Abstract
The Syk protein tyrosine kinase, a well-characterized regulator of immune cell function, plays an increasingly recognized role in tumorigenesis as a promoter of cell survival in both hematological and nonhematological malignancies. We show here that the expression of Syk in MCF7 or MDA-MB-231 breast cancer cells or in DG75 B-lymphoma cells protects cells from apoptosis induced by oxidative or genotoxic stress by stabilizing the mRNA for Bcl-xL, an antiapoptotic protein. Syk binds robustly to nucleolin and phosphorylates it on tyrosine, enhancing its ability to bind the Bcl-xL mRNA. Consequently, reducing the level of nucleolin by RNA interference attenuates the ability of Syk to protect cells from stress-induced cell death.
INTRODUCTION
In addition to its well-studied role in normal immune cell biology (1, 2), the Syk protein tyrosine kinase plays an increasingly recognized, albeit poorly understood, role in tumorigenesis. Constitutively active Syk has been reported to promote the survival of non-Hodgkin's lymphoma, acute lymphoblastic leukemia, chronic lymphocytic leukemia, and Epstein-Barr virus-associated B-cell lymphoma (3–11). The inhibition of Syk also promotes the differentiation of acute myeloid leukemia (AML) and attenuates the growth of AML cell lines and primary blasts (12, 13). The formation of a Tel-Syk fusion protein that results from a chromosomal translocation results in a myeloid proliferative disorder, while Itk-Syk fusion proteins are found in some T-cell lymphomas (14, 15). The aberrant expression of Syk itself is also found in a variety of peripheral T-cell lymphomas (16).
Even in nonhematological malignancies, Syk can play an important prosurvival function. Lung and pancreatic carcinomas that are dependent on activated K-Ras for viability are distinguished from those not dependent on K-Ras by the expression of Syk (17). These K-Ras-dependent cells undergo apoptosis in response to the inhibition of Syk activity or knockdown of Syk expression. Retinoblastoma cells in which the expression of Syk is induced by changes in gene methylation also undergo apoptosis in response to reductions in the activity or level of the kinase (18). The survival of breast and ovarian cancer cells is promoted by the alternative splicing of SYK transcripts in response to epidermal growth factor, which enhances expression of the long form of the kinase (19). While the mechanisms by which Syk promotes cancer cell survival are incompletely understood, these observations have led to the exploration of Syk inhibitors as antitumor agents (e.g., see references 18 and 20 to 22).
The ability to evade cell death is one of the fundamental hallmarks of a cancer cell (23). Programmed cell death in eukaryotic cells is regulated through the intrinsic pathway by members of the Bcl-2 family of proteins (24). These proteins function to modulate outer mitochondrial membrane channel opening and the release of cytochrome c necessary for the formation of apoptosomes. The Bcl-2 family includes both pro- and antiapoptotic members. Among these are Bcl-xL and Bcl-xS, which are products of alternatively spliced transcripts of the BCL2L1 gene (25). The product of the longer transcript, Bcl-xL, protects cells from apoptosis, while the smaller Bcl-xS protein promotes apoptosis by negatively regulating Bcl-xL and Bcl-2. The relative level of Bcl-xL and Bcl-xS in a cell is an important determinant of susceptibility to stress-induced cell death.
In this study, we explored the mechanism by which Syk enhances cell survival by examining its effect on the responses of cancer cells to induced stress. We found that the presence of Syk increases the resistance of several cancer cell types to H2O2-induced apoptosis by protecting Bcl-xL mRNA from degradation by a mechanism that involves the interaction of both Syk and the Bcl-xL mRNA with nucleolin (NCL). Reductions in the level of nucleolin destabilize the Bcl-xL message and inhibit the ability of Syk to protect cells from apoptosis induced by both oxidative and genotoxic stress.
MATERIALS AND METHODS
Plasmids and DNA constructs.
For constructing the tetracycline (Tet)-inducible enhanced green fluorescent protein (EGFP)-tagged Syk (Syk-EGFP) lentiviral vectors, cDNAs for Syk-EGFP, Syk-EGFP(K396R), Syk-EGFP(Y317F), Syk-EGFP(Y342F), Syk-EGFP(Y346F), Syk-EGFP(Y342F/Y346F), and Syk-EGFP(Y317F/Y342F/Y346F) were amplified by PCR from the corresponding EGFP-N2 (Clontech) constructs described previously (26). These were then cloned into the Tet-inducible lentiviral vector pLVX-Tight-Puro (Clontech) between the MluI and EcoRI restriction sites. Lentiviral pGIPZ short hairpin RNA (shRNA) sets for the knockdown of nucleolin and Syk were purchased from Thermo Scientific. The Bcl-xL expression plasmid pSFFV-neo Bcl-xL (27) was obtained from Addgene (plasmid 8749).
Cell lines.
A line of MCF7 cells lacking endogenous Syk (MCF7-BD) was described previously, as were MCF7-BD cells stably expressing exogenous Syk-EGFP (MCF7-Syk) (28). Syk-deficient MCF7-BD cells with tetracycline-regulated Syk-EGFP expression (MCF7-TRS) were constructed previously using a T-REx system (Invitrogen) (26). These cells were treated with 1 μg/ml doxycycline to induce Syk-EGFP expression. MDA-MB-231 breast cancer cells were obtained from ATCC. A line of MDA-MB-231 cells (MDA-MB-231-TRS) with inducible expression of Syk-EGFP was described previously (26).
MDA-MB-231 cells expressing Syk-EGFP, Syk-EGFP(K396R), or EGFP were constructed using a Lenti-X Tet-On advanced inducible expression system (Clontech). To constitutively express the tetracycline-controlled transactivator rtTA in the Tet-On inducible system, cells were first infected with viral particles with the pLVX Tet-On advanced regulator. Lentiviral particles were generated by cotransfecting HEK293T cells with 4 μg of pLVX-Tet-On, 4 μg of pHR′-CMV-ΔR8.20 vpr, and 2 μg of pHR′-CMV-VSVG using Lipofectamine 2000 (Invitrogen). Supernatants containing viral particles were harvested at 48 h posttransfection and used to infect MDA-MB-231 cells. Two days after infection, cells were selected with 500 μg/ml G418 and screened for rtTA expression. Cells constitutively expressing rtTA protein were infected with lentiviral particles packaged with pLVX-Tight-Puro-Syk-EGFP [or with Syk-EGFP(K396R) or EGFP] as described above. After 48 h, cells were selected with 1 μg/ml puromycin and screened for expression by Western blotting.
To establish nucleolin knockdown cells, MCF7-TRS, MDA-MB-231-TRS, and DG75 cells were stably infected by one of a set of eight lentiviral particles containing an shRNA sequence for NCL. Knockdown of Syk in DG75 cells was generated by stably infecting cells with one of a set of six lentiviral particles containing an shRNA sequence for human Syk. Cell lines were isolated from each lentiviral infection by selection with puromycin (1.0 μg/ml) and screened by Western blotting with antibodies against nucleolin (Abcam) or Syk (Cell Signaling Technology).
All breast cancer cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 7.5% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Human DG75 B-lymphoma cells (ATCC) were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 100 IU/ml penicillin G, and 100 μg/ml streptomycin.
DLBCL.
Fresh tumor tissue for ex vivo bioassays was obtained via surgical biopsy from pet dogs with naturally occurring diffuse large B-cell lymphoma (DLBCL). All dogs were seen as clinical patients at the Purdue University Veterinary Teaching Hospital. Written informed consent was obtained from the owners of all dogs, and the biopsy protocol was approved by the Purdue Animal Care and Use Committee. Dogs were anesthetized with propofol (10 mg/kg of body weight intravenously), and a surgical plane of anesthesia was maintained with inhaled isoflurane (1 to 5%). The surgical procedure consisted of an incisional wedge biopsy or complete extirpation of an affected peripheral lymph node. Postoperative analgesia was provided with hydromorphone (0.1 mg/kg subcutaneously). All dogs recovered from surgery uneventfully.
Lymph node biopsy specimens were handled aseptically and divided into sections. One section was placed into 10% neutral buffered formalin to be used for histopathologic diagnosis. Formalin-fixed, paraffin-embedded tissues were stained with hematoxylin-eosin and also prepared routinely for immunohistochemical analysis. The diagnosis of diffuse large B-cell lymphoma was based upon histomorphology and immunohistochemical detection of CD79a and/or CD20, as previously described (29). A second section was placed in RPMI 1640 and processed immediately for use in ex vivo bioassays. Cells were teased apart mechanically and cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 100 IU/ml penicillin G, and 100 μg/ml streptomycin and supplemented with 10 ng/ml soluble CD40L (InvivoGen).
