishing a global fund for NTDs, prize funds and advanced market commitments (AMCs), extending a manufacturer's market exclusivity through orphan disease policies, enhanced and targeted tax and trade incentives, socially responsible and humanitarian pharmaceutical licensing, and open access/source drug discovery development models (158, 321, 326, 348, 349).
International biomedical R&D treaty.
The establishment of an international binding biomedical treaty aimed at redirecting global resources toward health R&D priorities and creation of global public common goods (e.g., research tools, drugs, vaccines, diagnostics, biomedical databases, etc.) for NTDs and other diseases has been suggested as a potential policy since 2004 (321, 343, 348, 349). This concept has gained support from a number of stakeholders and would create the foundation of a new global health governance architecture for innovation under a proposed WHO treaty instrument that specifically prioritizes (i) global health needs-driven research, (ii) NTD R&D, (iii) global funding mechanisms based on a sliding scale related to the country's national income (e.g., 0.01% of gross domestic product [GDP]), (iv) ensuring equitable distribution and pricing of medicines, and (v) promoting open access to and exchange of information and ideas (including technology transfer) (321, 343, 349). Core to the treaty is the requirement that national governments have a legal obligation to provide a minimum investment in R&D through a centralized global financing structure ensuring sustainable financing (321). In 2012, the Consultative Expert Working Group (CEWG) and subsequently the WHA recommended that countries begin the process of negotiating a global medical R&D convention (321, 343).
However, obtaining member state agreement to binding treaty terms and ensuring that obligations do not conflict with other existing international intellectual property rights regimes such as the TRIPS agreement and free-trade agreements that include more favorable pharmaceutical innovation-related terms (i.e., “TRIPS-plus agreements”) will likely prove to be difficult, even though modification of these trade and IPR agreements to accommodate the proposal has been suggested (348). This is especially true given that only one health treaty (the WHO Framework Convention on Tobacco Control) has ever been established under the auspices of the normative powers of the WHO Constitution and given the reality that treaty negotiations are often lengthy, costly, and uncertain (350, 351). Further, reaching global consensus upon the core requirement of the treaty and other requirements appears to be difficult. These include the global “tax” or “contribution” requirement central to the function of the R&D treaty as well as requiring increased public sector drug discovery financing. As a reflection of these challenges in negotiation, diplomats have failed to make progress on negotiations and have instead supported a series of demonstration projects, which are described below (352).
Global Fund for NTDs.
A proposal for a global multistakeholder partnership modeled after the Global Fund has been suggested for NTDs (58). The Global Fund has emerged as a leading global health institution, funding mechanism, and successful large-scale PPP model that has attracted billions of dollars in financing and led to major advances in combating HIV/AIDS, malaria, and TB (58, 353). Translation of the Global Fund model to NTDs seems logical. These diseases impact the poorest and have a significant global burden of disease, require a sustainable funding mechanism and harmonization of stakeholder/donor activities, may be treated by low-cost drugs that can be available as generics, and require health system strengthening and scale-up of treatment delivery. Further, many NTD-focused partnerships have already engaged the private sector in drug discovery and medicine donations (58). All of these areas are within the operational expertise of the Global Fund, and this governance structure would seem apropos to the management issues for NTD efforts.
Though it is a plausible solution for enhancing funding and delivery of interventions for NTDs, the steps and political will required to create an “NTD Global Fund” are still underdeveloped. Possible strategies include expanding the current Global Fund's mandate to include NTD control and elimination or establishing a special and separate “NTD Fund” similar to structures such as the Global Fund, Stop TB Partnership, and Global Polio Eradication Initiative, with active participation of donors, WHO, and other NTD global partnerships in its planning and operation (58). There has also been a related proposal advocating for a “social offset” mechanism in NTD funding that would set aside resources for NTD related socio-environmental and health system issues (e.g., access to clean water and sanitation, community education for infection prevention, and vector control) to promote a more integrated approach for biomedical and socioeconomic determinants of health (354).
Prize funds and advanced market commitments.
Prize funds act as pull mechanisms for R&D by providing financial incentives for successful drug development and can be used to promote NTD innovation (343). This includes proposals for fixed awards, milestone requirements to space out payments, and prize amounts based on outcome measures such as impact on DALYs (326). Prize funds seek to delink R&D costs from the end price of the final product, often by including IPR management requirements in order to ensure accessibility (343).
However, specific calculation of correct prize value can be highly difficult. Payment terms must be carefully crafted to appropriately incentivize initial participation while mitigating potential overpayment for subsequent inconclusive research that does not lead to a finished product (326). Indeed, these programs often end up paying premiums to offset potential R&D investment failures (326).
AMCs are a vehicle similar to prize funds but may represent a more viable solution, as they operate through market creation or risk reduction (343). They provide specific criteria for future procurement of fixed drug quantities at agreed-upon pricing, ensuring an initial market for developed drugs (326). However, AMCs also suffer from the need for detailed but often unavailable, questionable, and/or incorrect information on costs. This may result from inherent difficulties in calculating future reference prices for approved, developed drugs versus estimated costs for industry R&D expenses (326). In fact, R&D expenditures may be variable over the drug development cycles and subject to market-based changes (e.g., inflation and foreign exchange fluctuations).
Both AMCs and prize funds require complex balancing of R&D cost calculations and future medication prices to ensure affordability for resource-poor populations. They ultimately rely on negotiation/agreement with industry partners, which can be extremely time-consuming and labor-intensive as well as heavily biased toward industry repeat players (326).
Orphan drug legislation.
Drug manufacturers sometimes engage in the development of products that target rare diseases, such as orphan drug indications (i.e., conditions that generally lack an approved treatment pathway and, as classified by U.S. law, afflict fewer than 200,000 patients), though they often require financial or market exclusivity incentives in order to engage in drug development for these needy populations (355). Extended drug market exclusivity provisions under orphan drug legislation (e.g., in the United States, Japan, Australia, and European Union) has been proposed as a possible mechanism to incentivize NTD drug R&D given its relative success in promoting development of drugs for rare diseases (355, 356). Common incentive mechanisms in existing orphan drug legislation include fast-track regulatory processes, protocol assistance for clinical trials, tax credits, exemption of registration fees, and access to research grants (356).
Orphan disease designation for NTDs has been used only a few times, and generally such incentives rely upon small biotechnology firms in niche markets rather than large multinational pharmaceutical manufacturers for utilization (356). However, orphan drug legislation strategies used in developed countries may not be translatable to resource-poor settings and can have unintended consequences for NTD medicine access. Specifically, drug development through orphan drug laws has been criticized because it provides private-sector benefits in the form of market exclusivity without ensuring patient access or affordability (158, 355). This approach is particularly risky for resource-poor countries, whose drug regulatory systems and authorizers may be unable to adequately control or obtain price concessions (158).
Tax and trade incentives.
In an attempt to address practical considerations of economic and trade issues that may negatively impact NTD R&D investment and medicine access, a global policy proposal has suggested more targeted use and coordination of national R&D tax credits and lowering of WTO trade and tariff barriers for NTD-related commodities (357). Some governments have applied this approach by providing additional tax credits to incentivize infectious disease research (343).
Specifically, the policy proposal would reform national tax codes for pharmaceutical R&D expenditures by requiring prequalification of incentives (that can come in the form of tax credits or subsidies) and targeting them for expenditures and organizations actively engaged in NTDs and other drug development for diseases that impact the poor and underserved (357). This could be accomplished by reducing or eliminating tax-based incentives for development of drugs that may be of lower global health need (e.g., lifestyle drugs or drug classes where first- or second-line therapy is already available) (357).
The policy proposal also calls for the elimination of trade-related barriers (including tariff and nontariff barriers) to NTD-associated products and commodities to ensure that interventions are delivered cost-effectively to the populations that need them (357). Prior studies have already identified that more than half of countries in sub-Saharan Africa impose drug tariffs and that 40 countries apply tariffs to imported vaccines, potentially limiting their accessibility (358). This can also augment efforts to harmonize, reduce, or eliminate variable taxes and tariff rates on insecticide-treated mosquito bed nets used as a public health intervention against malaria (359).
Though this policy proposal has the potential to incentivize elements of NTD R&D funding and ensuring affordable access to NTD treatments by lowering prices, global harmonization of tax incentives and trade and tariff policies is extremely complex and requires multistakeholder coordination and consensus building within and between both the public and private sectors. Further, tax incentives through credits and subsidies do not guarantee that pharmaceutical firms will engage in NTD R&D if those drugs will nevertheless be unprofitable and cannot be used by firms that are operating at a financial loss (343).
Socially responsible and humanitarian licensing.
Another emerging IPR management strategy to promote equitable access to NTD-related products is the practice of socially responsible licensing, also known as global access or humanitarian licensing, by academic and research institutions (360). This includes grass-roots advocacy in the movement for global access to medicines, specifically calling for research institutions to include equitable IPR management and technology transfer provisions in their licensing agreements when attempting to commercialize research (360). Socially responsible licensing principles often include establishing “equitable access licensing” policies at the institutional level, requiring nonexclusive and open licensing for developing countries and/or NTD innovation in technology transfer, having licensees allow “generic” production for low-income markets, requiring that licensees forego patent protection in developing countries, and developing partnerships to enhance access to medicines (360–364).
The Universities Allied for Essential Medicines (UAEM), a nonprofit international organization that advocates for global access to medicines and is led by university students, has acted as the primary advocate for these practices and has developed its own framework of principles on how institutional global access policies should be implemented (360, 365). UAEM has also highlighted NTDs as a programmatic priority and in 2010 held a Neglected Disease and Innovation Symposium that brought public and private stakeholders together to discuss future policy directions and advocacy for NTD innovation efforts (366). Indeed, NTDs are becoming a predominant theme in socially responsible licensing, and policies have included licensing requiring at-cost production and sale of a novel drugs to treat leishmaniasis in developing countries (360). Institutions that have adopted related policies include the University of British Columbia, Emory University, the University of California Berkeley, Boston University, the University of Edinburgh, and Oxford University (360, 365).
Barriers to more universal adoption include concerns about the negative financial impact of socially responsible licensing policies on institutions as well as industry concerns about diversion or reimportation of generic formulations back into developed countries, which could lead to price erosion (360, 365). Further, though UAEM has been active in advocacy efforts, only a small minority of universities have actual adopted comprehensive global access licensing programs as recommended by the organization (360).
Open-source drug discovery and development.
Open-source drug discovery and development models are a relatively new and innovative strategy focused on encouraging collaboration, sharing, and dissemination of research outcomes and deliverables through the public domain or a customized license (343). Open-source collaborations in science and medicine have led to well-known advancements, including human genome sequencing and open-source software (e.g., the Linux operating system) and are a growing trend in academic publishing as more peer-reviewed journals move to an open-access format to more broadly disseminate research findings (367–370).
The same open-access model can bring benefits in the drug discovery process for NTDs, especially if it can leverage the growing published data on sequenced genomes of organisms associated with NTDs and encourage broader open community research participation (368, 371, 372). Specifically, open-source drug discovery can have the potential to advance screening and identifying of potential protein targets/compounds/new chemical entities for drug discovery, target existing drugs (i.e., “drug repurposing”) for potential NTD drug candidates, enable more collaboration involving researchers in developed and developing countries, and potentially lower drug discovery-associated costs (64, 323, 372–376). Though it is an innovative concept and one that has shown success in other industries, open-source drug discovery is still in its relative infancy (371). Possible impediments to more widespread use of open-source drug discovery practices include the absence of a critical mass of participants and preexisting work necessary to build incremental innovation progress (371).
However, despite challenges, NTD drug development is at the forefront of the open-source drug discovery movement. This includes the Tropical Disease Initiative (TDI), which provides a decentralized, web-based, open-source environment for volunteer collaboration on NTD drug discovery (371). Other open-access resources for NTD development include TDRtargets.org, which provides genetic, biochemical, and pharmacological data and computational predictive models for prioritizing drug target candidates for NTD pathogens (372, 376). Regional open-source drug discovery NTD initiatives are also under way, including the African Network for Drugs and Diagnostics Innovation (ANDI), initiated by TDR in 2008 and now housed at the UN Economic Commission for Africa (377, 378). ANDI's primary goal is to promote African-led health product innovation to address African public health needs under regional governance and management (379).
WHO CEWG demonstration projects.
Many of the above-reviewed policy proposals for NTDs have also been assessed by the WHO-led CEWG on Research and Development, whose mandate was to analyze innovative financing mechanisms and coordination of health product and technological development, especially for those diseases for which access to medicines is lacking and that impact developing countries (36, 343, 344, 380). CEWG assessed a number of different proposals in this area and in April 2012, issued its final report recommending the further development of a global framework on research and development (e.g., an international treaty on R&D), open approaches to R&D and innovation (e.g., open drug discovery and socially responsible licensing), pooled funding (e.g., Global Fund for NTDs), direct grants to firms and milestone prizes and end prizes (e.g., prize funds and AMCs), and assessment of patent pool utilization (347).
