Abstract
All living organisms must repair DNA double-stranded breaks (DSBs) in order to survive. Many bacteria rely on nonhomologous end joining (NHEJ) when only a single copy of the genome is available and maintain NHEJ pathways with a minimum of two proteins. In this issue, Bhattarai and colleagues identify additional factors that can work together to aid in survival of stationary-phase cells with chromosomal breaks.
TEXT
A single DNA double-stranded break (DSB) is lethal if left unrepaired (1). Consequently, multiple pathways exist to address this potent form of damage. While multicellular eukaryotes tend to directly religate broken DNA ends using nonhomologous end joining (NHEJ), bacteria primarily rely on homologous recombination. In fact, the existence of NHEJ in some bacteria was discovered only during the past 13 years (2–5). NHEJ offers protection to species that live in stressful environments when only a single copy of the genome is available, such as after sporulation or during stationary phase (6, 7). This may have an impact on human health, since dormant Mycobacterium tuberculosis cells are bombarded by genotoxins from host macrophages, which presumably generate numerous DSBs that could be repaired by NHEJ (8–10). Furthermore, the variable environmental conditions occupied by bacteria suggest that DSBs will be induced by a diverse set of sources. This problem underscores a major challenge for NHEJ, since it must be capable of effectively repairing a wide variety of broken DNA ends.
The Ku-LigD model of bacterial NHEJ.
The NHEJ machinery in eukaryotes consists of multiple proteins, including a Ku70/Ku80 heterodimer that recognizes the DSB, as well as ligase IV, which subsequently seals the DNA backbone. At least four DNA modifying enzymes also dynamically associate with this core to “clean” the DSB ends, when necessary, to allow for ligation (11). In contrast, the NHEJ system in bacteria is thought to consist of only two proteins: a Ku homodimer and a multifunctional ligase (LigD), which has three enzymatic domains that may take the place of the multiple independent eukaryotic factors responsible for cleaning DNA ends (Fig. 1A). The LigD ligase domain (LigD-LIG) is stimulated by the presence of a 3′ ribonucleotide at the nick, distinguishing it from eukaryotic ATP-dependent ligases (12). It also has a polymerase domain (LigD-POL) that preferentially uses ribonucleoside triphosphates (NTPs) over deoxyribonucleoside triphosphates (dNTPs) and a phosphoesterase domain (LigD-PE) that resects RNA stretches until only a single ribonucleotide remains (13, 14). Although this suggests that LigD behaves as an assembly line, whereby LigD-POL (and LigD-PE, in certain situations) ensures the presence of a 3′ ribonucleotide to support the subsequent sealing by LigD-LIG, previous in vivo experiments were unable to detect a critical role for POL or PE in repair (15). In addition, a backup NHEJ operates when the ligase activity of LigD is disabled in Mycobacterium (4, 6, 15). Therefore, identifying the factors crucial for NHEJ in vivo remains a major goal for the field.
FIG 1.
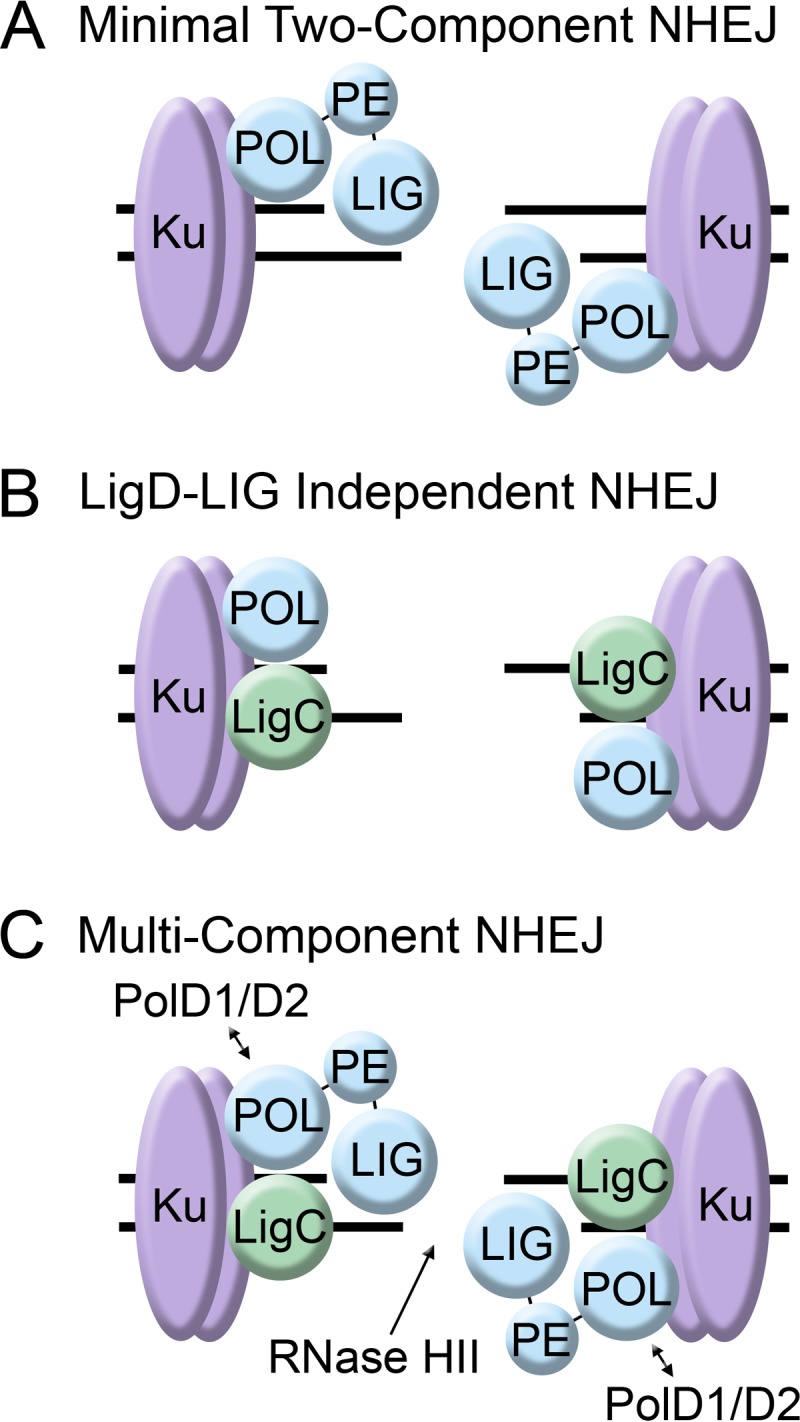
Models for bacterial NHEJ. (A) The minimal NHEJ system in bacteria has only two proteins, Ku (shown in purple) and LigD (shown in blue). LigD possesses all of the activities required to clean, process, and ligate DSBs. (B) The LigD-LIG-independent pathway consists of a polymerase (LigD-POL, PolD1, or PolD2) and LigC. (C) In M. smegmatis, NHEJ may require Ku and multiple polymerases (LigD-POL, PolD1, and PolD2) and ligases (LigD-LIG and LigC) in addition to RNase HII to remove the residual ribonucleotide left over after the DSB is repaired.
In this issue, Bhattarai and colleagues have addressed these problems by altering their experimental approach (16). Previous studies transformed bacteria with a digested plasmid and assayed for recircularization (15). Without any homology between the plasmid and the genome, ligation can occur only through NHEJ, allowing this pathway to be tested directly. Despite the utility of this approach, it presents the cell with DSB ends that do not require modification before ligation. Bhattarai et al. instead monitored survival of Mycobacterium smegmatis cells that were exposed to ionizing radiation (IR), a form of damage likely to generate DSBs with variable structures.
Role of LigD in chromosomal DSB repair.
Bhattarai and colleagues confirmed that although deleting either Ku or LigD renders stationary M. smegmatis cells hypersensitive to IR, a higher fraction of ΔligD mutants than ΔKu mutants survived due to a LigD-independent NHEJ pathway, as previously suggested (6). Inactivating the POL domain of LigD was as detrimental to cell survival as deleting the entire enzyme, suggesting that it is required to process all DSBs induced by IR. Intriguingly, expressing the isolated LigD-POL domain in the ΔligD mutant enhanced viability. Therefore, not only does this polymerase support the LigD ligase activity to which it is physically associated in a wild-type cell, but LigD-POL also supports the backup ligase that operates when LigD-LIG is not able to perform its function.