Antibodies and immunoprecipitation.
Antibodies against Syk, poly(ADP-ribose) polymerase (PARP), active caspase 3, and Bcl-xL were purchased from Cell Signaling Technology. Antinucleolin and anti-γ-tubulin were from Abcam, antiphosphotyrosine (4G10) was from Millipore, anti-green fluorescent protein (anti-GFP) was from Santa Cruz, and anti-GAPDH (anti-glyceraldehyde-3-phosphate dehydrogenase) was from Ambion.
For Syk and nucleolin coimmunoprecipitation assays, 5 × 106 MDA-MB-231 cells with inducible expression of Syk-EGFP, EGFP, or one of the site-directed Syk-EGFP mutants were treated with doxycycline (1 μg/ml) for 18 h. Cells were lysed in 20 mM Tris-HCl, pH 8, 137 mM NaCl, 2 mM EDTA, 1% NP-40, 10% glycerol, 1× protease inhibitor cocktail (Sigma), and 2 mM Na3VO4. EGFP or EGFP-tagged proteins were immunoprecipitated using GFP-Trap agarose beads (ChromoTek). Bound immune complexes were washed with lysis buffer, separated by SDS-PAGE, and analyzed by Western blotting with the indicated antibodies. Where indicated, immune complexes containing Syk-EGFP or Syk-EGFP(K396R) and associated nucleolin were incubated in 25 mM HEPES, pH 7.2, 5 mM MnCl2, 0.5 mM Na3VO4, 0.02 mg/ml leupeptin, and 0.02 mg/ml aprotinin with or without 1 mM ATP at 37°C for 10 min. Immune complexes were separated by SDS-PAGE. Nucleolin was detected by Western blotting.
RT-PCR analyses of BCL2L1 transcripts.
Total cellular RNA was isolated from 5 ×106 cells using the QIAzol reagent (Qiagen) according to the manufacturer's protocol. RNA (2 μg) from each sample was reverse transcribed and amplified using a SuperScript III Platinum One-Step quantitative reverse transcription-PCR (RT-PCR) system (Invitrogen) containing 0.1 μM each BCL2L1 forward and reverse primers in a 25-μl reaction mix. The primer sequences used were CATGGCAGCAGTAAAGCAAG and GCATTGTTCCCATAGAGTTCC for the human transcripts and GCATTGTTCCCGTAGAGTTCC and GATCATCTCGCGCTACTTG for the canine transcripts. The primers used to amplify the canine GAPDH transcripts were TGATTCTACCCACGGCAAATTC and TCATGGTTCACGCCCATCAC. The reverse transcription was performed at 50°C for 30 min. PCRs were optimized for semiquantification by activating Platinum Taq polymerase at 95°C for 2 min and carried out in 25 cycles of two-step conditions of 95°C for 15 s and 60°C for 30 s and finally ending with a 5-min cycle extension at 72°C. An aliquot (10 μl) of each reaction mixture was separated on a 2% agarose gel, visualized by ethidium bromide staining, and quantified with ImageJ software. Where indicated, cells were treated prior to lysis with H2O2 (5 mM).
RNA immunoprecipitation assay.
MDA-MB-231 cells were treated without or with 5 mM H2O2 and lysed in RNA immunoprecipitation (RIP) buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 0.5 mM dithiothreitol, 1% NP-40, 10 mM vanadyl ribonucleoside complex [NEB], 2 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail [Sigma]) for 15 min on ice. After centrifugation at 14,000 × g for 10 min, antibody to nucleolin was added to the supernatant and the mixture was incubated at 4°C for 4 h. Prior to precipitation, protein A-agarose beads (40 μl) were added and the mixture was incubated for 1 h at 4°C with gentle rotation. The beads were then washed three times with RIP buffer and once in phosphate-buffered saline. One-fourth of the precipitate was used to detect nucleolin by Western blotting following SDS-PAGE. RNAs in the nucleolin precipitates were isolated with 1 ml of QIAzol reagent according to the manufacturer's instructions. RNAs were resuspended in 20 μl nuclease-free water and subjected to semiquantitative RT-PCR to detect BCL2L1 transcripts.
PARP cleavage assay.
To detect the cleavage of PARP, lysates of 5 × 106 cells treated with 5 mM H2O2 or 1 μg/ml doxorubicin (Calbiochem) for the times indicated below were separated by SDS-PAGE and subjected to Western blot analysis using anti-PARP antibodies. Alternatively, activated caspase 3 was detected by Western blotting using antibodies specific for the cleaved caspase.
RESULTS
Syk stabilizes Bcl-xL mRNA in MCF7 breast cancer cells treated with H2O2.
MCF7 breast cancer cells have an epithelial morphology and express endogenous Syk (30). We had previously identified a line of MCF7 cells (MCF7-BD) that lacks Syk and is able to survive in its absence (28). We generated from these cells a new line in which the expression of Syk (as Syk-EGFP) could be induced by treatment with doxycycline (26). We then compared the responses of the Syk-deficient cells to those of cells induced to express Syk-EGFP to an external stress: exposure to H2O2. The activation of executioner caspases was monitored by Western blotting of cleavage products of poly(ADP-ribose) polymerase (PARP) (31). Cells expressing Syk-EGFP were more resistant to H2O2-induced PARP cleavage than were cells lacking the kinase (Fig. 1A). Consistent with this observation, treatment of Syk-deficient MCF7-BD cells with H2O2 caused them to round up and detach from the tissue culture plate, an effect that was attenuated in Syk-EGFP-expressing cells (see Fig. S1 in the supplemental material).
FIG 1.

Syk expression protects MCF7 cells from oxidative stress-induced apoptosis and degradation of Bcl-xL mRNA. (A) MCF7-BD cells lacking Syk (−) or stably expressing Syk-EGFP (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by SDS-PAGE and Western blotting with antibodies against PARP (top). The cleaved form of PARP is indicated by the arrow. The expression of Syk-EGFP was visualized by Western blotting of cell lysates (bottom). (B) MCF7-BD cells lacking Syk (−) or stably expressing Syk-EGFP (+) were exposed to 5 mM H2O2 for 30 min (pulse) and then moved to fresh medium for the indicated total incubation times or treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA (top) or by Western blotting to detect expressed Syk-EGFP (bottom). (C) Comparison of relative levels of Bcl-xL mRNA. Changes in the ratio of Bcl-xL mRNA to Bcl-xS mRNA were normalized to their relative levels of expression in Syk-deficient cells at time zero, which was set equal to a value of 1.0. Bars represent means ± SEMs from three replicate experiments. Significant differences between pairs were determined using an unpaired, two-tailed Student's t test. *, P < 0.01; **, P < 0.005; ***, P < 0.001.
Bcl-xL, the product of the longer BCL2L1 gene transcript, is a potent antiapoptotic factor in breast cancer cells (32, 33). To explore the mechanism by which Syk influences the response of MCF7 cells to oxidative stress, we measured the levels of mRNA for Bcl-xL and Bcl-xS in cells expressing or lacking Syk as a function of time of exposure to H2O2. The levels of each mRNA were equivalent in untreated cells whether they were lacking or expressing Syk (Fig. 1B and C). The treatment of MCF7-BD cells with H2O2 resulted in a striking reduction in the level of the longer Bcl-xL transcript. In cells expressing Syk-EGFP, the decrease in the level of mRNA for Bcl-xL in response to oxidative stress was markedly attenuated. This protective effect of Syk-EGFP was observed both in cells treated with a short 30-min pulse of H2O2 and then monitored over time and in cells in which H2O2 remained in the medium for 2 to 4 h. The protective effect of induced Syk-EGFP on Bcl-xL mRNA was retained in cells pretreated with actinomycin D, an inhibitor of gene transcription (see Fig. S2 in the supplemental material). Thus, Syk functioned to stabilize preexisting Bcl-xL mRNA rather than alter its rate of transcription.
Syk stabilizes Bcl-xL mRNA in MDA-MB-231 cells treated with H2O2.