In addition, CEWG also initiated additional meetings in March 2014 to assess and recommended a set of demonstration projects that demonstrate effectiveness of alternative, innovative, and sustainable financing and coordination approaches for diseases that disproportionately affect developing countries and where R&D has failed (381). Interestingly, all of the four CEWG-recommended demonstration projects address NTDs. The recommended demonstration projects include submissions from DNDi, the Medicines for Malaria Venture, the U.S. FDA and others, and ANDI (381). They focus specifically on innovation, development, and access to therapy for visceral leishmaniasis, open-access/source drug discovery for a range of NTD compound candidates, and development of affordable biomarkers for use in diagnostics for four parasitic NTDs (including for HAT) (381).
The recommended demonstration projects have met with some criticism, specifically that they lack novelty in favor of projects deemed more viable (e.g., low-risk), many of which are already under way, and may not have much of an impact (352, 382). Though these demonstration projects have yet to be fully implemented or endorsed by the WHO stakeholders, the emphasis on innovation for NTDs provides further evidence of raising global awareness and urgency for the need of tangible action and investment in NTDs.
CONCLUSION
The 21st century has created new and more challenging issues regarding infectious diseases, including concerns regarding their emergence and reemergence and their impact on the future of human development. Emergence and reemergence of old and new infectious diseases alike continue worldwide, are complex and multidisciplinary, involve a host of contributing factors, and are now accelerated by globalization, presenting unique challenges for collective global public health efforts and health security. At the same time, neglected tropical diseases continue to be a blight on human progress and remain critical impediments to alleviating worldwide poverty as envisioned by the international community through the MDGs.
Though scientific progress in addressing some infectious diseases is moving forward, the goals of elimination and eradication of all NTDs remain largely distant. Within this context, EReNTDs represent a subset of infectious disease that require close attention. These diseases have the potential to emerge/reemerge while remaining neglected in the global health priority setting. Though commitments have been made, there remains a dearth of necessary diagnostics, vaccines, and therapeutics needed to help populations affected by these terrible diseases, which can lead to significant morbidity and mortality in these populations. Global public health policies and interventions that hope to tackle the unique threats posed by EReNTDs need to be integrated and innovative to address the multifaceted factors associated with these diseases, which range from addressing vector control to alleviating poverty to addressing underlining social determinants, attending to climate change, blocking spread via international travel and trade, providing for more robust surveillance, and financing innovation.
Newly developed strategies that focus upon integration, such as the One Health concept, multiple stakeholder cooperation through public-private partnerships, and innovative financing and incentive mechanisms, all have a role to play in ensuring that the global control of EReNTDs moves apace. Continued policy advocacy, commitment, investment, and exploration of these strategies are critical to assisting the “bottom billion” out of the neglected disease trap and also preventing the continued spread of these diseases to other global populations. Only by these means can the global health community hope to alleviate the immense suffering caused by EReNTDs and work toward their ensured elimination and eradication now and for future generations.
Biographies

Tim K. Mackey, M.A.S., Ph.D., is the Director of the Global Health Policy Institute, an Assistant Professor of Anesthesiology and Global Public Health, and an Investigator at the San Diego Center for Patient Safety at the University of California, San Diego (UCSD), School of Medicine. He is also the Associate Director for the UCSD MAS Program in Health Policy & Law. He earned his B.A. in political science-international relations from UCSD, a master's degree from the UCSD Program in Health Policy & Law, and his Ph.D. in the Joint Global Public Health program with UCSD and San Diego State University. He has also completed an Executive Course in Global Health Diplomacy at the Graduate Institute, Geneva. His work focuses on global health policy, law, governance, diplomacy, and patient safety. He is specifically interested in strategies to improve the innovation economy for neglected tropical diseases following his work with the World Health Organization.

Bryan A. Liang, M.D., Ph.D., J.D., received his B.S. from MIT, his Ph.D. in Health Policy from the University of Chicago Harris School of Public Policy Studies, his M.D. from Columbia College of Physicians & Surgeons, and his J.D. from Harvard Law School. He is Professor of Anesthesiology and Director, San Diego Center for Patient Safety, University of California, San Diego School of Medicine. His policy work has also encompassed regulatory roles in the United States, including activities at the Federal Trade Commission, the Republic of China in its Department of Health, and the EU as a Faculty Fellow in the Instituut voor Sociaal Recht, Katholieke Universiteit Leuven. His research focuses upon patient safety, particularly as it relates to law, policy, and global health.

Raphael Cuomo, M.P.H., is a Ph.D. student in the Joint Doctoral Global Public Health Program at the University of California, San Diego, and San Diego State University. He obtained a B.S. in Biology at Lafayette College and completed a medical education summer program at Yale University and a medical externship at the Cardiac Arrhythmia Center in the Washington Hospital Center. He also holds a Masters in Public Health, with a concentration in Health Management and Policy, from San Diego State University. His master's thesis focused on the global prevention of maternal mortality. He has also completed graduate course work to obtain a Professional Certificate in Sustainability from Harvard University, and he holds a certification in public health from the National Board of Public Health Examiners. He has conducted public health research with several organizations, including the Institute for Behavioral and Community Health, Leidos, and the Worldwide Orphans Foundation.

Ryan Hafen, M.D., is currently an Education Chief Resident in Anesthesia at the University of California San Diego Hospital. He is entering his fourth year of residency and is also currently serving as a representative for the UCSD Resident Physician Council, where he represents the interests of anesthesia residents to the hospital administration. He is training as a combined resident in both anesthesiology and internal medicine. His clinical interests include perioperative critical care, surgical critical care, cardiac critical care, and medical critical care. He hopes to pursue fellowship training after residency in anesthesia critical care, where he would like to focus on the use of perioperative use of both transesophageal and transthoracic echocardiography for the assessment of the critically ill patient.

Kimberly C. Brouwer, Ph.D., is an Associate Professor in the Division of Global Public Health of the University of California, San Diego (UCSD). She graduated from the Johns Hopkins School of Hygiene and Public Health in 2000 with a Ph.D. focused on molecular epidemiology and parasitology. As part of her studies, she was awarded a Fulbright Fellowship to investigate genetic, environmental, and behavioral factors related to schistosomiasis disease severity in Zimbabwe. Dr. Brouwer subsequently worked as an Emerging Infectious Diseases Fellow and researcher in the Division of Parasitic Diseases at the U.S. Centers for Disease Control and Prevention, focusing on HIV/malaria coinfections. In 2004, she joined UCSD faculty, and she currently leads two NIH R01 grants, exploring factors affecting infectious disease transmission among marginalized populations at the Guatemala/Mexico and Mexico/U.S. borders. Having worked with neglected tropical or emerging infections for 2 decades, she welcomed the opportunity to contribute to this review.

Daniel E. Lee, M.D., Ph.D., completed his undergraduate degree in microbiology at the University of Illinois, Champaign/Urbana, and his M.D. and his Ph.D. in genetics and development at the University of Texas Southwestern Medical Center, Dallas, Texas. He completed pediatrics and anesthesiology residencies at the University of California, San Diego, and a fellowship in pediatric intensive care and pediatric anesthesia at Great Ormond Street Hospital, London, United Kingdom. He is currently an Associate Professor of Anesthesiology and Pediatrics at the University of California, San Diego. He has had a strong interest in infectious diseases since earning his undergraduate degree in microbiology in 1988. His specific interest in tropical diseases began in 1998 when he worked briefly as a pediatrician in South America and then in Mexico. His medical work and travels since then have also brought him to Africa and to Southeast Asia, where his current humanitarian medical efforts are concentrated.
REFERENCES
- 1.Mackey TK, Liang BA. 2012. Lessons from SARS and H1N1/A: employing a WHO-WTO forum to promote optimal economic-public health pandemic response. J. Public Health Policy. 33:119–130. 10.1057/jphp.2011.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frieden TR, Tappero JW, Dowell SF, Hien NT, Guillaume FD, Aceng JR. 2014. Safer countries through global health security. Lancet 383:764–766. 10.1016/S0140-6736(14)60189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451:990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, McKeever D, Mutua F, Young J, McDermott J, Pfeiffer DU. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. U. S. A. 110:8399–8404. 10.1073/pnas.1208059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy FA. 2008. Emerging zoonoses: the challenge for public health and biodefense. Prev. Vet. Med. 86:216–223. 10.1016/j.prevetmed.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymann DL, Dixon M. 2013. Infections at the animal/human interface: shifting the paradigm from emergency response to prevention at source. Curr. Top. Microbiol. Immunol. 366:207–215. 10.1007/82_2012_285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. 2010. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. WHO, Geneva, Switzerland: whqlibdoc.who.int [Google Scholar]
- 8.Mackey TK, Liang BA. 2012. Threats from emerging and re-emerging neglected tropical diseases (NTDs). Infect. Ecol. Epidemiol. 2:75–88. 10.3402/iee.v2i0.18667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morens DM, Folkers GK, Fauci AS. 2004. The challenge of emerging and re-emerging infectious diseases. Nature 430:242–249. 10.1038/nature02759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tognotti E. 2013. Lessons from the history of quarantine, from plague to influenza A. Emerg. Infect. Dis. 19:254–259. 10.3201/eid1902.120312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean HD, Fenton KA. 2010. Addressing social determinants of health in the prevention and control of HIV/AIDS, viral hepatitis, sexually transmitted infections, and tuberculosis. Public Health Rep. 125(Suppl 4):S1–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oramasionwu CU, Daniels KR, Labreche MJ, Frei CR. 2011. The environmental and social influences of HIV/AIDS in sub-Saharan Africa: a focus on rural communities. Int. J. Environ. Res. Public Health 8:2967–2979. 10.3390/ijerph8072967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailes E, Gao F, Bibollet-Ruche F, Courgnaud V, Peeters M, Marx PA, Hahn BH, Sharp PM. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713–1713. 10.1126/science.1080657 [DOI] [PubMed] [Google Scholar]
- 14.Hahn BH, Shaw GM, De Cock KM, Sharp PM. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607–614. 10.1126/science.287.5453.607 [DOI] [PubMed] [Google Scholar]
- 15.Kumar D. 2010. Emerging viruses in transplantation. Curr. Opin. Infect. Dis. 23:374–378. 10.1097/QCO.0b013e32833bc19d [DOI] [PubMed] [Google Scholar]