M. smegmatis has three additional ATP-dependent ligases that could potentially substitute for LigD-LIG activity in the backup pathway: LigC1, LigC2, and LigB. The authors show that only LigC1 is required, which is also stimulated by the presence of a 3′ ribonucleotide at the nick (12). Therefore, the role of the LigD-POL domain in the backup pathway may be to supply the critical 3′ ribonucleotide signal for LigC1. This model is supported by experiments in which Bhattarai and colleagues deleted two additional polymerases, PolD1 and PolD2, which also preferentially utilize NTPs over dNTPs (17). Cells that lack all three polymerase activities are unable to engage the backup pathway and behaved similarly to a ΔKu mutant when exposed to IR. However, LigD-POL is also responsible for binding to Ku at the site of damage, and therefore it cannot be ruled out that this domain helps recruit LigC1 in addition to providing the ribonucleotide (18).
Alternative ligases in NHEJ.
An intriguing result of these experiments is that LigC1 but not the homologous LigC2 enzyme supports backup NHEJ in a LigD-LIG point mutant. The authors clearly demonstrate that LigC2 is expressed but not maintained in vivo. Indeed, expression of the M. tuberculosis LigC enzyme, which is closely related to LigC2, partially restores NHEJ activity in M. smegmatis cells lacking all four endogenous ATP-dependent ligases. Consequently, LigC2-like enzymes per se are not restricted from participating in NHEJ if present at adequate levels in the cell. It will be interesting to determine if different conditions can alleviate the negative regulation of LigC2 in M. smegmatis and reveal an additional layer of redundancy in NHEJ.
This work clearly shows that the NHEJ pathway in M. smegmatis is more complex than the minimal Ku-LigD system thought to operate in bacteria (19). Instead, it may be more instructive to view NHEJ as consisting of a minimal number of activities, each of which is supplied by more than one protein. The recognition of the DSB by the Ku homodimer is strictly required, as is the generation of a single ribonucleotide at the 3′ end of the nick by a specialized polymerase (either LigD-POL, PolD1, or PolD2) and sealing of the DNA backbone by an ATP-dependent ligase (LigD-LIG, LigC1, or perhaps LigC2). Furthermore, whether LigC-type enzymes function in a truly alternative pathway in vivo is not yet clear. While the system used by Bhattarai and colleagues allowed for the identification of factors that function in NHEJ, the relative contribution of each protein awaits further experimentation with other clastogens. LigC1 clearly supports NHEJ after IR exposure if LigD ligase activity is not available (Fig. 1B), and deleting this enzyme does not impact viability in wild-type cells, which suggests it operates only as a backup. However, under different conditions, LigC1 may also act in concert with functional LigD to facilitate the repair of lesions not represented by IR-induced DSBs (Fig. 1C). Indeed, this situation would mirror the eukaryotic system, where DSB repair involves multiple pathways that functionally overlap. This flexibility may be a direct consequence of the heterogeneous nature of DSBs that must be contended with by all domains of life.
ACKNOWLEDGMENTS
We thank members of the Simmons lab for comments on the manuscript.
This work was supported by a grant from the NIH (GM107312) to L.A.S.