MDA-MB-231 breast cancer cells are highly invasive, have a mesenchymal phenotype, and lack endogenous Syk (30). A clone of cells transfected with the pcDNA6/TR regulatory plasmid was isolated (MDA-MB-231-TR), and from these we generated clones that contained a Tet-inducible plasmid expressing Syk-EGFP (MDA-MB-231-TRS) (26). The MDA-MB-231-TRS cells express low levels of Syk-EGFP in the absence of doxycycline due to leaky expression from the inducible plasmid but can be induced to express higher levels of Syk-EGFP upon treatment with doxycycline (Fig. 2A). The levels of Bcl-xL and Bcl-xS mRNA were comparable, regardless of the expression level of Syk. The treatment of MDA-MB-231-TR cells with H2O2 resulted in a reduction in the level of the longer transcript (Fig. 2A and B). The times of exposure to H2O2 required to produce this decreased level of Bcl-xL mRNA were longer in MDA-MB-231-TR cells than in MCF7-BD cells. The decrease in Bcl-xL mRNA level in Syk-deficient cells resulted in a concomitant reduction in the level of expression of Bcl-xL protein (Fig. 2C). The induction of Syk-EGFP protected the MDA-MB-231 cells from the oxidative stress-induced decrease in Bcl-xL mRNA (Fig. 2A and B). Even low levels of Syk-EGFP resulting from leaky transcription partially rescued the cells from the decrease in Bcl-xL mRNA caused by oxidative stress. This protective effect of Syk was again retained in cells pretreated with actinomycin D (see Fig. S2 in the supplemental material). The induced expression of Syk also protected MDA-MB-231 cells from H2O2-induced activation of caspase activity, as visualized by a decreased cleavage of PARP (Fig. 2D).
FIG 2.

Syk expression protects MDA-MB-231 cells from oxidative stress-induced apoptosis and degradation of Bcl-xL mRNA. (A) MDA-MB-231-TR (TR) cells lacking Syk or MDA-MB-231-TRS (TRS) cells either induced (+) or not induced (−) with doxycycline (Tet) to express Syk-EGFP were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA (top) or by Western blotting to detect expressed Syk-EGFP (bottom). (B) Comparison of relative levels of Bcl-xL mRNA to Bcl-xS mRNA. Ratios were normalized to a value of 1.0 for Syk-deficient cells at time zero. Bars represent means ± SEMs from three replicate experiments. *, P < 0.05; **, P < 0.01. (C) MDA-MB-231-TR cells (−) or doxycycline-induced MDA-MB-231-TRS cells (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by SDS-PAGE and Western blotting (WB) with antibodies against Bcl-xL (top) and Syk-EGFP (bottom). (D) MDA-MB-231-TR cells lacking Syk (−) or MDA-MB-231-TRS cells induced with doxycycline to express Syk-EGFP (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by SDS-PAGE and Western blotting with antibodies against PARP. The cleaved form of PARP is indicated by the arrow.
Syk stabilizes Bcl-xL mRNA in DG75 cells treated with H2O2.
To determine if Syk also plays a prosurvival role when expressed at normal endogenous levels, we examined the effects of a knockdown in the level of the kinase on Bcl-xL mRNA expression in a human Burkitt's lymphoma. DG75 B cells were infected with a lentivirus expressing shRNA targeted against human Syk mRNA. Of six lentiviruses tested, one resulted in a greater than 80% reduction in the level of the endogenous protein (Fig. 3A). The level of Bcl-xL mRNA was consistently lower in DG75 cells in which the expression of Syk was suppressed. Cells expressing either normal or reduced levels of Syk were treated with H2O2 for various periods of time and examined for changes in the levels of Bcl-xL and Bcl-xS mRNA. Cells with a reduced level of endogenous Syk exhibited a markedly reduced level of Bcl-xL mRNA when treated with H2O2 compared to that exhibited by cells expressing a normal level of Syk (Fig. 3A and B). Again, this effect was retained in cells pretreated with actinomycin D (see Fig. S2 in the supplemental material). DG75 cells with a reduced level of Syk also exhibited an increased sensitivity to oxidative stress, as measured by the cleavage of PARP (Fig. 3C). To confirm that this increased sensitivity resulted from a reduced level of Bcl-xL protein, we transfected the wild-type and Syk knockdown cells with a Bcl-xL expression plasmid to increase the intracellular level of the protein. Increasing the level of Bcl-xL reduced the sensitivity of the DG75 cells to oxidative stress-induced apoptosis, as measured by the cleavage of PARP in both cell types (Fig. 3D). Together, these results indicate that Syk functions to protect Bcl-xL mRNA from degradation in response to oxidative stress in multiple cell lines, thus protecting them from apoptosis.
FIG 3.

The knockdown of Syk expression sensitizes DG75 B lymphoma cells to oxidative stress-induced apoptosis and degradation of Bcl-xL mRNA. (A) DG75 B lymphoma cells (−) or DG75 cells stably expressing shRNA targeted against Syk (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA (top) or by Western blotting to detect endogenous Syk (bottom). (B) Comparison of relative levels of Bcl-xL to Bcl-xS mRNA. Ratios were normalized to a value of 1.0 for wild-type DG75 B cells at time zero. Bars represent means ± SEMs from three replicate experiments. *, P < 0.05; **, P < 0.01. (C) DG75 B lymphoma cells (−) or DG75 cells stably expressing shRNA targeted against Syk (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by SDS-PAGE and Western blotting with antibodies against PARP. The cleaved form of PARP is indicated by the arrow (top). The expression level of endogenous Syk was probed by Western blotting with antibodies against Syk (bottom). (D) DG75 B lymphoma cells (−) or DG75 cells stably expressing shRNA targeted against Syk (+) were transiently transfected with a Bcl-xL expression plasmid (+) or empty vector (−) and then treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by SDS-PAGE and Western blotting with antibodies against PARP (top), Syk (middle), or Bcl-xL (bottom). The cleaved form of PARP is indicated by the arrow.
Syk promotes the survival of DLBCL.
To determine if the protective effect of Syk on cell viability extended to clinical samples, we examined the effects of the Syk inhibitor R406 on biopsy specimens isolated from dogs suffering from naturally occurring diffuse large B-cell lymphoma (DLBCL). DLBCL cells die rapidly in culture, but their life span can be enhanced by the addition of CD40L (34). DLBCL cells expressed Syk, the activity of which could be inhibited by treatment with R406, as measured by Western blotting of whole-cell lysates with antibodies against phosphotyrosine (see Fig. S3A in the supplemental material). Treatment of DLBCL cells with R406 enhanced cell death, as judged by the approximately 3-fold increase in PARP cleavage measured 12 or 24 h after isolation (see Fig. S3C and D in the supplemental material), and enhanced the rate of loss of Bcl-xL mRNA in the absence or presence of oxidative stress (see Fig. S3E in the supplemental material).
The catalytic activity of Syk is required for stabilization of Bcl-xL mRNA.
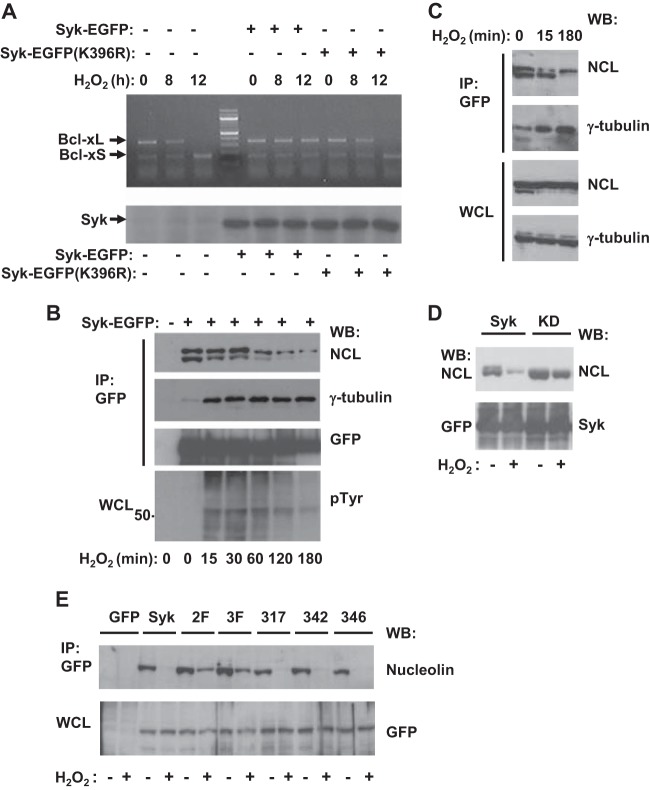
To determine if Syk-catalyzed protein phosphorylation was important for the protective effect of Syk on Bcl-xL mRNA, we generated two populations of MDA-MB-231 cells using a Lenti-X Tet-On inducible expression system in which either Syk-EGFP or a catalytically inactive mutant, Syk-EGFP(K396R), could be induced by treatment with doxycycline. This system provides more tightly controlled expression of inducible genes. We then compared the sensitivity of the Bcl-xL transcript to H2O2-stimulated degradation in cells induced or not induced to express either Syk-EGFP or Syk-EGFP(K396R). The expression of Syk-EGFP, but not that of Syk-EGFP(K396R), protected the Bcl-xL mRNA from stress-induced degradation (Fig. 4A). Thus, the ability of Syk to protect cells from the oxidative stress-induced loss of Bcl-xL mRNA required its catalytic activity. This suggests the involvement of one or more substrates of Syk in the regulation of Bcl-xL mRNA turnover.