- 16.Naggie S, Perfect JR. 2009. Molds: hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. Clin. Chest Med. 30:337–353. 10.1016/j.ccm.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 18.Lynch JB. 2013. Multidrug-resistant tuberculosis. Med. Clin. North Am. 97:553–557. 10.1016/j.mcna.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Paphitou NI. 2013. Antimicrobial resistance: action to combat the rising microbial challenges. Int. J. Antimicrob. Agents 42(Suppl):S25–S28. 10.1016/j.ijantimicag.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 20.Gould IM. 2008. The epidemiology of antibiotic resistance. Int. J. Antimicrob. Agents 32(Suppl 1:S2–S9. 10.1016/j.ijantimicag.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 21.Haines A, Kovats RS, Campbell-Lendrum D. 2006. Climate change and human health: impacts, vulnerability and public health. Public Health 120:585–596. 10.1016/j.puhe.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Miraglia M, Marvin HJP, Kleter GA, Battilani P, Brera C, Coni E, Cubadda F, Croci L, De Santis B, Dekkers S, Filippi L, Hutjes RWA, Noordam MY, Pisante M, Piva G, Prandini A, Toti L, van den Born GJ, Vespermann A. 2009. Climate change and food safety: an emerging issue with special focus on Europe. Food Chem. Toxicol. 47:1009–1021. 10.1016/j.fct.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 23.Bezirtzoglou C, Dekas K, Charvalos E. 2011. Climate changes, environment and infection: facts, scenarios and growing awareness from the public health community within Europe. Anaerobe 17:337–340. 10.1016/j.anaerobe.2011.05.016 [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie JS, Jeggo M. 2013. Reservoirs and vectors of emerging viruses. Curr. Opin. Virol. 3:170–179. 10.1016/j.coviro.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skovgaard N. 2007. New trends in emerging pathogens. Int. J. Food Microbiol. 120:217–224. 10.1016/j.ijfoodmicro.2007.07.046 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 1994. Addressing emerging infectious disease threats: a prevention strategy for the United States. Executive summary. MMWR Recommend. Rep. 43:1–18 [PubMed] [Google Scholar]
- 27.Butler CD. 2012. Infectious disease emergence and global change: thinking systemically in a shrinking world. Infect. Dis. Poverty 1:5. 10.1186/2049-9957-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohl KS, Arthur RR, O'Connor R, Fernandez J. 2012. Assessment of public health events through international health regulations, United States, 2007-2011. Emerg. Infect. Dis. 18:1047–1053. 10.3201/eid1807.120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milne-Price S, Miazgowicz KL, Munster VJ. 2014. The emergence of the Middle East Respiratory Syndrome coronavirus (MERS-CoV). Pathog. Dis. 71:119–134. 10.1111/2049-632X.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautret P, Benkouiten S, Salaheddine I, Parola P, Brouqui P. 2013. Preventive measures against MERS-CoV for Hajj pilgrims. Lancet Infect. Dis. 13:829–831. 10.1016/S1473-3099(13)70259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mertz D, Kim TH, Johnstone J, Lam P-P, Science M, Kuster SP, Fadel SA, Tran D, Fernandez E, Bhatnagar N, Loeb M. 2014. Populations at risk for severe or complicated avian influenza H5N1: a systematic review and meta-analysis. PLoS One 9:e89697. 10.1371/journal.pone.0089697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oi M. 2014. Guinea deaths: Ebola blamed for deadly fever outbreak. BBC News. http://www.bbc.com/news/world-africa-26703960 [Google Scholar]
- 33.Samb S. 2014. Guinea confirms fever is Ebola, has killed up to 59. http://www.reuters.com/article/2014/03/22/us-guinea-ebola-idUSBREA2L0MI20140322
- 34.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. 2007. Control of neglected tropical diseases. N. Engl. J. Med. 357:1018–1027. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 35.Fenwick A. 2012. The global burden of neglected tropical diseases. Public Health 126:233–236. 10.1016/j.puhe.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 36.Hotez PJ. 2013. NTDs V. 2.0: “blue marble health”—neglected tropical disease control and elimination in a shifting health policy landscape. PLoS Negl. Trop. Dis. 7:e2570. 10.1371/journal.pntd.0002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2014. EID journal background and goals. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 38.Centers for Disease Control and Prevention. Emerging infectious diseases. Disease information: NCID: CDC. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 39.Centers for Disease Control and Prevention. Emerging Infections Program (EIP)—DPEI—NCEZID. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 40.Breiman RF, Van Beneden CA, Farnon EC. 2013. Surveillance for respiratory infections in low- and middle-income countries: experience from the Centers for Disease Control and Prevention's Global Disease Detection International Emerging Infections Program. J. Infect. Dis. 208(Suppl 3):S167–S172. 10.1093/infdis/jit462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NIAID. List of emerging and re-emerging infectious diseases. NIAID, Bethesda, MD [Google Scholar]
- 42.Health Protection Agency. Emerging infections—list of agents. Health Protection Agency, London, United Kingdom [Google Scholar]
- 43.Liese B, Rosenberg M, Schratz A. 2010. Programmes, partnerships, and governance for elimination and control of neglected tropical diseases. Lancet 375:67–76. 10.1016/S0140-6736(09)61749-9 [DOI] [PubMed] [Google Scholar]
- 44.Molyneux DH. 2010. Neglected tropical diseases—beyond the tipping point? Lancet 375:3–4. 10.1016/S0140-6736(09)61914-0 [DOI] [PubMed] [Google Scholar]
- 45.WHO. 2013. Sustaining the drive to overcome the global impact of neglected tropical diseases. World Health Organization, Geneva, Switzerland [Google Scholar]
- 46.Baker MC, Mathieu E, Fleming FM, Deming M, King JD, Garba A, Koroma JB, Bockarie M, Kabore A, Sankara DP, Molyneux DH. 2010. Mapping, monitoring, and surveillance of neglected tropical diseases: towards a policy framework. Lancet 375:231–238. 10.1016/S0140-6736(09)61458-6 [DOI] [PubMed] [Google Scholar]
- 47.Conteh L, Engels T, Molyneux DH. 2010. Socioeconomic aspects of neglected tropical diseases. Lancet 375:239–247. 10.1016/S0140-6736(09)61422-7 [DOI] [PubMed] [Google Scholar]
- 48.Gubler DJ. 1998. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 4:442–450. 10.3201/eid0403.980326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutherst RW. 2004. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 17:136–173. 10.1128/CMR.17.1.136-173.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Berg H. 2009. Global status of DDT and its alternatives for use in vector control to prevent disease. Environ. Health Perspect. 117:1656–1663. 10.1289/ehp.0900785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Institute of Medicine Forum on Microbial Threats. 2011. The causes and impacts of neglected tropical and zoonotic diseases: opportunities for integrated intervention strategies. National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 52.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté L-A, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. 2013. Time to set the agenda for schistosomiasis elimination. Acta Trop. 128:423–440. 10.1016/j.actatropica.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 53.Mazigo HD, Nuwaha F, Kinung'hi SM, Morona D, de Moira AP, Wilson S, Heukelbach J, Dunne DW. 2012. Epidemiology and control of human schistosomiasis in Tanzania. Parasit. Vectors 5:274. 10.1186/1756-3305-5-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO. 2014. World Health Day: vector-borne diseases. World Health Organization, Geneva, Switzerland [Google Scholar]
- 55.UN News Centre. 2014. Ebola: UN health agency says more than 1 million people affected by outbreak. http://www.un.org/apps/news/story.asp?NewsID=48478#.U-0Kn0tEm3B
- 56.Hotez PJ, Pecoul B. 2010. “Manifesto” for advancing the control and elimination of neglected tropical diseases. PLoS Negl. Trop. Dis. 4:e718. 10.1371/journal.pntd.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gyapong JO, Gyapong M, Yellu N, Anakwah K, Amofah G, Bockarie M, Adjei S. 2010. Integration of control of neglected tropical diseases into health-care systems: challenges and opportunities. Lancet 375:160–165. 10.1016/S0140-6736(09)61249-6 [DOI] [PubMed] [Google Scholar]
- 58.Hotez PJ, Molyneux DH, Fenwick A, Savioli L, Takeuchi T. 2008. A global fund to fight neglected tropical diseases: is the G8 Hokkaido Toyako 2008 summit ready? PLoS Negl. Trop. Dis. 2:e220. 10.1371/journal.pntd.0000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotez P. 2011. A handful of ‘antipoverty' vaccines exist for neglected diseases, but the world's poorest billion people need more. Health Affairs 30:1080–1087. 10.1377/hlthaff.2011.0317 [DOI] [PubMed] [Google Scholar]
- 60.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. 2002. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet 359:2188–2194. 10.1016/S0140-6736(02)09096-7 [DOI] [PubMed] [Google Scholar]
- 61.Croft SL. 2005. Public-private partnership: from there to here. Trans. R. Soc. Trop. Med. Hyg. 99:9–14. 10.1016/j.trstmh.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 62.Trouiller P, Torreele E, Olliaro P, White N, Foster S, Wirth D, Pecoul B. 2001. Drugs for neglected diseases: a failure of the market and a public health failure? Trop. Med. Int. Health 6:945–951. 10.1046/j.1365-3156.2001.00803.x [DOI] [PubMed] [Google Scholar]
- 63.Maurer SM, Rai A, Sali A. 2004. Finding cures for tropical diseases: is open source an answer? PLoS Med. 1:e56. 10.1371/journal.pmed.0010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. 2009. Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl. Trop. Dis. 3:e440. 10.1371/journal.pntd.0000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biswas G, Sankara DP, Agua-Agum J, Maiga A. 2013. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Philos. Trans. R. Soc. B 368:20120146. 10.1098/rstb.2012.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feasey N, Wansbrough-Jones M, Mabey DCW, Solomon AW. 2010. Neglected tropical diseases. Br. Med. Bull. 93:179–200. 10.1093/bmb/ldp046 [DOI] [PubMed] [Google Scholar]
- 67.WHO. 2006. Neglected tropical diseases: hidden successes, emerging opportunities. WHO, Geneva, Switzerland [Google Scholar]
- 68.Allen T, Parker M. 2012. Will increased funding for neglected tropical diseases really make poverty history? Lancet 379:1097–1098. 10.1016/S0140-6736(12)60159-7 [DOI] [PubMed] [Google Scholar]
- 69.Liese BH, Schubert L. 2009. Official development assistance for health—how neglected are neglected tropical diseases? An analysis of health financing. Int. Health 1:141–147. 10.1016/j.inhe.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 70.Heymann DL. 2006. Control, elimination, eradication and re-emergence of infectious diseases: getting the message right. Bull. World Health Organ. 84:82. 10.2471/BLT.05.029512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moran M, Guzman J, Henderson K, Liyanage R, Wu L, Chin E, Chapman N, Abela-Oversteegen L, Gouglas D, Kwong D. 2012. Neglected disease research and development: a five year review. Policy Cures, Sydney, Australia [Google Scholar]
- 72.Musgrove P, Hotez PJ. 2009. Turning neglected tropical diseases into forgotten maladies. Health Affairs 28:1691–1706. 10.1377/hlthaff.28.6.1691 [DOI] [PubMed] [Google Scholar]
- 73.Bockarie MJ, Kelly-Hope LA, Rebollo M, Molyneux DH. 2013. Preventive chemotherapy as a strategy for elimination of neglected tropical parasitic diseases: endgame challenges. Philos. Trans. R. Soc. B 368:20120144. 10.1098/rstb.2012.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. 2013. Mass drug administration for the elimination of lymphatic filariasis—Port-au-Prince, Haiti, 2011-2012. MMWR Morb. Mortal. Wkly. Rep. 62:466–468 [PMC free article] [PubMed] [Google Scholar]
- 75.Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. 2003. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends Parasitol. 19:516–522. 10.1016/j.pt.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 76.Richards FO, Eigege A, Miri ES, Jinadu MY, Hopkins DR. 2006. Integration of mass drug administration programmes in Nigeria: the challenge of schistosomiasis. Bull. World Health Organ. 84:673–676. 10.2471/BLT.06.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Molyneux DH, Hotez PJ, Fenwick A. 2005. “Rapid-impact interventions”: how a policy of integrated control for Africa's neglected tropical diseases could benefit the poor. PLoS Med. 2:e336. 10.1371/journal.pmed.0020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canning D. 2006. Priority setting and the “neglected” tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 100:499–504. 10.1016/j.trstmh.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 79.Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, Aldrich S, Harrington T, Formenty P, Loh EH, Machalaba CC, Thomas MJ, Heymann DL. 2012. Ecology of zoonoses: natural and unnatural histories. Lancet 380:1936–1945. 10.1016/S0140-6736(12)61678-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO. 2005. The control of neglected zoonotic diseases. WHO, Geneva, Switzerland [Google Scholar]
- 81.WHO. 2011. Interagency meeting on planning the prevention and control of neglected zoonotic diseases (NZDs), Geneva, 5-6 July 2011. WHO, Geneva, Switzerland [Google Scholar]