Footnotes
Published ahead of print 21 July 2014
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Frankenberg-Schwager M, Frankenberg D. 1990. DNA double-strand breaks: their repair and relationship to cell killing in yeast. Int. J. Radiat. Biol. 58:569–575. 10.1080/09553009014551931 [DOI] [PubMed] [Google Scholar]
- 2.Aravind L, Koonin EV. 2001. Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res. 11:1365–1374. 10.1101/gr.181001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty AJ, Jackson SP, Weller GR. 2001. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett. 500:186–188. 10.1016/S0014-5793(01)02589-3 [DOI] [PubMed] [Google Scholar]
- 4.Gong C, Bongiorno P, Martins A, Stephanou NC, Zhu H, Shuman S, Glickman MS. 2005. Mechanism of nonhomologous end-joining in mycobacteria: a low-fidelity repair system driven by Ku, ligase D and ligase C. Nat. Struct. Mol. Biol. 12:304–312. 10.1038/nsmb915 [DOI] [PubMed] [Google Scholar]
- 5.Gong C, Martins A, Bongiorno P, Glickman M, Shuman S. 2004. Biochemical and genetic analysis of the four DNA ligases of mycobacteria. J. Biol. Chem. 279:20594–20606. 10.1074/jbc.M401841200 [DOI] [PubMed] [Google Scholar]
- 6.Pitcher RS, Green AJ, Brzostek A, Korycka-Machala M, Dziadek J, Doherty AJ. 2007. NHEJ protects mycobacteria in stationary phase against the harmful effects of desiccation. DNA Repair (Amst.) 6:1271–1276. 10.1016/j.dnarep.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 7.Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, Doherty AJ, Horneck G, Nicholson WL. 2007. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J. Bacteriol. 189:3306–3311. 10.1128/JB.00018-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuman S, Glickman MS. 2007. Bacterial DNA repair by non-homologous end joining. Nat. Rev. Microbiol. 5:852–861. 10.1038/nrmicro1768 [DOI] [PubMed] [Google Scholar]
- 9.Heaton B, Barkan D, Bongiorno P, Karakousis PC, Glickman MS. 2014. Deficiency of double strand DNA break repair does not impair Mycobacterium tuberculosis virulence in multiple animal models of infection. Infect. Immun. 82:3177–3185. 10.1128/IAI.01540-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brzostek A, Szulc I, Klink M, Brzezinska M, Sulowska Z, Dziadek J. 2014. Either non-homologous ends joining or homologous recombination is required to repair double-strand breaks in the genome of macrophage-internalized Mycobacterium tuberculosis. PLoS One 9:e92799. 10.1371/journal.pone.0092799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyman C, Kanaar R. 2006. DNA double-strand break repair: all's well that ends well. Annu. Rev. Genet. 40:363–383. 10.1146/annurev.genet.40.110405.090451 [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Shuman S. 2008. Bacterial nonhomologous end joining ligases preferentially seal breaks with a 3′-OH monoribonucleotide. J. Biol. Chem. 283:8331–8339. 10.1074/jbc.M705476200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H, Shuman S. 2005. Novel 3′-ribonuclease and 3′-phosphatase activities of the bacterial non-homologous end-joining protein, DNA ligase D. J. Biol. Chem. 280:25973–25981. 10.1074/jbc.M504002200 [DOI] [PubMed] [Google Scholar]
- 14.Pitcher RS, Brissett NC, Picher AJ, Andrade P, Juarez R, Thompson D, Fox GC, Blanco L, Doherty AJ. 2007. Structure and function of a mycobacterial NHEJ DNA repair polymerase. J. Mol. Biol. 366:391–405. 10.1016/j.jmb.2006.10.046 [DOI] [PubMed] [Google Scholar]
- 15.Aniukwu J, Glickman MS, Shuman S. 2008. The pathways and outcomes of mycobacterial NHEJ depend on the structure of the broken DNA ends. Genes Dev. 22:512–527. 10.1101/gad.1631908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattarai H, Gupta R, Glickman MS. 2014. DNA ligase C1 mediates the LigD-independent nonhomologous end-joining pathway of Mycobacterium smegmatis. J. Bacteriol. 196:3366–3376. 10.1128/JB.01832-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Bhattarai H, Yan HG, Shuman S, Glickman MS. 2012. Characterization of Mycobacterium smegmatis PolD2 and PolD1 as RNA/DNA polymerases homologous to the POL domain of bacterial DNA ligase D. Biochemistry 51:10147–10158. 10.1021/bi301202e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitcher RS, Tonkin LM, Green AJ, Doherty AJ. 2005. Domain structure of a NHEJ DNA repair ligase from Mycobacterium tuberculosis. J. Mol. Biol. 351:531–544. 10.1016/j.jmb.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 19.Della M, Palmbos PL, Tseng HM, Tonkin LM, Daley JM, Topper LM, Pitcher RS, Tomkinson AE, Wilson TE, Doherty AJ. 2004. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science 306:683–685. 10.1126/science.1099824 [DOI] [PubMed] [Google Scholar]