FIG 4.
Syk interacts with nucleolin. (A) MDA-MB-231 cells expressing rtTA but not Syk or MDA-MB-231 cells with Tet-regulated expression of Syk-EGFP or Syk-EGFP(K396R) (Lenti-X Tet-On) pretreated with doxycycline (+) were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA (top) or by Western blotting with anti-Syk antibodies to detect Syk-EGFP or Syk-EGFP(K396R) (bottom). (B) Tet-responsive MDA-MB-231 cells not induced (−) or induced with doxycycline to express Syk-EGFP (+) were treated with 5 mM H2O2 for the indicated times. Syk-EGFP was immunoprecipitated (IP) from cell lysates with GFP-nanotrap beads. Anti-GFP immune complexes were separated by SDS-PAGE and analyzed by Western blotting (WB) with antibodies against NCL, γ-tubulin, or GFP (to detect Syk-EGFP). Whole-cell lysates (WCL) were analyzed by Western blotting with antibodies against phosphotyrosine (pTyr) (bottom). The migration position of the 50-kDa molecular mass marker is indicated. (C) Syk-EGFP was immunoprecipitated with GFP-nanotrap beads from lysates of Tet-responsive MDA-MB-231 cells induced to express Syk-EGFP. Immune complexes and whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL or γ-tubulin. (D) Proteins were immunoprecipitated with GFP-nanotrap beads from lysates of Tet-responsive MDA-MB-231 cells induced to express Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD) and treated with (+) or without (−) 5 mM H2O2. Immune complexes and whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL (top) and, to detect Syk-GFP, antibodies against GFP (bottom). (E) Proteins were immunoprecipitated with GFP-nanotrap beads from lysates of Tet-responsive MDA-MB-231 cells induced to express EGFP (lane GFP), Syk-EGFP (lane Syk), Syk-EGFP(Y342F/Y346F) (lane 2F), Syk-EGFP(Y317F/Y342F/Y346F) (lane 3F), Syk-EGFP(Y317F) (lane 317), Syk-EGFP(Y342F) (lane 342), or Syk-EGFP(Y346F) (lane 346) and treated with (+) or without (−) 5 mM H2O2. Immune complexes and whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL (top) or GFP (bottom).
Syk interacts with nucleolin.
In a previous mass spectrometry-based analysis of Syk-interacting proteins, we identified nucleolin as a Syk-binding protein (35). Interestingly, nucleolin has been shown to stabilize Bcl-xL mRNA by binding directly to the AU-rich elements (AREs) in the 3′ untranslated region (UTR) that mediate its rapid turnover (36, 37). To confirm an interaction between Syk and nucleolin, we immunoprecipitated Syk-EGFP from the Tet-responsive MDA-MB-231 cells that had been induced with doxycycline to express the fusion protein and then searched the immune complex for associated nucleolin. Western blotting analyses of proteins present in the anti-Syk-EGFP immune complexes confirmed that nucleolin is a Syk-binding protein (Fig. 4B and C).
The treatment of cells that express Syk with H2O2 leads to its activation and to an increase in the phosphorylation of Syk substrates on tyrosine (38). As expected, treatment of MDA-MB-231 cells induced to express Syk-EGFP with H2O2 led to a transient increase in the level of proteins phosphorylated on tyrosine present in whole-cell lysates (Fig. 4B). The interaction between Syk-EGFP and nucleolin, as measured by coimmunoprecipitation, was the strongest in untreated cells and decreased as a function of time following the addition of H2O2. As a control, we also monitored the interaction of Syk with γ-tubulin, as the treatment of cells with H2O2 promotes the interaction of Syk with centrosomes (39). As expected, H2O2 treatment led to an increase in the amount of γ-tubulin in the anti-Syk immune complexes (Fig. 4B), indicating that H2O2 treatment did not, in general, block all protein-protein interactions involving Syk. Treatment of cells with H2O2 had no apparent effect on the overall level of expression of either nucleolin or γ-tubulin in the whole-cell lysate (Fig. 4C). These observations suggested that the interaction between Syk and nucleolin would be most robust when neither protein was phosphorylated on tyrosine. To test this, we immunoprecipitated either Syk-EGFP or the inactive (kinase-dead) Syk-EGFP(K396R) from the corresponding doxycycline-induced MDA-MB-231 cells treated with or without H2O2 and examined the resulting immune complexes for the presence of nucleolin. Indeed, the inactive version of Syk bound robustly to nucleolin, and this interaction was refractory to treatment with H2O2 compared to the interaction between Syk-EGFP and nucleolin (Fig. 4D).
The Syk-nucleolin interaction is disrupted by the phosphorylation of Syk in linker B.
The Syk molecule comprises an N-terminal tandem pair of SH2 domains separated by a linker (linker B) from the C-terminal catalytic domain. To explore further the mechanism of the H2O2-induced dissociation of Syk and nucleolin, we generated an additional series of MDA-MB-231 cells in which various EGFP-tagged variants of Syk could be inducibly expressed. These included a set of Syk mutants in which known sites of tyrosine phosphorylation in the linker B region (40) were replaced by phenylalanines: Syk-EGFP(Y317F), Syk-EGFP(Y342F), Syk-EGFP(Y346F), Syk-EGFP(Y342F/Y346F), and Syk-EGFP(Y317F/Y342F/Y346F). As a control, cells inducibly expressing EGFP alone were also generated. The various EGFP-tagged proteins were immunoprecipitated with an immobilized GFP-binding protein, and the presence of nucleolin in the immune complex was detected by Western blotting (Fig. 4E). No nucleolin coimmunoprecipitated with EGFP. The replacement of Y317, the binding site on Syk for Cbl-family proteins, had no significant effect on either the binding of Syk to nucleolin or its dissociation in response to H2O2. Forms of the kinase lacking single tyrosines at either position 342 or 346 bound nucleolin and largely dissociated from it in response to H2O2. Interestingly, the H2O2-induced dissociation from nucleolin of forms of Syk lacking both Y342 and Y346 or these two sites plus Y317 was compromised. Since the treatment of cells with H2O2 is known to result in the extensive phosphorylation of the linker B tyrosines on Syk (40), these results are consistent with the phosphorylation of Syk at both Y342 and Y346 disrupting the interaction between Syk and nucleolin.
Syk phosphorylates nucleolin.
The nucleolin associated with Syk appeared to undergo a change in its electrophoretic mobility when it was analyzed by SDS-PAGE (Fig. 4B and C) following the treatment of cells with H2O2, suggesting that it was covalently modified. To determine if nucleolin was phosphorylated on tyrosine in Syk-expressing cells, we treated MDA-MB-231 cells lacking or expressing Syk-EGFP with or without H2O2 and immunoprecipitated the tyrosine-phosphorylated proteins using antibodies against phosphotyrosine. Nucleolin could be detected in the antiphosphotyrosine immune complexes recovered from lysates of H2O2-treated cells that expressed Syk-EGFP (Fig. 5A) or endogenous Syk (see Fig. S3B in the supplemental material). Similarly, tyrosine-phosphorylated nucleolin could be isolated from lysates of DG75 B cells treated with H2O2 but not from cells also treated with the Syk inhibitor piceatannol or R406 (Fig. 5B and C). Nucleolin isolated from H2O2-treated MDA-MB-231-TRS cells expressing Syk-EGFP (Fig. 5D) or DG75 cells expressing normal levels of endogenous Syk (Fig. 5E) reacted on Western blots with antibodies against phosphotyrosine, indicating that nucleolin itself was directly phosphorylated on tyrosine in a Syk-dependent manner.