- 82.Rauyajin O, Kamthornwachara B, Yablo P. 1995. Socio-cultural and behavioural aspects of mosquito-borne lymphatic filariasis in Thailand: a qualitative analysis. Soc. Sci. Med. 41:1705–1713. 10.1016/0277-9536(95)00132-Q [DOI] [PubMed] [Google Scholar]
- 83.Wynd S, Melrose WD, Durrheim DN, Carron J, Gyapong M. 2007. Understanding the community impact of lymphatic filariasis: a review of the sociocultural literature. Bull. World Health Organ. 85:493–498. 10.2471/BLT.06.031047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mata L. 1982. Sociocultural factors in the control and prevention of parasitic diseases. Rev. Infect. Dis. 4:871–879. 10.1093/4.4.871 [DOI] [PubMed] [Google Scholar]
- 85.Utzinger J, de Savigny D. 2006. Control of neglected tropical diseases: integrated chemotherapy and beyond. PLoS Med. 3:e112 10.1371/journal.pmed.0030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones COH, Williams HA. 2004. The social burden of malaria: what are we measuring? Am. J. Trop. Med. Hyg. 71:156–161 [PubMed] [Google Scholar]
- 87.Gulati PV, Singh KP, Braganza C. 1977. Role of sociocultural and environmental factors in the cause of scabies. Int. J. Dermatol. 16:281–283. 10.1111/j.1365-4362.1977.tb04321.x [DOI] [PubMed] [Google Scholar]
- 88.McMichael AJ. 2004. Environmental and social influences on emerging infectious diseases: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359:1049–1058. 10.1098/rstb.2004.1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gubler DJ. 2002. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10:100–103. 10.1016/S0966-842X(01)02288-0 [DOI] [PubMed] [Google Scholar]
- 90.Dujardin J-C, Campino L, Cañavate C, Dedet J-P, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y, Boelaert M. 2008. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg. Infect. Dis. 14:1013–1018. 10.3201/eid1407.071589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CIBA Foundation Symposium. 2008. Environmental change and human health. John Wiley & Sons, New York, NY [Google Scholar]
- 92.Patz JA, Graczyk TK, Geller N, Vittor AY. 2000. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 30:1395–1405. 10.1016/S0020-7519(00)00141-7 [DOI] [PubMed] [Google Scholar]
- 93.Colón-González FJ, Fezzi C, Lake IR, Hunter PR. 2013. The effects of weather and climate change on dengue. PLoS Negl. Trop. Dis. 7:e2503. 10.1371/journal.pntd.0002503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunn FL. 1979. Behavioural aspects of the control of parasitic diseases. Bull. World Health Organ. 57:499–512 [PMC free article] [PubMed] [Google Scholar]
- 95.Gillies MT. 1953. The duration of the gonotrophic cycle in Anopheles gambiae and Anopheles funestus, with a note on the efficiency of hand catching. East Afr. Med. J. 30:129–135 [PubMed] [Google Scholar]
- 96.Lorenzo MG, Lazzari CR. 1999. Temperature and relative humidity affect the selection of shelters by Triatoma infestans, vector of Chagas disease. Acta Trop. 72:241–249. 10.1016/S0001-706X(98)00094-1 [DOI] [PubMed] [Google Scholar]
- 97.Guernier V, Hochberg ME, Guégan J-F. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2:e141. 10.1371/journal.pbio.0020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341:514–519. 10.1126/science.1239401 [DOI] [PubMed] [Google Scholar]
- 99.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. 2000. Climate change and vector-borne diseases: a regional analysis. Bull. World Health Organ. 78:1136–1147 [PMC free article] [PubMed] [Google Scholar]
- 100.Costa CHN. 2008. Characterization and speculations on the urbanization of visceral leishmaniasis in Brazil. Cad Saude Publica 24:2959–2963. 10.1590/S0102-311X2008001200027 [DOI] [PubMed] [Google Scholar]
- 101.Harhay MO, Olliaro PL, Costa DL, Costa CHN. 2011. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 27:403–409. 10.1016/j.pt.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 102.Srinivasan R, Jambulingam P, Vanamail P. 2013. Sand fly (Diptera:Psychodidae) abundance and species diversity in relation to environmental factors in parts of coastal plains of southern India. J. Med. Entomol. 50:758–763. 10.1603/ME12153 [DOI] [PubMed] [Google Scholar]
- 103.Ready PD. 2008. Leishmaniasis emergence and climate change. Rev. Sci. Tech. 27:399–412 [PubMed] [Google Scholar]
- 104.Gálvez R, Descalzo MA, Miró G, Jiménez MI, Martin O. 2010. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta Trop. 115:95–102. 10.1016/j.actatropica.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 105.Wasserberg G, Yarom I, Warburg A. 2003. Seasonal abundance patterns of the sandfly Phlebotomus papatasi in climatically distinct foci of cutaneous leishmaniasis in Israeli deserts. Med. Vet. Entomol. 17:452–456. 10.1111/j.1365-2915.2003.00461.x [DOI] [PubMed] [Google Scholar]
- 106.Inhorn MC, Brown PJ. 1990. The anthropology of infectious disease. Annu. Rev. Anthropol. 19:89–117. 10.1146/annurev.an.19.100190.000513 [DOI] [Google Scholar]
- 107.Rathgeber EM, Vlassoff C. 1993. Gender and tropical diseases: a new research focus. Soc. Sci. Med. 37:513–520. 10.1016/0277-9536(93)90286-D [DOI] [PubMed] [Google Scholar]
- 108.Coutinho EM, Abath FG, Barbosa CS, Domingues AL, Melo MC, Montenegro SM, Lucena MA, Romani SA, Souza WV, Coutinho AD. 1997. Factors involved in Schistosoma mansoni infection in rural areas of northeast Brazil. Mem. Inst. Oswaldo Cruz 92:707–715. 10.1590/S0074-02761997000500027 [DOI] [PubMed] [Google Scholar]
- 109.Taha HA, Soliman MI, Banjar SAN. 2013. Intestinal parasitic infections among expatriate workers in Al-Madina Al-Munawarah, Kingdom of Saudi Arabia. Trop. Biomed. 30:78–88 [PubMed] [Google Scholar]
- 110.Mohammad KAH, Koshak EAK. 2011. A prospective study on parasites among expatriate workers in Al-Baha from 2009-2011, Saudi Arabia. J. Egypt Soc. Parasitol. 41:423–432 [PubMed] [Google Scholar]
- 111.Vecchiato NL. 1997. Sociocultural aspects of tuberculosis control in Ethiopia. Med. Anthropol. Q. 11:183–201. 10.1525/maq.1997.11.2.183 [DOI] [PubMed] [Google Scholar]
- 112.Ho MJ. 2004. Sociocultural aspects of tuberculosis: a literature review and a case study of immigrant tuberculosis. Social Science & Medicine. 59:753–762. 10.1016/j.socscimed.2003.11.033 [DOI] [PubMed] [Google Scholar]
- 113.Boelaert M, Meheus F, Robays J, Lutumba P. 2010. Socio-economic aspects of neglected diseases: sleeping sickness and visceral leishmaniasis. Ann. Trop. Med. Parasitol. 104:535–542. 10.1179/136485910X12786389891641 [DOI] [PubMed] [Google Scholar]
- 114.Madeira NG, Macharelli CA, Pedras JF, Delfino MCN. 2002. Education in primary school as a strategy to control dengue. Rev. Soc. Bras. Med. Trop. 35:221–226. 10.1590/S0037-86822002000300004 [DOI] [PubMed] [Google Scholar]
- 115.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2:e300. 10.1371/journal.pntd.0000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvar J, Yactayo S, Bern C. 2006. Leishmaniasis and poverty. Trends Parasitol. 22:552–557. 10.1016/j.pt.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 117.Hotez PJ. 2008. Neglected infections of poverty in the United States of America. PLoS Negl. Trop. Dis. 2:e256. 10.1371/journal.pntd.0000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Narain JP, Dash AP, Parnell B, Bhattacharya SK, Barua S, Bhatia R, Savioli L. 2010. Elimination of neglected tropical diseases in the South-East Asia region of the World Health Organization. Bull. World Health Organ. 88:206–210. 10.2471/BLT.09.072322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosecrans K, Cruz-Martin G, King A, Dumonteil E. 2014. Opportunities for improved Chagas disease vector control based on knowledge, attitudes and practices of communities in the Yucatan Peninsula, Mexico. PLoS Negl. Trop. Dis. 8:e2763. 10.1371/journal.pntd.0002763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aagaard-Hansen J, Chaignat C. Neglected tropical diseases: equity and social determinants, p 135–157 In Blas E, Kurup AS. (ed), Equity, social determinants and public health programmes. WHO, Geneva, Switzerland [Google Scholar]
- 121.Briceno-Leon R. 1987. Rural housing for control of Chagas disease in Venezuela. Parasitol. Today 3:384–387. 10.1016/0169-4758(87)90252-3 [DOI] [PubMed] [Google Scholar]
- 122.Sachs JD, Mellinger AD, Gallup JL. 2001. The geography of poverty and wealth. Sci. Am. 284:70–75. 10.1038/scientificamerican0501-70 [DOI] [PubMed] [Google Scholar]
- 123.Thornthwaite CW. 1948. An approach toward a rational classification of climate. Geograph. Rev. 38:55–94. 10.2307/210739 [DOI] [Google Scholar]
- 124.Waide RB, Willig MR, Steiner CF. 1999. The relationship between productivity and species richness. Annu. Rev. Ecol. System. 30:257–300. 10.1146/annurev.ecolsys.30.1.257 [DOI] [Google Scholar]
- 125.Hajek AE, Olsen CH, Elkinton JS. 1999. Dynamics of airborne conidia of the gypsy moth (Lepidoptera:Lymantriidae) fungal pathogen Entomophaga maimaiga (Zygomycetes:Entomophthorales). Biol. Control 16:111–117. 10.1006/bcon.1999.0740 [DOI] [Google Scholar]
- 126.Thomas MB, Blanford S. 2003. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 7:344–350. 10.1016/S0169-5347(03)00069-7 [DOI] [Google Scholar]
- 127.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652. 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 19:547–551. 10.1016/j.pt.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 129.Gallup JL, Sachs JD. 2000. Agriculture, climate, and technology: why are the tropics falling behind? Am. J. Agric. Econ. 82:731–737. 10.1111/0002-9092.00071 [DOI] [Google Scholar]
- 130.Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 3:e412. 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brooker S, Hotez PJ, Bundy DAP. 2008. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl. Trop. Dis. 2:e291. 10.1371/journal.pntd.0000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olsen A. 2007. Efficacy and safety of drug combinations in the treatment of schistosomiasis, soil-transmitted helminthiasis, lymphatic filariasis and onchocerciasis. Trans. R. Soc. Trop. Med. Hyg. 101:747–758. 10.1016/j.trstmh.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 133.Barry MA, Simon GG, Mistry N, Hotez PJ. 2013. Global trends in neglected tropical disease control and elimination: impact on child health. Arch. Dis. Child. 98:635–641. 10.1136/archdischild-2012-302338 [DOI] [PubMed] [Google Scholar]
- 134.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. 2004. Hookworm infection. N. Engl. J. Med. 351:799–807. 10.1056/NEJMra032492 [DOI] [PubMed] [Google Scholar]
- 135.MacLeod CL. 1987. Parasitic infections in pregnancy and the newborn. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 136.Steketee RW. 2003. Pregnancy, nutrition and parasitic diseases. J. Nutr. 133:1661S–1667S [DOI] [PubMed] [Google Scholar]
- 137.Steketee RW, Nahlen BL, Parise ME, Menendez C. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28–35 [DOI] [PubMed] [Google Scholar]
- 138.King CH, Dickman K, Tisch DJ. 2005. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365:1561–1569. 10.1016/S0140-6736(05)66457-4 [DOI] [PubMed] [Google Scholar]
- 139.King CH, Dangerfield-Cha M. 2008. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 4:65–79. 10.1177/1742395307084407 [DOI] [PubMed] [Google Scholar]
- 140.Njenga SM, Wamae CN, Njomo DW, Mwandawiro CS, Molyneux DH. 2007. Chronic clinical manifestations related to Wuchereria bancrofti infection in a highly endemic area in Kenya. Trans. R. Soc. Trop. Med. Hyg. 101:439–444. 10.1016/j.trstmh.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 141.Latham MC, Stephenson LS, Kurz KM. 1990. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. Am. J. Trop. Med. Hyg. 43:170–179 [DOI] [PubMed] [Google Scholar]
- 142.Stephenson LS, Latham MC, Ottesen EA. 2000. Malnutrition and parasitic helminth infections. Parasitology 121(Suppl):S23–S38. 10.1017/S0031182000006491 [DOI] [PubMed] [Google Scholar]
- 143.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. 1993. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J. Nutr. 123:1036–1046 [DOI] [PubMed] [Google Scholar]
- 144.Hadju V, Stephenson LS, Abadi K, Mohammed HO, Bowman DD, Parker RS. 1996. Improvements in appetite and growth in helminth-infected schoolboys three and seven weeks after a single dose of pyrantel pamoate. Parasitology 113:497–504. 10.1017/S0031182000081579 [DOI] [PubMed] [Google Scholar]
- 145.Brooker S, Marriot H, Hall A, Adjei S, Allan E, Maier C, Bundy DA, Drake LJ, Coombes MD, Azene G, Lansdown RG, Wen ST, Dzodozmenyo M, Cobbinah J, Obro N, Kihamia CM, Issae W, Mwanri L, Mweta MR, Mwaikemwa A, Salimu M, Ntimbwa P, Kiwelu VM, Turuka A, Nkungu DR, Magingo J, Partnership for Child Development 2001. Community perception of school-based delivery of anthelmintics in Ghana and Tanzania. Trop. Med. Int. Health 6:1075–1083. 10.1046/j.1365-3156.2001.00806.x [DOI] [PubMed] [Google Scholar]
- 146.Luong TV. 2003. De-worming school children and hygiene intervention. Int. J. Environ. Health Res. 13(Suppl 1):S153–S159. 10.1080/0960312031000102912 [DOI] [PubMed] [Google Scholar]