FIG 5.
Syk phosphorylates nucleolin and promotes its binding to Bcl-xL mRNA. (A) Tet-responsive MDA-MB-231 cells pretreated without or with doxycycline to induce Syk-EGFP were treated without or with 5 mM H2O2 for 15 min. Tyrosine-phosphorylated proteins were immunoprecipitated from cell lysates with antibodies against phosphotyrosine. Immune complexes (top) and whole-cell lysates (WCL; bottom) were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL. (B) DG75 B cells were pretreated with 50 μM piceatannol (PIC; +) or dimethyl sulfoxide carrier alone (−) and then treated without or with 5 mM H2O2 for 15 min. Tyrosine-phosphorylated proteins were immunoprecipitated from cell lysates with antibodies against phosphotyrosine. Immune complexes (top) and whole-cell lysates (bottom) were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL. (C) DG75 cells were pretreated with the indicated concentrations of R406 and then treated without or with 5 mM H2O2 for 15 min. Tyrosine-phosphorylated proteins were immunoprecipitated from cell lysates with antibodies against phosphotyrosine. Immune complexes (top) and whole-cell lysates (bottom) were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL. (D) MDA-MB-231-TR (TR) or MDA-MB-231-TRS cells induced to express Syk-EGFP (TRS) were treated without or with 5 mM H2O2 for 15 min. Nucleolin was immunoprecipitated, and the resulting immune complexes were probed by Western blotting for phosphotyrosine (top) or NCL (bottom). (E) DG75 B cells (−) or DG75 cells stably expressing shRNA targeted against Syk (+) were treated without or with 5 mM H2O2 for 15 min. Nucleolin was immunoprecipitated, and the resulting immune complexes were probed by Western blotting for phosphotyrosine (top) or NCL (bottom). (F) Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD) was immunoprecipitated from the corresponding doxycycline-induced lines of MDA-MB-231 cells using GFP-nanotrap beads. The resulting immune complexes were incubated with buffer containing (+) or lacking (−) ATP. The immune complexes and whole-cell lysates were separated by SDS-PAGE and analyzed by Western blotting with antibodies against NCL. (G) Nucleolin was immunoprecipitated from Tet-responsive MDA-MB-231 cells either uninduced (−) or induced (+) with doxycycline to express Syk-EGFP and either treated with 5 mM H2O2 for 3 h or not treated. Immune complexes were examined for the presence of Bcl-xL mRNA by RT-PCR (top) and nucleolin by Western blotting (middle). The expression of Syk-EGFP was determined by Western blotting of whole-cell lysates with antibodies against Syk (bottom). (H) The relative amount of Bcl-xL mRNA associated with nucleolin, analyzed as described in the legend to panel G, was quantified. The data represent means ± SEMs from three replicate experiments. The level of mRNA bound to nucleolin in Syk-EGFP-expressing cells not treated with H2O2 was set equal to a value of 1.0.
To confirm further that the associated Syk could directly phosphorylate nucleolin, resulting in a shift in its electrophoretic mobility, we immunoprecipitated either Syk-EGFP or Syk-EGFP(K396R) from each Tet-induced MDA-MB-231 cell line along with the associated nucleolin and incubated the resulting immune complexes in vitro in a kinase reaction buffer containing ATP. The immune complexes were then analyzed by SDS-PAGE and Western blotting for the presence of nucleolin. Incubation with ATP induced a shift in the mobility of nucleolin in the immune complex containing active Syk-EGFP but not in the immune complex containing Syk-EGFP(K396R) (Fig. 5F). Together, these results indicate that Syk interacts with and phosphorylates nucleolin on tyrosine.
Nucleolin binds the Bcl-xL mRNA in a Syk-dependent manner.
Nucleolin has been reported to stabilize Bcl-xL mRNA through direct binding (37). To determine how Syk might influence such an interaction, we immunoprecipitated nucleolin from untreated or doxycycline-induced, Syk-EGFP-expressing MDA-MB-231 cells with antinucleolin antibodies, extracted RNA from the immune complexes, and analyzed it by semiquantitative PCR for the presence of the Bcl-xL mRNA. Interestingly, the association of nucleolin with the Bcl-xL mRNA was largely dependent on the expression of Syk-EGFP (Fig. 5G and H). Since the treatment of cells with H2O2 resulted in the phosphorylation of nucleolin on tyrosine, we examined the effect of H2O2 on the association of nucleolin with the Bcl-xL mRNA. The treatment of cells with H2O2 further enhanced the binding interaction between nucleolin and the Bcl-xL transcript selectively in Syk-expressing cells (Fig. 5G and H).
Nucleolin is required for the Syk-dependent stabilization of Bcl-xL mRNA.
To confirm that nucleolin plays a direct role in the ability of Syk to regulate Bcl-xL mRNA stability, we infected Tet-responsive, Syk-EGFP-expressing MDA-MB-231 cells with a series of lentiviruses coding shRNAs directed against nucleolin and selected populations of cells with a reduced level of the protein (Fig. 6A). These cells were then treated with H2O2 and examined for the levels of Bcl-xL mRNA. While the induced expression of Syk-EGFP protected Bcl-xL mRNA from H2O2-induced degradation, this effect was essentially abrogated in cells that had a reduced level of nucleolin (Fig. 6B). Similarly, the knockdown of nucleolin in DG75 B cells, which express endogenous Syk, increased their sensitivity to the oxidative stress-induced loss of Bcl-xL mRNA (Fig. 6C and D). The knockdown of nucleolin also reduced the ability of Syk-EGFP to protect Bcl-xL mRNA from H2O2-induced degradation in Tet-responsive MCF7 cells (Fig. 6E and F). Thus, the ability of Syk to stabilize Bcl-xL mRNA was dependent on a normal level of expression of nucleolin in all three cell types.
FIG 6.

Nucleolin is required for Syk-dependent stabilization of Bcl-xL mRNA. (A) Tet-responsive MDA-MB-231 cells were untreated (control [Ctrl]) or infected with one of a set of lentiviruses encoding shRNAs for nucleolin (shNCL). Nucleolin levels were measured by Western blotting. The level of GAPDH was measured as a loading control. Results from three different populations of infected cells are shown. (B) Tet-responsive MDA-MB-231 cells and two of the three sets of Tet-responsive cells carrying the nucleolin shRNA (shNCL2 and shNCL3) were either uninduced (−) or induced with doxycycline to express Syk-EGFP (+) and then treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA. (C) DG75 B cells were untreated (control) or infected with a lentivirus encoding shRNA directed against nucleolin. Nucleolin levels were measured by Western blotting. The level of GAPDH was measured as a loading control. (D) DG75 cells either infected (+) or not infected (−) with the lentivirus carrying the nucleolin shRNA were treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA. (E) Tet-responsive MCF7 cells were untreated (control) or infected with a set of lentiviruses encoding shRNAs for nucleolin. Nucleolin levels were measured by Western blotting. Results from two different populations of infected cells are shown. (F) Tet-responsive MCF7 cells and the two sets of Tet-responsive cells carrying the nucleolin shRNA (shNCL1 and shNCL2) were either uninduced (−) or induced with doxycycline to express Syk-EGFP (+) and then treated with 5 mM H2O2 for the indicated times. Cell lysates were analyzed by RT-PCR to measure the levels of Bcl-xL and Bcl-xS mRNA.
To determine if decreasing the level of nucleolin also increased the sensitivity of Syk-expressing cells to oxidative stress-induced apoptosis, we measured the cleavage of PARP in MCF7 cells in which the expression of Syk-EGFP was induced and the level of nucleolin was either normal or reduced by shRNA expression. The ability of expressed Syk-EGFP to inhibit H2O2-induced PARP cleavage was decreased by the knockdown of nucleolin (Fig. 7A). Syk-expressing cells with a reduced level of nucleolin also rounded up and lost cell-cell contacts in response to oxidative stress to a much greater extent than did cells with normal levels of the protein (see Fig. S1 in the supplemental material). A similar increased sensitivity to an apoptotic stimulus was seen for DG75 B cells, in which the knockdown of either Syk or nucleolin enhanced their susceptibility to PARP cleavage induced by exposure to H2O2 (Fig. 7B). For both cell types, the effects of Syk and nucleolin expression on PARP cleavage were observed in multiple biological replicates (Fig. 7E and F). Syk also protected cells from etoposide-induced cell death, as shown previously in MCF7 cells (41) and in MDA-MB-231 cells, as measured by the activation of caspase 3 (see Fig. S4 in the supplemental material).