- 147.Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M. 2013. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl. Trop. Dis. 7:e2397. 10.1371/journal.pntd.0002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lashley FR. 2004. Emerging infectious diseases: vulnerabilities, contributing factors and approaches. Expert Rev. Anti Infect. Ther. 2:299–316. 10.1586/14787210.2.2.299 [DOI] [PubMed] [Google Scholar]
- 149.Kotton CN. 2012. Travel and transplantation: travel-related diseases in transplant recipients. Curr. Opin. Organ Transplant. 17:594–600. 10.1097/MOT.0b013e328359266b [DOI] [PubMed] [Google Scholar]
- 150.Revich B, Tokarevich N, Parkinson AJ. 2012. Climate change and zoonotic infections in the Russian Arctic. Int. J. Circumpolar Health 71:18792. 10.3402/ijch.v71i0.18792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Centers for Disease Control and Prevention. National Center for Emerging and Zoonotic Infectious Diseases. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 152.WHO. Zoonoses and the human-animal-ecosystems interface. World Health Organization, Geneva, Switzerland [Google Scholar]
- 153.Smith DH, Pepin J, Stich AH. 1998. Human African trypanosomiasis: an emerging public health crisis. Br. Med. Bull. 54:341–355. 10.1093/oxfordjournals.bmb.a011692 [DOI] [PubMed] [Google Scholar]
- 154.Bäck AT, Lundkvist A. 2013. Dengue viruses—an overview. Infect. Ecol. Epidemiol. 3:33. 10.3402/iee.v3i0.19839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Normile D. 2013. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science 342:415. 10.1126/science.342.6157.415 [DOI] [PubMed] [Google Scholar]
- 156.Tang KF, Ooi EE. 2012. Diagnosis of dengue: an update. Expert Rev. Anti Infect. Ther. 10:895–907. 10.1586/eri.12.76 [DOI] [PubMed] [Google Scholar]
- 157.Yacoub S, Mongkolsapaya J, Screaton G. 2013. The pathogenesis of dengue. Curr. Opin. Infect. Dis. 26:284–289. 10.1097/QCO.0b013e32835fb938 [DOI] [PubMed] [Google Scholar]
- 158.Mrazek M. 2003. Stimulating pharmaceutical research and development for neglected diseases Health Policy 64:75–88. 10.1016/S0168-8510(02)00138-0 [DOI] [PubMed] [Google Scholar]
- 159.Guha-Sapir D. 2005. Dengue fever: new paradigms for a changing epidemiology. Emerg. Themes Epidemiol. 2:1. 10.1186/1742-7622-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Torresi J, Tapia-Conyer R, Margolis H. 2013. Preparing for dengue vaccine introduction: recommendations from the 1st Dengue v2V International Meeting. PLoS Negl. Trop. Dis. 7:e2261. 10.1371/journal.pntd.0002261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wilder-Smith A. 2012. Dengue infections in travellers. Paediatr. Int. Child Health 32(Suppl 1):S28–S32. 10.1179/2046904712Z.00000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carod-Artal FJ, Wichmann O, Farrar J, Gascón J. 2013. Neurological complications of dengue virus infection. Lancet Neurol. 12:906–919. 10.1016/S1474-4422(13)70150-9 [DOI] [PubMed] [Google Scholar]
- 163.Andraud M, Hens N, Marais C, Beutels P. 2012. Dynamic epidemiological models for dengue transmission: a systematic review of structural approaches. PLoS One 7:e49085. 10.1371/journal.pone.0049085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee C-Y, Tsai H-C, Lee SS-J, Lin C-K, Huang J-S, Chen Y-S. 2013. Dengue hemorrhagic fever presenting with hemorrhagic pancreatitis and an intramural hematoma of the duodenal wall: a case report and review of the literature. Southeast Asian J. Trop. Med. Public Health 44:400–408 [PubMed] [Google Scholar]
- 165.Undurraga EA, Halasa YA, Shepard DS. 2013. Use of expansion factors to estimate the burden of dengue in Southeast Asia: a systematic analysis. PLoS Negl. Trop. Dis. 7:e2056. 10.1371/journal.pntd.0002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shepard DS, Undurraga EA, Halasa YA. 2013. Economic and disease burden of dengue in Southeast Asia. PLoS Negl. Trop. Dis. 7:e2055. 10.1371/journal.pntd.0002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wilder-Smith A, Gubler DJ. 2008. Geographic expansion of dengue: the impact of international travel. Med. Clin. North Am. 92:1377–1390. 10.1016/j.mcna.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 168.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–S16. 10.1038/nrmicro2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Racloz V, Ramsey R, Tong S, Hu W. 2012. Surveillance of dengue fever virus: a review of epidemiological models and early warning systems. PLoS Negl. Trop. Dis. 6:e1648. 10.1371/journal.pntd.0001648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wattal C, Goel N. 2012. Infectious disease emergencies in returning travelers: special reference to malaria, dengue fever, and chikungunya. Med. Clin. North Am. 96:1225–1255. 10.1016/j.mcna.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 171.Thomas SJ. 2011. The necessity and quandaries of dengue vaccine development. J. Infect. Dis. 203:299–303. 10.1093/infdis/jiq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bouri N, Sell TK, Franco C, Adalja AA, Henderson DA, Hynes NA. 2012. Return of epidemic dengue in the United States: implications for the public health practitioner. Public Health Rep. 127:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boyce R, Lenhart A, Kroeger A, Velayudhan R, Roberts B, Horstick O. 2013. Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Trop. Med. Int. Health 18:564–577. 10.1111/tmi.12087 [DOI] [PubMed] [Google Scholar]
- 174.Schmitz J, Roehrig J, Barrett A, Hombach J. 2011. Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine 29:7276–7284. 10.1016/j.vaccine.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 175.Thomas SJ, Endy TP. 2011. Critical issues in dengue vaccine development. Curr. Opin. Infect. Dis. 24:442–450. 10.1097/QCO.0b013e32834a1b0b [DOI] [PubMed] [Google Scholar]
- 176.Quijano-Hernandez I, Dumonteil E. 2011. Advances and challenges towards a vaccine against Chagas disease. Vaccines 7:1184–1191. 10.4161/hv.7.11.17016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahalingam S, Herring BL, Halstead SB. 2013. Call to action for dengue vaccine failure. Emerg. Infect. Dis. 19:1335–1337. 10.3201/eid1908.121864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gupta B, Reddy BPN. 2013. Fight against dengue in India: progresses and challenges. Parasitol. Res. 112:1367–1378. 10.1007/s00436-013-3342-2 [DOI] [PubMed] [Google Scholar]
- 179.Sivagnaname N, Gunasekaran K. 2012. Need for an efficient adult trap for the surveillance of dengue vectors. Indian J. Med. Res. 136:739–749 [PMC free article] [PubMed] [Google Scholar]
- 180.Rasheed SB, Butlin RK, Boots M. 2013. A review of dengue as an emerging disease in Pakistan. Public Health 127:11–17. 10.1016/j.puhe.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 181.Rassi A, Marcondes de Rezende J. 2012. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North Am. 26:275–291. 10.1016/j.idc.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 182.Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 183.Nunes MCP, Dones W, Morillo CA, Encina JJ, Ribeiro AL, Council on Chagas Disease of the Interamerican Society of Cardiology 2013. Chagas disease: an overview of clinical and epidemiological aspects. J. Am. College Cardiol. 62:767–776. 10.1016/j.jacc.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 184.Bern C, Kjos S, Yabsley MJ, Mongtomery SP. 2011. Trypanosoma cruzi and Chagas' disease in the United States. Clin. Microbiol. Rev. 24:655–681. 10.1128/CMR.00005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lattes R, Lasala MB. 2014. Chagas disease in the immunosuppressed patient. Clin. Microbiol. Infect. 20:300–309. 10.1111/1469-0691.12585 [DOI] [PubMed] [Google Scholar]
- 186.Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. 2012. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin. Immunopathol. 34:753–770. 10.1007/s00281-012-0351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rodrigues JCF, Godinho JLP, de Souza W. 2014. Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell. Biochem. 74:1–42. 10.1007/978-94-007-7305-9_1 [DOI] [PubMed] [Google Scholar]
- 188.Jackson Y, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, Loutan L, Gervaix A. 2009. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg. Infect. Dis. 15:601–603. 10.3201/1504.080438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hashimoto K, Yoshioka K. 2012. Surveillance of Chagas disease. Adv. Parasitol. 79:375–428 [DOI] [PubMed] [Google Scholar]
- 190.Zofou D, Nyasa RB, Nsagha DS, Ntie-Kang F, Meriki HD, Assob JCN, Kuete V. 2014. Control of malaria and other vector-borne protozoan diseases in the tropics: enduring challenges despite considerable progress and achievements. Infect. Dis. Poverty 3:1. 10.1186/2049-9957-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Njogu PM, Chibale K. 2013. Recent developments in rationally designed multitarget antiprotozoan agents. Curr. Med. Chem. 20:1715–1742. 10.2174/0929867311320130010 [DOI] [PubMed] [Google Scholar]
- 192.Pinazo M-J, Espinosa G, Cortes-Lletget C, Posada E de J, Aldasoro E, Oliveira I, Muñoz J, Gállego M, Gascón J. 2013. Immunosuppression and Chagas disease: a management challenge. PLoS Negl. Trop. Dis. 7:e1965. 10.1371/journal.pntd.0001965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bestetti RB, Cardinalli-Neto A. 2012. Device therapy in Chagas disease heart failure. Expert Rev. Cardiovasc. Ther. 10:1307–1317. 10.1586/erc.12.115 [DOI] [PubMed] [Google Scholar]
- 194.Araújo-Jorge TC, Waghabi MC, Bailly S, Feige J-J. 2012. The TGF-β pathway as an emerging target for Chagas disease therapy. Clin. Pharmacol. Ther. 92:613–621. 10.1038/clpt.2012.102 [DOI] [PubMed] [Google Scholar]
- 195.Hotez PJ, Dumonteil E, Heffernan MJ, Bottazzi ME. 2013. Innovation for the “bottom 100 million”: eliminating neglected tropical diseases in the Americas. Adv. Exp. Med. Biol. 764:1–12 [DOI] [PubMed] [Google Scholar]
- 196.Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME. 2013. New vaccines for neglected parasitic diseases and dengue. Transl. Res. 162:144–155. 10.1016/j.trsl.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 197.Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P, Interamerican Society of Cardiology 2013. The reality of heart failure in Latin America. J. Am. College Cardiol. 62:949–958. 10.1016/j.jacc.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 198.Dumonteil E, Bottazzi ME, Zhan B, Heffernan MJ, Jones K, Valenzuela JG, Kamhawi S, Ortega J, Rosales SP de, Lee LBY, Bacon KM, Fleischer B, Slingsby BT, Cravioto MB, Tapia-Conyer R, Hotez PJ. 2012. Accelerating the development of a therapeutic vaccine for human Chagas disease: rationale and prospects. Expert Rev. Vaccines 11:1043–1055. 10.1586/erv.12.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Benaim G, Paniz Mondolfi AE. 2012. The emerging role of amiodarone and dronedarone in Chagas disease. Nat. Rev. Cardiol. 9:605–609. 10.1038/nrcardio.2012.108 [DOI] [PubMed] [Google Scholar]
- 200.Weant KA, Baker SN. 2013. Review of human rabies prophylaxis and treatment. Crit. Care Nurs. Clin. North Am. 25:225–242. 10.1016/j.ccell.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 201.Meslin F-X, Briggs DJ. 2013. Eliminating canine rabies, the principal source of human infection: what will it take? Antiviral Res. 98:291–296. 10.1016/j.antiviral.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 202.Bader MS, McKinsey DS. 2013. Postexposure prophylaxis for common infectious diseases. Am. Fam. Physician 88:25–32 [PubMed] [Google Scholar]
- 203.Shantavasinkul P, Wilde H. 2011. Postexposure prophylaxis for rabies in resource-limited/poor countries. Adv. Virus Res. 79:291–307. 10.1016/B978-0-12-387040-7.00013-5 [DOI] [PubMed] [Google Scholar]
- 204.Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. 2013. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 12:498–513. 10.1016/S1474-4422(13)70038-3 [DOI] [PubMed] [Google Scholar]
- 205.Warrell MJ. 2012. Current rabies vaccines and prophylaxis schedules: preventing rabies before and after exposure. Travel Med. Infect. Dis. 10:1–15. 10.1016/j.tmaid.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 206.Huang G, Liu H, Tang Q, Yu P, Shen X, Zhang Y, Liu X, Cao Q, Fu C, Liu B, Wang M. 2014. Making rabies prophylaxis more economical: Immunogenicity and safety results from a preliminary study using a 2-1 intramuscular regimen in healthy volunteers. Hum. Vaccin. Immunother. 10:114–119. 10.4161/hv.26264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hampson K, Cleaveland S, Briggs D. 2011. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl. Trop. Dis. 5:e982. 10.1371/journal.pntd.0000982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Warrell MJ. 2003. The challenge to provide affordable rabies post-exposure treatment. Vaccine 21:706–709. 10.1016/S0264-410X(02)00585-6 [DOI] [PubMed] [Google Scholar]
- 209.Hu R, Tang Q, Tang J, Fooks AR. 2009. Rabies in China: an update. Vector Borne Zoonotic Dis. 9:1–12. 10.1089/vbz.2008.0046 [DOI] [PubMed] [Google Scholar]