FIG 7.
Nucleolin is required for Syk-dependent protection of cells from stress-induced apoptosis. (A) Tet-responsive MCF7 cells or Tet-responsive cells carrying the nucleolin shRNA were either uninduced or induced with doxycycline to express Syk-EGFP and then treated with 5 mM H2O2 for the indicated times. Cell lysates were separated by SDS-PAGE and probed with antibodies against PARP (top), nucleolin (middle), or Syk (bottom). The cleaved form of PARP is indicated by the arrow. (B) DG75 cells or DG75 cells expressing the shRNA targeting either Syk (Syk-shRNA) or nucleolin (NCL-shRNA) were treated with 5 mM H2O2 for the indicated times. Cell lysates were separated by SDS-PAGE and probed with antibodies against PARP (top), nucleolin (middle), or Syk (bottom). The cleaved form of PARP is indicated by the arrow. (C) Tet-responsive MCF7 cells or Tet-responsive cells carrying the nucleolin shRNA were either uninduced or induced with doxycycline to express Syk-EGFP and then treated with 1 μg/ml doxorubicin (Dox) for the indicated times. Cell lysates were separated by SDS-PAGE and probed with antibodies against PARP (top), nucleolin (middle), or Syk (bottom). The cleaved form of PARP is indicated by the arrow. (D) DG75 cells or DG75 cells expressing either the shRNA targeting Syk (Syk-shRNA) or nucleolin (NCL-shRNA) were treated with 1 μg/ml doxorubicin for the indicated times. Cell lysates were separated by SDS-PAGE and probed with antibodies against PARP (top), nucleolin (middle), or Syk (bottom). The cleaved form of PARP is indicated by the arrow. (E) The degree of PARP cleavage was quantified from Western blots of lysates of MCF7 cells lacking Syk (no Syk), expressing Syk-EGFP (SykGFP), or expressing Syk-EGFP and shRNA for nucleolin (shNCL) and treated for 24 h with 5 mM H2O2 (left) or 1 μg/ml doxorubicin (right). The data represent means ± SEMs from three replicate experiments. **, P < 0.005 (compared to Syk-EGFP-expressing cells); ***, P < 0.001 (compared to Syk-EGFP-expressing cells). (F) The degree of PARP cleavage was quantified from Western blots of lysates of DG75 cells expressing shRNA for Syk (shSyk), wild-type DG75 cells (WT), or wild-type cells expressing shRNA for nucleolin (shNCL) and treated for 24 h with 5 mM H2O2 (left) or 1 μg/ml doxorubicin (right). The data represent means ± SEMs from three replicate experiments. *, P < 0.05 (compared to wild-type cells); **, P < 0.005 (compared to wild-type cells); ***, P < 0.001 (compared to wild-type cells).
Nucleolin is required for Syk-dependent resistance to genotoxic stress.
We had shown previously that the expression of Syk in MCF7 cells protected them from apoptosis induced by exposure to the genotoxic agents doxorubicin and etoposide (41). To determine if nucleolin plays a role in the capacity of Syk to protect cells from genotoxic agents, we monitored the ability of induced Syk-EGFP to protect Syk-deficient MCF7 cells from doxorubicin-induced PARP cleavage in cells expressing either a normal or a reduced level of nucleolin. As shown in Fig. 7C, the ability of Syk to protect MCF7 cells from doxorubicin-induced apoptosis was attenuated in cells with a reduced level of nucleolin. This protective role for Syk was also seen in DG75 cells, in which the knockdown of either Syk or nucleolin by shRNA increased their susceptibility to the doxorubicin-induced cleavage of PARP (Fig. 7D). This effect was observed in multiple biological replicates (Fig. 7E). Thus, Syk stabilizes Bcl-xL mRNA against the actions of genotoxic agents in a manner also dependent on the expression of normal levels of nucleolin.
DISCUSSION
The Syk protein tyrosine kinase is best known for its roles in hematopoietic cells, where it is required for signaling through immune recognition receptors that contain immunoreceptor tyrosine-based activation motifs (ITAMs) (1, 2). Examples include the antigen receptor on B cells (BCR), the high-affinity IgE receptor of mast cells, and IgG receptors on neutrophils and macrophages. Receptor clustering leads to the phosphorylation of a pair of ITAM tyrosines, creating a high-affinity docking site for the tandem pair of Syk SH2 domains. The binding of Syk to the receptor activates the enzyme, which becomes phosphorylated on tyrosine and functions as both a kinase and a scaffold to couple the receptor to downstream effectors of multiple signaling pathways. In certain malignancies of B-cell origin, it is tonic signaling from the BCR that is proposed to activate Syk to promote cell survival (4, 7, 8). Interestingly, the repertoire of cells in which Syk functions as a prosurvival factor extends to tumors of B-cell origin that have not yet rearranged immunoglobulin genes, hematological malignancies not of B-cell origin, and nonhematological cancers, such as retinoblastoma and certain carcinomas of the lung and pancreas (12–22). Whether ITAM-bearing receptors other than the BCR are involved in sending tonic signals via Syk in these cell types is as yet unclear.
In some cancer cell types, including AML, retinoblastoma, and Ras-addicted lung and pancreatic carcinomas, cell survival is dependent on the expression of Syk, such that inhibitors of the kinase alone are sufficient to induce cell death (13, 17, 18). It is clear, however, that Syk is not essential for the survival of all cancer cells. In fact, Syk is frequently absent from certain tumor types, such as highly metastatic breast cancer, hepatocellular carcinoma, and melanoma, as a result of gene silencing by promoter methylation (30, 42–48). The exogenous expression of Syk in such cells decreases cellular motility, invasion, and metastasis (but does not induce apoptosis [30]). In all cell types examined in our study, a nonaggressive breast carcinoma, a B-cell lymphoma, naturally occurring DLBCL, and a highly aggressive breast carcinoma that does not normally express the kinase, Syk functions as a prosurvival factor. This is true even for MDA-MB-231 breast cancer cells, in which the expression of Syk is silenced and in which Syk, when exogenously expressed, has been described to be a tumor suppressor (30). Syk is unlikely to be the driver of transformation in all of the cancer cell types in which it is expressed but instead offers protection from stress-induced apoptosis regardless of the molecular mechanism by which the cells became transformed. In cells lacking Syk, it is likely that other antiapoptotic proteins function to protect the cells from external stress. For example, the MCF7-BD cells that lack Syk express much higher levels of Bcl-2 than do MCF7 cells that express endogenous Syk (41). In addition to protecting cells from oxidative stress, Syk also protects cells from certain inducers of genotoxic stress (41). Thus, the expression of Syk in a tumor cell could lead to drug resistance. These results indicate that Syk inhibitors might be useful in combination with genotoxic agents for the treatment of cancers in which the kinase is expressed.
The enhanced expression of one or more members of the Bcl-2 family of antiapoptotic proteins is a frequent occurrence in malignant cells (49, 50). Our studies indicate that Syk functions as a prosurvival factor, at least in part, by stabilizing the mRNA for Bcl-xL. The longer Bcl-xL transcript has a decreased half-life compared to that of the shorter transcript that encodes Bcl-xS due to the presence of AREs located in the 3′ UTR. It is here that nucleolin binds to stabilize the message. This was first demonstrated in HeLa cells, in which the downregulation of nucleolin was shown to shorten the half-life of the Bcl-xL message, while overexpression lengthened the half-life of the Bcl-xL message (37). In this study, nucleolin was shown to bind to an ARE on the Bcl-xL transcript in conjunction with poly(A) binding protein (PABP). Interestingly, in a previous mass spectrometric analysis of Syk-binding proteins, we found that both nucleolin and PABP coimmunoprecipitated with Syk, suggesting that all three proteins may be part of the same ribonucleoprotein complex (35). Nucleolin also modulates Bcl-xL expression in primary lung endothelial cells (51). In these cells, treatment with angiotensin II induces apoptosis through the activation of the tyrosine phosphatase SHP-2, which inhibits the interaction of nucleolin with the Bcl-xL mRNA. Thus, a tyrosine kinase (Syk) and a tyrosine phosphatase (SHP-2) have opposing effects on the ability of nucleolin to stabilize the Bcl-xL transcript. This suggests the existence of one or more critical substrates that modulate the turnover of Bcl-xL mRNA, whose phosphorylation on tyrosine is regulated in a positive or negative fashion by Syk or SHP-2, respectively. It was suggested that nucleolin itself might be the SHP-2 target. Our studies indicate that nucleolin is, in fact, phosphorylated on tyrosine by Syk in a manner that correlates with its enhanced ability to bind the Bcl-xL mRNA and protect it from degradation.
We propose a model by which Syk associates with nucleolin, which is an intriguingly pleiotropic molecule with functions ranging from ribosome biogenesis to chromatin remodeling, transcriptional regulation, and binding to lipoproteins at the cell surface (52, 53). This Syk-nucleolin association likely occurs prior to any activation of Syk or phosphorylation of either component of the complex since catalytically inactive Syk binds robustly. Treatment of cells with hydrogen peroxide causes dissociation of the Syk-nucleolin complex. Site-directed mutagenesis studies indicate that the dissociation of Syk from nucleolin occurs as a consequence of its phosphorylation on tyrosines 342 and 346, which are located within the linker B region of the kinase. We did not observe an ATP-dependent dissociation of nucleolin from Syk-EGFP in anti-EGFP immune complexes. Thus, it is likely that the phosphorylation of both Y342 and Y346 on Syk within an intact cell generates a high-affinity docking site for a protein or proteins that contain an SH2 domain that binds to this region to disrupt the interaction between Syk and nucleolin. This hypothesis is consistent with the fact that several SH2 domains found on Syk-interacting proteins contain two phosphotyrosine-binding pockets and these interact with Syk preferentially when both Y342 and Y346 are phosphorylated (54, 55).
Active Syk phosphorylates nucleolin on tyrosine to promote its binding to the Bcl-xL mRNA. This interaction protects cells from the loss of Bcl-xL resulting from exposure to inducers of oxidative or genotoxic stress. It is of interest to note that both Syk and Bcl-xL are important prosurvival factors in pre-B cells that have undergone gene rearrangements necessary for expression of a pre-B-cell antigen receptor, an event essential for B-cell survival in vivo (56, 57). Bcl-xL is also elevated in germinal center B cells undergoing class switching and somatic hypermutation (58), conditions under which DNA strand breaks are likely to occur (and which are events also induced in cells treated with doxorubicin or etoposide). Thus, the prosurvival role of Syk in cancer is perhaps an unintended manifestation of one of its normal functions in B-cell development and is exploited by the cancer cell to escape from signals that would otherwise lead to cell death.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Services grant AI098132 from the National Institute of Allergy and Infectious Diseases. The DNA sequencing facility was supported by NCI CCSG CA23168 to the Purdue University Center for Cancer Research.
Footnotes
Published ahead of print 4 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00937-14.
REFERENCES
- 1.Geahlen RL. 2009. Syk and pTyr'd: signaling through the B cell antigen receptor. Biochim. Biophys. Acta 1793:1115–1127. 10.1016/j.bbamcr.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mócsai A, Ruland J, Tybulewicz VL. 2010. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10:387–402. 10.1038/nri2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinaldi A, Kwee I, Taborelli M, Largo C, Uccella S, Martin V, Poretti G, Gaidano G, Calabrese G, Martinelli G, Baldini L, Pruneri G, Capella C, Zucca E, Cotter FE, Cigudosa JC, Catapano CV, Tibiletti MG, Bertoni F. 2006. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. Br. J. Haematol. 132:303–316. 10.1111/j.1365-2141.2005.05883.x [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE, Habermann TM, Kutok JL, Shipp MA. 2008. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111:2230–2237. 10.1182/blood-2007-07-100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young RM, Hardy IR, Clarke RL, Lundy N, Pine P, Turner BC, Potter TA, Refaeli Y. 2009. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood 113:2508–2516. 10.1182/blood-2008-05-158618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholomé K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG, Jumaa H, Veelken H, Dierks C, Zirlik K. 2009. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 69:5424–5432. 10.1158/0008-5472.CAN-08-4252 [DOI] [PubMed] [Google Scholar]
- 7.Baudot AD, Jeandel PY, Mouska X, Maurer U, Tartare-Deckert S, Raynaud SD, Cassuto JP, Ticchioni M, Deckert M. 2009. The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKCδ and proteasome-dependent regulation of Mcl-1 expression. Oncogene 28:3261–3273. 10.1038/onc.2009.179 [DOI] [PubMed] [Google Scholar]
- 8.Gobessi S, Laurenti L, Longo PG, Carsetti L, Bemo V, Sica S, Leone G, Effremov DG. 2009. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 23:686–697. 10.1038/leu.2008.346 [DOI] [PubMed] [Google Scholar]
- 9.Uckun FM, Ek RO, Jan ST, Chen CL, Qazi S. 2010. Targeting SYK kinase-dependent anti-apoptotic resistance pathway in B-lineage acute lymphoblastic leukaemia (ALL) cells with a potent SYK inhibitory pentapeptide mimic. Br. J. Haematol. 149:508–517. 10.1111/j.1365-2141.2010.08106.x [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lu P, Pandey A, Melnick AM, Sinha U, Wang YL. 2011. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood 118:6342–6352. 10.1182/blood-2011-02-333773 [DOI] [PubMed] [Google Scholar]
- 11.Hatton O, Lambert SL, Phillips LK, Vaysberg M, Natkunam Y, Esquivel CO, Krams SM, Martinez OM. 2013. Syk-induced phosphatidylinositol-3-kinase activation in Epstein-Barr virus posttransplant lymphoproliferative disorder. Am. J. Transplant. 13:883–890. 10.1111/ajt.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaian L, Ball ED. 2006. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia 20:2093–2101. 10.1038/sj.leu.2404437 [DOI] [PubMed] [Google Scholar]
- 13.Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC, Ross L, Galinsky I, Davis TN, Silver SJ, Root DE, Stone RM, DeAngelo DJ, Carroll M, Hahn WC, Carr SA, Golub TR, Kung AL, Stegmaier K. 2009. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell 16:281–294. 10.1016/j.ccr.2009.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuno Y, Abe A, Emi N, Iida M, Yokozawa T, Towatari M, Tanimoto M, Saito H. 2001. Constitutive kinase activation of the TEL-Syk fusion gene in myelodysplastic syndrome with t(9;12)(q22;p12). Blood 97:1050–1055. 10.1182/blood.V97.4.1050 [DOI] [PubMed] [Google Scholar]
- 15.Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. 2006. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20:313–318. 10.1038/sj.leu.2404045 [DOI] [PubMed] [Google Scholar]
- 16.Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, Fink SR, Vrana JA, Caron BL, Morice WG, Remstein ED, Grogg KL, Kurtin PJ, Macon WR, Dogan A. 2008. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia 22:1139–1143. 10.1038/leu.2008.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. 2009. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell 15:489–500. 10.1016/j.ccr.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X, Ulyanov A, Wu G, Wilson M, Wang J, Brennan R, Rusch M, Manning AL, Ma J, Easton J, Shurtleff S, Mullighan C, Pounds S, Mukatira S, Gupta P, Neale G, Zhao D, Lu C, Fulton RS, Fulton LL, Hong X, Dooling DJ, Ochoa K, Naeve C, Dyson NJ, Mardis ER, Bahrami A, Ellison D, Wilson RK, Downing JR, Dyer MA. 2012. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481:329–334. 10.1038/nature10733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinos P, Garneau D, Lucier J-F, Gendron D, Couture S, Boivin M, Brosseau J-P, Lapointe E, Thibault P, Durand M, Tremblay K, Gervais-Bird J, Nwilati H, Klinck R, Chabot B, Perreault J-P, Wellinger RJ, Elela SA. 2011. Alternative splicing of SYK regulates mitosis and cell survival. Nat. Struct. Mol. Biol. 18:673–680. 10.1038/nsmb.2040 [DOI] [PubMed] [Google Scholar]
- 20.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, LaCasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. 2010. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115:2578–2585. 10.1182/blood-2009-08-236471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman SEM, Barr PM, McAuley EM, Liu D, Wiestner A, Friedberg JW. 2013. Fostamatinib inhibits B-cell receptor signaling, cellular activation and tumor proliferation in patients with relapsed and refractory chronic lymphocytic leukemia. Leukemia 27:1769–1773. 10.1038/leu.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geahlen RL. 2014. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol. Sci. 35:414–422. 10.1016/j.tips.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144:646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 24.Youle RJ, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9:47–59. 10.1038/nrm2308 [DOI] [PubMed] [Google Scholar]