- 210.Wu X, Hu R, Zhang Y, Dong G, Rupprecht CE. 2009. Reemerging rabies and lack of systemic surveillance in People's Republic of China. Emerg. Infect. Dis. 15:1159. 10.3201/eid1508.081426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Malerczyk C, Nel LH, Gniel D, Blumberg L. 2010. Rabies in South Africa and the FIFA Soccer World Cup: travelers' awareness for an endemic but neglected disease. Vaccines 6:385–389. 10.4161/hv.6.5.11713 [DOI] [PubMed] [Google Scholar]
- 212.Taylor L, Partners for Rabies Prevention 2013. Eliminating canine rabies: the role of public-private partnerships. Antiviral Res. 98:314–318. 10.1016/j.antiviral.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 213.Sibunruang S, Tepsumethanon S, Raksakhet N, Tantawichien T. 2013. Rabies immunization of travelers in a canine rabies endemic area. J. Travel Med. 20:159–164. 10.1111/jtm.12023 [DOI] [PubMed] [Google Scholar]
- 214.Shwiff S, Hampson K, Anderson A. 2013. Potential economic benefits of eliminating canine rabies. Antiviral Res. 98:352–356. 10.1016/j.antiviral.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Davlin SL, Vonville HM. 2012. Canine rabies vaccination and domestic dog population characteristics in the developing world: a systematic review. Vaccine 30:3492–3502. 10.1016/j.vaccine.2012.03.069 [DOI] [PubMed] [Google Scholar]
- 216.WHO. Rabies—bulletin—Europe. http://www.who-rabies-bulletin.org/
- 217.Macpherson C. (ed). 2012. Dogs, zoonoses and public health. CABI Publishing, Wallingford, United Kingdom [Google Scholar]
- 218.Morters MK, Restif O, Hampson K, Cleaveland S, Wood JLN, Conlan AJK. 2013. Evidence-based control of canine rabies: a critical review of population density reduction. J. Anim. Ecol. 82:6–14. 10.1111/j.1365-2656.2012.02033.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tenzin Sharma B, Dhand NK, Timsina N, Ward MP. 2010. Reemergence of rabies in Chhukha district, Bhutan, 2008. Emerg. Infect. Dis. 16:1925–1930. 10.3201/eid1612.100958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gautret P, Lim PL, Shaw M, Leder K. 2011. Rabies post-exposure prophylaxis in travellers returning from Bali, Indonesia, November 2008 to March 2010. Clin. Microbiol. Infect. 17:445–447. 10.1111/j.1469-0691.2010.03271.x [DOI] [PubMed] [Google Scholar]
- 221.Kim CH, Lee CG, Yoon HC, Nam HM, Park CK, Lee JC, Kang MI, Wee SH. 2006. Rabies, an emerging disease in Korea. J. Vet. Med. Ser. B 53:111–115. 10.1111/j.1439-0450.2006.00928.x [DOI] [PubMed] [Google Scholar]
- 222.David D, Dveres N, Yakobson BA, Davidson I. 2009. Emergence of dog rabies in the northern region of Israel. Epidemiol. Infect. 137:544–548. 10.1017/S0950268808001180 [DOI] [PubMed] [Google Scholar]
- 223.Schneider MC, Romijn PC, Uieda W, Tamayo H, da Silva DF, Belotto A, da Silva JB, Leanes LF. 2009. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev. Panam. Salud Publica 25:260–269 [DOI] [PubMed] [Google Scholar]
- 224.Marano N, Arguin PM, Pappaioanou M. 2007. Impact of globalization and animal trade on infectious disease ecology. Emerg. Infect. Dis. 13:1807–1809. 10.3201/eid1312.071276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Warrell M. 2010. Rabies and African bat lyssavirus encephalitis and its prevention. Int. J. Antimicrob. Agents 36(Suppl 1):S47–S52. 10.1016/j.ijantimicag.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 226.Tregle RW, Loe CL, Earhart RH, d'Autremont SB. 2011. Cercopithecine herpesvirus 1 risk in a child bitten by a Bonnet Macaque monkey. J. Emerg. Med. 41:e89–90. 10.1016/j.jemermed.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 227.Schatz J, Fooks AR, McElhinney L, Horton D, Echevarria J, Vázquez-Moron S, Kooi EA, Rasmussen TB, Müller T, Freuling CM. 2013. Bat rabies surveillance in Europe. Zoonoses Public Health 60:22–34. 10.1111/zph.12002 [DOI] [PubMed] [Google Scholar]
- 228.Tenzin , Ward MP. 2012. Review of rabies epidemiology and control in South, South East and East Asia: past, present and prospects for elimination. Zoonoses Public Health 59:451–467. 10.1111/j.1863-2378.2012.01489.x [DOI] [PubMed] [Google Scholar]
- 229.Wieten RW, Leenstra T, van Ghiel PP, van Vugt M, Stijnis C, Goorhuis A, Grobusch MP. 2013. Rabies vaccinations: are abbreviated intradermal schedules the future? Clin. Infect. Dis. 56:414–419. 10.1093/cid/cis853 [DOI] [PubMed] [Google Scholar]
- 230.Briggs DJ. 2012. The role of vaccination in rabies prevention. Curr. Opin. Virol. 2:309–314. 10.1016/j.coviro.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 231.Permpalung N, Wongrakpanich S, Korpaisarn S, Tanratana P, Angsanakul J. 2013. Trend of human rabies prophylaxis in developing countries: toward optimal rabies immunization. Vaccine 31:4079–4083. 10.1016/j.vaccine.2013.06.083 [DOI] [PubMed] [Google Scholar]
- 232.Fooks AR, Johnson N, Freuling CM, Wakeley PR, Banyard AC, McElhinney LM, Marston DA, Dastjerdi A, Wright E, Weiss RA, Müller T. 2009. Emerging technologies for the detection of rabies virus: challenges and hopes in the 21st century. PLoS Negl. Trop. Dis. 3:e530. 10.1371/journal.pntd.0000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sorvillo F. 2011. Public health implications of cysticercosis acquired in the United States. Emerg. Infect. Dis 17:1–6. 10.3201/eid1701.101210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kraft R. 2007. Cysticercosis: an emerging parasitic disease. Am. Fam. Physician 76:91–96 [PubMed] [Google Scholar]
- 235.Nash TE, Mahanty S, Garcia HH. 2013. Neurocysticercosis—more than a neglected disease. PLoS Negl. Trop. Dis. 7:e1964. 10.1371/journal.pntd.0001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Garcia HH, Del Brutto OH. 2005. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 4:653–661. 10.1016/S1474-4422(05)70194-0 [DOI] [PubMed] [Google Scholar]
- 237.Michelet L, Dauga C. 2012. Molecular evidence of host influences on the evolution and spread of human tapeworms. Biol. Rev. Camb. Philos. Soc. 87:731–741. 10.1111/j.1469-185X.2012.00217.x [DOI] [PubMed] [Google Scholar]
- 238.Rodriguez S, Wilkins P, Dorny P. 2012. Immunological and molecular diagnosis of cysticercosis. Pathog. Glob. Health 106:286–298. 10.1179/2047773212Y.0000000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wallin MT, Kurtzke JF. 2004. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 63:1559–1564. 10.1212/01.WNL.0000142979.98182.FF [DOI] [PubMed] [Google Scholar]
- 240.Sciutto E, Fragoso G, de Aluja AS, Hernández M, Rosas G, Larralde C. 2008. Vaccines against cysticercosis. Curr. Top. Med. Chem. 8:415–423. 10.2174/156802608783790839 [DOI] [PubMed] [Google Scholar]
- 241.Luo X, Zheng Y, Hou J, Zhang S, Cai X. 2009. Protection against Asiatic Taenia solium induced by a recombinant 45W-4B protein. Clin. Vaccine Immunol. 16:230–232. 10.1128/CVI.00367-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gonzalez AE, Gauci CG, Barber D. 2005. Vaccination of pigs to control human neurocysticercosis. Am. J. Trop. Med. Hyg. 72:837–839 [PubMed] [Google Scholar]
- 243.Molinari JL, Rodríguez D, Tato P, Soto R, Arechavaleta F, Solano S. 1997. Field trial for reducing porcine Taenia solium cysticercosis in Mexico by systematic vaccination of pigs. Vet. Parasitol. 69:55–63. 10.1016/S0304-4017(96)01102-8 [DOI] [PubMed] [Google Scholar]
- 244.Molinari JL, Soto R, Tato P, Rodríguez D, Retana A, Sepulveda J, Palet A. 1993. Immunization against porcine cysticercosis in an endemic area in Mexico: a field and laboratory study. Am. J. Trop. Med. Hyg. 49:502–512 [DOI] [PubMed] [Google Scholar]
- 245.Huerta M, de Aluja AS, Fragoso G, Toledo A, Villalobos N, Hernández M, Gevorkian G, Acero G, Díaz A, Alvarez I, Avila R, Beltrán C, Garcia G, Martinez JJ, Larralde C, Sciutto E. 2001. Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine 20:262–266. 10.1016/S0264-410X(01)00249-3 [DOI] [PubMed] [Google Scholar]
- 246.Jayashi CM, Kyngdon CT, Gauci CG, Gonzalez AE, Lightowlers MW. 2012. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet. Parasitol. 188:261–267. 10.1016/j.vetpar.2012.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gonzalez AE, Gavidia C, Falcon N, Bernal T, Verastegui M, Garcia HH, Gilman RH, Tsang VC, Cysticercosis Working Group in Peru 2001. Protection of pigs with cysticercosis from further infections after treatment with oxfendazole. Am. J. Trop. Med. Hyg. 65:15–18 [DOI] [PubMed] [Google Scholar]
- 248.Sciutto E, Fragoso G, Hernandez M, Rosas G, Marinez JJ, Fleury A, Cervantes J, Aluja A, Larralde C. 2013. Development of the S3Pvac vaccine against porcine Taenia solium cysticercosis: a historical review. J. Parasitol. 99:686–692. 10.1645/GE-3102.1 [DOI] [PubMed] [Google Scholar]
- 249.Sciutto E, Rosas G, Hernández M, Morales J, Cruz-Revilla C, Toledo A, Manoutcharian K, Gevorkian G, Blancas A, Acero G, Hernández B, Cervantes J, Bobes RJ, Goldbaum FA, Huerta M, Diaz-Orea A, Fleury A, de Aluja AS, Cabrera-Ponce JL, Herrera-Estrella L, Fragoso G, Larralde C. 2007. Improvement of the synthetic tri-peptide vaccine (S3Pvac) against porcine Taenia solium cysticercosis in search of a more effective, inexpensive and manageable vaccine. Vaccine 25:1368–1378. 10.1016/j.vaccine.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 250.MacLean L, Myburgh E, Rodgers J, Price HP. 2013. Imaging African trypanosomes. Parasite Immunol. 35:283–294. 10.1111/pim.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lutje V, Seixas J, Kennedy A. 2013. Chemotherapy for second-stage human African trypanosomiasis. Cochrane Database Syst. Rev. 6:CD006201. 10.1002/14651858.CD006201.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brun R, Blum J, Chappuis F, Burri C. 2010. Human African trypanosomiasis. Lancet 375:148–159. 10.1016/S0140-6736(09)60829-1 [DOI] [PubMed] [Google Scholar]
- 253.Bardosh K, Waiswa C, Welburn SC. 2013. Conflict of interest: use of pyrethroids and amidines against tsetse and ticks in zoonotic sleeping sickness endemic areas of Uganda. Parasites Vectors 6:204. 10.1186/1756-3305-6-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fèvre EM, von Wissman B, Welburn SC, Lutumba P. 2008. The burden of human African trypanosomiasis. PLoS Negl. Trop. Dis. 2:e333. 10.1371/journal.pntd.0000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.WHO. 2014. The transmission cycle. World Health Organization, Geneva, Switzerland [Google Scholar]
- 256.Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Fèvre EM, Mattioli RC, Jannin JG. 2012. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl. Trop. Dis. 6:e1859. 10.1371/journal.pntd.0001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Simarro PP, Diarra A, Ruiz-Postigo JA, Franco JR, Jannin JG. 2011. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000-2009: the way forward. PLoS Negl. Trop. Dis. 5:e1007. 10.1371/journal.pntd.0001007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.WHO. 2014, Human African trypanosomiasis. World Health Organization, Geneva, Switzerland [Google Scholar]
- 259.Lindner AK, Priotto G. 2010. The unknown risk of vertical transmission in sleeping sickness—a literature review. PLoS Negl. Trop. Dis. 4:e783. 10.1371/journal.pntd.0000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mwanakasale V, Songolo P, Babaniyi O, Simarro P. 2014. Clinical presentation of human African trypanosomiasis in Zambia is linked to the existence of strains of Trypanosoma brucei rhodesiense with varied virulence: two case reports. J. Med. Case Rep. 8:53. 10.1186/1752-1947-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goodhead I, Capewell P, Bailey JW, Beament T, Chance M, Kay S, Forrester S, MacLeod A, Taylor M, Noyes H, Hall N. 2013. Whole-genome sequencing of Trypanosoma brucei reveals introgression between subspecies that is associated with virulence. mBio 4(4):e00197-13. 10.1128/mBio.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yansouni CP, Bottieau E, Lutumba P, Winkler AS, Lynen L, Büscher P, Jacobs J, Gillet P, Lejon V, Alirol E, Polman K, Utzinger J, Miles MA, Peeling RW, Muyembe J-J, Chappuis F, Boelaert M. 2013. Rapid diagnostic tests for neurological infections in central Africa. Lancet Infect. Dis. 13:546–558. 10.1016/S1473-3099(13)70004-5 [DOI] [PubMed] [Google Scholar]
- 263.Lejon V, Bentivoglio M, Franco JR. 2013. Human African trypanosomiasis. Handb. Clin. Neurol. 114:169–181. 10.1016/B978-0-444-53490-3.00011-X [DOI] [PubMed] [Google Scholar]
- 264.Fairlamb AH. 2003. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol. 19:488–494. 10.1016/j.pt.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 265.Stein J, Mogk S, Mudogo CN, Sommer BP, Scholze M, Meiwes A, Huber M, Gray A, Duszenko M. 2014. Drug development against sleeping sickness: old wine in new bottles? Curr. Med. Chem. 21:1713–1727. 10.2174/0929867320666131119121636 [DOI] [PubMed] [Google Scholar]
- 266.Stich A, Ponte-Sucre A, Holzgrabe U. 2013. Do we need new drugs against human African trypanosomiasis? Lancet Infect. Dis. 13:733–734. 10.1016/S1473-3099(13)70191-9 [DOI] [PubMed] [Google Scholar]
- 267.Babokhov P, Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF, Iriemenam NC. 2013. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 107:242–252. 10.1179/2047773213Y.0000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.World Health Organization. 2013. Control and surveillance of human African trypanosomiasis. World Health Organ. Tech. Rep. Ser. 2013:1–237 [PubMed] [Google Scholar]
- 269.Burri C. 2010. Chemotherapy against human African trypanosomiasis: is there a road to success? Parasitology 137:1987–1994. 10.1017/S0031182010001137 [DOI] [PubMed] [Google Scholar]
- 270.Ferrins L, Rahmani R, Baell JB. 2013. Drug discovery and human African trypanosomiasis: a disease less neglected? Future Med. Chem. 5:1801–1841. 10.4155/fmc.13.162 [DOI] [PubMed] [Google Scholar]
- 271.Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Mattioli RC, Jannin JG. 2014. Mapping the capacities of fixed health facilities to cover people at risk of gambiense human African trypanosomiasis. Int. J. Health Geogr. 13:4. 10.1186/1476-072X-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ndeledje N, Bouyer J, Stachurski F, Grimaud P, Belem AMG, Molélé Mbaïndingatoloum F, Bengaly Z, Oumar Alfaroukh I, Cecchi G, Lancelot R. 2013. Treating cattle to protect people? Impact of footbath insecticide treatment on tsetse density in Chad. PLoS One 8:e67580. 10.1371/journal.pone.0067580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Torr SJ, Vale GA. 2011. Is the even distribution of insecticide-treated cattle essential for tsetse control? Modelling the impact of baits in heterogeneous environments. PLoS Negl. Trop. Dis. 5:e1360. 10.1371/journal.pntd.0001360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cecchi G, Courtin F, Paone M, Diarra A, Franco JR, Mattioli RC, Simarro PP. 2009. Mapping sleeping sickness in Western Africa in a context of demographic transition and climate change. Parasite 16:99–106. 10.1051/parasite/2009162099 [DOI] [PubMed] [Google Scholar]
- 275.Wengert O, Kopp M, Siebert E, Stenzel W, Hegasy G, Suttorp N, Stich A, Zoller T. 2014. Human African trypanosomiasis with 7-year incubation period: clinical, laboratory and neuroimaging findings. Parasitol. Int. 63:557–560. 10.1016/j.parint.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 276.Neuberger A, Meltzer E, Leshem E, Dickstein Y, Stienlauf S, Schwartz E. 2014. The changing epidemiology of human African trypanosomiasis among patients from nonendemic countries—1902-2012. PLoS One 9:e88647. 10.1371/journal.pone.0088647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Simarro PP, Franco JR, Cecchi G, Paone M, Diarra A, Ruiz-Postigo JA, Jannin JG. 2012. Human African trypanosomiasis in non-endemic countries (2000-2010). J. Travel Med. 19:44–53. 10.1111/j.1708-8305.2011.00576.x [DOI] [PubMed] [Google Scholar]
- 278.Norman FF, Pérez de Ayala A, Pérez-Molina J-A, Monge-Maillo B, Zamarrón P, López-Vélez R. 2010. Neglected tropical diseases outside the tropics. PLoS Negl. Trop. Dis. 4:e762. 10.1371/journal.pntd.0000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mahmoud A, Zerhouni E. 2009. Neglected tropical diseases: moving beyond mass drug treatment to understanding the science. Health Affairs 28:1726–1733. 10.1377/hlthaff.28.6.1726 [DOI] [PubMed] [Google Scholar]
- 280.Keenan JD, Hotez PJ, Amza A, Stoller NE, Gaynor BD, Porco TC, Lietman TM. 2013. Elimination and eradication of neglected tropical diseases with mass drug administrations: a survey of experts. PLoS Negl. Trop. Dis. 7:e2562. 10.1371/journal.pntd.0002562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kumar S. 2001. Biowarfare fears in India. Lancet Infect. Dis. 1:216. 10.1016/S1473-3099(01)00107-4 [DOI] [PubMed] [Google Scholar]
- 282.Sharma R. 2001. India wakes up to threat of bioterrorism. BMJ 323:714. 10.1136/bmj.323.7315.714a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Powell K, Jayaraman KS. 2002. Mosquito researchers deny plotting secret biowarfare test. Nature 419:867. 10.1038/419867a [DOI] [PubMed] [Google Scholar]
- 284.Bossi P, Tegnell A, Baka A, Van Loock F, Hendriks J, Werner A, Maidhof H, Gouvras G, Task Force on Biological and Chemical Agent Threats, Public Health Directorate, European Commission, Luxembourg 2004. Bichat guidelines for the clinical management of haemorrhagic fever viruses and bioterrorism-related haemorrhagic fever viruses. Euro Surveill. 9(12):pii=504 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=504 [DOI] [PubMed] [Google Scholar]
- 285.Rigaudeau S, Bricaire F, Bossi P. 2005. Haemorrhagic fever viruses, possible bioterrorist use. Presse Med. 34:169–176. 10.1016/S0755-4982(05)83898-9 [DOI] [PubMed] [Google Scholar]
- 286.Yap FB-B. 2011. Cutaneous larva migrans in Hospital Kuala Lumpur, Malaysia: rate of correct diagnosis made by the referring primary care doctors. Trans. R. Soc. Trop. Med. Hyg. 105:405–408. 10.1016/j.trstmh.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 287.Needham DM, Foster SD, Tomlinson G, Godfrey-Faussett P. 2001. Socio-economic, gender and health services factors affecting diagnostic delay for tuberculosis patients in urban Zambia. Trop. Med. Int. Health 6:256–259. 10.1046/j.1365-3156.2001.00709.x [DOI] [PubMed] [Google Scholar]
- 288.Siddiqui MR, Velidi NR, Pati S, Rath N, Kanungo AK, Bhanjadeo AK, Rao BB, Ojha BM, Krishna Moorthy K, Soutar D, Porter JDH, Ranganadha Rao PV. 2009. Integration of leprosy elimination into primary health care in Orissa, India. PLoS One 4:e8351. 10.1371/journal.pone.0008351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Haines A, Sanders D, Lehmann U, Rowe AK, Lawn JE, Jan S, Walker DG, Bhutta Z. 2007. Achieving child survival goals: potential contribution of community health workers. Lancet 369:2121–2131. 10.1016/S0140-6736(07)60325-0 [DOI] [PubMed] [Google Scholar]
- 290.Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, van Wyk BE, Odgaard-Jensen J, Johansen M, Aja GN, Zwarenstein M, Scheel IB. 2010. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst. Rev. CD004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.West MA, Hirst G, Richter A. 2004. Twelve steps to heaven: successfully managing change through developing innovative teams. Eur. J. Work Organ. Psychol. 13:269–299. 10.1080/13594320444000092 [DOI] [Google Scholar]
- 292.Hotez PJ, Brown AS. 2009. Neglected tropical disease vaccines. Biologicals 37:160–164. 10.1016/j.biologicals.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 293.Duthie MS, Gillis TP, Reed SG. 2011. Advances and hurdles on the way toward a leprosy vaccine. Vaccines 7:1172–1183. 10.4161/hv.7.11.16848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, Petri W, Reed S, Valenzuela JG, Hotez PJ. 2011. Vaccines to combat the neglected tropical diseases. Immunol. Rev. 239:237–270. 10.1111/j.1600-065X.2010.00976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Siddiqui AA, Siddiqui BA, Ganley-Leal L. 2011. Schistosomiasis vaccines. Vaccines 7:1192–1197. 10.4161/hv.7.11.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Basso B, Moretti E, Fretes R. 2008. Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection. Mem. Inst. Oswaldo Cruz 103:370–374. 10.1590/S0074-02762008000400010 [DOI] [PubMed] [Google Scholar]
- 297.Noazin S, Modabber F, Khamesipour A, Smith PG. 2008. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine 26:6759–6767. 10.1016/j.vaccine.2008.09.085 [DOI] [PubMed] [Google Scholar]
- 298.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. 2013. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine 31:1616–1622. 10.1016/j.vaccine.2012.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.McManus DP, Loukas A. 2008. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 21:225–242. 10.1128/CMR.00046-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rodrigues LC, Lockwood DN. 2011. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect. Dis. 11:464–470. 10.1016/S1473-3099(11)70006-8 [DOI] [PubMed] [Google Scholar]
- 301.Lammie P, Solomon A, Secor E, Peeling R. 2011. Diagnostic needs for NTD programs, p 346. In Choffnes ER, Relman DA. (ed), The causes and impacts of neglected tropical and zoonotic diseases. National Academies Press, Washington, DC [Google Scholar]
- 302.Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen J-X, Chen J-H, Churcher TS, Drakeley CJ, Edwards T, Fenwick A, French M, Gabrielli AF, Grassly NC, Harding-Esch EM, Holland MJ, Koukounari A, Lammie PJ, Leslie J, Mabey DC, Rhajaoui M, Secor WE, Stothard JR, Wei H, Willingham AL, Zhou X-N, Peeling RW. 2012. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl. Trop. Dis. 6:e1746. 10.1371/journal.pntd.0001746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Afonso AM, Ebell MH, Tarleton RL. 2012. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl. Trop. Dis. 6:e1881. 10.1371/journal.pntd.0001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Thomas Dorlo PC, Eggelte TA, Schoone GJ, de Vries PJ, Beijnen JH. 2012. A poor-quality generic drug for the treatment of visceral leishmaniasis: a case report and appeal. PLoS Negl. Trop. Dis. 6:e1544. 10.1371/journal.pntd.0001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mackey TK, Liang BA. 2013. Improving global health governance to combat counterfeit medicines: a proposal for a UNODC-WHO-Interpol trilateral mechanism. BMC Med. 11:233. 10.1186/1741-7015-11-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mackey TK, Liang BA. 2011. The global counterfeit drug trade: patient safety and public health risks. J. Pharm. Sci. 100:4571–4579. 10.1002/jps.22679 [DOI] [PubMed] [Google Scholar]
- 307.Attaran A, Barry D, Basheer S, Bate R, Benton D, Chauvin J, Garrett L, Kickbusch I, Kohler JC, Midha K, Newton PN, Nishtar S, Orhii P, McKee M. 2012. How to achieve international action on falsified and substandard medicines. BMJ 345:e7381. 10.1136/bmj.e7381 [DOI] [PubMed] [Google Scholar]
- 308.Dorlo TPC, Ravinetto RM, Beijnen JH, Boelaert M. 2012. Substandard medicines are the priority for neglected tropical diseases. BMJ 345:e7518. 10.1136/bmj.e7518 [DOI] [PubMed] [Google Scholar]
- 309.Montgomery MA, Elimelech M. 2007. Water and sanitation in developing countries: including health in the equation. Environ. Sci. Technol. 41:17–24. 10.1021/es072435t [DOI] [PubMed] [Google Scholar]
- 310.Martens W, Jetten TH, Rotmans J. 1995. Climate change and vector-borne diseases: a global modelling perspective. Global Environ. Change 5:195–209. 10.1016/0959-3780(95)00051-O [DOI] [Google Scholar]
- 311.Samson J, Berteaux D, McGill BJ. 2011. Geographic disparities and moral hazards in the predicted impacts of climate change on human populations. Global Ecol. Biogeogr. 20:532–544. 10.1111/j.1466-8238.2010.00632.x [DOI] [Google Scholar]
- 312.Green JF. 2010. Private standards in the climate regime: the greenhouse gas protocol. Business Politics 12:issue 3. 10.2202/1469-3569.1318 [DOI] [Google Scholar]
- 313.Kolk A, Levy D, Pinkse J. 2008. Corporate responses in an emerging climate regime: the institutionalization and commensuration of carbon disclosure. Eur. Account. Rev. 17:719–745. 10.1080/09638180802489121 [DOI] [Google Scholar]
- 314.Molyneux DH, Malecela MN. 2011. Neglected tropical diseases and the millennium development goals: why the “other diseases” matter: reality versus rhetoric. Parasit. Vectors 4:234. 10.1186/1756-3305-4-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.WHO. 2012. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. WHO, Geneva, Switzerland [Google Scholar]
- 316.Nishtar S. 2004. Public-private “partnerships” in health—a global call to action. Health Res. Policy Syst. 2:5. 10.1186/1478-4505-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.WHO. Public-private partnerships for health. World Health Organization, Geneva, Switzerland [Google Scholar]
- 318.Buse K, Harmer AM. 2007. Seven habits of highly effective global public-private health partnerships: practice and potential. Soc. Sci. Med. 64:259–271. 10.1016/j.socscimed.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 319.Chatelain E, Ioset J-R. 2011. Drug discovery and development for neglected diseases: the DNDi model. Drug Des. Dev. Ther. 5:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nwaka S, Ridley RG. 2003. Virtual drug discovery and development for neglected diseases through public-private partnerships. Nat. Rev. Drug Discov. 2:919–928. 10.1038/nrd1230 [DOI] [PubMed] [Google Scholar]
- 321.Moon S, Bermudez J, Hoen E' 2012. Innovation and access to medicines for neglected populations: could a treaty address a broken pharmaceutical R&D system? PLoS Med. 9:e1001218. 10.1371/journal.pmed.1001218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gustavsen K, Hanson C. 2009. Progress in public-private partnerships to fight neglected diseases. Health Affairs 28:1745–1749. 10.1377/hlthaff.28.6.1745 [DOI] [PubMed] [Google Scholar]
- 323.Nwaka S, Hudson A. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941–955. 10.1038/nrd2144 [DOI] [PubMed] [Google Scholar]