- 25.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. 1993. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608. 10.1016/0092-8674(93)90508-N [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Shrikhande U, Alicie BM, Zhou Q, Geahlen RL. 2009. Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol. Cancer Res. 7:634–644. 10.1158/1541-7786.MCR-08-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. 1995. Bcl-XL and Bcl-2 repress a common pathway of cell death. J. Exp. Med. 182:821–828. 10.1084/jem.182.3.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Geahlen RL. 2009. The protein-tyrosine kinase Syk interacts with TRAF-interacting protein TRIP in breast epithelial cells. Oncogene 28:1348–1356. 10.1038/onc.2008.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos-Vara JA, Miller MA, Valli VE. 2007. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet. Pathol. 44:875–884. 10.1354/vp.44-6-875 [DOI] [PubMed] [Google Scholar]
- 30.Coopman PJP, Do MTH, Barth M, Bowden ET, Hayes AJ, Basyuk E, Blancato JK, Vezza PR, McLeskey SW, Mangeat PH, Mueller SC. 2000. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 406:742–747. 10.1038/35021086 [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. 1993. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53:3976–3985 [PubMed] [Google Scholar]
- 32.Olapade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB, Recant WM. 1997. Overexpression of Bcl-x protein in primary breast cancer is associated with high grade and nodal metastases. Cancer J. Sci. Am. 3:230–237 [PubMed] [Google Scholar]
- 33.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. 2006. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer 6:213. 10.1186/1471-2407-6-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito D, Frantz AM, Williams C, Thomas R, Burnett RC, Avery AC, Breen M, Mason NJ, O'Brien TD, Modiano JF. 2012. CD40 ligand is necessary and sufficient to support primary diffuse large B-cell lymphoma cells in culture: a tool for in vitro preclinical studies with primary B-cell malignancies. Leuk. Lymphoma 53:1390–1398. 10.3109/10428194.2011.654337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galan JA, Paris LL, Zhang H-J, Adler J, Geahlen RL, Tao WA. 2011. Proteomic studies of Syk-interacting proteins using a novel amine-specific isotope tag and GFP nanotrap. J. Am. Soc. Mass Spectrom. 22:319–328. 10.1007/s13361-010-0030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachelor MA, Bowden GT. 2004. Ultraviolet A-induced modulation of Bcl-XL by p38 MAPK in human keratinocytes. Post-transcriptional regulation through the 3′-untranslated region. J. Biol. Chem. 279:42658–42668. 10.1074/jbc.M406626200 [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Tsaprailis G, Bowden GT. 2008. Nucleolin stabilizes Bcl-XL messenger RNA in response to UVA irradiation. Cancer Res. 68:1046–1054. 10.1158/0008-5472.CAN-07-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schieven GL, Kirihara JM, Burg DL, Geahlen RL, Ledbetter JA. 1993. p72syk tyrosine kinase is activated by oxidizing conditions that induce lymphocyte tyrosine phosphorylation and Ca2+ signals. J. Biol. Chem. 268:16688–16692 [PubMed] [Google Scholar]
- 39.Xue L, Wang W-H, Iliuk A, Hu L, Galan JA, Yu S, Hans M, Geahlen RL, Tao WA. 2012. Sensitive kinase assay linked with phosphoproteomics for identifying direct kinase substrates. Proc. Natl. Acad. Sci. U. S. A. 109:5615–5620. 10.1073/pnas.1119418109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshvara LM, Isaacson C, Yankee TM, Sarac R, Harrison ML, Geahlen RL. 1998. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B-cell activation includes a site that negatively regulates signaling. J. Immunol. 161:5276–5283 [PubMed] [Google Scholar]
- 41.Yu S, Huang H, Iliuk A, Wang W-H, Jayasundera KB, Tao WA, Post CB, Geahlen RL. 2013. Syk inhibits the activity of protein kinase A by phosphorylating tyrosine 330 of the catalytic subunit. J. Biol. Chem. 288:10870–10881. 10.1074/jbc.M112.426130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minobe K, Onda M, Iida A, Kasumi F, Sakamoto G, Nakamura Y, Emi M. 1998. Allelic loss on chromosome 9q is associated with lymph node metastasis of primary breast cancer. Jpn. J. Cancer Res. 89:916–922. 10.1111/j.1349-7006.1998.tb00649.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y, Mendez R, Sahin A, Dai JL. 2001. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res. 61:5558–5561 [PubMed] [Google Scholar]
- 44.Moroni M, Soldatenkov V, Zhang L, Zhang Y, Stoica G, Gehan E, Rashidi B, Singh B, Ozdemirli M, Mueller SC. 2004. Progressive loss of Syk and abnormal proliferation in breast cancer cells. Cancer Res. 64:7346–7354. 10.1158/0008-5472.CAN-03-3520 [DOI] [PubMed] [Google Scholar]
- 45.Hoeller C, Thallinger C, Pratscher B, Bister MD, Schicher N, Loewe R, Heere-Ress E, Roka F, Sexl V, Pehamberger H. 2005. The non-receptor-associated tyrosine kinase Syk is a regulator of metastatic behavior in human melanoma cells. J. Investig. Dermatol. 124:1293–1299. 10.1111/j.0022-202X.2005.23685.x [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y, Wang J, Li J, Wang L, Li M, Yang Z, Zhang C, Dai JL. 2006. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin. Cancer Res. 12:6687–6695. 10.1158/1078-0432.CCR-06-0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthusamy V, Duraisamy S, Bradbury CM, Hobbs C, Curley DP, Nelson B, Bosenberg M. 2006. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 66:11187–11193. 10.1158/0008-5472.CAN-06-1274 [DOI] [PubMed] [Google Scholar]
- 48.Layton T, Stalens C, Gunderson F, Goodison S, Silletti S. 2009. Syk tyrosine kinase acts as a pancreatic adenocarcinoma tumor suppressor by regulating cellular growth and invasion. Am. J. Pathol. 175:2625–2636. 10.2353/ajpath.2009.090543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip KW, Reed JC. 2008. Bcl-2 family proteins and cancer. Oncogene 27:6398–6406. 10.1038/onc.2008.307 [DOI] [PubMed] [Google Scholar]
- 50.Kelly PN, Strasser A. 2011. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 18:1414–1424. 10.1038/cdd.2011.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YH, Mungunsukh O, Tutino RL, Marquez AP, Day RM. 2010. Angiotensin-II-induced apoptosis requires regulation of nucleolin and Bcl-xL by SHP-2 in primary lung endothelial cells. J. Cell Sci. 123:1634–1643. 10.1242/jcs.063545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mongelard F, Bouvet P. 2007. Nucleolin: a multifaceted protein. Trends Cell Biol. 17:80–88. 10.1016/j.tcb.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 53.Srivastava M, Pollard HB. 1999. Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 13:1911–1922 [PubMed] [Google Scholar]
- 54.Groesch TD, Zhou F, Matilla S, Geahlen RL, Post CB. 2006. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J. Mol. Biol. 356:1222–1236. 10.1016/j.jmb.2005.11.095 [DOI] [PubMed] [Google Scholar]
- 55.Chen C-H, Martin VA, Gorenstein NM, Geahlen RL, Post CB. 2011. Two closely-spaced tyrosines regulate NFAT signaling in B cells via Syk association with Vav. Mol. Cell. Biol. 31:2984–2996. 10.1128/MCB.05043-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VLJ. 1995. Perinatal lethality and a block in the development of B cells in mice lacking the tyrosine kinase p72syk. Nature 378:298–302. 10.1038/378298a0 [DOI] [PubMed] [Google Scholar]
- 57.Grillot DAM, Merino R, Pena JC, Fanslow WC, Finkelman FD, Thompson CB, Núñez G. 1996. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J. Exp. Med. 183:381–391. 10.1084/jem.183.2.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuscano JM, Druey KM, Riva A, Pena J, Thompson CB, Kehrl JH. 1996. Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood 88:1359–1364 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.