- 324.Grépin KA, Reich MR. 2008. Conceptualizing integration: a framework for analysis applied to neglected tropical disease control partnerships. PLoS Negl. Trop. Dis. 2:e174. 10.1371/journal.pntd.0000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Morel CM. 2005. Health innovation networks to help developing countries address neglected diseases. Science 309:401–404. 10.1126/science.1115538 [DOI] [PubMed] [Google Scholar]
- 326.Maurer SA. 2006. Choosing the right incentive strategy for research and development in neglected diseases. Bull. World Health Organ. 84:376–381. 10.2471/BLT.06.029835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lembo T, Partners for Rabies Prevention 2012. The blueprint for rabies prevention and control: a novel operational toolkit for rabies elimination. PLoS Negl. Trop. Dis. 6:e1388. 10.1371/journal.pntd.0001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Bansal S. 2012. United we stand: the new approach in fighting neglected tropical diseases (NTDs). http://www.forbes.com/sites/sarikabansal/2012/01/31/united-we-stand-a-new-mentality-in-the-fight-against-neglected-tropical-diseases-ntds/
- 329.Pappaioanou M, Spencer H. 2008. “One Health” initiative and ASPH. Public Health Rep. 123:261. [PMC free article] [PubMed] [Google Scholar]
- 330.Lee K, Brumme ZL. 2013. Operationalizing the One Health approach: the global governance challenges. Health Policy Plan. 28:778–785. 10.1093/heapol/czs127 [DOI] [PubMed] [Google Scholar]
- 331.Kaplan B, Kahn LH, Monath TP, Woodall J. 2009. “ONE HEALTH” and parasitology. Parasit. Vectors 2:36. 10.1186/1756-3305-2-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, Pfeiffer DU, Stärk K, Rweyemamu M. 2011. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 11:326–331. 10.1016/S1473-3099(10)70312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Gibbs EPJ. 2014. The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec. 174:85–91. 10.1136/vr.g143 [DOI] [PubMed] [Google Scholar]
- 334.Michell AR. 2014. Developing one health. Vet. Rec. 174:124–125. 10.1136/vr.g1176 [DOI] [PubMed] [Google Scholar]
- 335.Heymann DL, Dar OA. 2014. Prevention is better than cure for emerging infectious diseases. BMJ 348:g1499–g1499. 10.1136/bmj.g1499 [DOI] [PubMed] [Google Scholar]
- 336.Mwacalimba KK, Green J. 14 February 2014. “One health” and development priorities in resource-constrained countries: policy lessons from avian and pandemic influenza preparedness in Zambia. Health Policy Plan. 10.1093/heapol/czu001 [DOI] [PubMed] [Google Scholar]
- 337.Gardner CA, Acharya T, Yach D. 2007. Technological and social innovation: a unifying new paradigm for global health affairs. Health Aff. 26:1052–1061. 10.1377/hlthaff.26.4.1052 [DOI] [PubMed] [Google Scholar]
- 338.One Health Initiative. onehealthinitiative.com
- 339.Aenishaenslin C, Simon A, Forde T, Ravel A, Proulx J-F, Fehlner-Gardiner C, Picard I, Bélanger D. 2014. Characterizing rabies epidemiology in remote Inuit communities in Québec, Canada: a “One Health” approach. Ecohealth 11:343–355. 10.1007/s10393-014-0923-1 [DOI] [PubMed] [Google Scholar]
- 340.Mannion CJ, Shepherd K. 2014. One Health approach to dog bite prevention. Vet. Rec. 174:151–152. 10.1136/vr.g1370 [DOI] [PubMed] [Google Scholar]
- 341.Wall P. 2014. One Health and the food chain: maintaining safety in a globalised industry. Vet. Rec. 174:189–192. 10.1136/vr.g1512 [DOI] [PubMed] [Google Scholar]
- 342.WHO. 2009. Global strategy and plan of action on public health, innovation and intellectual property. WHA62.16. 62nd World Health Assembly WHO, Geneva, Switzerland [Google Scholar]
- 343.WHO, WIPO, WTO. 2013. Promoting access to medical technologies and innovation. Intersections between public health, intellectual property and trade. WHO, Geneva, Switzerland [Google Scholar]
- 344.Mackey TKT, Liang BAB. 2012. Promoting global health: utilizing WHO to integrate public health, innovation and intellectual property. Drug Discov. Today 17:1254–1257. 10.1016/j.drudis.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 345.Mackey TK, Liang BA. 2012. Patent and exclusivity status of essential medicines for non-communicable disease. PLoS One 7:e51022. 10.1371/journal.pone.0051022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Cohen JC, Illingworth P. 2003. The dilemma of intellectual property rights for pharmaceuticals: the tension between ensuring access of the poor to medicines and committing to international agreements. Dev. World Bioeth. 3:27–48. 10.1111/1471-8847.00058 [DOI] [PubMed] [Google Scholar]
- 347.WHO. 2012. Research and development to meet health needs in developing countries: strengthening global financing and coordination. WHO, Geneva, Switzerland [Google Scholar]
- 348.Hubbard T, Love J. 2004. A new trade framework for global healthcare R&D. PLoS Biol. 2:e52. 10.1371/journal.pbio.0020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Dentico N, Ford N. 2005. The courage to change the rules: a proposal for an essential health R&D treaty. PLoS Med. 2:e14. 10.1371/journal.pmed.0020014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mackey TK. 2013. Global health diplomacy and the governance of counterfeit medicines: a mapping exercise of institutional approaches. http://www.ghd-net.org/abstracts/volume-1/5
- 351.Mackey TK, Liang BA, Novotny TE. 2013. Evolution of tobacco labeling and packaging: international legal considerations and health governance. Am. J. Public Health 103:e39–43. 10.2105/AJPH.2012.301029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hayden EC. 2014. Projects set to tackle neglected diseases. Nat. News 505:142. 10.1038/505142a [DOI] [PubMed] [Google Scholar]
- 353.McCoy D, Chand S, Sridhar D. 2009. Global health funding: how much, where it comes from and where it goes. Health Policy Plan. 24:407–417. 10.1093/heapol/czp026 [DOI] [PubMed] [Google Scholar]
- 354.Spiegel JM, Dharamsi S, Wasan KM, Yassi A, Singer B, Hotez PJ, Hanson C, Bundy DAP. 2010. Which new approaches to tackling neglected tropical diseases show promise? PLoS Med. 7:e1000255. 10.1371/journal.pmed.1000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liang BA, Mackey T. 2010. Health care policy. Reforming off-label promotion to enhance orphan disease treatment. Science 327:273–274. 10.1126/science.1181567 [DOI] [PubMed] [Google Scholar]
- 356.Villa S, Compagni A, Reich MR. 2009. Orphan drug legislation: lessons for neglected tropical diseases. Int. J. Health Plan. Manage. 24:27–42. 10.1002/hpm.930 [DOI] [PubMed] [Google Scholar]
- 357.Mackey TK, Liang BA. 2012. Global health policy coordination to address neglected tropical diseases. Trop. Med. Int. Health 17:1053–1056. 10.1111/j.1365-3156.2012.03049.x [DOI] [PubMed] [Google Scholar]
- 358.Stevens P, Linfield H. 2010. Death and taxes: government mark-ups on the price of drugs. International Policy Network, London, United Kingdom [Google Scholar]
- 359.Alilio M, Mwenesi H, Barat LM, Payes RM, Prysor-Jones S, Diara M, McGuire D, Shaw W. 2007. Broken promise? Taxes and tariffs on insecticide treated mosquito nets. Am. J. Trop. Med. Hyg. 77:227–231 [PubMed] [Google Scholar]
- 360.Chen CE, Gilliland CT, Purcell J, Kishore SP. 2010. The silent epidemic of exclusive university licensing policies on compounds for neglected diseases and beyond. PLoS Negl. Trop. Dis. 4:e570. 10.1371/journal.pntd.0000570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sterckx S. 2011. Patenting and licensing of university research: promoting innovation or undermining academic values? Sci. Eng. Ethics 17:45–64. 10.1007/s11948-009-9168-8 [DOI] [PubMed] [Google Scholar]
- 362.Roose-Snyder B, Doyle MK. 2009. The global health licensing program: a new model for humanitarian licensing at the university level. Am. J. Law Med. 35:281–309 [DOI] [PubMed] [Google Scholar]
- 363.Kishore SP, Tavera G, Hotez PJ. 2010. The global health crisis and our nation's research universities. PLoS Negl. Trop. Dis. 4:e635. 10.1371/journal.pntd.0000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Chokshi DA, Rajkumar R. 2007. Leveraging university research to advance global health. JAMA 298:1934–1936. 10.1001/jama.298.16.1934 [DOI] [PubMed] [Google Scholar]
- 365.Chokshi DA. 2006. Improving access to medicines in poor countries: the role of universities. PLoS Med. 3:e136. 10.1371/journal.pmed.0030136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Musselwhite LW, Maciag K, Lankowski A, Gretes MC, Wellems TE, Tavera G, Goulding RE, Guillen E. 2012. First Universities Allied for Essential Medicines (UAEM) neglected diseases and innovation symposium. Am. J. Trop. Med. Hyg. 86:65–74. 10.4269/ajtmh.2012.11-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Masum H, Lackman R, Bartleson K. 2013. Developing global health technology standards: what can other industries teach us? Global Health 9:49. 10.1186/1744-8603-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Bhardwaj A, Scaria V, Raghava GPS, Lynn AM, Chandra N, Banerjee S, Raghunandanan MV, Pandey V, Taneja B, Yadav J, Dash D, Bhattacharya J, Misra A, Kumar A, Ramachandran S, Thomas Z, Open Source Drug Discovery Consortium Brahmachari SK 2011. Open source drug discovery–a new paradigm of collaborative research in tuberculosis drug development. Tuberculosis (Edinb.) 91:479–486. 10.1016/j.tube.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 369.Van Noorden R. 2013. Open access: the true cost of science publishing. Nat. News 495:426–429. 10.1038/495426a [DOI] [PubMed] [Google Scholar]
- 370.Munroe R. 2013. The rise of open access. Science 342:58–59. 10.1126/science.342.6154.58 [DOI] [PubMed] [Google Scholar]
- 371.Ortí L, Carbajo RJ, Pieper U, Eswar N, Maurer SM, Rai AK, Taylor G, Todd MH, Pineda-Lucena A, Sali A, Marti-Renom MA. 2009. A kernel for open source drug discovery in tropical diseases. PLoS Negl. Trop. Dis. 3:e418. 10.1371/journal.pntd.0000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Agüero F, Al-Lazikani B, Aslett M, Berriman M, Buckner FS, Campbell RK, Carmona S, Carruthers IM, Chan AWE, Chen F, Crowther GJ, Doyle MA, Hertz-Fowler C, Hopkins AL, McAllister G, Nwaka S, Overington JP, Pain A, Paolini GV, Pieper U, Ralph SA, Riechers A, Roos DS, Sali A, Shanmugam D, Suzuki T, Van Voorhis WC, Verlinde CLMJ. 2008. Genomic-scale prioritization of drug targets: the TDR Targets database. Nat. Rev. Drug Discov. 7:900–907. 10.1038/nrd2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Kraus CN. 2008. Low hanging fruit in infectious disease drug development. Curr. Opin. Microbiol. 11:434–438. 10.1016/j.mib.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 374.Allarakhia M. 2013. Open-source approaches for the repurposing of existing or failed candidate drugs: learning from and applying the lessons across diseases. Drug Des. Dev. Ther. 7:753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Nwaka S, Besson D, Ramirez B, Maes L, Matheeussen A, Bickle Q, Mansour NR, Yousif F, Townson S, Gokool S, Cho-Ngwa F, Samje M, Misra-Bhattacharya S, Murthy PK, Fakorede F, Paris J-M, Yeates C, Ridley R, Van Voorhis WC, Geary T. 2011. Integrated dataset of screening hits against multiple neglected disease pathogens. PLoS Negl. Trop. Dis. 5:e1412. 10.1371/journal.pntd.0001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Crowther GJ, Shanmugam D, Carmona SJ, Doyle MA, Hertz-Fowler C, Berriman M, Nwaka S, Ralph SA, Roos DS, Van Voorhis WC, Agüero F. 2010. Identification of attractive drug targets in neglected-disease pathogens using an in silico approach. PLoS Negl. Trop. Dis. 4:e804. 10.1371/journal.pntd.0000804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nwaka S, Ilunga TB, Da Silva JS, Rial Verde E, Hackley D, De Vré R, Mboya-Okeyo T, Ridley RG. 2010. Developing ANDI: a novel approach to health product R&D in Africa. PLoS Med. 7:e1000293. 10.1371/journal.pmed.1000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Mboya-Okeyo T, Ridley RG, Nwaka S, Task Force ANDI. 2009. The African Network for Drugs and Diagnostics Innovation. Lancet 373:1507–1508. 10.1016/S0140-6736(09)60838-2 [DOI] [PubMed] [Google Scholar]
- 379.Jakobsen PH, Wang M-W, Nwaka S. 2011. Innovative partnerships for drug discovery against neglected diseases. PLoS Negl. Trop. Dis. 5:e1221. 10.1371/journal.pntd.0001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.CEWG. 2011. Report of the second meeting of the Consultative Expert Working Group on Research And development: financing and coordination. WHO, Geneva, Switzerland [Google Scholar]
- 381.WHO. Identification of health R&D demonstration projects. World Health Organization, Geneva, Switzerland [Google Scholar]
- 382.Moran M. 2014. WHO plans for neglected diseases are wrong. Nat. News 506:267–267. 10.1038/506267a [DOI] [PubMed] [Google Scholar]
- 383.Dent J, Ramamoorthi R, Graef K, Nelson LM, Wichard JC. 2013. WIPO Re:Search: a consortium catalyzing research and product development for neglected tropical diseases. Pharm. Pat. Anal. 2:591–596. 10.4155/ppa.13.49 [DOI] [PubMed] [Google Scholar]
- 384.Gustavsen KM, Bradley MH, Wright AL. 2009. GlaxoSmithKline and Merck: private-sector collaboration for the elimination of lympahtic filariasis. Ann. Trop. Med. Parasitol. 103(Suppl 1):S11–S15. 10.1179/000349809X12502035776478 [DOI] [PubMed] [Google Scholar]